Published online Aug 27, 2022. doi: 10.4240/wjgs.v14.i8.855
Peer-review started: May 26, 2022
First decision: June 19, 2022
Revised: June 27, 2022
Accepted: August 6, 2022
Article in press: August 6, 2022
Published online: August 27, 2022
Processing time: 90 Days and 1.8 Hours
Endoscopic ultrasound (EUS)-guided transluminal drainage is an advanced technique used to treat pancreatic fluid collections (PFCs). However, gastric varices and intervening vessels may be associated with a high risk of bleeding and are, therefore, listed as relative contraindications. Herein, we report two patients who underwent interventional embolization before EUS-guided drainage.
Two 32-year-old males developed symptomatic PFCs after acute pancreatitis and came to our hospital for further treatment. One patient suffered from intermittent abdominal pain and vomiting, and computed tomography (CT) imaging showed an encapsulated cyst 7.93 cm × 6.13 cm in size. The other patient complained of a mass inside the abdomen, which gradually became enlarged. Gastric varices around the ideal puncture site were detected by EUS when we evaluated the possibility of endoscopic drainage in both patients. Interventional embolization was recommended as the first procedure to decrease the risk of bleeding. After that, EUS-guided transluminal drainage was successfully conducted, without vascular rupture. No postoperative complications occurred during hospitalization, and no recurrence was detected at the last follow-up CT scan performed at 1 mo.
Interventional embolization is a safe, preoperative procedure that is performed before EUS-guided drainage in PFC patients with gastric varices or at high risk of bleeding.
Core Tip: Endoscopic ultrasound-guided drainage has previously proved to be an excellent method to cure pancreatic fluid collections (PFCs). However, it is not recommended for PFCs with the gastric varices and the abundant surrounding vessels because of the high bleeding risk. Preoperative interventional embolization decreases the possibility of hemorrhage when a transluminal tunnel is established between the stomach and cyst. In our cases, the patients underwent this new preoperative arrangement and transgastric drainage was performed. No bleeding or other intraoperative complications occurred. We recommend this modality as a new strategy for PFCs drainage in patients with high bleeding risk.
- Citation: Xu N, Li LS, Yue WY, Zhao DQ, Xiang JY, Zhang B, Wang PJ, Cheng YX, Linghu EQ, Chai NL. Interventional radiology followed by endoscopic drainage for pancreatic fluid collections associated with high bleeding risk: Two case reports. World J Gastrointest Surg 2022; 14(8): 855-861
- URL: https://www.wjgnet.com/1948-9366/full/v14/i8/855.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i8.855
Pancreatic fluid collections (PFCs), including walled-off necrosis (WON) and pancreatic pseudocysts (PPCs), are local complications of acute or chronic pancreatitis according to the updated Atlanta classification[1]. European Society of Gastrointestinal Endoscopy (ESEG) recommends endoscopic or percutaneous drainage as a first-line therapy for symptomatic PFCs[2]. A previous study found that endoscopic transmural drainage is more effective than surgery because of its minimal invasiveness[3]. However, the gastric varices and the abundant vessels surrounding PFCs might be ruptured while establishing the tunnel between the stomach and cyst, thus resulting in uncontrollable bleeding that is unresponsive to endoscopic clips or electrocoagulation[4]. In the two patients described here, lumen-metal apposing stents were successfully placed to drain PFCs under endoscopic ultrasound (EUS) guidance during preoperative embolization of potential bleeding vessels. Herein, we share our successful experience in the form of two case reports to help endoscopists prevent bleeding during the endoscopic drainage procedure.
Case 1: A 32-year-old male was admitted to our department with the symptoms of abdominal pain and vomiting.
Case 2: A 32-year-old male with abdominal distension was referred to our hospital for therapeutic management.
Case 1: The patient experienced continuous abdominal pain and vomiting and was sent to the emergency department of our hospital. The symptoms gradually disappeared after fasting and acid suppression. Abdominal ultrasound indicated the presence of cystic lesions in the body of the pancreas. Then, he was transferred to our inpatient area.
Case 2: In December 2020, the patient who was diagnosed with PPC from an outside hospital was admitted to the Department of Hepatobiliary Surgery to undergo open surgery. However, he was unsuitable for the surgical operation because of renal insufficiency. He came to our department for further treatment of PPC until renal function returned to normal in September 2021.
Case 1: Three years ago, he was admitted to a local hospital to receive treatment for severe acute pancreatitis.
Case 2: The patient suffered from acute pancreatitis for the first time five years prior to hospitalization, and recovered after symptomatic treatment. Intermittent pancreatitis occurred frequently between 2017 and 2020. The patient was hospitalized in the intensive care unit, at least once, for severe abdominal pain combined with continuous vomiting and fever.
Cases 1 and 2: The personal and family histories were unremarkable.
Case 1: Abdominal distension was visible even when the patient lay flat.
Case 2: An obvious mass was palpable in the left upper abdomen, but the size of the mass might not have been evaluated accurately.
Case 1: No pancreatitis-related abnormalities were found by blood biochemical examination.
Case 2: A slight increase in the carbohydrate antigen 125 level was detected by blood biochemical examination, as well as a sharp increase in the carbohydrate antigen 19-9 level. Amylase (501 U/L) and lipase levels (559 U/L) were much higher than normal (normal ranges: 0-150 U/L and 13-60 U/L).
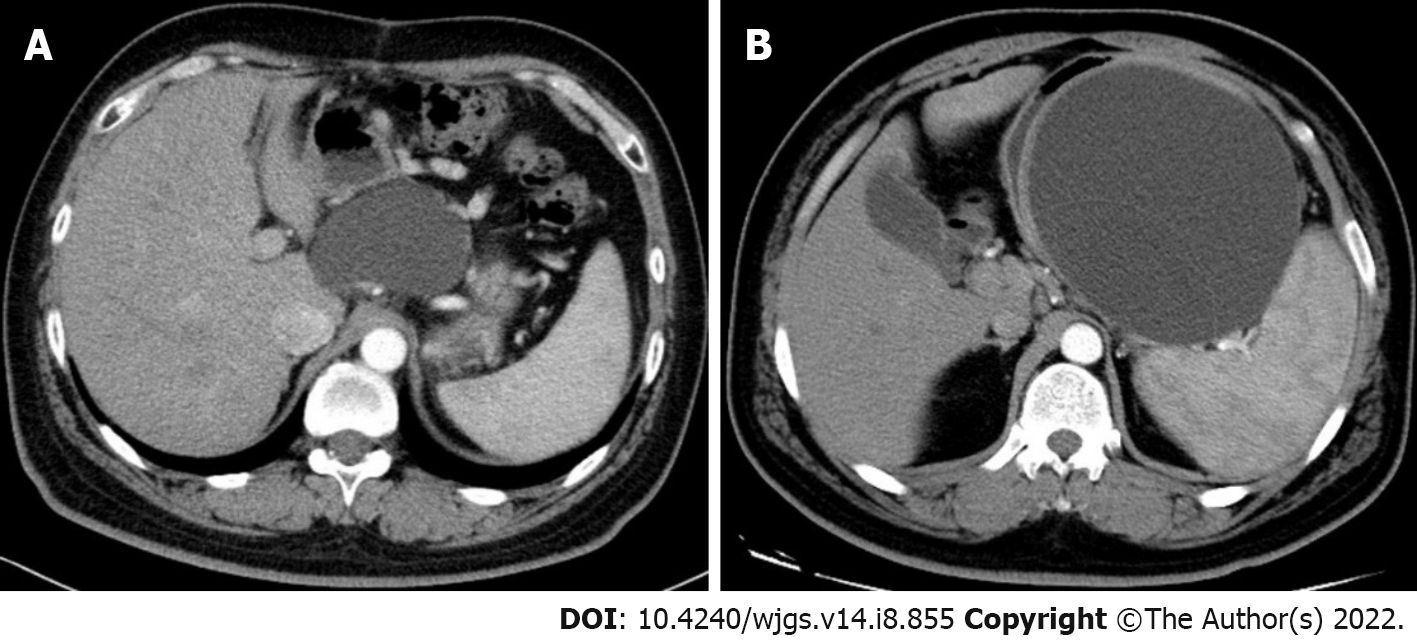
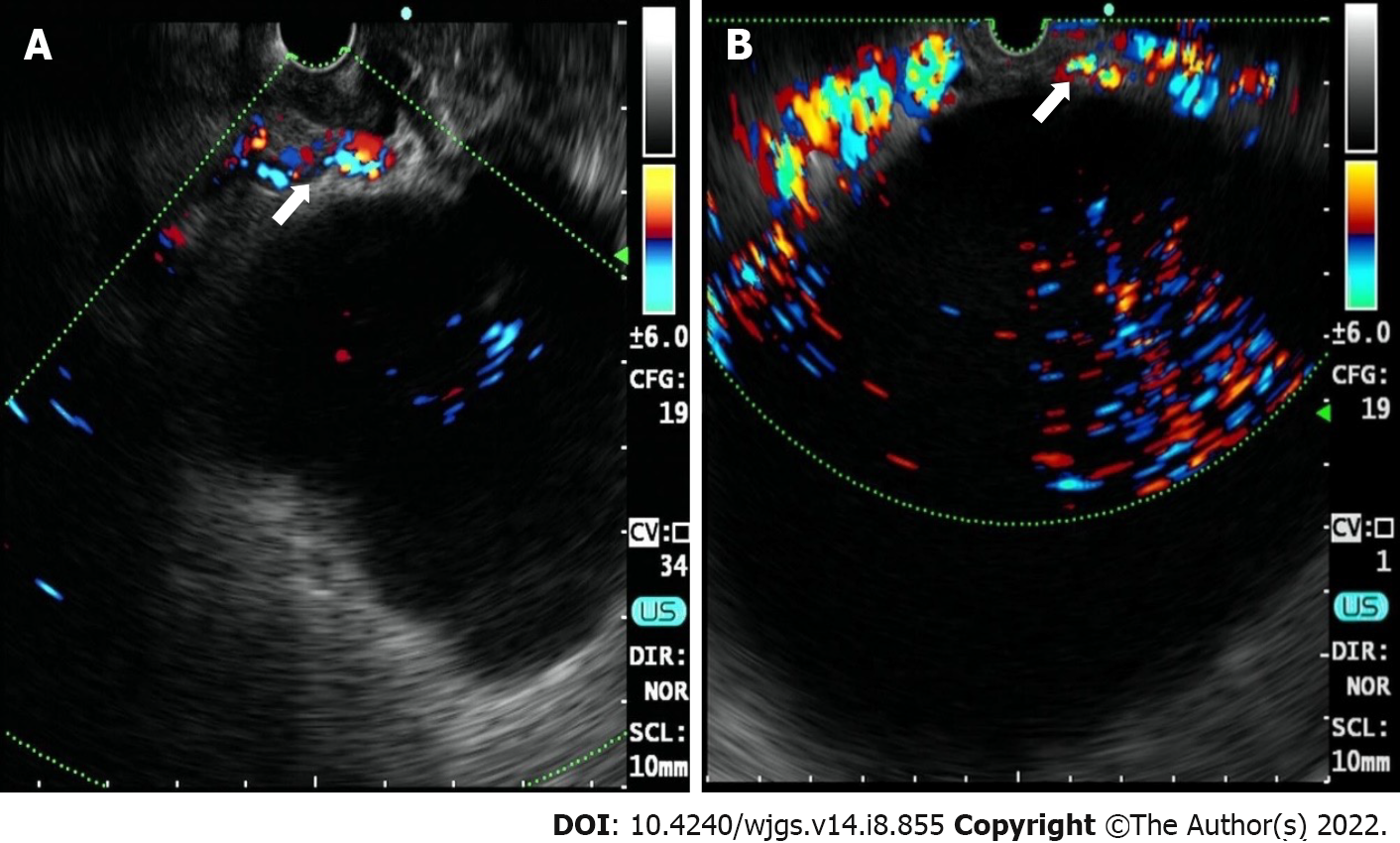
Case 1: Contrast-enhanced abdominal computed tomography (CECT) showed a cystic lesion in the body of the pancreas, with a size of 7.93 cm × 6.13 cm (Figure 1A). A cystic lesion of the same size and the presence of blood vessels around the cyst were observed on linear EUS (Figure 2A).
Case 2: A cyst with a maximum diameter of 14 cm was detected by CECT (Figure 1B). Linear EUS showed signs of several vessels around the fundus of the stomach, which may have been a potential puncture site (Figure 2B).
Based on the patient’s history of illness and the direct endoscopic visualization of the cystic cavity contents, his diagnosis ultimately concluded as being WON.
According to the characterization of the cystic cavity contents, he was diagnosed with PPC.
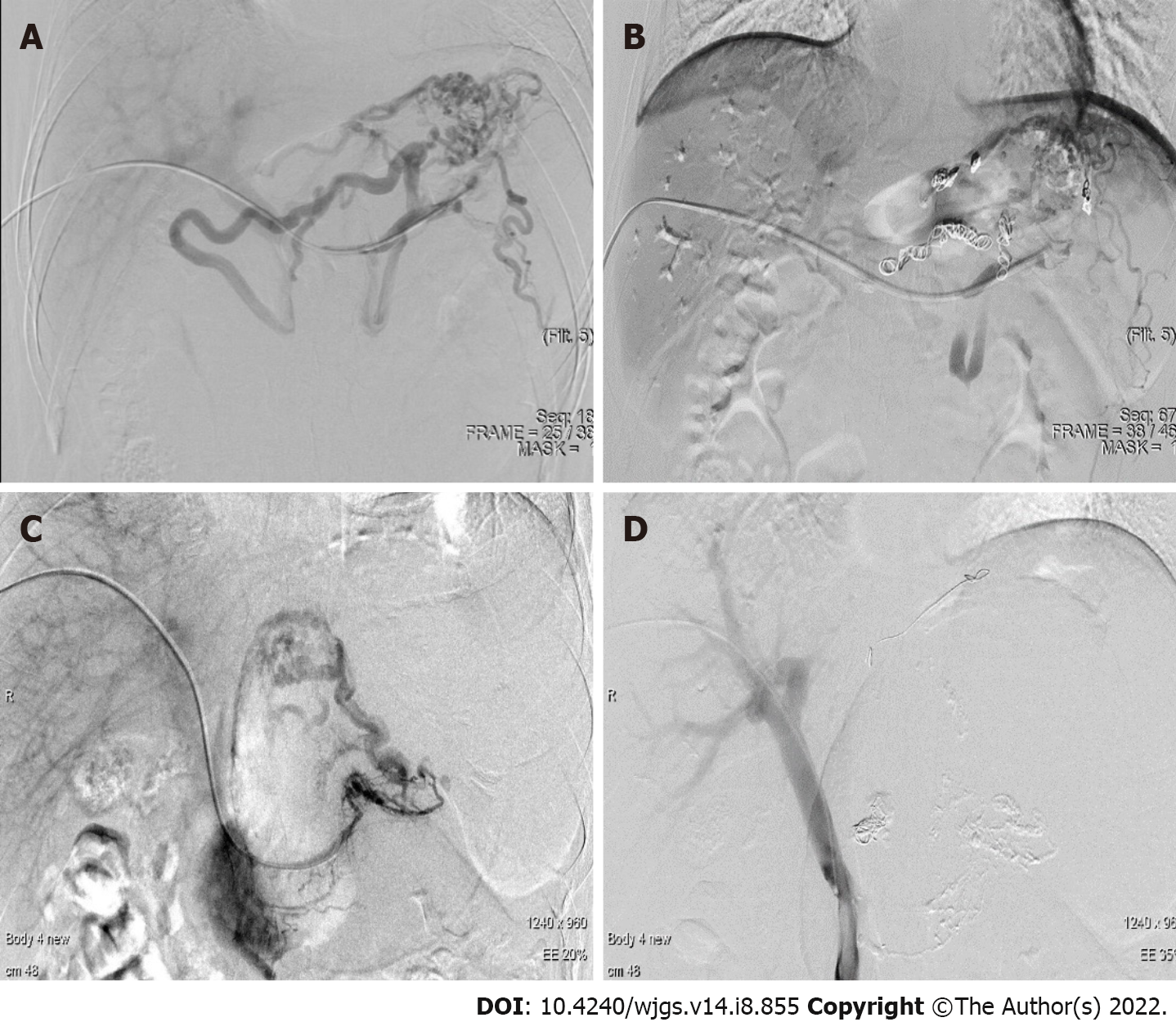
Coil embolization was performed before the endoscopic drainage (Figure 3A and B). Then the patient was prepared to undergo EUS-guided cystogastrostomy and a lumen-metal apposing stent (LAMS: 16 mm × 26 mm, Micro-Tech Co., Ltd., Nanjing, Jiangsu Province, China) placement.
Subsequent monitoring showed that the patient’s temperature was maintained within the normal range. However, he experienced unexplained nausea and vomiting during hospitalization after the LAMS was placed. Four days after stent placement, postoperative endoscopic observation showed that the contents were almost fully discharged to the stomach cavity. Thus, after irrigation of the cystic cavity with sterile water only, the stent was retrieved, and thereby eliminated all discomforting symptoms. One month after endoscopic drainage, CECT of the abdomen revealed that WON in the patient has resolved.
The patient’s vital signs were stable during hospitalization. Postoperative endoscopy was used to perform direct endoscopic necrosectomy. Sterile water was used to rinse the small amount of liquid content that remained in the cystic cavity followed by withdrawal of the stent. CECT obtained one month after the procedure showed shrinkage of the PPC. No abdominal symptoms or postoperative complications were observed.
PFCs are local complications of acute pancreatitis that frequently occur more than 4 wk after the onset of pancreatitis[5]. Some PFCs patients might suffer from symptoms of abdominal pain, vomiting, and other digestive-related discomfort, but the majority of patients are asymptomatic and their symptoms resolve spontaneously[6]. For symptomatic PFCs, especially those that seriously affect normal life, drainage of the collections is vital for effective treatment[7,8]. Although there are other drainage methods, endoscopic drainage is minimally invasive and has improved safety and efficacy when compared to open surgery or percutaneous drainage, so endoscopic drainage is recommended as the first-line treatment.
Endoscopic drainage is a well-established therapy for PFCs; however, bleeding complications still haunt endoscopists[9,10]. In the past, PFCs associated with gastric varices or abundant surrounding vessels were referred to the surgical department for further treatment[11]. Previous studies have reported attempts to treat PFC-associated diseases with high bleeding risks, such as arterial pseudoaneurysms, with a combination of minimally invasive endoscopic and radiological interventions[12,13]. However, this combined treatment is rare because of its association with the gastric varices or the surrounding vessels, thus limiting is applicability due to the demand for expertise in interventional radiology and therapeutic endoscopy.
Endovascular embolization, an advanced technique, is the preferred treatment of choice for esophageal or gastric varices and has been widely used to stop and prevent bleeding[14,15]. However, clinicians have limited experience in the clinical management of PFCs that present with gastric varices. Moreover, ideal management depends on the patient’s hemodynamic stability[16]. The development of interventional radiological techniques has led to better outcomes of hemostasis with angioembolization. One report indicated that angioembolization alone is an effective treatment for a pseudocyst associated with pseudoaneurysms[17].
In the presence of gastric varices or pseudoaneurysms, EUS-guided endoscopic drainage is contraindicated because of the increased risk of vessel rupture[18]. In our study, we show that endoscopic drainage combined with coil embolization is an effective treatment for varices. These two patients underwent EUS-guided puncture and a small incision was made in the wall of the stomach and PFC cysts after interventional radiology. No intraoperative complications, such as bleeding or infection, occurred. We did not encounter any complications while removing the necrotic solid debris or the metal stent. However, we did not determine the cause of intermittent nausea and vomiting that occurred in one patient. All symptoms disappeared after the stent was removed.
One limitation is associated with this combined treatment method. For patients with PFCs less than 6 cm, a LAMS cannot be used to establish a tunnel between the two lumens[19]. Therefore, EUS-guided endoscopic drainage combined with interventional radiology would not be feasible.
The application of endovascular embolization before EUS-guided endoscopic drainage prevents vessel rupture. This combined treatment has the potential to be a solution for PFC patients with high bleeding risks and warrants further investigation to substantiate its use.
The authors thank Dr. Yuan K for providing the imaging data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; Brigode WM, United States; Lee S, South Korea; Shami V, United States S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4664] [Article Influence: 358.8] [Reference Citation Analysis (48)] |
| 2. | Arvanitakis M, Dumonceau JM, Albert J, Badaoui A, Bali MA, Barthet M, Besselink M, Deviere J, Oliveira Ferreira A, Gyökeres T, Hritz I, Hucl T, Milashka M, Papanikolaou IS, Poley JW, Seewald S, Vanbiervliet G, van Lienden K, van Santvoort H, Voermans R, Delhaye M, van Hooft J. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy. 2018;50:524-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 327] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 3. | Tyberg A, Karia K, Gabr M, Desai A, Doshi R, Gaidhane M, Sharaiha RZ, Kahaleh M. Management of pancreatic fluid collections: A comprehensive review of the literature. World J Gastroenterol. 2016;22:2256-2270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | Ishikawa T, Takami T. Therapeutic Strategy Using Interventional Radiology for Refractory Esophageal Varices Resistant to Endoscopic Treatment. Intern Med. 2022;61:771-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Zaheer A, Singh VK, Qureshi RO, Fishman EK. The revised Atlanta classification for acute pancreatitis: updates in imaging terminology and guidelines. Abdom Imaging. 2013;38:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Fugazza A, Sethi A, Trindade AJ, Troncone E, Devlin J, Khashab MA, Vleggaar FP, Bogte A, Tarantino I, Deprez PH, Fabbri C, Aparicio JR, Fockens P, Voermans RP, Uwe W, Vanbiervliet G, Charachon A, Packey CD, Benias PC, El-Sherif Y, Paiji C, Ligresti D, Binda C, Martínez B, Correale L, Adler DG, Repici A, Anderloni A. International multicenter comprehensive analysis of adverse events associated with lumen-apposing metal stent placement for pancreatic fluid collection drainage. Gastrointest Endosc. 2020;91:574-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Singla V, Garg PK. Role of diagnostic and therapeutic endoscopic ultrasonography in benign pancreatic diseases. Endosc Ultrasound. 2013;2:134-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 8. | Umapathy C, Gajendran M, Mann R, Boregowda U, Theethira T, Elhanafi S, Perisetti A, Goyal H, Saligram S. Pancreatic fluid collections: Clinical manifestations, diagnostic evaluation and management. Dis Mon. 2020;66:100986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Auriemma F, Anderloni A, Carrara S, Fugazza A, Maselli R, Troncone E, Repici A. Cyanoacrylate Hemostasis for Massive Bleeding After Drainage of Pancreatic Fluid Collection by Lumen-apposing Metal Stent. Am J Gastroenterol. 2018;113:1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Brimhall B, Han S, Tatman PD, Clark TJ, Wani S, Brauer B, Edmundowicz S, Wagh MS, Attwell A, Hammad H, Shah RJ. Increased Incidence of Pseudoaneurysm Bleeding With Lumen-Apposing Metal Stents Compared to Double-Pigtail Plastic Stents in Patients With Peripancreatic Fluid Collections. Clin Gastroenterol Hepatol. 2018;16:1521-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 11. | Czernik M, Stefańczyk L, Szubert W, Chrząstek J, Majos M, Grzelak P, Majos A. Endovascular treatment of pseudoaneurysms in pancreatitis. Wideochir Inne Tech Maloinwazyjne. 2014;9:138-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Michimoto K, Higuchi T, Enoki K, Matsui Y, Takenaga S, Saeki C. Percutaneous puncture and embolisation for pancreatitis-related pseudoaneurysm: the feasibility of thrombin injection even in collection of fluid surrounding the pseudoaneurysm. Pol J Radiol. 2018;83:e510-e513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Rana SS, Kumar A, Lal A, Sharma R, Kang M, Gorsi U, Gupta R. Safety and efficacy of angioembolisation followed by endoscopic ultrasound guided transmural drainage for pancreatic fluid collections associated with arterial pseudoaneurysm. Pancreatology. 2017;17:658-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Lee EW, Shahrouki P, Alanis L, Ding P, Kee ST. Management Options for Gastric Variceal Hemorrhage. JAMA Surg. 2019;154:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Ryan BM, Stockbrugger RW, Ryan JM. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004;126:1175-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Bhasin DK, Rana SS, Sharma V, Rao C, Gupta V, Gupta R, Kang M, Singh K. Non-surgical management of pancreatic pseudocysts associated with arterial pseudoaneurysm. Pancreatology. 2013;13:250-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Elton E, Howell DA, Amberson SM, Dykes TA. Combined angiographic and endoscopic management of bleeding pancreatic pseudoaneurysms. Gastrointest Endosc. 1997;46:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Zabicki B, Limphaibool N, Holstad MJV, Juszkat R. Endovascular management of pancreatitis-related pseudoaneurysms: A review of techniques. PLoS One. 2018;13:e0191998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Zhu HY, Xie P, Song YX, Li ZS, Jin ZD, Du YQ. Lumen-apposing metal stents (LAMS) versus plastic stents for EUS-guided drainage of walled-off necrosis (WON) (LVPWON): study protocol for a multicenter randomized controlled trial. Trials. 2018;19:549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |