Published online Feb 27, 2023. doi: 10.4240/wjgs.v15.i2.211
Peer-review started: September 15, 2022
First decision: December 12, 2022
Revised: December 14, 2022
Accepted: December 31, 2022
Article in press: December 31, 2022
Published online: February 27, 2023
Processing time: 164 Days and 19.9 Hours
Remnant gastric cancer (GC) is defined as GC that occurs five years or more after gastrectomy. Systematically evaluating the preoperative immune and nutritional status of patients and analyzing its prognostic impact on postoperative remnant gastric cancer (RGC) patients are crucial. A simple scoring system that combines multiple immune or nutritional indicators to identify nutritional or immune status before surgery is necessary.
To evaluate the value of preoperative immune-nutritional scoring systems in predicting the prognosis of patients with RGC.
The clinical data of 54 patients with RGC were collected and analyzed retro
The median age of this cohort was 70.5 years (ranging from 39 to 87 years). No significant correlation was found between most pathological features and immune-nutritional status (P > 0.05). Patients with a PNI score < 45, CONUT score or NPS score ≥ 3 were considered to be at high immune-nutritional risk. The areas under the receiver operating characteristic curves of PNI, CONUT, and NPS systems for predicting postoperative survival were 0.611 [95% confidence interval (CI): 0.460–0.763; P = 0.161], 0.635 (95%CI: 0.485–0.784; P = 0.090), and 0.707 (95%CI: 0.566–0.848; P = 0.009), respectively. Cox regression analysis showed that the three immune-nutritional scoring systems were significantly correlated with OS (PNI: P = 0.002; CONUT: P = 0.039; NPS: P < 0.001). Survival analysis revealed a significant difference in OS between different immune-nutritional groups (PNI: 75 mo vs 42 mo, P = 0.001; CONUT: 69 mo vs 48 mo, P = 0.033; NPS: 77 mo vs 40 mo, P < 0.001).
These preoperative immune-nutritional scores are reliable multidimensional prognostic scoring systems for predicting the prognosis of patients with RGC, in which the NPS system has relatively effective predictive performance.
Core Tip: Three preoperative immune-nutritional scores of patients with remnant gastric cancer (RGC) were calculated, including prognostic nutritional index (PNI), controlled nutritional status (CONUT), and Naples prognostic score (NPS). Patients were divided into groups according to the immune-nutritional risk. The three immune-nutritional scoring systems were significantly correlated with overall survival (OS) (PNI: P = 0.002; CONUT: P = 0.039; NPS: P < 0.001). Survival analysis revealed a significant difference in OS between different immune-nutritional groups (PNI: 75 mo vs 42 mo, P = 0.001; CONUT: 69 mo vs 48 mo, P = 0.033; NPS: 77 mo vs 40 mo, P < 0.001). These preoperative immune-nutritional scores are reliable multidimensional RGC prognostic scoring systems.
- Citation: Zhang Y, Wang LJ, Li QY, Yuan Z, Zhang DC, Xu H, Yang L, Gu XH, Xu ZK. Prognostic value of preoperative immune-nutritional scoring systems in remnant gastric cancer patients undergoing surgery. World J Gastrointest Surg 2023; 15(2): 211-221
- URL: https://www.wjgnet.com/1948-9366/full/v15/i2/211.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i2.211
The incidence rate of gastric cancer (GC) ranks fifth among all malignancies, with an annual incidence of more than one million people. Thus far, GC is still one of the diseases that seriously affect the health system[1]. Many high-risk factors contribute to the occurrence of GC, one of which is the remnant stomach after gastrectomy[2]. Remnant GC (RGC) is defined as GC that occurs five years or more after gastrectomy due to benign or malignant lesions. Reports have shown that approximately 2%–3% of remnant stomachs will develop RGC[3,4]. The mechanism of the occurrence and development of RGC remains unclear. Bile reflux, the loss of vagus nerve, and the change in gastric mucosal microenvironment may play important roles in RGC carcinogenesis[5-7].
The treatment of RGC is often comprehensively based on surgery[8,9]. However, the prognosis of RGC is often worse than that of primary GC even after radical gastrectomy[10,11]. Notably, patients often have malnutrition and poor immune status[12,13] after gastrectomy, which may be one of the factors leading to the poor prognosis of RGC patients. Therefore, systematically evaluating the preoperative immune and nutritional status of patients and analyzing its prognostic impact on postoperative RGC patients are crucial.
An increasing number of studies have shown that the immune system plays a crucial role in the tumor microenvironment[14-16]. Meanwhile, the nutritional status of patients often affects tumor growth, metastasis, angiogenesis, and the efficacy of antitumor therapy[17,18]. Prognostic factors related to inflammation and nutrition, including neutrophil to lymphocyte ratio (NLR), platelet-to-lymphocyte ratio, lymphocyte to monocyte ratio (LMR), serum albumin (ALB), and total cholesterol (TC), are associated with the prognosis of a variety of cancers, including GC, rectal cancer, and breast cancer[19-23].
However, prognostic prediction based on a single marker is often inaccurate and may even be misleading. Therefore, a simple scoring system that combines multiple immune or nutritional indicators to identify nutritional or immune status before surgery is significantly better than single inflammatory or nutritional markers. The prognostic nutritional index (PNI), controlled nutritional status (CONUT), and a new inflammation related prognostic system named Naples prognostic score (NPS) established in recent years by combining preoperative TC content, serum ALB content, LMR, and NLR have been widely used to predict the prognosis of a variety of tumors[24-26]. However, studies on the prognosis of RGC patients predicted by preoperative immune-nutritional score systems are few.
Therefore, this retrospective cohort study aimed to determine the prognostic value of three preoperative immune-nutritional scoring systems, namely, PNI, CONUT, and NPS, in patients with RGC, and to examine their relationship with other clinicopathological features.
The medical data of 43 patients with RGC at the First Affiliated Hospital of Nanjing Medical University (Jiangsu Province Hospital) and 11 patients at the Affiliated Suzhou Hospital of Nanjing Medical University (Suzhou Municipal Hospital) from January 2009 to July 2019 were collected. The inclusion criteria of this study were as follows: (1) The patient had a previous history of gastrectomy; (2) The interval from the occurrence of residual GC was five years or more; (3) After admission, the patient underwent radical resection of residual GC; (4) The postoperative pathological diagnosis was gastric adenocarcinoma; (5) The patient did not receive any anticancer treatment from the diagnosis of RGC to surgery; (6) The patient had detailed and extractable medical data and laboratory results; and (7) The patient had survival follow-up data of three years or more. By contrast, participants who met any of the following criteria were excluded from the final analysis: (1) The patient had any clinical evidence of infection or inflammatory disease. Infection was defined in this study as preoperative body temperature ≥ 37.5 °C or increased preoperative C-reactive-protein levels; and (2) The patient had a history of malignant tumors other than GC. This study was approved by the medical ethics committee of Nanjing Medical University. The data were anonymous; therefore, relevant informed consent was not required.
PNI score is defined as serum ALB (g/L) + 5 × Lymphocyte count (× 109); a PNI score < 45 indicated that the patient had immune-nutritional risk.
The CONUT score is defined as the sum of the three scores based on serum ALB concentration, lymphocyte count, and TC concentration. A score ≥ 3 was considered to be at immune-nutritional risk. Serum ALB concentration was grouped as > 35, 30–34.9, 25–29.9, and < 25 (g/L), and the scores of the four groups were 0, 2, 4, and 6, respectively. Lymphocyte count was grouped as ≥ 1.6, 1.2–1.5, 0.8–1.1, and < 0.8 (× 109), respectively, which had scores of 0, 1, 2, and 3, respectively. TC concentration was divided into groups ≥ 180, 140–179, 100–139, and < 100 mg/dL, with scores of 0, 1, 2, and 3, respectively.
NPS is defined on the basis of the following four parameters: Serum ALB, TC, LMR, and NLR. NLR and LMR are calculated by dividing the neutrophil count by lymphocyte and monocyte counts in routine blood tests, respectively. Patients with serum ALB lower than 40 g/L, TC lower than 180 mg/dL, LMR lower than 4.44, or NLR higher than 2.96 will obtain 1 point; otherwise, it will be regarded as 0. The sum of the scores of the four parameters is an NPS score. Patients with an NPS score of 0 were considered to have non-immune-nutritional risks, those with an NPS of 1 or 2 were regarded have mild immune-nutritional risks, and patients with an NPS of 3 or 4 were considered to have severe immune-nutritional risks. In the actual grouping, patients with an NPS score of 0 (6/54) are few due to the generally poor nutritional status of patients with RGC; however, this score cannot be analyzed alone. Therefore, patients with an NPS score of 0–2 (no or mild immune nutritional risk) were regarded as one group.
The clinical characteristics and pathological parameters of the patients, including gender, age, histological type, pathological stage, and laboratory data, were retrospectively collected from the hospital information system. Among them, the data of neutrophils, lymphocytes, and monocytes were from routine blood tests, the levels of serum ALB and TC were respectively from liver and kidney function tests, and all blood samples were fasting blood samples. All patients were followed up regularly after radical gastrectomy. This study mainly obtained the survival information of patients through postoperative medical examination or telephone contact. The follow-up interval was once every 6 mo. The patient was followed up to death (event) or the last follow-up (censored).
IBM SPSS statistics 23.0 (SPSS, Inc., Chicago, Illinois, United States) and GraphPad prism software (version 5.0) were used for statistical analyses and mapping, respectively. T-test or chi-square test was used to analyze the differences in statistical data between various groups. The receiver operating characteristic (ROC) curve was generated to evaluate the difference in survival prediction capability between different scoring systems. The Kaplan–Meier method was used for survival analysis, and log-rank test was employed to compare the difference in prognosis between various immune-nutritional system groups. P < 0.05 was considered statistically significant.
According to the inclusion and exclusion criteria, a total of 54 patients with RGC were included in this study. Among these patients, 42 were male (77.8%) and 12 were female (22.2%), and the median age was 70.5 years (ranging from 39 to 87 years). A total of 28 patients (51.9%) and 26 patients (48.1%) had better or worse histological differentiation types, respectively. In addition, 26 patients had lymph node metastasis, accounting for 48.1% of all patients. Table 1 describes the potential different immune-nutritional system scoring groups among populations with different clinical characteristics. The results show that no significant correlation existed between most pathological features and immune-nutritional status in patients with RGC.
| Characteristics | Immune–nutritional score systems | ||||||
| Values (n = 54) | PNI (n = 27/27) | P value | CONUT (n = 34/20) | P value | NPS (n = 32/22) | P value | |
| Age (years) | 1.000 | 0.368 | 0.047 | ||||
| < 70 | 26 | 13/13 | 18/8 | 19/7 | |||
| ≥ 70 | 28 | 14/14 | 16/12 | 13/15 | |||
| Gender | 1.000 | 0.713 | 0.216 | ||||
| Male | 42 | 21/21 | 27/15 | 23/19 | |||
| Female | 12 | 6/6 | 7/5 | 9/3 | |||
| Primary tumor size | 0.790 | 0.581 | 1.000 | ||||
| ≤ 3 cm | 27 | 13/14 | 18/9 | 16/11 | |||
| > 3 cm | 27 | 14/13 | 16/11 | 16/11 | |||
| Gross type | 0.412 | 0.313 | 0.386 | ||||
| Non-ulcerative type | 21 | 12/9 | 15/6 | 14/7 | |||
| Ulcerative type | 33 | 15/18 | 19/14 | 18/15 | |||
| Differentiation | 0.285 | 0.449 | 0.189 | ||||
| Well/Moderate | 28 | 16/12 | 19/9 | 19/9 | |||
| Poor | 26 | 11/15 | 15/11 | 13/13 | |||
| T stage | 0.588 | 0.519 | 0.984 | ||||
| T1/T2 | 22 | 12/10 | 15/7 | 13/9 | |||
| T3/T4 | 32 | 15/17 | 19/13 | 19/13 | |||
| Lymph node metastasis | 0.106 | 0.449 | 0.445 | ||||
| No | 28 | 17/11 | 19/9 | 18/10 | |||
| Yes | 26 | 10/16 | 15/11 | 14/12 | |||
| cTNM status | 0.282 | 0.538 | 0.075 | ||||
| I/II | 30 | 17/13 | 20/10 | 21/9 | |||
| III | 24 | 10/14 | 14/10 | 11/13 | |||
Fasting blood indicators of the patients were used to calculate the immune-nutritional scores. Among these indicators, the average level of serum ALB was 38.75 g/L [95% confidence interval (CI): 35.98–38.93 g/L], TC was 168 mg/dL (95%CI: 157–179 mg/dL), and the average lymphocyte count was 1.30 × 109/L (95%CI: 1.16–1.44 × 109/L); the average monocyte count was 0.40 × 109/L (95%CI: 0.35–0.44 × 109/L), the average neutrophil count was 3.61 × 109/L (95%CI: 3.04–4.17 × 109/L), the calculated NLR was 3.22 (95%CI: 2.45–4.00), and the LMR was 3.71 (95%CI: 2.21–4.21).
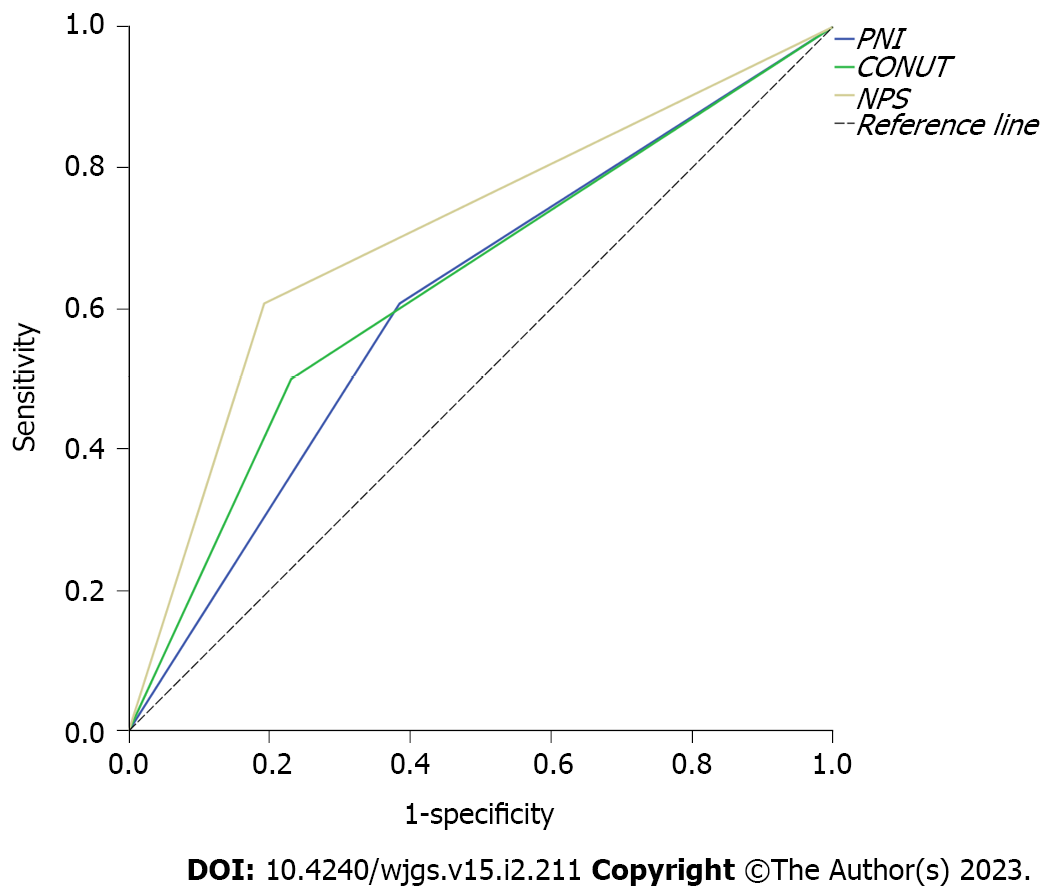
The curves of the three immune-nutritional scores for predicting postoperative survival were plotted (Figure 1). NPS was found to have the largest area under the curve (AUC = 0.707; 95%CI: 0.566–0.848; P = 0.009). The AUC values of PNI and CONUT were 0.611 (95%CI: 0.460–0.763; P = 0.161) and 0.635 (95%CI: 0.485–0.784; P = 0.090), respectively.
Cox regression analysis showed that tumor gross classification (P = 0.014), pathological differentiation type (P = 0.032), lymph node metastasis (P < 0.001), clinical Tumor-Node-Metastasis status (P = 0.001), and three immune-nutritional scoring systems, namely, PNI (P = 0.002), CONUT (P = 0.039), and NPS (P < 0.001), were significantly correlated with OS. In addition, no significant correlation was found between age, sex, primary tumor size, and T stage and OS (P > 0.05 for all) (Table 2).
| Characteristic | OS | ||
| HR | 95%CI | P value | |
| Age (years) | 0.500 | ||
| < 70 | 1.000 | 0.605-2.796 | |
| ≥ 70 | 1.301 | ||
| Gender | 0.451 | ||
| Male | 1.000 | 0.261-1.1.816 | |
| Female | 0.689 | ||
| Primary tumor size | 0.926 | ||
| ≤ 3 cm | 1.000 | 0.479-2.242 | |
| > 3 cm | 1.037 | ||
| Gross type | 0.014 | ||
| Non-ulcerative type | 1.000 | 1.285-8.932 | |
| Ulcerative type | 3.388 | ||
| Differentiation | 0.032 | ||
| Well/Moderate | 1.000 | 1.083-5.629 | |
| Poor | 2.469 | ||
| T stage | 0.289 | ||
| T1/T2 | 1.000 | 0.671-3.823 | |
| T3/T4 | 1.601 | ||
| Lymph node metastasis | <0.001 | ||
| No | 1.000 | 1.963-10.472 | |
| Yes | 4.534 | ||
| cTNM stage | 0.001 | ||
| I/II | 1.000 | 1.734-9.117 | |
| III | 3.976 | ||
| PNI group | 0.002 | ||
| Low risk | 1.000 | 1.724-10.030 | |
| High risk | 4.158 | ||
| CONUT group | 0.039 | ||
| Low risk | 1.000 | 1.041-4.666 | |
| High risk | 2.204 | ||
| NPS group | < 0.001 | ||
| Low risk | 1.000 | 1.879-9.425 | |
| High risk | 4.208 | ||
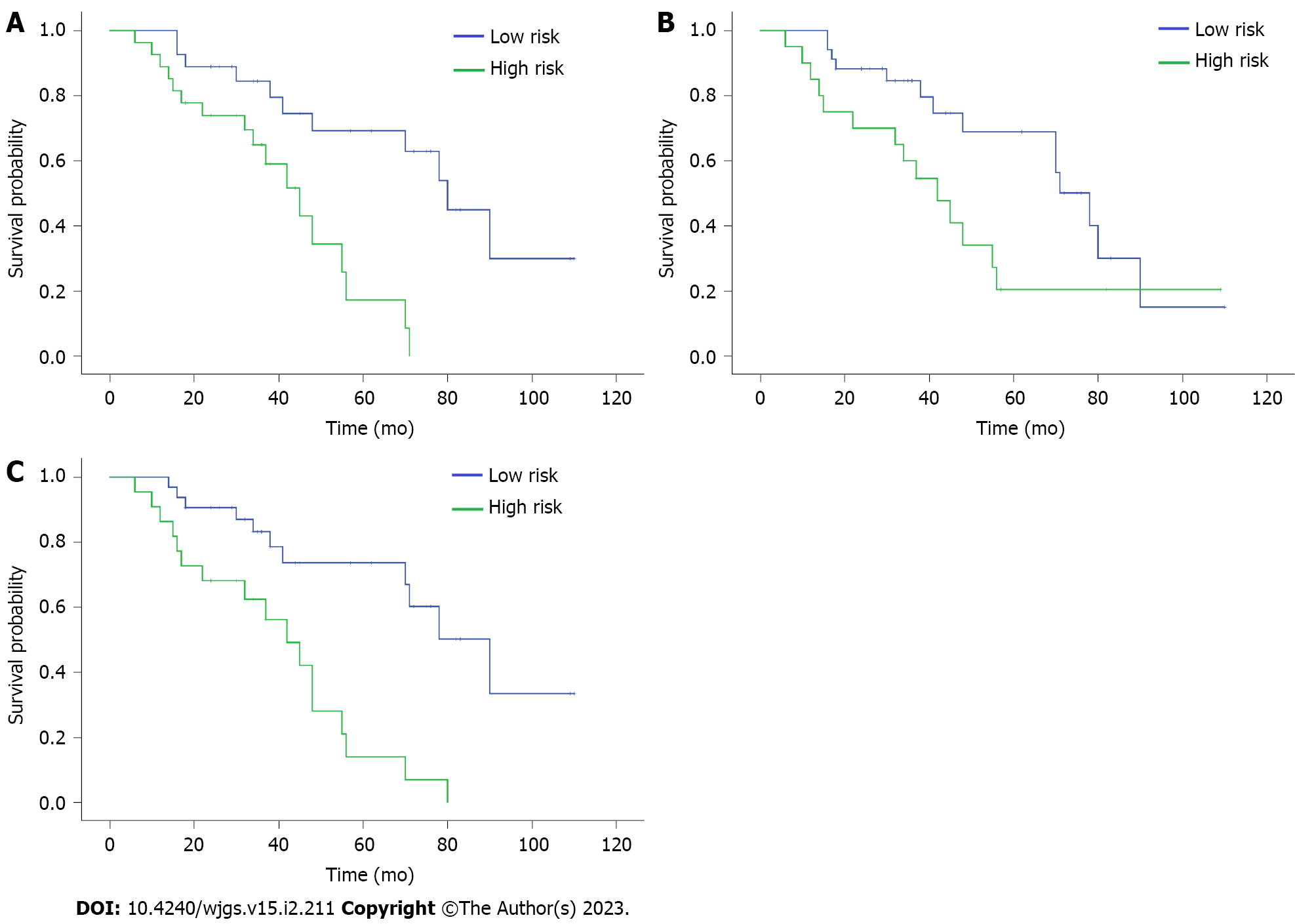
Kaplan–Meier analysis was used to analyze the relationship between PNI, CONUT, and NPS scores and prognosis. The analysis showed that the median OS of patients with a low immune-nutritional risk was significantly higher than that of patients with a high immune-nutritional risk (PNI: 75 mo vs 42 mo, P = 0.001; CONUT: 69 mo vs 48 mo, P = 0.033; NPS: 77 mo vs 40 mo; P < 0.001) (Figure 2). This finding suggests that the three immune-nutritional systems can significantly predict the prognosis of patients. Of note, the NPS system demonstrated the best prediction capability.
An increasing number of studies have shown that immunity and nutrition are closely related to the occurrence and development of cancer, which has led to the research and development of biomarkers or prognostic scoring systems based on immunity and nutrition[14-16]. The nutritional status of patients is often worse after partial gastrectomy and frequently combined with poor immune status, leading to the crucial evaluation of immune-nutritional indicators in patients with RGC[12,13]. Appropriate treatment strategies can be formulated by evaluating the relationship between immune-nutritional systems and the postoperative prognosis of patients with RGC. Three immune-nutritional systems are analyzed in the current study based on the calculation of inflammatory cells in routine blood tests and nutritional indicators, such as ALB and TC. The results showed that PNI, CONUT, and NPS can accurately predict the postoperative OS of patients with RGC. Among the three scoring systems, NPS has a superior accuracy.
Inflammatory cells participate in the destruction of tumor cells and angiogenesis in the tumor microenvironment and regulate the sensitivity of tumors to radiotherapy and chemotherapy drugs. Lymphocytes are the main antitumor cells and play an important role in cell-mediated immune response by recognizing and killing cancer cells[27]. Lymphopenia is related to the adverse reactions and prognosis of a variety of malignant tumors, including GC[28,29]. However, patients with malignant tumor with increased neutrophil infiltration often have poor clinical outcomes. Neutrophils can promote tumor formation by releasing cytokines and stimulate tumor cell proliferation and metastasis[30,31]. Tumor-associated macrophages and blood monocytes are also involved in tumor progression and metastasis and the improvement of tumor microenvironment through a variety of mechanisms[32,33]. Thus far, an increasing number of immune cell-based prognostic parameters, including NLR and LMR, have been studied and reported[19,20,34]. NLR and LMR are objective markers that reflect the inflammatory and immune status of the host. The increase in NLR and the decrease in LMR in patients are usually associated with a poor prognosis[21,35].
Malnutrition is closely related to tumor growth, angiogenesis, and progression. Serum ALB concentration is an important marker of nutrition. In a variety of tumors, patients with hypoalbuminemia usually represent a high degree of malignancy, which often indicates a poor prognosis[23,36]. Almost all nutritional prognosis scoring systems cover serum ALB levels, such as C-reactive protein to ALB ratio, Glasgow diagnostic score, as well as PNI, CONUT, and NPS discussed in this study, due to its important significance in malignant tumors[37,38]. Simultaneously, TC content is also one of the indicators of tumor prognosis. Hypocholesterolemia is associated with a poor prognosis in many tumors, including prostate cancer and non-small cell lung cancer[22,39]. Cholesterol integrates into specialized lipoprotein membrane domains, forms signal transduction mechanisms, and participates in a variety of key cellular signaling pathways[40].
The scoring system formed by combining immune and nutritional indicators can effectively reflect the physical condition of patients and improve the efficacy of predicting prognosis. This study calculated the PNI, CONUT, and NPS scores of patients according to their blood indicators. Survival analysis revealed that the three scoring systems can show good prediction efficiency. PNI, an index related to ALB concentration and lymphocyte count, has been used to predict the risk of postoperative complications and the OS of patients with GC and other cancers[24,41,42]. The CONUT score is an index related to ALB concentration, lymphocyte count, and TC concentration. Studies have shown that the CONUT score is strongly correlated with the survival rate of patients with thyroid cancer; it is also an independent risk factor for lung cancer prognosis[25,43]. NPS is an index related to serum ALB concentration, TC concentration, LMR, and NLR and has been proven to be a predictor of OS in a variety of tumors. NPS has better predictive value than clinical prognostic parameters alone in patients with resected pancreatic cancer[26]. In esophageal cancer, high NPS is associated with a poor prognosis in locally advanced patients[44]. NPS can also predict the prognosis of endometrial cancer patients and may play an important role in clinical guidance[45].
In RGC, the clinical data of immune-nutritional scoring systems are still lacking; particularly, no relevant literature is available for the prediction of postoperative OS. PNI, CONUT, and NPS are comprehensive predictive evaluation methods that are easy to obtain. They represent the entire systemic inflammation and nutritional status of patients with RGC from many aspects. Meanwhile, the results show that NPS has the strongest prediction efficiency among the three prediction systems according to survival analysis.
This study has some limitations. First, the current study is retrospective. Although the data of two institutions were included, the sample size of patients was relatively small and the selection deviation was inevitable due to the low incidence and radical resection rate of RGC, respectively. For example, this study found significant differences in gender between different NPS groups, which may represent a selection bias. This bias may reduce the universality of the research results. Second, the cutoff points of laboratory indicators were obtained from previous literature reports. A new prediction system has not been developed. This system should be established by creating new cutoff points based on RGC, possibly leading to the weakening of the prediction capability of immune-nutritional indicators. However, the predictive significance of the three immune-nutritional systems in the prognosis of patients with RGC still shows considerable value.
In this study, the immune-nutritional score systems PNI, CONUT, and NPS were the factors that affected the prognosis of patients with RGC. The results showed that poor immune-nutritional scores were associated with a poor OS. Among the three immune-nutritional scoring systems, the NPS scoring system can more accurately evaluate the prognosis of patients with RGC.
The mechanism of the occurrence and development of remnant gastric cancer (RGC) remains unclear. Systematically evaluating the preoperative immune and nutritional status of patients and analyzing its prognostic impact on postoperative RGC patients are crucial.
In RGC, the clinical data of immune-nutritional scoring systems are still lacking; particularly, no relevant literature is available for the prediction of postoperative overall survival (OS). Prognostic nutritional index (PNI), controlled nutritional status (CONUT), and Naples prognostic score system (NPS) are comprehensive predictive evaluation methods that are easy to obtain. They represent the entire systemic inflammation and nutritional status of patients with RGC from many aspects.
This retrospective cohort study aimed to determine the prognostic value of three preoperative immune-nutritional score systems, namely, PNI, CONUT, and NPS, in patients with RGC, and to examine their relationship with other clinicopathological features.
The curves of the three immune-nutritional scores for predicting postoperative survival were plotted. Kaplan–Meier analysis was used to analyze the relationship between PNI, CONUT, and NPS scores and prognosis.
NPS was found to have the largest area under the curve [AUC = 0.707; 95% confidence interval (CI): 0.566–0.848; P = 0.009]. The AUC values of PNI and CONUT were 0.611 (95%CI: 0.460–0.763; P = 0.161) and 0.635 (95%CI: 0.485–0.784; P = 0.090), respectively. The three immune-nutritional scoring systems, PNI (P = 0.002), CONUT (P = 0.039), and NPS (P < 0.001), were significantly correlated with OS. Median OS of patients with a low immune-nutritional risk was significantly higher than that of patients with a high immune-nutritional risk (PNI: 75 mo vs 42 mo, P = 0.001; CONUT: 69 mo vs 48 mo, P = 0.033; NPS: 77 mo vs 40 mo, P < 0.001).
Poor immune-nutritional scores are associated with a poor OS. Among the three immune-nutritional scoring systems, the NPS scoring system can more accurately evaluate the prognosis of patients with RGC.
The finding shows that the three immune-nutritional systems can significantly predict the prognosis of patients. Of note, the NPS system demonstrates the best prediction capability.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68565] [Article Influence: 13713.0] [Reference Citation Analysis (201)] |
| 2. | Hanyu T, Wakai A, Ishikawa T, Ichikawa H, Kameyama H, Wakai T. Carcinoma in the Remnant Stomach During Long-Term Follow-up After Distal Gastrectomy for Gastric Cancer: Analysis of Cumulative Incidence and Associated Risk Factors. World J Surg. 2018;42:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Nunobe S, Ohyama S, Miyata S, Matsuura M, Hiki N, Fukunaga T, Seto Y, Ushijima M, Yamaguchi T. Incidence of gastric cancer in the remnant stomach after proximal gastrectomy. Hepatogastroenterology. 2008;55:1855-1858. [PubMed] |
| 4. | Mak TK, Guan B, Peng J, Chong TH, Wang C, Huang S, Yang J. Prevalence and characteristics of gastric remnant cancer: A systematic review and meta-analysis. Asian J Surg. 2021;44:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Oymaci E, Sari E, Uçar AD, Erkan N, Yildirim M. Gastric Remnant Cancer: Continuing Serious and Insidious Problem for Surgeons. Hepatogastroenterology. 2015;62:727-731. [PubMed] |
| 6. | Saarinen T, Räsänen J, Salo J, Loimaala A, Pitkonen M, Leivonen M, Juuti A. Bile Reflux Scintigraphy After Mini-Gastric Bypass. Obes Surg. 2017;27:2083-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Nunobe S, Takahashi M, Kinami S, Fujita J, Suzuki T, Suzuki A, Tanahashi T, Kawaguchi Y, Oshio A, Nakada K. Evaluation of postgastrectomy symptoms and daily lives of small remnant distal gastrectomy for upper-third gastric cancer using a large-scale questionnaire survey. Ann Gastroenterol Surg. 2022;6:355-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Liao G, Wen S, Xie X, Wu Q. Laparoscopic gastrectomy for remnant gastric cancer: Risk factors associated with conversion and a systematic analysis of literature. Int J Surg. 2016;34:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Ramos MFKP, Pereira MCM, Oliveira YS, Pereira MA, Barchi LC, Dias AR, Zilberstein B, Ribeiro Junior U, Cecconello I. Surgical results of remnant gastric cancer treatment. Rev Col Bras Cir. 2020;47:e20202703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Ohira M, Toyokawa T, Sakurai K, Kubo N, Tanaka H, Muguruma K, Yashiro M, Onoda N, Hirakawa K. Current status in remnant gastric cancer after distal gastrectomy. World J Gastroenterol. 2016;22:2424-2433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Chen QY, Zhong Q, Zhou JF, Qiu XT, Dang XY, Cai LS, Su GQ, Xu DB, Lin GT, Guo KQ, Liu ZY, Chen QX, Li P, Li TW, Xie JW, Lin SM, Wang JB, Lin JX, Lu J, Cao LL, Lin M, Zheng CH, Lin W, He QL, Huang CM. Conditional survival and recurrence of remnant gastric cancer after surgical resection: A multi-institutional study. Cancer Sci. 2020;111:502-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Davis JL, Ripley RT. Postgastrectomy Syndromes and Nutritional Considerations Following Gastric Surgery. Surg Clin North Am. 2017;97:277-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (2)] |
| 13. | Ramos MFKP, Pereira MA, de Castria TB, Ribeiro RRE, Cardili L, de Mello ES, Zilberstein B, Ribeiro-Júnior U, Cecconello I. Remnant gastric cancer: a neglected group with high potential for immunotherapy. J Cancer Res Clin Oncol. 2020;146:3373-3383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 14. | Giannini R, Zucchelli G, Giordano M, Ugolini C, Moretto R, Ambryszewska K, Leonardi M, Sensi E, Morano F, Pietrantonio F, Cremolini C, Falcone A, Fontanini G. Immune Profiling of Deficient Mismatch Repair Colorectal Cancer Tumor Microenvironment Reveals Different Levels of Immune System Activation. J Mol Diagn. 2020;22:685-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Zeng Z, Li J, Zhang J, Li Y, Liu X, Chen J, Huang Z, Wu Q, Gong Y, Xie C. Immune and stromal scoring system associated with tumor microenvironment and prognosis: a gene-based multi-cancer analysis. J Transl Med. 2021;19:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Zheng X, Li L, Yu C, Yang J, Zhao Y, Su C, Yu J, Xu M. Establishment of a tumor immune microenvironment-based molecular classification system of breast cancer for immunotherapy. Aging (Albany NY). 2021;13:24313-24338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Flores-Pérez JA, de la Rosa Oliva F, Argenes Y, Meneses-Garcia A. Nutrition, Cancer and Personalized Medicine. Adv Exp Med Biol. 2019;1168:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Wijler LA, Raats DAE, Elias SG, Dijk FJ, Quirindongo H, May AM, Furber MJW, Dorresteijn B, van Dijk M, Kranenburg O. Specialized nutrition improves muscle function and physical activity without affecting chemotherapy efficacy in C26 tumour-bearing mice. J Cachexia Sarcopenia Muscle. 2021;12:796-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Pan YC, Jia ZF, Cao DH, Wu YH, Jiang J, Wen SM, Zhao D, Zhang SL, Cao XY. Preoperative lymphocyte-to-monocyte ratio (LMR) could independently predict overall survival of resectable gastric cancer patients. Medicine (Baltimore). 2018;97:e13896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Hamid HKS, Davis GN, Trejo-Avila M, Igwe PO, Garcia-Marín A. Prognostic and predictive value of neutrophil-to-lymphocyte ratio after curative rectal cancer resection: A systematic review and meta-analysis. Surg Oncol. 2021;37:101556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Song D, Li X, Zhang X. Expression and prognostic value of ratios of platelet lymphocyte, neutrophil lymphocyte and lymphocyte monocyte in breast cancer patients. Am J Transl Res. 2022;14:3233-3239. [PubMed] |
| 22. | Hirano H, Ide H, Lu Y, Inoue Y, Okada H, Horie S. Impact of Pretreatment Total Cholesterol Level Is Associated With Metastasis of Prostate Cancer. Am J Mens Health. 2020;14:1557988320918788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Bekos C, Polterauer S, Seebacher V, Bartl T, Joura E, Reinthaller A, Sturdza A, Horvat R, Schwameis R, Grimm C. Pre-operative hypoalbuminemia is associated with complication rate and overall survival in patients with vulvar cancer undergoing surgery. Arch Gynecol Obstet. 2019;300:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Ding P, Guo H, Sun C, Yang P, Kim NH, Tian Y, Liu Y, Liu P, Li Y, Zhao Q. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: a prospective study. BMC Gastroenterol. 2022;22:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 25. | Dalmiglio C, Brilli L, Campanile M, Ciuoli C, Cartocci A, Castagna MG. CONUT Score: A New Tool for Predicting Prognosis in Patients with Advanced Thyroid Cancer Treated with TKI. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Nakagawa N, Yamada S, Sonohara F, Takami H, Hayashi M, Kanda M, Kobayashi D, Tanaka C, Nakayama G, Koike M, Fujiwara M, Kodera Y. Clinical Implications of Naples Prognostic Score in Patients with Resected Pancreatic Cancer. Ann Surg Oncol. 2020;27:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Li T, Wu B, Yang T, Zhang L, Jin K. The outstanding antitumor capacity of CD4(+) T helper lymphocytes. Biochim Biophys Acta Rev Cancer. 2020;1874:188439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Tatara T, Suzuki S, Kanaji S, Yamamoto M, Matsuda Y, Hasegawa H, Yamashita K, Matsuda T, Oshikiri T, Nakamura T, Kakeji Y. Lymphopenia predicts poor prognosis in older gastric cancer patients after curative gastrectomy. Geriatr Gerontol Int. 2019;19:1215-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Eberst G, Vernerey D, Laheurte C, Meurisse A, Kaulek V, Cuche L, Jacoulet P, Almotlak H, Lahourcade J, Gainet-Brun M, Fabre E, Le Pimpec-Barthes F, Adotevi O, Westeel V. Prognostic value of CD4+ T lymphopenia in non-small cell lung Cancer. BMC Cancer. 2022;22:529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 30. | Guan X, Lu Y, Zhu H, Yu S, Zhao W, Chi X, Xie C, Yin Z. The Crosstalk Between Cancer Cells and Neutrophils Enhances Hepatocellular Carcinoma Metastasis via Neutrophil Extracellular Traps-Associated Cathepsin G Component: A Potential Therapeutic Target. J Hepatocell Carcinoma. 2021;8:451-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 31. | Xia X, Zhang Z, Zhu C, Ni B, Wang S, Yang S, Yu F, Zhao E, Li Q, Zhao G. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat Commun. 2022;13:1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 168] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 32. | Wang YH, Shen CY, Lin SC, Kuo WH, Kuo YT, Hsu YL, Wang WC, Lin KT, Wang LH. Monocytes secrete CXCL7 to promote breast cancer progression. Cell Death Dis. 2021;12:1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Chen Z, Wu J, Wang L, Zhao H, He J. Tumor-associated macrophages of the M1/M2 phenotype are involved in the regulation of malignant biological behavior of breast cancer cells through the EMT pathway. Med Oncol. 2022;39:83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Duan J, Pan L, Yang M. Preoperative elevated neutrophil-to-lymphocyte ratio (NLR) and derived NLR are associated with poor prognosis in patients with breast cancer: A meta-analysis. Medicine (Baltimore). 2018;97:e13340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Kim JH, Lee JH, Lee HS, Shin SJ, Park EJ, Cho ES, Baik SH, Lee KY, Kang J. Elevated Neutrophil-to-Lymphocyte Ratio in Perioperative Periods is Suggestive of Poor Prognosis in Patients with Colorectal Cancer. J Inflamm Res. 2021;14:4457-4466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Hardt J, Pilz L, Magdeburg J, Kienle P, Post S, Magdeburg R. Preoperative hypoalbuminemia is an independent risk factor for increased high-grade morbidity after elective rectal cancer resection. Int J Colorectal Dis. 2017;32:1439-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Bai X, Feng L. Correlation between Prognostic Nutritional Index, Glasgow Prognostic Score, Systemic Inflammatory Response, and TNM Staging in Colorectal Cancer Patients. Nutr Cancer. 2020;72:1170-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Liu X, Guo X, Zhang Z. Preoperative Serum Hypersensitive-c-Reactive-Protein (Hs-CRP) to Albumin Ratio Predicts Survival in Patients with Luminal B Subtype Breast Cancer. Onco Targets Ther. 2021;14:4137-4148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Zhang G, Zhang D, Wu J, Zhang F, Zhu Z, Chen K, Zhang N, Jin J, Feng J, Lin N, Zhang Y, Yu H, Su D, Ying L. Low Serum Levels of Pre-Surgical Total Cholesterol are Associated with Unfavorable Overall Survival in Patients with Operable Non-Small Cell Lung Cancer. Clin Lab. 2018;64:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Vona R, Iessi E, Matarrese P. Role of Cholesterol and Lipid Rafts in Cancer Signaling: A Promising Therapeutic Opportunity? Front Cell Dev Biol. 2021;9:622908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 41. | Xishan Z, Ye Z, Feiyan M, Liang X, Shikai W. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci Rep. 2020;10:17373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 42. | Momokita M, Abe A, Shibata K, Hayashi H, Furuta H, Taniguchi S, Nakayama A. Prognostic Nutritional Index in Patients With End-Stage Oral Cancer. Am J Hosp Palliat Care. 2022;10499091221102581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Shao J, Li J, Zhang XL, Wang G. Prognostic Significance of the Preoperative Controlled Nutritional Status Score in Lung Cancer Patients Undergoing Surgical Resection. Nutr Cancer. 2021;73:2211-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Feng JF, Zhao JM, Chen S, Chen QX. Naples Prognostic Score: A Novel Prognostic Score in Predicting Cancer-Specific Survival in Patients With Resected Esophageal Squamous Cell Carcinoma. Front Oncol. 2021;11:652537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | Li Q, Cong R, Wang Y, Kong F, Ma J, Wu Q, Ma X. Naples prognostic score is an independent prognostic factor in patients with operable endometrial cancer: Results from a retrospective cohort study. Gynecol Oncol. 2021;160:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dilek ON, Turkey; Senchukova M, Russia S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR