Published online Jan 27, 2023. doi: 10.4240/wjgs.v15.i1.9
Peer-review started: September 20, 2022
First decision: October 21, 2022
Revised: November 20, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 27, 2023
Processing time: 119 Days and 19.8 Hours
The post-hepatectomy recurrence rate of hepatocellular carcinoma (HCC) is persistently high, affecting the prognosis of patients. An effective therapeutic option is crucial for achieving long-term survival in patients with postoperative recurrences. Local ablative therapy has been established as a treatment option for resectable and unresectable HCCs, and it is also a feasible approach for recurrent HCC (RHCC) due to less trauma, shorter operation times, fewer complications, and faster recovery. This review focused on ablation techniques, description of potential candidates, and therapeutic and prognostic implications of ablation for guiding its application in treating intrahepatic RHCC.
Core Tip: The high recurrence rate of hepatocellular carcinoma (HCC) remains a global health challenge, which urges close surveillance following hepatectomy for earlier detection of recurrent HCC. Unlike primary HCC, recurrent HCCs are usually detected in the early stage but are not amenable to repeat hepatectomy after comprehensive evaluation. The value of ablation as a minimally invasive but curative method is an increasing concern. We herein discuss the role of various ablation modalities and procedures in treating intrahepatic recurrent HCC for guiding its better application.
- Citation: Cong R, Ma XH, Wang S, Feng B, Cai W, Chen ZW, Zhao XM. Application of ablative therapy for intrahepatic recurrent hepatocellular carcinoma following hepatectomy. World J Gastrointest Surg 2023; 15(1): 9-18
- URL: https://www.wjgnet.com/1948-9366/full/v15/i1/9.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i1.9
Hepatocellular carcinoma (HCC), with high morbidity, mortality, and recurrence rates, remains a global health challenge[1]. Surgical resection is considered the main strategy for long-term survival of patients with HCC. However, the incidence of recurrence reaches approximately 70% 5 years after hepatectomy, even in patients with a single tumor ≤ 2 cm[2]. Advances in preoperative prediction and postoperative follow-up strategies have facilitated the earlier detection of recurrent HCC (RHCC)[3-5], allowing for more treatment options. Thus, an appropriate therapeutic option is crucial for achieving long-term survival of patients with recurrence after surgery, which requires a comprehensive understanding of possible treatments and thorough evaluation of the patient.
With the necessity to fully consider the initial treatment, the clinicopathologic characteristics of primary HCC, recurrence interval, the characteristics of RHCC, general condition of the patient's liver, and other factors[6,7], treating RHCC cannot exactly follow the guidelines for primary HCC. Considering that inadequacy of residual liver volume, postoperative liver decompensation, intra-abdominal adhesions and anatomical variation following initial resection increase difficulty and risk of re-resection, only about 19% of well-selected patients can receive secondary surgery for a definite survival benefit in clinical practice[8,9]. Ablation as a curative but less invasive treatment may be considered in the management of RHCC.
Local ablative therapy has been established as a treatment option for resectable and unresectable HCCs according to current clinical guidelines[3,10], which can provide a sustained complete response, a lower complication rate, and a 5-year survival rate of 68.5% for early HCC, even initially operable HCC[11]. The extensive and promising application of ablation in primary HCC makes it a feasible approach for the treatment of intrahepatic RHCC. This review demonstrated the role of ablation in treating RHCC, focusing on different ablative techniques, descriptions of potential candidates, as well, therapeutic and prognostic implications for guiding its better application.
Radiofrequency ablation (RFA) is the most commonly used modality for treating both primary and recurrent HCC. Meanwhile, RFA has gained an increasing role owing to its efficacy and safety. When the electrode tip is inserted into the selected tissue to generate electric current, RFA induces ionic agitation, local heat, and subsequent coagulation necrosis[12]. Some factors, such as centrifugal heat propagation, “heat-sink effect” mediated by blood perfusion, and increased impedance due to tissue charring limit the size of the ablation zone and reduce the efficacy[13]. These also have driven continuous device and procedure improvements: Multi-tined expandable electrodes, internally cooled electrodes, multipolar ablation using bipolar electrodes, and simultaneous vessel obstruction[13-15].
For intrahepatic recurrent HCC after hepatectomy, the indications for RFA[16-18] are as follows: Within the Milan criteria at recurrence, satisfying a single lesion (≤ 5 cm in diameter) or three or fewer lesions (each ≤ 3 cm in diameter) without macrovascular invasion or distant metastasis; Child-Pugh grade A or B liver function; Eastern Cooperative Oncology Group performance score of 0 to 1; no uncorrectable coagulation status; no severe varices and intractable ascites; and an acceptable and safe path evaluated by imaging.
Bai et al[18] analyzed the long-term survival of solitary RHCC of 5 cm or less after RFA, and the rates of primary technical success, local tumor progression (LTP), and 1-, 3-, 5-, and 10-year overall survival (OS) post ablation were 94.8%, 11.2%, 94.0%, 71.8%, 54.5%, and 33.7%, respectively, in the RHCC following hepatectomy subgroup, which was similar to primary HCC of 5 cm or less after RFA. The safety and efficacy of RFA for RHCC are being gradually affirmed by clinical studies, and an increasing number of retrospective studies comparing repeat hepatectomy and RFA, especially for early stage RHCC, have been reported in recent years. The comparison outcomes of survival between the two groups are conflicting, with inherent selection biases, either equivocal or favorable for one. The majority reported that RFA provided similar OS to repeat hepatectomy for RHCC, with 5-year OS rates of 26%-71%, but with fewer major complications (0%-1.6% vs 2.6%-9.1%) and shorter hospital stays (3-5 d vs 8-14 d)[19-25].
Xia et al[17] conducted a randomized clinical trial for comparing long-term survival results following repeat hepatectomy with those following percutaneous RFA in 240 patients with early stage RHCC. They found no significant difference in the 1-, 3-, and 5-year OS rates between the two groups (92.5%, 65.8%, and 43.6% vs 87.5%, 52.5%, and 38.5%, respectively). However, RFA was linked to a greater risk of local repeat recurrence and early repeat recurrence than repeat hepatectomy, consistent with the findings of a retrospective multicenter study[25] which concluded that repeat hepatectomy for RHCC within the Milan criteria resulted in longer recurrence-free survival and less frequent early repeat recurrence (less than 12 mo). The rate of inaccurate ablation and the possibility of the presence of satellite nodules increase as the target size of RFA increases in general, leading to an inferior to repeat hepatectomy for local tumor control and a tendency toward a shorter recurrence-free survival of RFA.
A number of factors reported previously were associated with worse survival of RHCC following treatment, including larger and multiple resected tumors, the presence of microvascular invasion (MVI) at initial hepatectomy stage, time to recurrence (TTR) ≤ 1 year, poor Child-Pugh class, portal hypertension, serum-fetoprotein (AFP) level greater than 200 ng/mL, larger and multiple RHCC at recurrent stage, etc[18,21-26]. These factors resulted in a higher tumor burden, poorer liver function, and more aggressive behavior, which needed to be considered for appropriate therapeutic strategies.
Xia et al[17] found that percutaneous RFA ablation was related to worse local tumor control and OS than repeat hepatectomy in patients with target diameter > 3 cm or AFP level > 200 ng/mL. Small ablated tumors (≤ 3 cm) can achieve higher complete response rates of > 95%[16,26,27]. For larger tumors (> 3 cm), an overlapping ablation strategy, other ablation modalities, or combination of transarterial chemoembolization (TACE) and RFA were required to produce ablation zones more reliably and sufficiently[28].
A previous study[29] focused on RHCC with MVI-positivity at initial hepatectomy and concluded that repeat surgery/RFA can provide a better survival outcome for selected BCLC stage 0-A patients than TACE, which was contrary to the results of Meniconi et al[6] and Jin et al[30] They concluded that TACE seemed more appropriate than curative treatments in a small sample of early stage MVI-positive HCC. Early recurrence (TTR ≤ 1 or 2 years) is generally related to intrahepatic metastases, MVI, and microsatellite lesions generated by primary HCC, with poor survival after hepatectomy[31]. Yang et al[32] reported that patients with late recurrence (> 1 year) had better survival outcomes after RFA than those with early recurrence (≤ 1 year). The comparison between repeat hepatectomy and RFA for RHCC with different TTR was conducted in a limited number of studies. Liang et al[19] and Xia et al[17] found that the OS was similar between the two treatments in patients with a TTR ≤ 1 year or > 1 year. Lu et al[33] showed that the post-recurrence survival rates for the repeat hepatectomy group were better than those for the RFA group of patients with early recurrence (TTR ≤ 2 years). However, no significant difference was found in the late recurrence group (TTR > 2 years). Sequential TACE and RFA were found to offer a better OS for patients with recurrence ≤ 1 year than RFA alone, but not for those with recurrence for more than 1 year[28]. With the different results of limited studies, treatments for these particular populations will be required further investigation.
The morbidity and mortality of RFA are obviously lower than those observed following repeat hepatectomy for RHCC, while the rate of complications increases when performing more aggressive procedures for larger tumors and targets at-risk location or at poor liver and general condition. Pain and fever post-ablation are common but remain short after symptomatic treatment. The major complications of RFA include pneumonia, pneumothorax, pleural effusion, hemoperitoneum, ascites, liver hematoma, liver abscess, subdiaphragmatic abscess, liver failure, injury or perforation of adjacent structures such as diaphragm, gallbladder, colon or stomach, ileus, wound or puncture site infection and tumor seeding[17,18,25]. A reasonable RFA protocol for well-selected patients is crucial for protecting surrounding tissues and preventing complications.
Microwave ablation (MWA), an emerging alternative modality to RFA, causes thermal coagulation by utilizing microwaves at a frequency of 2450 MHz to induce the vibration and rotation of water molecules within the tissue and subsequent heat generation[34]. MWA have theoretical advantages over RFA including a higher temperature, a faster heating of a larger target, a less “heat-sink effect” and insensitivity to tissue conductance[13]. The first-generation MWA was initially limited by technical problems related to sub-optimal power handling, large antenna diameter and antenna shaft heating. Its resulting ablation zone is small and more elliptic[35,36]. Thus new-generation MWA have developed and simultaneous power delivery technique of multiple antennas has been tried for producing reliable and large spherical ablation zone[37,38]. Zhang et al[39] evaluated the efficacy of US-guided percutaneous MWA for RHCC measuring ≤ 5 cm and get 5- and 7-year OS rates of 39.6% and 17.3%, respectively. Ryu et al[40] performed MWA during open surgery in 75 patients with intrahepatic recurrence after hepatectomy and identified MWA as a safe and feasible procedure, which provided a 5-year survival rate of 55.4%, comparable to results reported previously for re-resection, RFA, and MWA for primary HCC. The application of MWA in RHCC was slowly being recognized, and more data will be needed to demonstrate its value for larger RHCC and its efficacy over RFA.
Ethanol injected into the tissue induces coagulation necrosis mainly because of its dehydronative and protein degenerative effects and partly because of its thromboembolic effect[41]. Percutaneous ethanol injection (PEI) could be precisely applied to ablate HCC ≤ 2 cm in diameter, but the necrosis rate is reduced and the local recurrence rate increases for larger tumors[42]. Compared to thermal ablation, it is inexpensive and has a low rate of adverse effects even for patients with Child-Pugh class C or tumors at risk locations; however, repeated injections are often required for effective treatment. These characteristics have promoted its application in combination therapies[43]. Yin et al[27] treated 288 patients with post-hepatectomy RHCC (maximum diameter ≤ 7 cm and number ≤ 5) using PEI, RFA, MWA, or PEI combined with RFA. The incidence of LTP in the PEI group was 19.5% and no significant difference was found among the four ablative modalities. However, selection bias existed, and the authors did not focus on comparing the efficiencies of the different techniques.
High-intensity focused ultrasound ablation (HIFU) ablation is an extracorporeal conformal therapy that can achieve heat-induced coagulation necrosis without the need for surgical exposure or probe insertion. Heat generation is mediated by focusing high-intensity ultrasound beams on the target using the extracorporeal motion of a multi-element ultrasound transducer. HIFU, which is noninvasive and conformal, can ablate a large volume of tumor with no worry of tumor seeding along the needle tract[44]. The value of HIFU or HIFU combined with TACE in unresectable HCC has been previously reported[44,45]. A study[46] showed that HIFU was a safe and feasible treatment modality for RHCC with an acceptably low morbidity rate and a comparable survival outcome to RFA, which was conducted among a small number of patients meeting the Milan criteria. HIFU have not get widespread adoption yet, probably as ultrasound propagation influenced by different tissues, ultrasound artifacts and respiration motion add time consumption and technical challenge relative to other ablation modalities[47]. There is no additional clinical data with HIFU for RHCC currently.
Cryoablation (CRA) is a thermal technique that uses cryoprobes to transfer low temperatures caused by the Joule-Thomson effect with super-cooled gas or liquid expansion, and achieves tissue necrosis by alternating cycles of freezing and thawing, which induces denaturation of cellular proteins, cell membrane rupture, cell dehydration, and ischemic hypoxia[48]. Cryoshock, a severe adverse event associated with multiorgan failure post-CRA, has been reported in previous studies, but the new generation of cryoablation systems with ultrathin cryoprobes that use argon-helium may lead to a low risk of bleeding and cryoshock[49]. The main advantage of CRA over heat-based ablation modalities is a well-visualized ice ball on ultrasound (US), computed tomography (CT), or magnetic resonance imaging (MRI) during ablation for precise monitoring, which contributes to the potential value of cryoablation for targets larger or close to important structures[48]. A multicenter randomized controlled trial showed a significantly lower LTP after CRA than after RFA for HCCs sized 3.1-4.0 cm[50]. For RHCC, Chen et al[51] used percutaneous CRA to treat 76 tumors (≤ 7 cm) in 26 recurrent patients and confirmed its efficacy with 1- and 3-year OS rates of 70.2% and 28.8%, respectively; however, further research is insufficient.
Irreversible electroporation (IRE) works by short pulses of high intensity delivered between two electrodes (convergent centripetal technique), which produce irreversible pores in the cellular bilayer membrane for cell death, while the connective tissue, blood vessels, and bile ducts are preserved. It is a nonthermal ablative method with no influence of the “heat-sink effect”, a lower risk of thermal injury, and less frequent liver failure[13]. Therefore, it can be considered for the treatment of dangerous sites and poor liver function[52]. This procedure can only be performed in patients with normal cardiac rhythm, because high-intensity pulses can cause myoclonia and severe arrhythmias. Overall, IRE could be indicated for a wider range of candidates than thermal techniques with consideration of patient condition, cost, and operational complexity, although more clinical data are required to validate its efficacy.
Various ablation modalities have their advantages and limitations (Table 1). RFA has been confirmed to be effective and used for RHCC with an increasing frequency; however, available data on other ablation modalities are insufficient, and limited studies have sought to directly compare the effects of various ablation techniques for treating RHCC.
| Ablation modalities | Advantages | Limitations |
| RFA[13,14] | Most widely used and mature technology | Limited zone of monopolar centrifugal ablation |
| Multibipolar RFA for larger and more modulable ablation zones | Sensitive to heat sink effect | |
| Influenced by tissue conductance | ||
| MWA[13,14] | Higher temperature and faster heating of larger target over RFA | Complex and technically demanding operation |
| Less sensitive to heat sink effect | Thermal injury from higher temperature | |
| Less influenced by tissue conductance | ||
| PEI[42] | Simple to perform, inexpensive | Small size of ablation zone |
| Chemo-ablation: No thermal injury | High local recurrence rate | |
| HIFU[47] | Noninvasive operation: No worry of needle tract seeding | Time consuming |
| Influenced by ultrasoundpropagation and artifacts, respiration motion | ||
| CRA[13,48] | Less pain | High cost |
| Well-visualized ice ball on imaging for precise monitoring | Cryoshock (more often in early device) | |
| IRE[13,14] | Nonthermal ablation: low risk of thermal injury | Risk of myoclonia and arrhythmias |
| Less sensitive to heat-sink effect | Limited clinical data | |
| Well preserved connective tissue, blood vessels and bile ducts | ||
| Less frequent liver failure |
Various combinations of treatments have been explored to improve the local tumor control and survival outcomes of ablation. The available experience with ablation combination therapy for RHCC has mainly focused on RFA.
Ethanol injection can reduce the “heat-sink effect” by destroying vessels within or around the tumors and promoting thermal conduction by lowering the extent of carbonization of the tissue. Therefore, RFA started after PEI completion could induce an enlarged ablation zone with an adequate safety margin compared with RFA alone, improving local control and reducing distant recurrence[53,54]. Chen et al[43] retrospectively compared the efficacy and safety of RFA and PEI (RFA-PEI) with repeat hepatectomy in elderly patients (≥ 70 years) with RHCC within the Milan criteria after initial surgery. The 1-, 3-, and 5-year OS and RFS rates after RFA-PEI were 78.2%, 40.8%, and 36.7%, and 69.5%, 37.8%, and 33.1%, respectively, comparable to those of repeat hepatectomy. They confirmed the good efficacy and high safety of RFA-PEI for RHCC, even for patients with poor performance status who urgently require minimally invasive treatments.
Because occlusion of blood flow by TACE before RFA reduces the “heat-sink effect” and the hyper
The combination with systemic therapy has been considered effective to impede rapid progression of residual tumors due to inadequate RFA and control advanced HCC[57]. Peng et al[58] investigated the role of Sorafenib combined with TACE-RFA in the treatment of advanced RHCC after initial hepatectomy and proved its safety, efficacy and superior survival outcomes over sorafenib alone. These benefits might be due to Sorafenib suppressing angiogenesis induced by TACE or inadequate RFA. The combination of RFA and immunotherapy is also considered rationale. Ablation boosts the T cell immune response to improve the efficacy of immunotherapy and immune checkpoint inhibitors block immune escape to reduce recurrence after ablation[59]. A retrospective study[60] reported that patients with RHCC had significantly better RFS and OS outcomes in the RFA plus anti-PD-1 group than in the RFA alone group. However, additional trials are required to confirm these interesting findings.
Ablation procedures can be performed percutaneously, laparoscopically, or at open surgery, using various imaging guidance techniques, including US, CT, or MRI. In general, ablation is appropriate for treating lesions within the Milan criteria and distant from the adjacent organs. In addition to the above-mentioned ablation modalities and combination treatments, multiple options of performing paths, guidance strategies, and other technical advances may allow extensive access to curative ablation therapy, especially for patients with a poor profile and tumors with large size, invisibility on US, or risk location.
Laparoscopy and laparotomy over percutaneous RFA provide greater exposure and more direct observation of the tumor and surrounding structures and can be used to temporarily occlude blood flow to increase the ablation zone. Santambrogio et al[61] performed laparoscopic thermal ablation for the treatment of intrahepatic RHCCs (within Milan criteria) that required repeated punctures or adjacent to visceral structures. Laparoscopic ablation was proposed as a safe and effective treatment for RHCC, leading to survival and DFS rates similar to those of primary HCC patients undergoing laparoscopic ablation without increasing morbidity. Contrast-enhanced US, CT, MRI, and image fusion can better delineate the target and final extent of the ablation zone, remedying the limitation of lesion invisibility in conventional US. Song et al[62] and Zhao et al[63] performed US-CT/MRI fusion–guided RFA for recurrent HCC that was subcentimeter or invisible on US, and both achieved technical success and efficacy rates of over 94%. Lin et al[64] conducted MWA guided by enhanced liver-specific MRI in 18 patients with small RHCC and achieved 100% technical success rate.
Furthermore, the creation of artificial ascites or artificial pleural effusion, balloon catheter interposition, three-dimensional visualization technology, fluoroscopic real-time guidance, and other assistive techniques are all effective in ablation safety, a high rate of success, and expansion of indications for ablation[65-68].
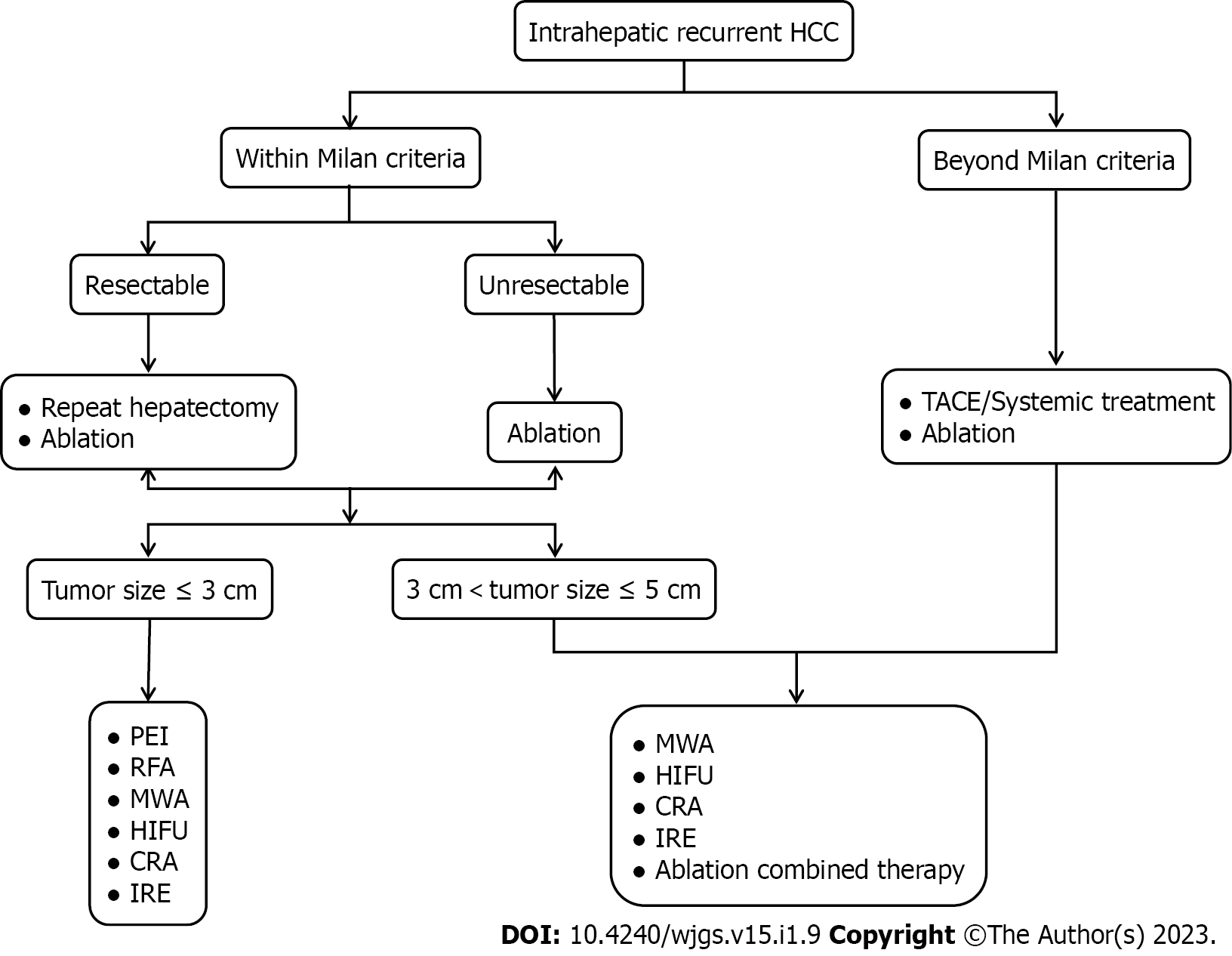
The role of ablation in intrahepatic RHCC was shown in Figure 1. Unlike primary HCC, RHCCs are usually detected in the early stage but are not amenable to repeat hepatectomy with consideration of inadequate liver remnants, limited liver function reserves, and technical difficulties due to adhesions following initial surgery. The value of ablation as a minimally invasive but curative method is an increasing concern. For patients who are eligible for ablation and repeat hepatectomy, clinicians need to balance the worse local control and lower major complication rates or shorter hospital stays when making ablation decisions. Various ablation modalities and procedures are continuously improving, and combination strategies may add additional benefits, which promote the extended application of ablative therapy. Further exploration of a particular population with risk prognostic factors and sufficient experience on the efficacy of different ablation modalities and techniques in treating RHCC are required and based on randomized clinical trials with larger sample sizes. Moreover, evidence that ablation could boost the immune response raises expectations for its combination with immunotherapy for advanced RHCC.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56688] [Article Influence: 7086.0] [Reference Citation Analysis (135)] |
| 2. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4366] [Article Influence: 545.8] [Reference Citation Analysis (6)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6423] [Article Influence: 802.9] [Reference Citation Analysis (9)] |
| 4. | Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, Tada T, Chong CCN, Xiang BD, Li LQ, Lai PBS, Mazzaferro V, García-Fiñana M, Kudo M, Kumada T, Roayaie S, Johnson PJ. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69:1284-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 427] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 5. | Ji GW, Zhu FP, Xu Q, Wang K, Wu MY, Tang WW, Li XC, Wang XH. Radiomic Features at Contrast-enhanced CT Predict Recurrence in Early Stage Hepatocellular Carcinoma: A Multi-Institutional Study. Radiology. 2020;294:568-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 6. | Meniconi RL, Komatsu S, Perdigao F, Boëlle PY, Soubrane O, Scatton O. Recurrent hepatocellular carcinoma: a Western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery. 2015;157:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Zou Q, Li J, Wu D, Yan Z, Wan X, Wang K, Shi L, Lau WY, Wu M, Shen F. Nomograms for Pre-operative and Post-operative Prediction of Long-Term Survival of Patients Who Underwent Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma. Ann Surg Oncol. 2016;23:2618-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Yoh T, Seo S, Taura K, Iguchi K, Ogiso S, Fukumitsu K, Ishii T, Kaido T, Uemoto S. Surgery for Recurrent Hepatocellular Carcinoma: Achieving Long-term Survival. Ann Surg. 2021;273:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 9. | Goh BKP, Syn N, Teo JY, Guo YX, Lee SY, Cheow PC, Chow PKH, Ooi LLPJ, Chung AYF, Chan CY. Perioperative Outcomes of Laparoscopic Repeat Liver Resection for Recurrent HCC: Comparison with Open Repeat Liver Resection for Recurrent HCC and Laparoscopic Resection for Primary HCC. World J Surg. 2019;43:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 3135] [Article Influence: 783.8] [Reference Citation Analysis (61)] |
| 11. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 834] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 12. | Hong K, Georgiades C. Radiofrequency ablation: mechanism of action and devices. J Vasc Interv Radiol. 2010;21:S179-S186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Nault JC, Sutter O, Nahon P, Ganne-Carrié N, Séror O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol. 2018;68:783-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 295] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 14. | Seror O. Ablative therapies: Advantages and disadvantages of radiofrequency, cryotherapy, microwave and electroporation methods, or how to choose the right method for an individual patient? Diagn Interv Imaging. 2015;96:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Kobayashi M, Ikeda K, Kawamura Y, Hosaka T, Sezaki H, Yatsuji H, Akuta N, Suzuki F, Suzuki Y, Arase Y, Kumada H. Randomized controlled trial for the efficacy of hepatic arterial occlusion during radiofrequency ablation for small hepatocellular carcinoma--direct ablative effects and a long-term outcome. Liver Int. 2007;27:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Choi D, Lim HK, Rhim H, Kim YS, Yoo BC, Paik SW, Joh JW, Park CK. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14:2319-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, Yang T, Yan Z, Lei Z, Si A, Wan X, Zhang H, Gao C, Cheng Z, Pawlik TM, Wang H, Lau WY, Wu M, Shen F. Long-term Effects of Repeat Hepatectomy vs Percutaneous Radiofrequency Ablation Among Patients With Recurrent Hepatocellular Carcinoma: A Randomized Clinical Trial. JAMA Oncol. 2020;6:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 18. | Bai XM, Cui M, Yang W, Wang H, Wang S, Zhang ZY, Wu W, Chen MH, Yan K, Goldberg SN. The 10-year Survival Analysis of Radiofrequency Ablation for Solitary Hepatocellular Carcinoma 5 cm or Smaller: Primary versus Recurrent HCC. Radiology. 2021;300:458-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Liang HH, Chen MS, Peng ZW, Zhang YJ, Zhang YQ, Li JQ, Lau WY. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol. 2008;15:3484-3493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Chan AC, Poon RT, Cheung TT, Chok KS, Chan SC, Fan ST, Lo CM. Survival analysis of re-resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg. 2012;36:151-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Song KD, Lim HK, Rhim H, Lee MW, Kim YS, Lee WJ, Paik YH, Gwak GY, Kim JM, Kwon CH, Joh JW. Repeated Hepatic Resection versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma after Hepatic Resection: A Propensity Score Matching Study. Radiology. 2015;275:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Sun WC, Chen IS, Liang HL, Tsai CC, Chen YC, Wang BW, Lin HS, Chan HH, Hsu PI, Tsai WL, Cheng JS. Comparison of repeated surgical resection and radiofrequency ablation for small recurrent hepatocellular carcinoma after primary resection. Oncotarget. 2017;8:104571-104581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Yin X, Hua T, Liang C, Chen Z. Efficacy of re-resection versus radiofrequency ablation for recurrent Barcelona Clinic Liver Cancer stage 0/A hepatocellular carcinoma (HCC) after resection for primary HCC. Transl Cancer Res. 2019;8:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Feng Y, Wu H, Huang DQ, Xu C, Zheng H, Maeda M, Zhao X, Wang L, Xiao F, Lv H, Liu T, Qi J, Li J, Zhong N, Wang C, Feng H, Liang B, Ren W, Qin C, Nguyen MH, Zhu Q. Radiofrequency ablation versus repeat resection for recurrent hepatocellular carcinoma (≤ 5 cm) after initial curative resection. Eur Radiol. 2020;30:6357-6368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 25. | Zhong JH, Xing BC, Zhang WG, Chan AW, Chong CCN, Serenari M, Peng N, Huang T, Lu SD, Liang ZY, Huo RR, Wang YY, Cescon M, Liu TQ, Li L, Wu FX, Ma L, Ravaioli M, Neri J, Cucchetti A, Johnson PJ, Li LQ, Xiang BD. Repeat hepatic resection versus radiofrequency ablation for recurrent hepatocellular carcinoma: retrospective multicentre study. Br J Surg. 2021;109:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Lu MD, Yin XY, Xie XY, Xu HX, Xu ZF, Liu GJ, Kuang M, Zheng YL. Percutaneous thermal ablation for recurrent hepatocellular carcinoma after hepatectomy. Br J Surg. 2005;92:1393-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Yin XY, Xie XY, Lu MD, Kuang M, Liu GJ, Xu ZF, Xu HX, Wang Z. Percutaneous ablative therapies of recurrent hepatocellular carcinoma after hepatectomy: proposal of a prognostic model. Ann Surg Oncol. 2012;19:4300-4306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 29. | Xiao H, Chen ZB, Jin HL, Li B, Xu LX, Guo Y, Chen SL, Li HP, Peng ZW, Shen JX. Treatment selection of recurrent hepatocellular carcinoma with microvascular invasion at the initial hepatectomy. Am J Transl Res. 2019;11:1864-1875. [PubMed] |
| 30. | Jin YJ, Lee JW, Lee OH, Chung HJ, Kim YS, Lee JI, Cho SG, Jeon YS, Lee KY, Ahn SI, Shin WY. Transarterial chemoembolization versus surgery/radiofrequency ablation for recurrent hepatocellular carcinoma with or without microvascular invasion. J Gastroenterol Hepatol. 2014;29:1056-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 758] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 32. | Yang W, Chen MH, Yin SS, Yan K, Gao W, Wang YB, Huo L, Zhang XP, Xing BC. Radiofrequency ablation of recurrent hepatocellular carcinoma after hepatectomy: therapeutic efficacy on early- and late-phase recurrence. AJR Am J Roentgenol. 2006;186 Suppl 5:S275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Lu LH, Mei J, Kan A, Ling YH, Li SH, Wei W, Chen MS, Zhang YF, Guo RP. Treatment optimization for recurrent hepatocellular carcinoma: Repeat hepatic resection versus radiofrequency ablation. Cancer Med. 2020;9:2997-3005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192-S203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 508] [Article Influence: 31.8] [Reference Citation Analysis (2)] |
| 35. | Meloni MF, Chiang J, Laeseke PF, Dietrich CF, Sannino A, Solbiati M, Nocerino E, Brace CL, Lee FT Jr. Microwave ablation in primary and secondary liver tumours: technical and clinical approaches. Int J Hyperthermia. 2017;33:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 36. | Head HW, Dodd GD 3rd. Thermal ablation for hepatocellular carcinoma. Gastroenterology. 2004;127:S167-S178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Harari CM, Magagna M, Bedoya M, Lee FT Jr, Lubner MG, Hinshaw JL, Ziemlewicz T, Brace CL. Microwave Ablation: Comparison of Simultaneous and Sequential Activation of Multiple Antennas in Liver Model Systems. Radiology. 2016;278:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Imajo K, Tomeno W, Kanezaki M, Honda Y, Kessoku T, Ogawa Y, Yoshida K, Yoneda M, Kirikoshi H, Ono M, Kaneta T, Inoue T, Teratani T, Saito S, Nakajima A. New microwave ablation system for unresectable liver tumors that forms large, spherical ablation zones. J Gastroenterol Hepatol. 2018;33:2007-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Zhang TT, Luo HC, Cui X, Zhang W, Zhang LY, Chen XP, Li KY. Ultrasound-guided percutaneous microwave ablation treatment of initial recurrent hepatocellular carcinoma after hepatic resection: long-term outcomes. Ultrasound Med Biol. 2015;41:2391-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Ryu T, Takami Y, Wada Y, Hara T, Sasaki S, Saitsu H. Efficacy of surgical microwave ablation for recurrent hepatocellular carcinoma after curative hepatectomy. HPB (Oxford). 2020;22:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Shiina S, Tagawa K, Unuma T, Terano A. Percutaneous ethanol injection therapy for the treatment of hepatocellular carcinoma. AJR Am J Roentgenol. 1990;154:947-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 432] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 43. | Chen S, Peng Z, Xiao H, Lin M, Chen Z, Jiang C, Hu W, Xie X, Liu L, Peng B, Kuang M. Combined radiofrequency ablation and ethanol injection versus repeat hepatectomy for elderly patients with recurrent hepatocellular carcinoma after initial hepatic surgery. Int J Hyperthermia. 2018;34:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Wu F, Wang ZB, Chen WZ, Zhu H, Bai J, Zou JZ, Li KQ, Jin CB, Xie FL, Su HB. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol. 2004;11:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 189] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 45. | Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, Li KQ, Jin CB, Xie FL, Su HB. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology. 2005;235:659-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Chan AC, Cheung TT, Fan ST, Chok KS, Chan SC, Poon RT, Lo CM. Survival analysis of high-intensity focused ultrasound therapy versus radiofrequency ablation in the treatment of recurrent hepatocellular carcinoma. Ann Surg. 2013;257:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Dubinsky TJ, Cuevas C, Dighe MK, Kolokythas O, Hwang JH. High-intensity focused ultrasound: current potential and oncologic applications. AJR Am J Roentgenol. 2008;190:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 48. | Song KD. Percutaneous cryoablation for hepatocellular carcinoma. Clin Mol Hepatol. 2016;22:509-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Lee SM, Won JY, Lee DY, Lee KH, Lee KS, Paik YH, Kim JK. Percutaneous cryoablation of small hepatocellular carcinomas using a 17-gauge ultrathin probe. Clin Radiol. 2011;66:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Wang C, Wang H, Yang W, Hu K, Xie H, Hu KQ, Bai W, Dong Z, Lu Y, Zeng Z, Lou M, Gao X, Chang X, An L, Qu J, Li J, Yang Y. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 51. | Chen HW, Lai EC, Zhen ZJ, Cui WZ, Liao S, Lau WY. Ultrasound-guided percutaneous cryotherapy of hepatocellular carcinoma. Int J Surg. 2011;9:188-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Liu ZG, Chen XH, Yu ZJ, Lv J, Ren ZG. Recent progress in pulsed electric field ablation for liver cancer. World J Gastroenterol. 2020;26:3421-3431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (8)] |
| 53. | Zhang YJ, Liang HH, Chen MS, Guo RP, Li JQ, Zheng Y, Zhang YQ, Lau WY. Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: a prospective randomized trial. Radiology. 2007;244:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Huang G, Lin M, Xie X, Liu B, Xu Z, Lencioni R, Lu M, Kuang M. Combined radiofrequency ablation and ethanol injection with a multipronged needle for the treatment of medium and large hepatocellular carcinoma. Eur Radiol. 2014;24:1565-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Peng Z, Wei M, Chen S, Lin M, Jiang C, Mei J, Li B, Wang Y, Li J, Xie X, Kuang M. Combined transcatheter arterial chemoembolization and radiofrequency ablation versus hepatectomy for recurrent hepatocellular carcinoma after initial surgery: a propensity score matching study. Eur Radiol. 2018;28:3522-3531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Yang W, Chen MH, Wang MQ, Cui M, Gao W, Wu W, Wu JY, Dai Y, Yan K. Combination therapy of radiofrequency ablation and transarterial chemoembolization in recurrent hepatocellular carcinoma after hepatectomy compared with single treatment. Hepatol Res. 2009;39:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 661] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 58. | Peng Z, Chen S, Wei M, Lin M, Jiang C, Mei J, Li B, Wang Y, Li J, Xie X, Chen M, Qian G, Kuang M. Advanced Recurrent Hepatocellular Carcinoma: Treatment with Sorafenib Alone or in Combination with Transarterial Chemoembolization and Radiofrequency Ablation. Radiology. 2018;287:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 59. | Dumolard L, Ghelfi J, Roth G, Decaens T, Macek Jilkova Z. Percutaneous Ablation-Induced Immunomodulation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Wang X, Liu G, Chen S, Bi H, Xia F, Feng K, Ma K, Ni B. Combination therapy with PD-1 blockade and radiofrequency ablation for recurrent hepatocellular carcinoma: a propensity score matching analysis. Int J Hyperthermia. 2021;38:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 61. | Santambrogio R, Costa M, Barabino M, Zuin M, Bertolini E, De Filippi F, Bruno S, Opocher E. Recurrent hepatocellular carcinoma successfully treated with laparoscopic thermal ablation. Surg Endosc. 2012;26:1108-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Song KD, Lee MW, Rhim H, Kang TW, Cha DI, Sinn DH, Lim HK. Percutaneous US/MRI Fusion-guided Radiofrequency Ablation for Recurrent Subcentimeter Hepatocellular Carcinoma: Technical Feasibility and Therapeutic Outcomes. Radiology. 2018;288:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 63. | Zhao QY, Xie LT, Chen SC, Xu X, Jiang TA, Zheng SS. Virtual navigation-guided radiofrequency ablation for recurrent hepatocellular carcinoma invisible on ultrasound after hepatic resection. Hepatobiliary Pancreat Dis Int. 2020;19:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Lin ZY, Fang Y, Chen J, Lin QF, Yan Y, Li YL. Feasibility and efficacy study of microwave ablation of recurrent small HCC guided by enhanced liver-specific magnetic resonance imaging contrast agent. Int J Hyperthermia. 2020;37:1330-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 65. | Koda M, Ueki M, Maeda Y, Mimura K, Okamoto K, Matsunaga Y, Kawakami M, Hosho K, Murawaki Y. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. AJR Am J Roentgenol. 2004;183:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 66. | Liu F, Liang P, Yu X, Lu T, Cheng Z, Lei C, Han Z. A three-dimensional visualisation preoperative treatment planning system in microwave ablation for liver cancer: a preliminary clinical application. Int J Hyperthermia. 2013;29:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Li Q, Chen K, Huang W, Ma H, Zhao X, Zhang J, Zhang Y, Fang C, Nie L. Minimally invasive photothermal ablation assisted by laparoscopy as an effective preoperative neoadjuvant treatment for orthotopic hepatocellular carcinoma. Cancer Lett. 2021;496:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 68. | Yamakado K, Nakatsuka A, Akeboshi M, Takeda K. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Interv Radiol. 2003;14:1183-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dogrul AB, Turkey; Elshimi E, Egypt; Hoyos S, Colombia; Masuzaki R, Japan; Shomura M, Japan; Yue T, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL