Published online Nov 27, 2023. doi: 10.4240/wjgs.v15.i11.2470
Peer-review started: August 15, 2023
First decision: August 31, 2023
Revised: September 5, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 27, 2023
Processing time: 104 Days and 0.2 Hours
Colon cancer is a common malignant tumor in the gastrointestinal tract that is typically treated surgically. However, postradical surgery is prone to complications such as anastomotic fistulas.
To investigate the risk factors for postoperative anastomotic fistulas and their impact on the prognosis of patients with colon cancer.
We conducted a retrospective analysis of 488 patients with colon cancer who underwent radical surgery. This study was performed between April 2016 and April 2019 at a tertiary hospital in Wuxi, Jiangsu Province, China. A t-test was used to compare laboratory indicators between patients with and those without postoperative anastomotic fistulas. Multiple logistic regression analysis was performed to identify independent risk factors for postoperative anastomotic fistulas. The Functional Assessment of Cancer Therapy-Colorectal Cancer was also used to assess postoperative recovery.
Binary logistic regression analysis revealed that age [odds ratio (OR) = 1.043, P = 0.015], tumor, node, metastasis stage (OR = 2.337, P = 0.041), and surgical procedure were independent risk factors for postoperative anastomotic fistulas. Multiple linear regression analysis showed that the development of postoperative anastomotic fistula (P = 0.000), advanced age (P = 0.003), and the presence of diabetes mellitus (P = 0.015), among other factors, independently affected prognosis.
Postoperative anastomotic fistulas significantly affect prognosis and survival rates. Therefore, focusing on the clinical characteristics and risk factors and immediately implementing individualized preventive measures are important to minimize their occurrence.
Core Tip: The incidence of anastomotic fistulas after radical colon cancer surgery is high and significantly affects patient prognosis. Additional targeted interventions should be supplemented to reduce the occurrence of anastomotic fistulas and improve the prognosis.
- Citation: Wang J, Li MH. Risk factors for anastomotic fistula development after radical colon cancer surgery and their impact on prognosis. World J Gastrointest Surg 2023; 15(11): 2470-2481
- URL: https://www.wjgnet.com/1948-9366/full/v15/i11/2470.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i11.2470
Colon cancer is a malignant tumor that originates from the colonic epithelium and often occurs at the sigmoid colorectal junction. While its etiology remains unclear, most colon cancers develop from adenomatous polyps and progress to carcinoma. As one of the most prevalent cancers worldwide, colon cancer is the second and third leading cause of cancer-related deaths globally[1] and in the United States, respectively[2]. In China, it is one of the most frequently diagnosed tumors, with an increasing incidence rate[3]. A recent report from the National Cancer Center in China published in the Journal of the National Cancer Center revealed that colon cancer is the third and fourth most commonly occurring cancer in women and men, respectively, in China[4]. An increase in the incidence of colon cancer has been reported among younger individuals, with approximately 11% of the cases occurring in those aged < 50 years. This incidence rate also increases by 1%-2% annually[5,6]. The statistics emphasize the current threat of colon cancer poses to human health. Additionally, a study indicated that the 5-year survival rates for patients with colon cancer aged 18-65 years with stages I, II, III, and IV are 91%, 82%, 66%, and 10%, respectively[7]. Furthermore, Dekker et al[8] reported that in 2017, approximately 140000 people were diagnosed with colon cancer, and approximately 50000 died from this disease.
Clinical symptoms of colon cancer primarily include abdominal distension, indigestion, and bloody stools. The initial mild symptoms, such as indigestion, bloody stools, and constipation, can progress to edema, jaundice, and ascites in advanced stages. Surgery remains the primary treatment option for patients with early-stage colon cancer, with approximately 20% of patients diagnosed with distant metastases ineligible for surgical resection[9]. Surgical resection is the mainstay of treatment for colon cancer[10]. In recent years, laparoscopic radical colon cancer surgery has become the preferred approach over open surgery because of its advantages including reduced trauma and postoperative pain[11]. However, regardless of the surgical method used, patients with colon cancer are prone to developing anastomotic fistulas following surgery[12]. Anastomotic fistulas are a common and severe postoperative complication following radical colon cancer surgery. If not promptly treated, they can result in permanent stomas, increase the risk of recurrence, and even lead to death[13]. The development of anastomotic fistulas can be attributed to various factors, including poor blood flow due to tight anastomotic sutures, inadequate preoperative intestinal preparation, poor postoperative nutritional status, and improper patient care during the postoperative period. These factors increase the risk of postoperative abdominal infection, further exacerbating the patient’s condition[14,15]. Previous studies examined the risk factors associated with the development of anastomotic fistulas after radical colon cancer surgery, but their findings were inconsistent, and none of them investigated the prognostic implications of anastomotic fistulas on patients with colon cancer[12,16]. Therefore, this study aimed to identify independent risk factors for the development of anastomotic fistulas after radical colon cancer surgery and to investigate the effects of these fistulas on patient prognosis. The findings of this study may contribute to improving the postoperative well-being of patients, prolonging their life expectancy, and improving their prognosis.
A total of 488 patients who underwent radical colon cancer surgery at the Affiliated Hospital of Jiangnan University between April 2016 and April 2019 were included in the study.
The inclusion criteria were as follows: (1) All patients underwent elective surgery; (2) pathological stage of tumor, node, metastasis (TNM) stages I-III; (3) postoperative pathology confirming colon cancer; (4) radical resection of colon cancer surgery; (5) preoperative imaging ruling out liver, lung, and other distant metastases; (6) availability of detailed medical records and complete postoperative pathological data; and (7) informed consent signed by patients and family members.
The exclusion criteria included: (1) Patients who died during hospitalization or were discharged automatically and terminated treatment; (2) patients with a planned stoma; (3) patients with severe coagulation abnormalities; (4) patients with confirmed unresectable tumor invasion of surrounding organs or advanced tumors with distant metastases, eligible only for palliative resection; (5) pregnant and lactating women; (6) patients who underwent emergency surgery for bleeding, perforation, and intestinal obstruction; and (7) patients with incomplete medical records during the treatment process that affected result evaluation (Figure 1).
The sample size was calculated using the following formula: n = (Z1-α/2/δ)2P(1-P), with the postoperative development of an anastomotic fistula as the primary outcome index. Based on our clinical experience, the incidence of intestinal fistula (P) in patients after radical colon cancer surgery was approximately 7%. Taking α as 3% and δ as 0.05 (bilateral), and considering a 10% sample attrition rate, the sample size was determined to be n = 306 cases. Using a similar approach, the study population included 510 patients. After excluding 22 patients and accounting for loss to follow-up, 488 cases were finally included.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and informed consent was obtained from all patients.
The general information questionnaire collected demographic (e.g., sex and age) and clinical data (e.g., occurrence of anastomotic fistula, presence of hypertension and diabetes, site of lesion, type of tissue, type of pathology, TNM stage, surgical approach, presence of lymph node metastasis, presence of adjuvant chemotherapy, postoperative intensive care unit (ICU) stay, operative time, intraoperative bleeding, postoperative time to exhaustion, hospitalization time, preoperative and postoperative levels of carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), albumin (Alb), total protein (TP), hemoglobin (Hb), blood potassium (K), and platelet).
Anastomotic leak is defined as the drainage of colonic contents through a drain, wound, or abnormal orifice. It is typically diagnosed using computed tomography (CT) scan or surgery. The specific diagnostic methods used were as follows: Limited or diffuse abdominal pain, turbid purulent drainage fluid or presence of gas, liquid, or fecal discharge, abdominal incision with pus, or even fecal-like fluid overflowing from the abdominal cavity. For low rectal anastomotic fistula, it can be detected through rectal examination and presence of generalized fever; elevated C-reactive protein (CRP) levels in routine blood tests; computed tomography examination showing bubbles or inflammatory edema around the anastomosis, blurring of the surrounding fat planes, or suspected abdominal abscesses associated with the intestine; dilute barium enema imaging showing contrast agent leakage or injection of contrast agent through the drainage tube revealing the flow of contrast agent into the intestinal cavity; and endoscopy or re-operation assisting in confirming the diagnosis.
The Functional Assessment of Cancer Therapy-Colorectal (FACT-C) questionnaire is a validated and reliable measure of health-related quality of life in patients with colorectal cancer[17]. It consists of five subscales: physical well-being (seven items; score range 0-28), social well-being (seven items; score range 0-28), emotional well-being (six items; score range 0-24), functional well-being (seven items; score range 0-28), and a colorectal cancer subscale (seven items; score range 0-28). The FACT-C questionnaire comprises a total of 34 items with an overall score range of 0-136.
The scores obtained for each subscale were entered into a computer for conversion. All the statistical analyses were performed using SPSS version 26 (IBM Corp., Armonk, NY, United States). Measurement data are presented as means and standard deviations, and count data are expressed as frequencies and percentages. Intergroup comparisons were performed using the t-test and chi-square test, and binary logistic regression analysis was used to identify independent risk factors for postoperative anastomotic fistula. Multiple linear regression analysis was performed to determine independent risk factors for assessing patient prognosis. Statistical significance was defined as a two-sided P value of < 0.05.
Among the 488 patients included in this study, postoperative anastomotic fistulas developed in 38 patients (7.8%). The chi-square test and t-test revealed significant differences between patients with and those without postoperative anastomotic fistulas in terms of age, presence of diabetes, TNM stage, surgical method, preoperative radiotherapy, and postoperative ICU stay (P < 0.05). The mean age of patients with postoperative anastomotic fistula was 58.95 ± 11.91 years, and that of patients without fistula was 52.90 ± 12.7 years. Among the patients with and without postoperative anastomotic fistula (38 and 450, respectively), 16 (42.1%) and 110 (24.4%) had diabetes mellitus, 20 (52.6%) and 323 (71.8%) had TNM stage I-II, 9 (23.7%) and 278 (61.8%) underwent laparoscopic radical surgery, and 29 (76.3%) and 172 (38.2%) underwent conventional radical surgery, 13 (34.2%) and 85 (18.9%) received preoperative radiotherapy, and 5 (13.2%) and 20 (4.4%) were transferred to the ICU postoperatively, respectively (Table 1).
| Item, n (%) | Anastomotic fistula group | Non-anastomotic fistula group | t/χ2 | P value |
| Gender | ||||
| Male | 19 (50.0) | 236 (52.4) | 0.084 | 0.772 |
| Female | 19 (50.0) | 214 (47.6) | ||
| Age (yr) | 58.95 ± 11.91 | 52.90 ± 12.7 | -2.921 | 0.004 |
| Hypertension or not | ||||
| Yes | 17 (44.7) | 171 (38.0) | 0.672 | 0.413 |
| No | 21 (55.3) | 279 (62.0) | ||
| Diabetes or not | ||||
| Yes | 16 (42.1) | 110 (24.4) | 5.706 | 0.017 |
| No | 22 (57.9) | 340 (75.6) | ||
| Site of lesion | ||||
| Left hemi-colon | 17 (44.7) | 227 (50.4) | 0.892 | 0.640 |
| Right hemi-colon | 15 (39.5) | 144 (25.3) | ||
| Transverse colon | 6 (15.8) | 79 (17.6) | ||
| Type of organization | ||||
| Low to moderately differentiated adenocarcinoma | 27 (71.1) | 325 (72.2) | 0.024 | 0.877 |
| Highly differentiated adenocarcinoma | 11 (28.9) | 125 (27.8) | ||
| Type of TNM | ||||
| Type of I-II | 20 (52.6) | 323 (71.8) | 6.151 | 0.013 |
| Type of III | 18 (47.4) | 127 (28.2) | ||
| Surgery method | ||||
| Laparoscopic radical surgery | 9 (23.7) | 278 (61.8) | 20.991 | 0.000 |
| Traditional radical surgery | 29 (76.3) | 172 (38.2) | ||
| Lymph node metastasis or not | ||||
| Yes | 11 (28.9) | 122 (27.1) | 0.060 | 0.807 |
| No | 27 (71.1) | 328 (72.9) | ||
| With preoperative adjuvant chemotherapy or not | ||||
| Yes | 13 (34.2) | 85 (18.9) | 5.125 | 0.024 |
| No | 25 (65.8) | 365 (81.1) | ||
| Post-operative ICU stay | ||||
| Yes | 5 (13.2) | 20 (4.4) | 5.474 | 0.019 |
| No | 33 (86.8) | 430 (95.6) | ||
| Type of pathology | ||||
| Ductal gland | 30 (78.9) | 332 (73.8) | 0.489 | 0.484 |
| Mucus gland | 8 (21.1) | 118 (26.2) | ||
The t-test demonstrated significant differences between patients with and those without postoperative anastomotic fistulas in terms of operative time, intraoperative bleeding, and postoperative hospital stay (P < 0.05). The mean operative time, intraoperative bleeding, and postoperative hospital stay for patients with postoperative anastomotic fistula were 187.39 ± 16.31 min, 229.21 ± 60.81 mL, and 11.76 ± 2.57 d, respectively. Patients without fistula had a mean operative time of 179.96 ± 21.32 min, intraoperative bleeding of 187.51 ± 60.51 mL, and postoperative hospital stay of 10.76 ± 2.11 d (Table 2).
| Item (mean ± SD) | Operating time (min) | Intraoperative bleeding (mL) | Post-operative time to exhaustion (d) | Postoperative hospitalization time (d) |
| Anastomotic fistula group | 187.39 ± 16.31 | 229.21 ± 60.81 | 2.37 ± 0.91 | 11.76 ± 2.57 |
| Non-anastomotic fistula group | 179.96 ± 21.32 | 187.51 ± 60.51 | 2.21 ± 0.90 | 10.76 ± 2.11 |
| t value | -2.098 | -4.078 | -1.053 | -2.777 |
| P value | 0.036 | 0.000 | 0.293 | 0.006 |
The t-test revealed statistically significant differences between patients with and those without postoperative anastomotic fistulas in terms of preoperative and postoperative Alb, postoperative TP, postoperative Hb, and postoperative K levels (P < 0.05). In patients with and without postoperative anastomotic fistula, the preoperative Alb, postoperative Alb, postoperative TP, postoperative Hb, and postoperative K were 33.87 ± 4.74 g/L and 36.22 ± 4.72 g/L, 27.70 ± 3.24 g/L and 29.92 ± 3.56 g/L, 55.32 ± 8.83 g/L and 58.37 ± 8.67 g/L, 115.57 ± 14.00 g/L and 58.37 ± 8.67 g/L, and 4.89 ± 0.49 mmol/L and 4.71 ± 0.47 mmol/L, respectively (Table 3).
| Item (mean ± SD) | CEA (ng/mL) | CA125 (U/mL) | Alb (g/L) | TP (g/L) | Hb (g/L) | K (mmol/L) |
| Preoperative group | ||||||
| Anastomotic fistula group | 7.71 ± 0.77 | 51.51 ± 10.78 | 33.87 ± 4.74 | 69.13 ± 9.31 | 135.18 ± 15.21 | 3.96 ± 0.18 |
| Non-anastomotic fistula group | 7.74 ± 0.81 | 52.53 ± 10.54 | 36.22 ± 4.72 | 69.77 ± 8.04 | 133.46 ± 13.64 | 3.94 ± 0.17 |
| t value | 0.240 | 0.574 | 2.951 | 0.465 | -0.739 | -0.549 |
| P value | 0.811 | 0.567 | 0.003 | 0.642 | 0.460 | 0.583 |
| Postoperative group | ||||||
| Anastomotic fistula group | 5.12 ± 0.90 | 39.34 ± 10.00 | 27.70 ± 3.24 | 55.32 ± 8.83 | 115.57 ± 14.00 | 4.89 ± 0.49 |
| Non-anastomotic fistula group | 5.01 ± 0.87 | 39.93 ± 10.11 | 29.92 ± 3.56 | 58.37 ± 8.67 | 120.90 ± 14.06 | 4.71 ± 0.47 |
| t value | -0.749 | 0.344 | 3.716 | 2.045 | 2.246 | -2.266 |
| P value | 0.454 | 0.731 | 0.000 | 0.047 | 0.025 | 0.024 |
The t-test demonstrated significant differences in all components of the FACT-C score between patients with and those without postoperative anastomotic fistulas (P < 0.05). The mean scores for physical well-being, social well-being, emotional well-being, functional well-being, colorectal cancer subscale, and total scores for patients with postoperative anastomotic fistula were 12.50 ± 3.80, 18.24 ± 3.77, 15.50 ± 3.45, 12.66 ± 3.78, 15.89 ± 4.59, and 74.79 ± 11.86, respectively, and those for patients without fistula were 15.62 ± 3.94, 20.06 ± 3.54, 18.32 ± 3.36, 15.53 ± 4.39, 19.52 ± 3.98, and 89.05 ± 13.32, respectively (Table 4).
| Item (mean ± SD) | First group | Second group | ||||
| PWB | SWB | EWB | FWB | CCS | Total | |
| Anastomotic fistula group | 12.50 ± 3.80 | 18.24 ± 3.77 | 15.50 ± 3.45 | 12.66 ± 3.78 | 15.89 ± 4.59 | 74.79 ± 11.86 |
| Non-anastomotic fistula group | 15.62 ± 3.94 | 20.06 ± 3.54 | 18.32 ± 3.36 | 15.53 ± 4.39 | 19.52 ± 3.98 | 89.05 ± 13.32 |
| t value | 4.705 | 3.024 | 4.962 | 3.909 | 4.724 | 6.389 |
| P value | 0 | 0.003 | 0 | 0 | 0 | 0 |
The chi-square test revealed that the presence or absence of a postoperative anastomotic fistula significantly influenced the survival rate of patients 1 year after surgery (89.5% vs 96.7%, respectively; P < 0.05). However, no significant differences were observed in the 1- to 3-year recurrence and 2- to 3- survival rates between patients with and those without postoperative anastomotic fistulas (P > 0.05; Table 5).
| Item, n (%) | Recurrence 1 yr after surgery | Recurrence 2 yr after surgery | Recurrence 3 yr after surgery | Survival 1 yr after surgery | Survival 2 yr after surgery | Survival 3 yr after surgery | ||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Anastomotic fistula group | 4 (10.5) | 34 (89.5) | 6 (15.8) | 32 (84.2) | 7 (18.4) | 31 (81.6) | 34 (89.5) | 4 (10.5) | 32 (84.2) | 6 (15.8) | 28 (73.7) | 10 (26.3) |
| Non-anastomotic fistula group | 22 (4.9) | 428 (95.1) | 40 (8.9) | 410 (91.1) | 50 (11.1) | 400 (88.9) | 435 (96.7) | 15 (3.3) | 412 (91.6) | 38 (8.4) | 382 (84.9) | 68 (15.1) |
| t value | 2.208 | 1.954 | 1.815 | 4.845 | 2.304 | 3.276 | ||||||
| P value | 0.137 | 0.162 | 0.178 | 0.028 | 0.129 | 0.07 | ||||||
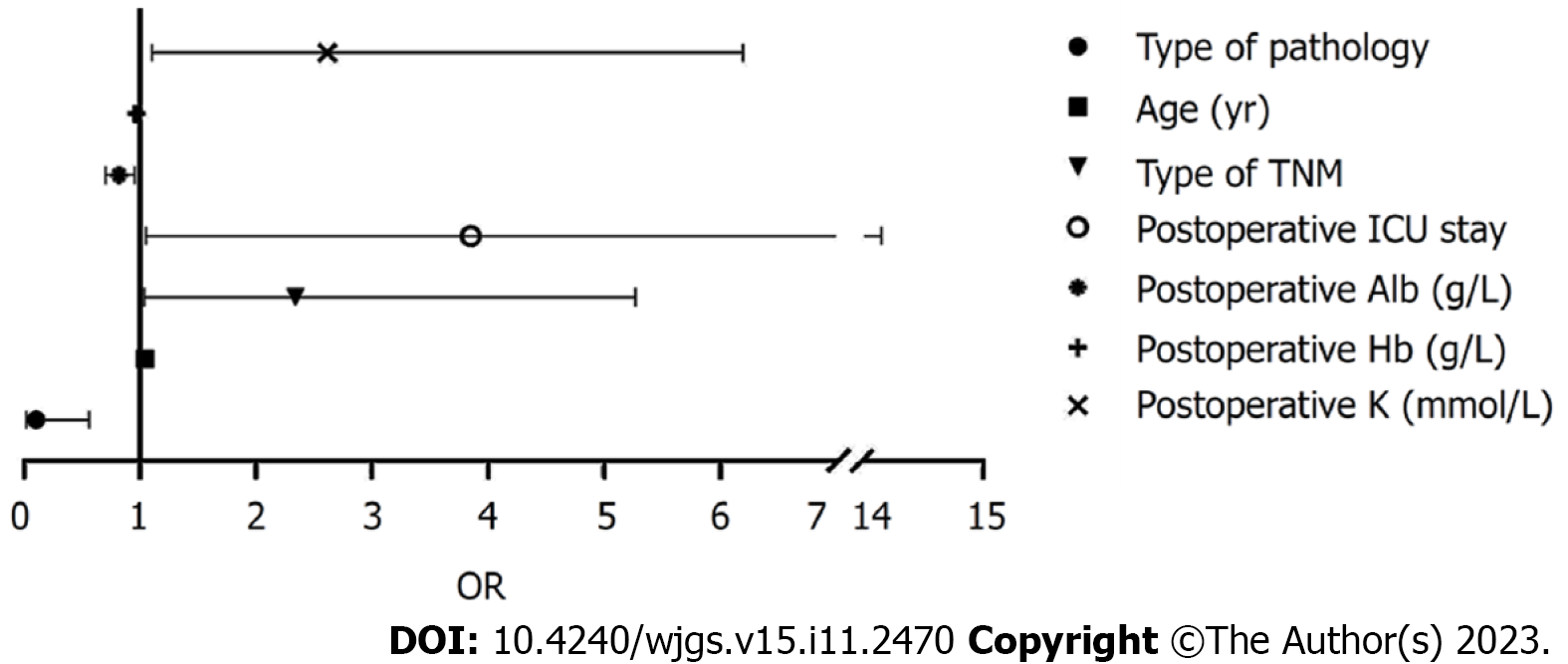
Binary logistic regression analysis identified patient age, TNM stage, surgical procedure, postoperative ICU stay after surgery, and postoperative Alb, Hb, and potassium levels as independent risk factors for postoperative anastomotic fistulas (P < 0.05; Table 6, Figure 2).
| Related factor | B | SE | Wald | P value | OR | 95%CI | |
| Upper | Lower | ||||||
| Surgery method | -2.304 | 0.878 | 6.890 | 0.009 | 0.100 | 0.558 | 0.018 |
| Age (yr) | 0.042 | 0.017 | 5.882 | 0.015 | 1.043 | 1.079 | 1.008 |
| Diabetes | 0.602 | 0.413 | 2.127 | 0.145 | 1.827 | 4.105 | 0.813 |
| Type of TNM | 0.849 | 0.415 | 4.187 | 0.041 | 2.337 | 5.268 | 1.036 |
| Preoperative adjuvant chemotherapy | 0.687 | 0.457 | 2.261 | 0.133 | 1.987 | 4.865 | 0.812 |
| Post-operative ICU stay | 1.348 | 0.664 | 4.124 | 0.042 | 3.850 | 14.144 | 1.048 |
| Operating time (min) | -0.015 | 0.013 | 1.424 | 0.233 | 0.985 | 1.010 | 0.960 |
| Intraoperative bleeding (mL) | -0.005 | 0.006 | 0.561 | 0.454 | 0.995 | 1.007 | 0.984 |
| Postoperative hospitalization time (d) | 0.160 | 0.093 | 2.993 | 0.084 | 1.174 | 1.408 | 0.979 |
| Preoperative Alb (g/L) | -0.023 | 0.051 | 0.210 | 0.647 | 0.977 | 1.080 | 0.883 |
| Postoperative Alb (g/L) | -0.203 | 0.078 | 6.693 | 0.010 | 0.817 | 0.952 | 0.701 |
| Postoperative TP (g/L) | -0.038 | 0.025 | 2.406 | 0.121 | 0.963 | 1.010 | 0.917 |
| Postoperative Hb (g/L) | -0.030 | 0.015 | 4.194 | 0.041 | 0.970 | 0.999 | 0.943 |
| Postoperative K (mmol/L) | 0.960 | 0.441 | 4.742 | 0.029 | 2.612 | 6.198 | 1.101 |
Multiple linear regression analysis revealed that postoperative anastomotic fistula, advanced age, presence of diabetes, lymph node metastasis, pathological mucinous glands, high postoperative CEA and CA125 Levels, and high preoperative CA125 Levels independently influenced the postoperative prognosis of the patients (P < 0.05; Table 7).
| Related factor | B | SE | t value | P value |
| Anastomotic fistula | -11.180 | 2.506 | -4.461 | 0.000 |
| Surgery method | 0.060 | 3.204 | 0.019 | 0.985 |
| Age (yr) | -0.148 | 0.049 | -3.012 | 0.003 |
| Gender | 0.221 | 1.155 | 0.191 | 0.848 |
| Hypertension | 0.383 | 1.183 | 0.323 | 0.747 |
| Diabetes | -3.292 | 1.348 | -2.443 | 0.015 |
| Site of lesion | -0.172 | 0.765 | -0.224 | 0.823 |
| Type of organization | -0.172 | 1.288 | -0.134 | 0.894 |
| Type of TNM | -2.414 | 1.322 | -1.826 | 0.069 |
| Lymph node metastasis | -2.694 | 1.286 | -2.094 | 0.037 |
| Preoperative adjuvant chemotherapy | -0.196 | 1.511 | -0.130 | 0.897 |
| Post-operative ICU stay | 0.480 | 2.665 | 0.180 | 0.857 |
| Type of pathology | -6.303 | 1.322 | -4.768 | 0.000 |
| Operating time (min) | 0.040 | 0.036 | 1.116 | 0.265 |
| Intraoperative bleeding (mL) | -0.014 | 0.024 | -0.572 | 0.567 |
| Post-operative time to exhaustion (d) | 0.731 | 0.748 | 0.977 | 0.329 |
| Postoperative hospitalization time (d) | -0.242 | 0.270 | -0.894 | 0.372 |
| Preoperative CEA (ng/mL) | 0.912 | 0.709 | 1.287 | 0.199 |
| Postoperative CEA (ng/mL) | -1.778 | 0.782 | -2.274 | 0.023 |
| Preoperative CA125 (U/mL) | 0.488 | 0.170 | 2.865 | 0.004 |
| Postoperative CA125 (U/mL) | -0.416 | 0.178 | -2.333 | 0.020 |
| Preoperative Alb (g/L) | 0.077 | 0.158 | 0.490 | 0.624 |
| Postoperative Alb (g/L) | 0.381 | 0.209 | 1.818 | 0.070 |
| Preoperative TP (g/L) | 0.020 | 0.178 | 0.114 | 0.910 |
| Postoperative TP (g/L) | 0.018 | 0.168 | 0.108 | 0.914 |
| Preoperative Hb (g/L) | 0.060 | 0.132 | 0.455 | 0.649 |
| Postoperative Hb (g/L) | -0.068 | 0.131 | -0.523 | 0.601 |
| Preoperative K (mmol/L) | 0.244 | 3.504 | 0.070 | 0.945 |
| Postoperative K (mmol/L) | 0.437 | 1.239 | 0.353 | 0.724 |
Colon cancer is a malignancy that is associated with high global incidence and mortality rates[1]. In 2014, colon cancer accounted for approximately one out of every 10 cancers in China[18]. In the United States, colon cancer is among the top three cancers in terms of incidence and mortality[1]. The treatment of colon cancer treatment involves the implementation of various modalities, which are carefully planned based on factors such as the patient’s physical condition, tumor pathology, and invasion scope. Individualized and comprehensive treatment approaches are crucial in achieving efficient outcomes. Radical colon cancer surgery is a common clinical procedure that effectively removes diseased tissue. However, this procedure can lead to complications, such as anastomotic fistula, which significantly affects both the quality of life and survival rate of the patient[19]. Previous studies identified anastomotic fistulas as one of the most serious complications of colon surgery, resulting in prolonged hospital stays and increased treatment costs. Consistent with these findings, our study also found that patients with postoperative anastomotic fistula had a significantly longer average hospital stay compared with those without postoperative anastomotic fistula[20]. The incidence of anastomotic fistula after colon cancer surgery varies from 2.7% to 15.9%[21], with reported postoperative fistula-related mortality rates ranging from 0.8% to 27%[22-24]. Early identification of anastomotic fistulas is, therefore, crucial to reduce subsequent adverse events.
In the present study, we observed an incidence of postoperative anastomotic fistula of 7.8%, which is consistent with previous research findings. The incidence rates of anastomotic fistulas after laparoscopic and open surgeries were 5.1% and 11.8%, respectively, with a statistically significant difference between the two approaches. Our binary logistic regression analysis further confirmed that the choice of surgical approach independently influenced the incidence of postoperative anastomotic fistula. This can be attributed to the advantages of laparoscopic surgery, including smaller incisions, reduced risk of postoperative infection, and faster wound healing, compared with open surgery. The use of carbon dioxide pneumoperitoneum and laparoscopic magnification provides a clearer intraoperative field of view, allowing for more precise tumor localization and avoidance of important structures, such as the blood vessels and nerves. These factors contribute to a lower risk of postoperative anastomotic fistula[25]. Additionally, our binary logistic regression analysis identified patient age, TNM stage, postoperative stay in the ICU, and postoperative Alb, Hb, and K levels as independent risk factors for postoperative anastomotic fistula. Increasing age has been recognized as an important risk factor for surgical patients, with a linear increase in the risk of postoperative complications among patients aged 18 to 69 years and a nearly 10-fold increase in those aged 70 years and older. A consensus exists among domestic and international scholars that surgical tolerance decreases as patients age, leading to increased procedural uncontrollability. The elderly body gradually experiences a decline in the ability to absorb nutrients and perform metabolic functions, resulting in difficulties absorbing the essential nutrients necessary for postsurgical recovery. This difficulty in healing wounds can contribute to the development of anastomotic fistulas. TNM stage serves as an important index for assessing the severity of colon cancer. Higher TNM stages indicate more severe tumor infiltration, invasion of surrounding organs, and a decline in overall body function, indicating a higher risk of postoperative complications[26]. Research has shown that, during the recovery process after radical colorectal surgery, cells require a significant amount of oxygen and nutrients for adequate energy production. Serum Alb, which is associated with protein synthesis and plasma osmolality, is widely used in clinical practice to assess the nutritional level of patients. A lower postoperative Alb level is indicative of poorer nutritional status[27]. Amino acids and proteins play an important role in wound scar stabilization during the remodeling phase[28]. In patients with colon cancer, who often experience chronic wasting, surgical trauma, perioperative fasting, and significant fluid dilution through intravenous rehydration, the postoperative serum Alb level is further reduced. In the state of low Alb level, surgical wound exudate is increased. Prolonged fluid accumulation, coupled with the postoperative inflammatory state, affects the healing of the surgical wound and anastomosis, consequently increasing the risk of anastomotic fistulas. Therefore, in clinical practice, medical staff needs to have a clear understanding of the indications for surgery and ensure timely supplementation of proteins and energy to reduce the risk of postoperative anastomotic fistulas.
Furthermore, the results of the multiple linear regression analysis in the present study revealed that several factors independently influenced the prognosis of patients postoperatively, including the occurrence of postoperative anastomotic fistula, advanced age, presence of diabetes mellitus, lymph node metastasis, pathological type of the mucinous gland, high postoperative CEA and CA125 Levels, and high preoperative CA125 Levels. The presence of postoperative anastomotic fistula significantly reduced the survival rate of patients at one year postoperatively. Diabetes mellitus, a metabolic disease, can lead to abnormal protein metabolism, water-electrolyte imbalance, acid-base disorders, and abnormal fat metabolism. Uncontrolled or unstable diabetes disrupts glucose metabolism, resulting in insufficient energy supply to tissue cells, weakened biological barrier function, and compromised bactericidal ability of the immune system[29]. Surgical procedures themselves, both mental and physical as well as the effects of anesthetic drugs, can increase blood glucose levels, exacerbating the negative impact on patient prognosis. Studies have demonstrated that patients with tubular adenocarcinoma show the highest cumulative survival rates at 1, 3, and 5 years postoperatively, whereas those with mucinous adenocarcinoma and papillary carcinoma have lower cumulative survival rates at 3 and 5 years postoperatively[30]. Lymph node metastasis and mucinous adenocarcinoma contribute to poorer prognosis due to their higher malignancy, faster progression, and greater invasiveness.
In conclusion, numerous risk factors contribute to the development of anastomotic fistulas after radical colon cancer surgery. Therefore, healthcare professionals should carefully evaluate the patients’ clinical data before surgery. If a high-risk postoperative anastomotic fistula is identified, prompt communication with patients and their families is essential to consider alternative surgical approaches and improve patient prognosis.
This study has two main limitations. First, it is a retrospective cohort study, which may be susceptible to selection bias. Second, it is a single-center clinical study with a relatively small sample size. Future multicenter clinical studies are warranted to validate these findings.
Postoperative anastomotic fistula has a significant impact on prognosis and survival rates. Careful consideration of the clinical characteristics and risk factors associated with anastomotic fistula and implementation of individualized preventive measures at an early stage are crucial to reduce its occurrence. In this manner, we can improve patients’ prognosis and prolong their life expectancy.
Patients are prone to complications such as anastomotic fistula after radical colon cancer surgery.
Postoperative complications such as anastomotic fistulas have a significant negative impact on patient prognosis.
This study aimed to investigate the risk factors for postoperative anastomotic fistulas and their impact on the prognosis of patients with colon cancer.
This retrospective analysis of 488 patients with colon cancer who underwent radical surgery between April 2016 and April 2019 at our research center was summarized, and the risk factors for the development of anastomotic fistula and the impact of anastomotic fistula occurrence on patient prognosis were analyzed.
A total of 38 (7.8%) of 488 patients who underwent radical surgery for colon cancer had complications of postoperative anastomotic fistula with a mean Functional Assessment of Cancer Therapy-Colorectal score of 74.79 ± 11.86.
Based on the results of our study, we present the independent risk factors affecting the development of anastomotic fistulas and the prognosis of patients with colon cancer after radical surgery. The main causes and preventive measures are also described.
Based on the clinical data comparing patients who developed anastomotic fistulas with those who did not, the factors influencing the development of anastomotic fistulas in patients postoperatively were analyzed, and the prognoses of the two groups of patients were compared.
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11995] [Article Influence: 2998.8] [Reference Citation Analysis (9)] |
| 2. | Fabregas JC, Ramnaraign B, George TJ. Clinical Updates for Colon Cancer Care in 2022. Clin Colorectal Cancer. 2022;21:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 84] [Reference Citation Analysis (2)] |
| 3. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13332] [Article Influence: 1333.2] [Reference Citation Analysis (4)] |
| 4. | Gao W, Zheng Y, Zhang R, Liu G, Jian Y, Zhou H, Zhang Z, Chen S, Wu S, Chen W. Incidence of multiple myeloma in Kailuan cohort: A prospective community-based study in China. Cancer Epidemiol. 2022;78:102168. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (2)] |
| 5. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1856] [Reference Citation Analysis (5)] |
| 6. | Siegel RL, Jakubowski CD, Fedewa SA, Davis A, Azad NS. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am Soc Clin Oncol Educ Book. 2020;40:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 7. | Su Y, Tian X, Gao R, Guo W, Chen C, Jia D, Li H, Lv X. Colon cancer diagnosis and staging classification based on machine learning and bioinformatics analysis. Comput Biol Med. 2022;145:105409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (1)] |
| 8. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3439] [Article Influence: 491.3] [Reference Citation Analysis (4)] |
| 9. | Hou W, Yi C, Zhu H. Predictive biomarkers of colon cancer immunotherapy: Present and future. Front Immunol. 2022;13:1032314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 10. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Garrido-Laguna I, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedman-Cass DA. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 725] [Article Influence: 90.6] [Reference Citation Analysis (3)] |
| 11. | Ouyang M, Luo Z, Wu J, Zhang W, Tang S, Lu Y, Hu W, Yao X. Comparison of outcomes of complete mesocolic excision with conventional radical resection performed by laparoscopic approach for right colon cancer. Cancer Manag Res. 2019;11:8647-8656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 12. | Zouari A, Masmoudi A, Khanfir F, Ketata S, Rejab H, Bouzid A, Loukil I, Zribi I, Talbi S, Abdelhedi A, Abid B, Boujelben S. [Predictive factors for anastomotic leakage after colon cancer surgery]. Pan Afr Med J. 2022;42:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 13. | Kulikov EP, Kaminsky YD, Klevtsova SV, Nosov SA, Kholchev MY, Aristarkhov VG, Mertsalov SA. [Prevention of colorectal anastomotic leakage in patients with rectal cancer]. Khirurgiia (Mosk). 2019;64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 14. | Pop MG, Fit AM, Vesa SC, Bartos A, Bartos DM, Corpadean AG, Puia C, Al-Hajjar N, Cornel I. Predictors of 1-year postoperative mortality in radical colon cancer surgery. Ann Ital Chir. 2018;89:507-512. [PubMed] |
| 15. | Guo Y, Wang D, He L, Zhang Y, Zhao S, Zhang L, Sun X, Suo J. Marginal artery stump pressure in left colic artery-preserving rectal cancer surgery: a clinical trial. ANZ J Surg. 2017;87:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Zarnescu EC, Zarnescu NO, Costea R. Updates of Risk Factors for Anastomotic Leakage after Colorectal Surgery. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 17. | AlFayyad I, Al-Tannir M, Howaidi J, AlTannir D, Abu-Shaheen A. Health-related quality of life of breast and colorectal cancer patients undergoing active chemotherapy treatment: Patient-reported outcomes. Qual Life Res. 2022;31:2673-2680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 18. | Wang X, Wei Q, Gao J, Li J, Gong J, Li Y, Shen L. Clinicopathologic features and treatment efficacy of Chinese patients with BRAF-mutated metastatic colorectal cancer: a retrospective observational study. Chin J Cancer. 2017;36:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Ozmen I, Grupa VEM, Bedrikovetski S, Dudi-Venkata NN, Huisman DE, Reudink M, Slooter GD, Sammour T, Kroon HM, Daams F; LekCheck Study Group. Risk Nomogram Does Not Predict Anastomotic Leakage After Colon Surgery Accurately: Results of the Multi-center LekCheck Study. J Gastrointest Surg. 2022;26:900-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 20. | Limaiem F, Azzabi S, Sassi A, Mzabi S, Bouraoui S. Colorectal cancer in young adults: a retrospective study of 32 tunisian patients. Pan Afr Med J. 2018;31:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Marra F, Steffen T, Kalak N, Warschkow R, Tarantino I, Lange J, Zünd M. Anastomotic leakage as a risk factor for the long-term outcome after curative resection of colon cancer. Eur J Surg Oncol. 2009;35:1060-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Akasu T, Takawa M, Yamamoto S, Yamaguchi T, Fujita S, Moriya Y. Risk factors for anastomotic leakage following intersphincteric resection for very low rectal adenocarcinoma. J Gastrointest Surg. 2010;14:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Yu XN, Xu LM, Bin YW, Yuan Y, Tian SB, Cai B, Tao KX, Wang L, Wang GB, Wang Z. Risk Factors of Anastomotic Leakage After Anterior Resection for Rectal Cancer Patients. Curr Med Sci. 2022;42:1256-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Boccola MA, Lin J, Rozen WM, Ho YH. Reducing anastomotic leakage in oncologic colorectal surgery: an evidence-based review. Anticancer Res. 2010;30:601-607. [PubMed] |
| 25. | Trencheva K, Morrissey KP, Wells M, Mancuso CA, Lee SW, Sonoda T, Michelassi F, Charlson ME, Milsom JW. Identifying important predictors for anastomotic leak after colon and rectal resection: prospective study on 616 patients. Ann Surg. 2013;257:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 240] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 26. | Imran J, Yao JJ, Madni T, Huerta S. Current concepts on the distal margin of resection of rectal cancer tumors after neoadjuvant chemoradiation. Curr Colorectal Cancer Rep. 2017;13:1-9. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 27. | Bauer JD, Isenring E, Waterhouse M. The effectiveness of a specialised oral nutrition supplement on outcomes in patients with chronic wounds: a pragmatic randomised study. J Hum Nutr Diet. 2013;26:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Quain AM, Khardori NM. Nutrition in Wound Care Management: A Comprehensive Overview. Wounds. 2015;27:327-335. [PubMed] |
| 29. | Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2142] [Cited by in RCA: 2089] [Article Influence: 139.3] [Reference Citation Analysis (1)] |
| 30. | Shi Z, Mei X, Li C, Chen Y, Zheng H, Wu Y, Liu L, Marcantonio ER, Xie Z, Shen Y. Postoperative Delirium Is Associated with Long-term Decline in Activities of Daily Living. Anesthesiology. 2019;131:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kolli S, United States; Silveira FC, United States S-Editor: Yan JP L-Editor: A P-Editor: Zhang YL