Published online Aug 27, 2022. doi: 10.4240/wjgs.v14.i8.765
Peer-review started: March 4, 2022
First decision: June 12, 2022
Revised: June 21, 2022
Accepted: July 20, 2022
Article in press: July 20, 2022
Published online: August 27, 2022
Processing time: 173 Days and 4.4 Hours
Approximately 20 percent of patients with a tumour localized in the low rectum still encounter the possibility of requiring permanent stoma (PS), which can cause drastic changes in lifestyle and physical perceptions.
To determine the risk factors for PS and to develop a prediction model to predict the probability of PS in rectal cancer patients after sphincter-saving surgery.
A retrospective cohort of 421 rectal cancer patients who underwent radical surgery at Taipei Medical University Hospital between January 2012 and Dece
The PS rate after sphincter-saving surgery was 15.1% (59/391) in our study after a median follow-up of 47.3 mo (range 7–114 mo). Multivariate logistic regression analysis demonstrated that local recurrence, perirectal abscess, anastomosis site stenosis, perineural invasion, tumor size and operative time were independent risk factors for PS. These identified risk factors were incorporated into the nomogram, and the concordance index of this model was 0.903 (95%CI: 0.851-0.955). According to the calibration curves, the nomogram represents a perfect prediction model.
Several risk factors for PS after sphincter-saving surgery were identified. Our nomogram exhibited perfect predictive ability and will improve a physician’s ability to communicate the benefits and risks of various treatment options in shared decision making.
Core Tip: Approximately 20 percent of patients with a tumour localized in the low rectum still encounter the possibility of requiring permanent stoma (PS), which can cause drastic changes in lifestyle and physical perceptions. The study aimed to identify the risk factors for PS in rectal cancer patients after sphincter-saving surgery. Our results showed that the predictive models constructed by clinicopathological features exhibited perfect predictive ability and will allow physicians to inform patients about the possibility of PS prior to surgery.
- Citation: Kuo CY, Wei PL, Chen CC, Lin YK, Kuo LJ. Nomogram to predict permanent stoma in rectal cancer patients after sphincter-saving surgery. World J Gastrointest Surg 2022; 14(8): 765-777
- URL: https://www.wjgnet.com/1948-9366/full/v14/i8/765.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i8.765
Shared decision making (SDM) is a structured process that incorporates available scientific evidence, patient values, preferences, and life situation into screening decisions[1]. The benefits of SDM include improved medical quality, improved patient satisfaction, increased patient compliance to medical treatment, and reduced patient anxiety during treatment; SDM also helps patients understand the issues with which they should be familiar before they undergo treatment[2,3]. This discussion is particularly important in cancer treatment since patients are often provided with more than one available treatment strategy[4].
Despite innovative advancements, the management of rectal cancer remains a formidable endeavor, especially distally located rectal cancer[5]. It is extremely challenging to work in the low and narrow pelvis with laparoscopic straight instruments. Male sex, high body mass index (BMI), low rectal cancer, bulky tumor, and advanced stage are well known to increase the technical difficulty[6]. Moreover, a certain percentage of anastomosis-related complications will occur after colorectal surgery. Anastomosis complications, such as anastomotic leakage, perirectal abscess, and anastomotic stenosis, often lead to permanent stoma (PS). According to previous studies, 3%-24% of rectal cancer patients experience anastomosis complications after sphincter-saving surgery[7-9].
A nomogram is a statistical tool that can transform a complex regression equation result into a simple and visual graph[10]. Thus, the results of prediction models become more readable and valuable. The aim of this study was to develop and validate a nomogram that incorporated both the clinical and pathologic risk factors for individual preoperative prediction of PS in patients with rectal cancer who underwent sphincter-saving surgery.
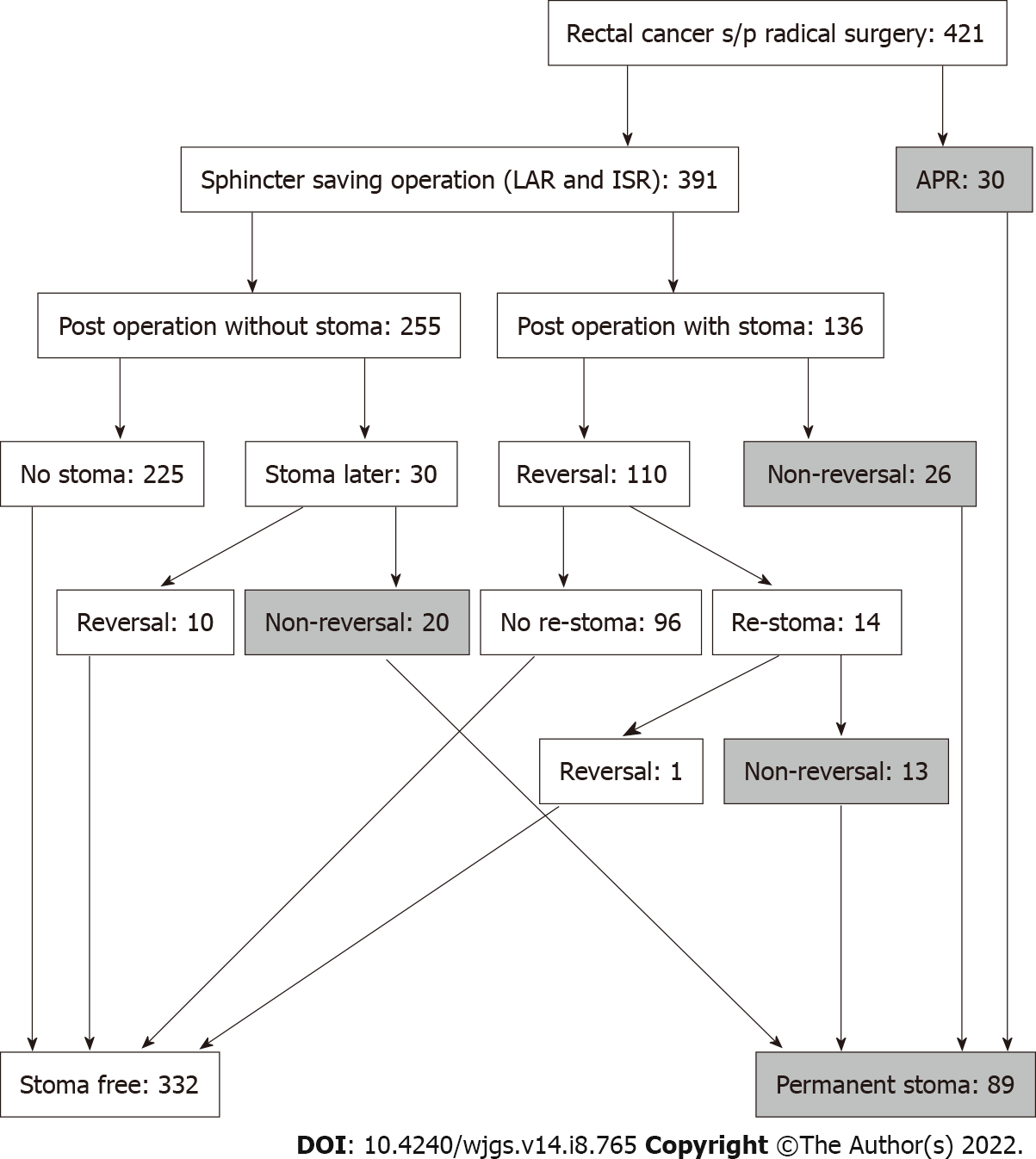
We retrospectively reviewed records of patients with rectal cancer who underwent surgery at Taipei Medical University Hospital from January 2012 to December 2020. The inclusion criteria were as follows: (1) Patients older than 18 years; (2) Underwent radical surgery [low anterior resection, intersphincteric resection, or abdominoperineal resection (APR)]; (3) Pathological diagnosis of malignancy; and (4) lesion located within 12 cm from the anal verge. The exclusion criteria were as follows: (1) Patients with stage IV disease; (2) Those who underwent emergency surgery; and (3) Those who underwent other organ resection during primary surgery. Defunctioning stoma was performed if any of the following conditions applied: (1) Positive air leak test; (2) Patient received preoperative chemoradiotherapy (CRT); (3) Anastomosis had tension or poor blood supply; (4) Presence of incomplete anastomotic ring; (5) Very low anastomosis; (6) Patients’ clinical condition indicated defunctioning stoma; and (7) The surgeon elected to perform this procedure based on his/her experience. The condition of PS included non-reversal temporary stoma and stoma re-creation after reversal surgery (Figure 1). This study was approved by the Joint Institutional Review Board of Taipei Medical University (TMU-JIRB No: N202103023).
Patient demographics and potential risk factors for PS were retrospectively collected and included sex, age, BMI, comorbidities (diabetes mellitus, hypertension, heart disease, chronic obstructive pulmonary disease, chronic kidney disease, liver disease), smoking status, clinical tumor-node-metastasis stage, whether the patient received neoadjuvant CRT, American Society of Anesthesiologists (ASA) score, tumor location (distance from the anal verge), tumor markers, such as carcinoembryonic antigen (CEA), preoperative lab data (hemoglobin and albumin), surgical approach, blood loss, operative time, stoma status, postoperative hospital stay, histologic grade, lymph vascular invasion, perineural invasion, circumferential resection margin (CRM) status, whether the patient received adjuvant chemotherapy, local recurrence, postoperative leakage, anastomosis site stenosis, perirectal abscess, and recto-visceral fistula.
Anastomotic leakage was defined as peritonitis that was clinically apparent (discharge containing pus or fecal material) or radiologically evident (contrast leakage or abscess around the anastomosis). Perirectal abscess (late anastomotic leak) was defined as a leak that was diagnosed more than 30 d after surgery. Anastomotic stricture was defined as the inability of a 12-mm proctoscope to pass through the anastomosis. A PS was defined when a closure procedure had not been performed or scheduled within the follow-up period (median, 47 mo; range, 7–114 mo).
Patients were followed-up every 3 mo during the first 2 years and then every 6 mo until the fifth year. Clinical examination and serum CEA testing were performed during each follow-up visit. Surveillance colonoscopy was performed within 12 mo after the initial surgery and every other year thereafter. Contrast-enhanced computed tomography scan of the thorax, abdomen, and pelvis was performed annually for 3 years and subsequently only when clinically indicated.
Categorical variables are presented as counts and percentages, while continuous variables are depicted as the mean ± SD. Differences between both groups were assessed with the chi-square test or Fisher exact test depending on the sample size. Univariate analyses for risk factors related to a PS were performed. Multivariate logistic regression was conducted to identify the independent risk factors. A two-tailed P value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS v9.4 (Cary, NY)
Statistical analyses of the nomogram were conducted using SAS v 9.4 and R (ver. 3.0.1, Vienna, Austria). The rms package in R was used to plot the nomogram as a graphical calculating device that visualizes an approximation of mathematical function. Features of the nomogram are based on logistic regression models. The nomogram function in the rms package was adopted to generate nomograms from the fitted logistic statistical model. As a result, the performance of the nomogram is dependent on the regression models. We assessed the predictive power of the nomogram using receiver operating characteristic curve analysis. Calibration curves were used to explore the performance of the nomogram.
In all, 421 patients who underwent radical surgery are included in our study, including 391 (92.9%) who underwent sphincter-saving surgery and 30 (7.1%) who underwent APR. Moreover, 136/391 (34.8%) patients who underwent a sphincter-saving procedure had a temporary stoma after primary surgery. After a median follow-up of 47.3 mo (range 7–114 mo), 59/391 (15.1%) patients were confirmed to have PS, and the details of the stoma condition are shown in Figure 1. According to our data, 332 patients are in the stoma free group, while 89 patients are in the PS group. In summary, the PS rate after sphincter-saving surgery at our hospital from January 2012 to December 2020 is 15.1% (59/391), and the total sphincter-saving rate is 78.9% (89/421). All data compared between the stoma free and PS groups are presented in Table 1.
| Characteristic | Stoma free (n = 332) | Permanent stoma (n = 89) | P value |
| Age, yr | 60.78 ± 12.80 | 60.56 ± 12.60 | 0.888 |
| Sex (n) | 0.716 | ||
| Male | 196 (59.04%) | 50 (56.18%) | |
| Female | 136 (40.96%) | 39 (43.82%) | |
| Body mass index, kg/m2 | 24.00 ± 3.97 | 24.47 ± 4.32 | 0.331 |
| Comorbidity (n) | |||
| DM | 68 (20.48%) | 14 (15.73%) | 0.393 |
| Hypertension | 103 (31.02%) | 33 (7.08%) | 0.339 |
| Heart disease | 25 (7.53%) | 8 (8.99%) | 0.816 |
| COPD | 2 (0.60%) | 2 (2.25%) | 0.421 |
| Chronic kidney disease | 36 (10.84%) | 9 (10.11%) | 0.996 |
| Liver disease | 39 (11.75%) | 10 (11.24%) | 1 |
| Smoker (n) | 49 (14.76%) | 9 (10.11%) | 0.339 |
| Distance to anus verge, cm | 7.06 ± 3.52 | 4.68 ± 3.96 | < 0.001 |
| Clinical T stage (n) | 0.002 | ||
| T0 | 8 (2.41%) | 1 (1.13%) | |
| T1 | 12 (3.61%) | 1 (1.13%) | |
| T2 | 50 (15.06%) | 8 (8.98%) | |
| T3 | 218 (65.66%) | 56 (62.92%) | |
| T4 | 20 (6.03%) | 17 (19.10%) | |
| Data loss | 24 (7.23%) | 6 (6.74%) | |
| Clinical N stage (n) | 0.44 | ||
| N0 | 108 (32.53%) | 23 (25.84%) | |
| N1 | 100 (30.12%) | 31 (34.83%) | |
| N2 | 100 (30.12%) | 29 (32.59%) | |
| Data loss | 24 (7.23%) | 6 (6.74%) | |
| AJCC c TNM stage (n) | 0.002 | ||
| Stage 0 | 8 (2.41%) | 1 (1.13%) | |
| Stage I | 49 (14.76%) | 7 (7.86%) | |
| Stage II | 52 (15.66%) | 15 (16.85%) | |
| Stage III | 199 (59.94%) | 60 (67.42%) | |
| Data loss | 24 (7.23%) | 6 (6.74%) | |
| NACR (n) | 222 (66.87%) | 69 (77.53%) | 0.026 |
| Hb, g/dL | 12.78 ± 1.57 | 12.52 ± 1.72 | 0.169 |
| Albumin, g/dL | 4.14 ± 0.36 | 4.08 ± 0.37 | 0.19 |
| CEA, ng/mL | 4.81 ± 8.58 | 6.15 ± 8.69 | 0.198 |
| ASA score (n) | 0.182 | ||
| I | 26 (7.83%) | 3 (3.37%) | |
| II | 271 (81.63%) | 73 (82.02%) | |
| III | 30 (9.03%) | 12 (13.48%) | |
| Data loss | 5 (1.51%) | 1 (1.13%) |
Data from the univariate and multivariate analyses for PS are provided in Table 2. According to the multivariate logistic regression analysis, seven features were significantly related to PS. The independent risk factors for PS by multivariate logistic regression were local recurrence [odd ratio (OR), 111.578; 95%CI: 7.964-> 999; P < 0.001], perirectal abscess (OR, 369.397; 95%CI: 17.137-> 999; P < 0.001), anastomosis site stenosis (OR, 211.256; 95%CI: 13.705-> 999; P < 0.001), perineural invasion (OR, 7.674; 95%CI: 1.138-51.745; P = 0.036), tumor size (OR, 1.076; 95%CI: 1.015-1.14; P = 0.014), liver disease (OR, 0.054; 95%CI: 0.004-0.698; P = 0.025), and operative time (min) (OR, 1.008; 95%CI: 1.002-1.014; P = 0.01). We excluded liver disease because of OR < 1. Thus, these six variables were selected to construct the nomogram.
| Variable | Univariable analysis OR (95%CI) | P value | Multivariable analysis OR (95%CI) | P value |
| Age, yr | 0.99 (0.969-1.012) | 0.369 | 0.959 (0.895-1.027) | 0.232 |
| Sex (Ref. = female) | ||||
| Male | 0.822 (0.472-1.443) | 0.491 | 0.273 (1.044-1.7) | 0.164 |
| Body mass index, kg/m2 | 1.022 (0.953-1.092) | 0.532 | 0.949 (0.807-1.116) | 0.525 |
| DM (Ref. = No) | ||||
| Yes | 0.792 (0.363-1.586) | 0.532 | 0.307 (0.032-2.9) | 0.303 |
| Hypertension (Ref. = No) | ||||
| Yes | 1.229 (0.678-2.179) | 0.488 | 0.819 (0.121-5.542) | 0.838 |
| Heart disease (Ref. = No) | ||||
| Yes | 0.893 (0.256-2.413) | 0.84 | 0.229 (0.008-6.382) | 0.385 |
| COPD (Ref. = No) | ||||
| Yes | 5.795 (0.684-49.02) | 0.082 | 451.125 (0.376->999) | 0.091 |
| CKD (Ref. = No) | ||||
| Yes | 0.931 (0.34-2.172) | 0.878 | 0.421 (0.019-9.234) | 0.583 |
| Liver disease (Ref. = No) | ||||
| Yes | 1.179 (0.488-2.55) | 0.694 | 0.054 (0.004-0.698) | 0.025 |
| Smoker (Ref. = No) | ||||
| Yes | 0.906 (0.379-1.932) | 0.81 | 0.125 (0.007-2.148) | 0.152 |
| Distance to anus verge, cm | 0.838 (0.758-0.921) | < 0.001 | 0.834 (0.618-1.127) | 0.238 |
| Clinical T stage (Ref. = T0) | ||||
| T1 | < 0.001 (NA-4.239) | 0.98 | 1.081 (< 0.001-> 999) | 0.999 |
| T2 | 1.28 (0.193-25.357) | 0.827 | > 999 (< 0.001-> 999) | 0.968 |
| T3 | 1.394 (0.246-26.24) | 0.757 | > 999 (< 0.001-> 999) | 0.976 |
| T4 | 3.2 (0.468-64.31) | 0.308 | > 999 (< 0.001-> 999) | 0.971 |
| Clinical N stage (Ref. = N0) | ||||
| N1 | 1.697 (0.831-3.568) | 0.152 | 0.017 (< 0.001-> 999) | 0.986 |
| N2 | 1.466 (0.701-3.129) | 0.313 | 0.003 (< 0.001-> 999) | 0.981 |
| AJCC c TNM stage (Ref. = Stage 0) | ||||
| Stage I | 0.98 (0.139-19.76) | 0.986 | 0.015 (< 0.001-> 999) | 0.986 |
| Stage II | 1.077 (0.159-21.492) | 0.948 | 0.007 (< 0.001-> 999) | 0.983 |
| Stage III | 1.648 (0.291-30.993) | 0.642 | NA | NA |
| Pre-operative CCRT (Ref. = No) | ||||
| Yes | 1.332 (0.731-2.533) | 0.364 | 1.873 (0.137-25.575) | 0.638 |
| Hb, g/dL | 0.987 (0.832-1.18) | 0.887 | 1.404 (0.768-2.568) | 0.27 |
| Albumin, g/dL | 0.821 (0.361-1.928) | 0.643 | 0.66 (0.041-10.497) | 0.769 |
| CEA, ng/mL | 1.011 (0.978-1.038) | 0.443 | 0.936 (0.804-1.09) | 0.396 |
| ASA score (Ref. = I) | ||||
| II | 2.02 (1.046-3.891) | 0.036 | 7.967 (0.64-99.127) | 0.107 |
| III | NA | NA | NA | NA |
| Surgical Approach way (Ref. = 0) | ||||
| LPS (1) | NA | NA | > 999 (< 0.001-> 999) | 0.859 |
| Robotic (2) | NA | NA | > 999 (< 0.001-> 999) | 0.872 |
| Type of operation (Ref. = LAR) | ||||
| CAA | 3.46 (1.958-6.266) | < 0.001 | 0.221 (0.027-1.796) | 0.158 |
| Estimated blood loss | 1.002 (1-1.005) | 0.072 | 1.001 (0.987-1.016) | 0.889 |
| Operative time | 1.004 (1.002-1.007) | < 0.001 | 1.011 (1.001-1.02) | 0.026 |
| Histologic tumor grade (Ref. = Grade I) | ||||
| Grade II | 1.622 (0.883-3.05) | 0.124 | 1.203 (0.22-6.586) | 0.831 |
| Grade III | 2.507 (0.645-8.203) | 0.147 | 1.53 (0.038-61.785) | 0.822 |
| Tumor size, mm | 1.026 (1.011-1.041) | < 0.001 | 1.076 (1.015-1.14) | 0.014 |
| Circumferential resection margin (Ref. = No) | ||||
| Yes | 6.575 (2.955-14.604) | < 0.001 | 0.936 (0.064-13.699) | 0.961 |
| Lymph vascular invasion (Ref. = No) | ||||
| Yes | 1.99 (1.071-3.617) | 0.026 | 0.94 (0.132-6.715) | 0.951 |
| Perineural invasion (Ref. = No) | ||||
| Yes | 3.085 (1.726-5.518) | < 0.001 | 7.674 (1.138-51.745) | 0.036 |
| Postoperative hospital stays | 1.05 (1.02-1.083) | 0.001 | 1.003 (0.911-1.104) | 0.953 |
| Postoperative chemotherapy (Ref. = No) | ||||
| Yes | 1.907 (0.963-4.134) | 0.079 | 4.281 (0.247-74.107) | 0.318 |
| Anastomosis site stenosis (Ref. = No) | ||||
| Yes | 11.648 (5.499-25.374) | < 0.001 | 211.256 (13.705-> 999) | < 0.001 |
| Local recurrence (Ref. = No) | ||||
| Yes | 12.584 (5.874-27.885) | < 0.001 | 111.578 (7.964-> 999) | < 0.001 |
| Postoperative leakage (Ref. = No) | ||||
| Yes | 2.659 (0.982-6.557) | 0.041 | 0.743 (0.047-11.833) | 0.833 |
| Perirectal abscess (Ref. = No) | ||||
| Yes | 11.037 (3.22-43.367) | < 0.001 | 369.397 (17.137-> 999) | < 0.001 |
| Recto visceral fistula (Ref. = No) | ||||
| Yes | 44.557 (7.71-841.643) | < 0.001 | > 999 (< 0.001-> 999) | 0.963 |
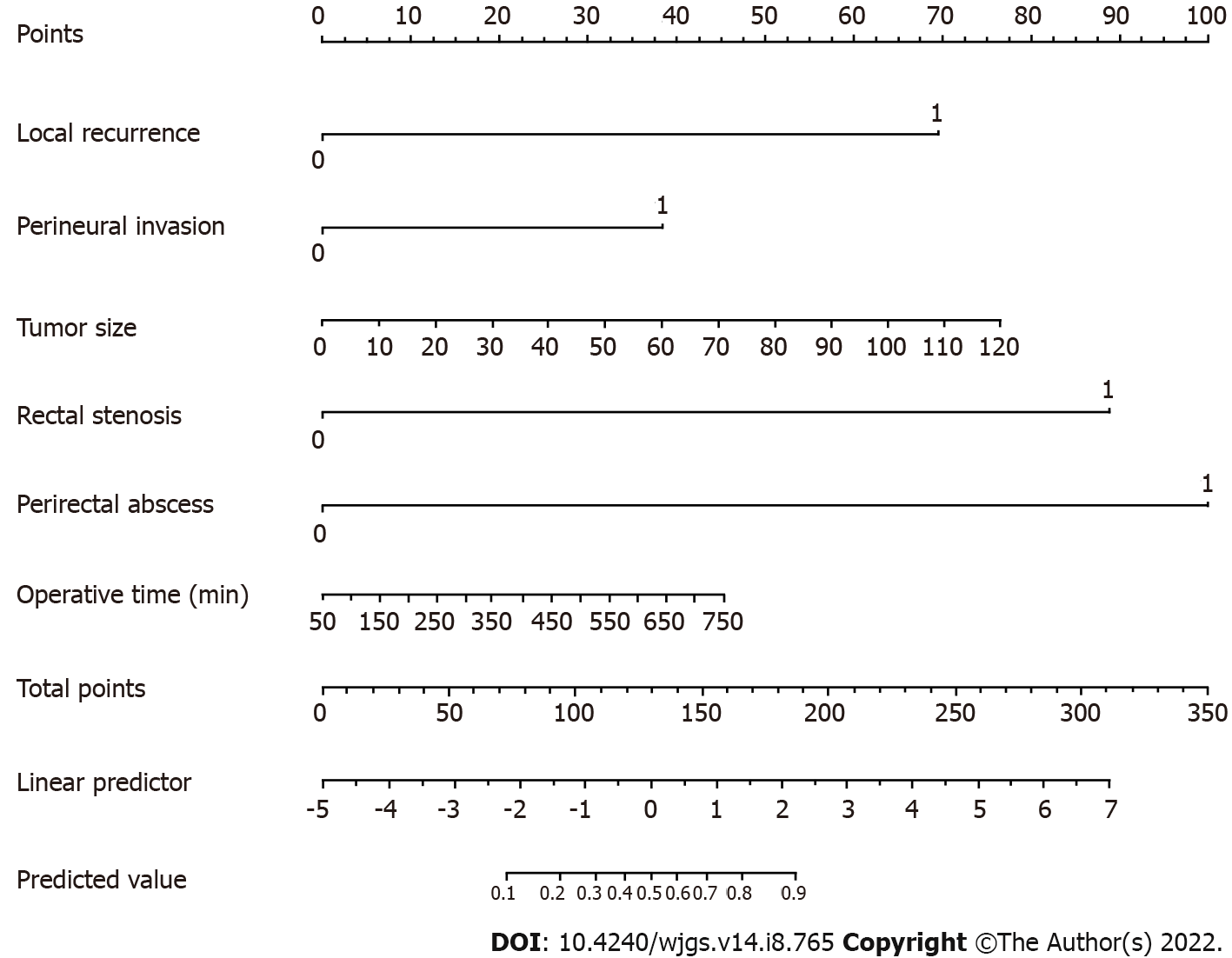
The prognostic nomogram that integrated all potential risk factors for PS in the cohort is shown in Figure 2. The nomogram model was validated by computing the concordance index (C-index) of the nomogram sample. The nomogram provides a visualization of accumulated risk by mapping the predicted probabilities into points on a scale from 0 to 1 in a graphical interface. The total points accumulated by each covariate correspond to the predicted probability in a given patient. To further illustrate this, the point system functions by ranking the effect estimates, regardless of statistical significance, and this ranking is influenced by the presence of other covariates. Despite statistical significance, the risk factor whose absolute value has the largest regression coefficient will be assigned 100 points on the scale, while the remaining variables are assigned a smaller number of points proportional to their effect size. As shown in Figure 2, perirectal abscess has the highest effect, and thus, this variable is assigned 100 points. Whereas a patient with perirectal abscess would be assigned 100 points, a patient without perirectal abscess would be assigned 0 points. Similarly, a patient with perineural invasion would be assigned 40 points, while a patient with a tumor size of 20 mm would be assigned 10 points. For example, a patient with perirectal abscess, perineural invasion, and a tumor size of 20 mm would be assigned 150 points overall, which is mapped to an approximate predicted probability of 70%.
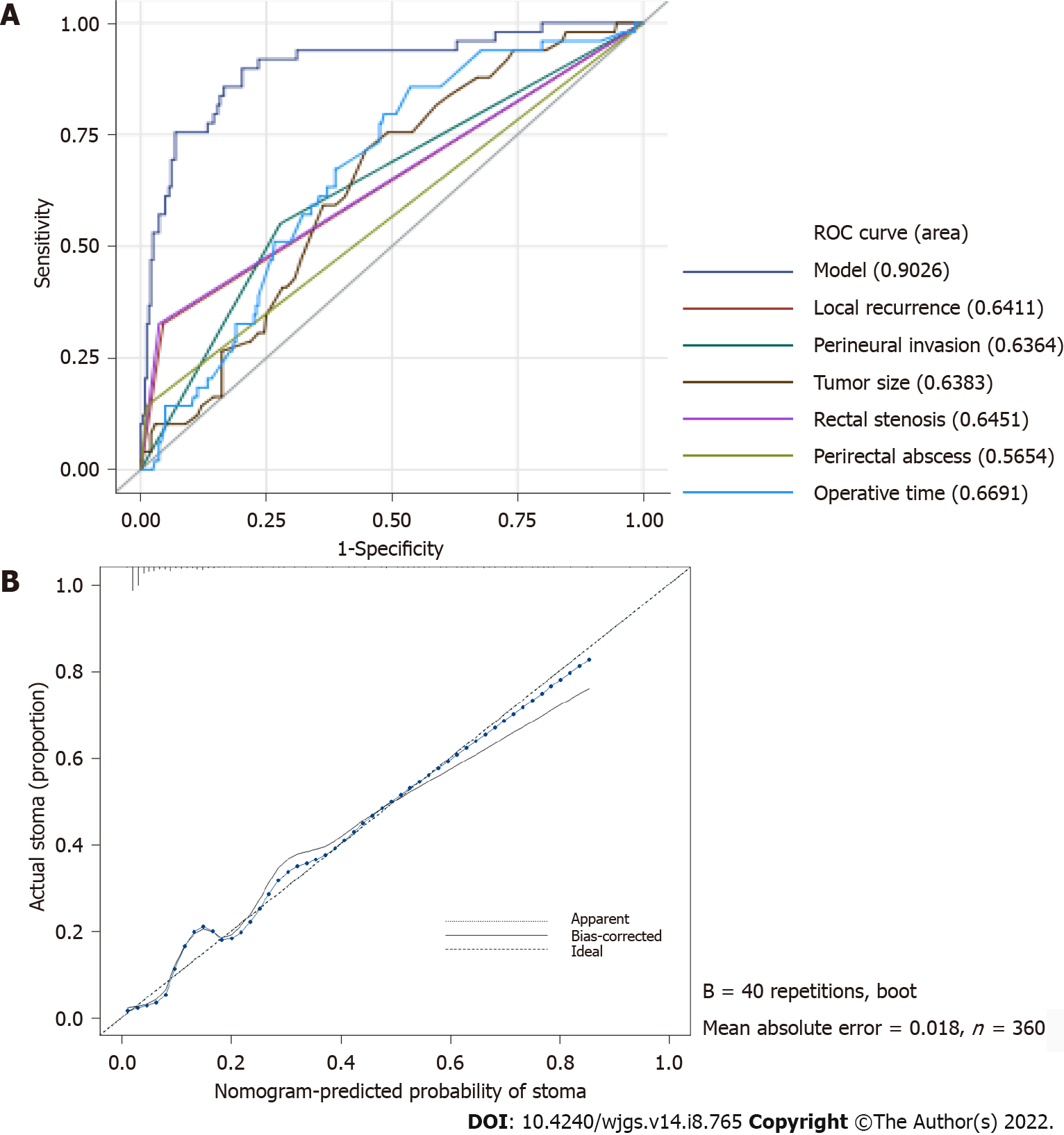
After these six factors were incorporated, the nomogram achieved an outstanding C-index of 0.903 (95%CI: 0.851–0.955). The area under the receiver operating characteristic curve of our model (0.903) was higher than that of any single factor (local recurrence: 0.641; perineural invasion: 0.636; tumor size: 0.638; rectal stenosis: 0.645; perirectal abscess: 0.565; operative time: 0.669), which indicates that this model was more accurate than other models (Figure 3A). According to the calibration curve, the nomogram calibration plot demonstrated high reliability (Figure 3B). Predicted PS rates based on the model and the observed outcomes on calibration fit best at PS probability rates above 40%. However, the nomogram showed less consistent but high performance in the lower PS rate ranges, as the calibration curve fluctuates below 40% probability.
For the past three decades, dramatic improvements have been made in rectal cancer treatment, including advances in surgical pathology, refinements in surgical techniques and instrumentation, new imaging modalities, and the widespread use of neoadjuvant therapy[11]. No matter how advanced the surgical technique, restoration of bowel continuity in patients with rectal cancer is still currently a challenge. Whenever possible, sphincter preservation should be sought. The sphincter can generally be preserved if the tumor can be resected with a 1-cm distal margin[12]. However, not all patients meet the surgical indications for sphincter-saving surgery. Even if patients undergo resection for rectal cancer, a common dilemma faced by surgeons is whether or not to create a defunctioning stoma. According to a recent meta-analysis published in 2017, which included ten studies consisting of 8568 patients, the rate of non-reversal of temporary stoma was 19%[13]. Patients still encounter multiple possible complications and the risk of perioperative mortality after surgery. Anastomotic complications are the primary reason for the necessity of a PS, and thus, these complications are more frequent than local recurrence[14-16]. Therefore, surgical decision making in the setting of rectal cancer is often complex, and detailed meetings for SDM are necessary. Patients and physicians arrive at treatment decisions together based on clinical evidence within the context of a patient’s personal preferences[4]. Prior to surgery, patients should be informed that a certain percentage of postoperative anastomosis complications may occur, which in turn may lead to PS. In addition, the physician should carefully judge whether sphincter-saving surgery or APR should be performed. Many factors should be carefully considered, including the effects of neoadjuvant CRT, sufficient tumor resection margins, the patient’s functional status/comorbid disease, and his or her personal wishes[17]. If patients who are at a higher risk of a PS after surgery can be identified, a physician’s ability to communicate the benefits and risks of various treatment options in an SDM setting will be improved.
Postoperative leakage and stricture are the most well-known anastomotic healing complications that have continued to plague surgeons. Both are primary reasons for PS. Although numerous studies have attempted to determine the healing process of colorectal anastomoses, the pathophysiologic mechanisms that govern the process of anastomotic regeneration remain poorly understood[18]. One major obstacle has been the lack of access to observe, sample, and analyze an anastomosis as it heals. Traditional dogma suggests that the most common factors implicated in anastomotic healing include tissue perfusion/ischemia, tissue tension, and patient nutritional status[19]. However, surgeons still cannot predict which anastomoses will leak or undergo stenosis. Even a well-constructed anastomosis by the most skilled surgeon with good perfusion and no tension can still develop leakage or stricture. Therefore, many retrospective studies attempt to determine the incidence and potential risk factors of anastomotic complications, which can help us predict the probability of PS. According to recent studies, the incidence of anastomotic leakage in the literature varies from 1% to 29%[20], and over half of patients with symptomatic anastomotic leakage will have PS[21,22]. A systematic search by Qu et al[23] indicated that common risk factors for anastomotic leakage include male gender, high BMI, high ASA score, large tumor size, preoperative chemotherapy, intraoperative adverse events, and low rectal anastomosis. While many studies have thoroughly analyzed the risk factors of anastomotic leakage, relatively few studies have focused on risk factors of anastomotic stricture. Rates have been shown to vary from 2%–30% in the literature, but these rates are usually under-reported due to the requirement for long-term follow-up[24]. In addition, while high-grade strictures are immediately recognized due to patient symptoms, low-grade strictures are not always identified[18]. According to recent studies, neoadjuvant CRT, clinical anastomotic leakage, and hand-sewn coloanal anastomosis have all been shown to be associated with independent risk factors of anastomotic stricture[25,26]. Endoscopic balloon dilation is the most common and effective way to treat symptomatic anastomotic stricture, but the recurrence rates after this procedure range from 6%–25%[27]. Some patients with recurrent anastomotic stricture have to accept PS to avoid the symptoms of anastomotic stricture and maintain a good quality of life.
Histology and pathology have played an important role in cancer diagnosis and prognostic prediction for decades. Some markers may potentially reflect the biological aggressiveness of the tumor, such as tumor type, tumor differentiation, growth pattern, tumor budding, and involvement of the serosa, nerves, lymphatic vessels, intramural, and extramural veins[28]. Patients with these high-risk tumor patterns may easily develop local recurrence (LR), which can lead to PS. Perineural invasion and lymphovascular invasion have been demonstrated to be independent prognostic factors of recurrence in many cancers. Perineural invasion is characterized by tumor invasion of nervous structures and spread along nerve sheaths, while lymphovascular invasion is characterized by tumor invasion of small lymphatic or blood vessels[29]. According to a study in rectal cancer by Peng et al[30], the 5-year LR rate of the perineural invasion-positive group was more than 2.5-fold higher than that of the perineural invasion-negative group (22.7% vs 7.9%; P = 0.017). In addition, in terms of lymphovascular invasion, Dresen et al[28] indicated that the presence of lymphovascular invasion (OR 4.66, P < 0.001) was associated with an increased risk for the development of local recurrence in patients with rectal cancer. Another key factor for the development of local recurrence is positive CRM. Agger et al[31] reported that the local recurrence rate was 17.0% in patients without any microscopic margin (CRM 0 mm) and 6.7% in patients with a CRM of 0–1 mm. With advancements in surgical techniques, the ratio of CRM has continued to decrease. In the study by Quentin et al[32], the rate of positive CRM decreased significantly after perineal dissection compared with after abdominal rectal dissection (4% vs 18%; P = 0.025). Moreover, it was beyond our expectations that tumor size was an independent risk factor for PS according to the results of the multivariate analysis. In previous studies, the results of the correlation between tumor size and the prognosis of rectal cancer are often contradictory, and multivariate analyses are seldom performed. However, in more recent studies, Kornprat et al[33] indicated that tumors larger than 4.5 cm are associated with high T and N classification, UICC stage, and tumor grade. Moreover, Chen et al[34] reported that pathological tumor size ≥ 5 cm is an independent prognostic factor for local recurrence in rectal adenocarcinoma. In our current study, the univariate analysis revealed that the independent risk factors for PS were lymphovascular invasion (OR, 1.99; 95%CI: 1.071–3.617; P = 0.026) and positive CRM (OR, 6.575; 95%CI: 2.955–14.604; P < 0.001), while the multivariate analysis revealed that the independent risk factors for PS were perineural invasion (OR, 3.085; 95%CI: 1.726–5.518; P < 0.001) and tumor size (OR, 1.076; 95%CI: 1.015-1.14; P = 0.014). The above four factors have been confirmed to be related to tumor recurrence, which can cause intestinal obstruction and affect intestinal continuity. The patient has no choice but to accept PS when the disease recurs because it is impossible for the physician to close the stoma in these patients.
Here, we developed a nomogram to predict the incidence of PS in patients with rectal cancer who undergo sphincter-saving surgery. To our knowledge, nomograms are widely used in many cancers to predict patient prognosis and cancer behavior (e.g., lymph node metastasis, recurrence, and distant metastasis)[35-37]. In addition, some studies have used nomograms to predict the rate of postoperative complications, such as infection, anastomotic leakage, and stenosis[38,39]. Currently, only a few predictive models of PS for patients with rectal cancer have been published[40-42]. We collected 391 cases for analysis, which is the largest case number to date among all relevant studies. The C-index for the nomogram is 0.903 (95%CI: 0.851–0.955), which indicates a perfect prediction model. According to the calibration curve, the nomogram calibration plot demonstrated high reliability. Patients with these risk factors would be classified as high-risk patients with PS, and they should be informed of their status prior to surgery. We propose that this nomogram provides more individualized outcome predictions and could aid clinicians and patients in the treatment decision making process.
The present study has some limitations. First, this was a retrospective study and was not randomized in nature. In some incomplete patient records, the details of stoma complications after hospital discharge may be difficult to evaluate. Second, the study period was relatively long, and differences may exist in surgeon discretion and surgical techniques. Finally, this analysis was based on data from a single center. External validation using data from other centers is needed to certify the discriminatory ability of this model. More representative prediction models can be developed using data from multiple centers.
This study reports that risk factors leading to PS were highly correlated with local recurrence, perirectal abscess, anastomosis site stenosis, perineural invasion, tumor size and operative time (min). Our established nomogram enables a relatively accurate assessment of the risk of PS after sphincter-saving surgery. The ease of use of this nomogram can improve a physician’s ability to communicate the benefits and risks of various treatment options in SDM.
Despite innovative advancements, the management of rectal cancer remains a formidable endeavor, especially distally located rectal cancer. According to previous studies, 3%-24% of rectal cancer patients experience anastomosis complications after sphincter-saving surgery, which may lead to permanent stoma (PS).
Patients fail to achieve stoma closure can cause drastic changes in lifestyle and physical perceptions.
The purpose of this study was to determine the risk factors for PS and to develop a prediction model to predict the probability of PS in rectal cancer patients after sphincter-saving surgery.
A retrospective cohort of 421 rectal cancer patients who underwent radical surgery at Taipei Medical University Hospital between January 2012 and December 2020 was included in this study. Univariate and multivariate analyses were performed to identify the independent risk factors for PS. A nomogram was developed according to the independent risk factors obtained in the multivariate analysis. The performance of the nomogram was assessed using a receiver operating characteristic curve and a calibration curve.
The PS stoma rate after sphincter-saving surgery was 15.1% (59/391) in our study after a median follow-up of 47.3 mo (range 7-114 mo). Multivariate logistic regression analysis demonstrated that local recurrence, perirectal abscess, anastomosis site stenosis, perineural invasion, tumor size, liver disease, and operative time were independent risk factors for PS. After exclude liver disease, these identified risk factors were incorporated into the nomogram, and the concordance index of this model was 0.903 (95%CI: 0.851-0.955). According to the calibration curves, the nomogram represents a perfect prediction model.
This study reports that risk factors leading to PS were highly correlated with local recurrence, perirectal abscess, anastomosis site stenosis, perineural invasion, tumor size and operative time (min). Our established nomogram enables a relatively accurate assessment of the risk of PS after sphincter-saving surgery. The ease of use of this nomogram can improve a physician’s ability to communicate the benefits and risks of various treatment options in shared decision making.
The present study has some limitations. First, this was a retrospective study and was not randomized in nature. In some incomplete patient records, the details of stoma complications after hospital discharge may be difficult to evaluate. Second, the study period was relatively long, and differences may exist in surgeon discretion and surgical techniques. Finally, this analysis was based on data from a single center. External validation using data from other centers is needed to certify the discriminatory ability of this model. More representative prediction models can be developed using data from multiple centers.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bustamante-Lopez LA, Brazil; Dimofte GM, Romania; Sano W, Japan; Wang LH, China; Yoshimatsu K, Japan S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Steffensen KD, Vinter M, Crüger D, Dankl K, Coulter A, Stuart B, Berry LL. Lessons in Integrating Shared Decision-Making Into Cancer Care. J Oncol Pract. 2018;14:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Thomson R, Trevena L. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 1420] [Article Influence: 157.8] [Reference Citation Analysis (1)] |
| 3. | Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366:780-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2000] [Cited by in RCA: 2334] [Article Influence: 166.7] [Reference Citation Analysis (0)] |
| 4. | Tamirisa NP, Goodwin JS, Kandalam A, Linder SK, Weller S, Turrubiate S, Silva C, Riall TS. Patient and physician views of shared decision making in cancer. Health Expect. 2017;20:1248-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, Ueno M, Miyata S, Yamaguchi T. Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery. 2009;146:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Targarona EM, Balague C, Pernas JC, Martinez C, Berindoague R, Gich I, Trias M. Can we predict immediate outcome after laparoscopic rectal surgery? Ann Surg. 2008;247:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Seo SI, Yu CS, Kim GS, Lee JL, Yoon YS, Kim CW, Lim SB, Kim JC. Characteristics and risk factors associated with permanent stomas after sphincter-saving resection for rectal cancer. World J Surg. 2013;37:2490-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Mak JCK, Foo DCC, Wei R, Law WL. Sphincter-Preserving Surgery for Low Rectal Cancers: Incidence and Risk Factors for Permanent Stoma. World J Surg. 2017;41:2912-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Holmgren K, Kverneng Hultberg D, Haapamäki MM, Matthiessen P, Rutegård J, Rutegård M. High stoma prevalence and stoma reversal complications following anterior resection for rectal cancer: a population-based multicentre study. Colorectal Dis. 2017;19:1067-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1306] [Cited by in RCA: 2414] [Article Influence: 134.1] [Reference Citation Analysis (0)] |
| 11. | Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015;33:1797-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Shirouzu K, Isomoto H, Kakegawa T. Distal spread of rectal cancer and optimal distal margin of resection for sphincter-preserving surgery. Cancer. 1995;76:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Zhou X, Wang B, Li F, Wang J, Fu W. Risk Factors Associated With Nonclosure of Defunctioning Stomas After Sphincter-Preserving Low Anterior Resection of Rectal Cancer: A Meta-Analysis. Dis Colon Rectum. 2017;60:544-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Dinnewitzer A, Jäger T, Nawara C, Buchner S, Wolfgang H, Öfner D. Cumulative incidence of permanent stoma after sphincter preserving low anterior resection of mid and low rectal cancer. Dis Colon Rectum. 2013;56:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Celerier B, Denost Q, Van Geluwe B, Pontallier A, Rullier E. The risk of definitive stoma formation at 10 years after low and ultralow anterior resection for rectal cancer. Colorectal Dis. 2016;18:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Song O, Kim KH, Lee SY, Kim CH, Kim YJ, Kim HR. Risk factors of stoma re-creation after closure of diverting ileostomy in patients with rectal cancer who underwent low anterior resection or intersphincteric resection with loop ileostomy. Ann Surg Treat Res. 2018;94:203-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Weiser MR, Quah HM, Shia J, Guillem JG, Paty PB, Temple LK, Goodman KA, Minsky BD, Wong WD. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009;249:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Guyton KL, Hyman NH, Alverdy JC. Prevention of Perioperative Anastomotic Healing Complications: Anastomotic Stricture and Anastomotic Leak. Adv Surg. 2016;50:129-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Lam A, Fleischer B, Alverdy J. The Biology of Anastomotic Healing-the Unknown Overwhelms the Known. J Gastrointest Surg. 2020;24:2160-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD. Postoperative complications following surgery for rectal cancer. Ann Surg. 2010;251:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 373] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 21. | Lindgren R, Hallböök O, Rutegård J, Sjödahl R, Matthiessen P. What is the risk for a permanent stoma after low anterior resection of the rectum for cancer? Dis Colon Rectum. 2011;54:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Jutesten H, Draus J, Frey J, Neovius G, Lindmark G, Buchwald P, Lydrup ML. High risk of permanent stoma after anastomotic leakage in anterior resection for rectal cancer. Colorectal Dis. 2019;21:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Qu H, Liu Y, Bi DS. Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc. 2015;29:3608-3617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 24. | Clifford RE, Fowler H, Manu N, Vimalachandran D. Management of benign anastomotic strictures following rectal resection: a systematic review. Colorectal Dis. 2021;23:3090-3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Lee SY, Kim CH, Kim YJ, Kim HR. Anastomotic stricture after ultralow anterior resection or intersphincteric resection for very low-lying rectal cancer. Surg Endosc. 2018;32:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Qin Q, Ma T, Deng Y, Zheng J, Zhou Z, Wang H, Wang L, Wang J. Impact of Preoperative Radiotherapy on Anastomotic Leakage and Stenosis After Rectal Cancer Resection: Post Hoc Analysis of a Randomized Controlled Trial. Dis Colon Rectum. 2016;59:934-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 27. | Biraima M, Adamina M, Jost R, Breitenstein S, Soll C. Long-term results of endoscopic balloon dilation for treatment of colorectal anastomotic stenosis. Surg Endosc. 2016;30:4432-4437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Dresen RC, Peters EE, Rutten HJ, Nieuwenhuijzen GA, Demeyere TB, van den Brule AJ, Kessels AG, Beets-Tan RG, van Krieken JH, Nagtegaal ID. Local recurrence in rectal cancer can be predicted by histopathological factors. Eur J Surg Oncol. 2009;35:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV, Nagle RB, Nielsen ML, Sargent DJ, Taylor CR, Welton M, Willett C. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 869] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 30. | Peng J, Sheng W, Huang D, Venook AP, Xu Y, Guan Z, Cai S. Perineural invasion in pT3N0 rectal cancer: the incidence and its prognostic effect. Cancer. 2011;117:1415-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Agger EA, Jörgren FH, Lydrup MA, Buchwald PL. Risk of local recurrence of rectal cancer and circumferential resection margin: population-based cohort study. Br J Surg. 2020;107:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Denost Q, Adam JP, Rullier A, Buscail E, Laurent C, Rullier E. Perineal transanal approach: a new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann Surg. 2014;260:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 33. | Kornprat P, Pollheimer MJ, Lindtner RA, Schlemmer A, Rehak P, Langner C. Value of tumor size as a prognostic variable in colorectal cancer: a critical reappraisal. Am J Clin Oncol. 2011;34:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Chen CH, Hsieh MC, Hsiao PK, Lin EK, Lu YJ, Wu SY. A critical reappraisal for the value of tumor size as a prognostic variable in rectal adenocarcinoma. J Cancer. 2017;8:1927-1934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 998] [Cited by in RCA: 964] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 36. | Lambert LA, Ayers GD, Hwang RF, Hunt KK, Ross MI, Kuerer HM, Singletary SE, Babiera GV, Ames FC, Feig B, Lucci A, Krishnamurthy S, Meric-Bernstam F. Validation of a breast cancer nomogram for predicting nonsentinel lymph node metastases after a positive sentinel node biopsy. Ann Surg Oncol. 2006;13:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, Bonnetain F, Bosset JF, Bujko K, Cionini L, Gerard JP, Rödel C, Sainato A, Sauer R, Minsky BD, Collette L, Lambin P. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29:3163-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 417] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 38. | Yao J, Jiang Y, Ke J, Lu Y, Hu J, Zhi M. A Validated Prognostic Model and Nomogram to Predict Early-Onset Complications Leading to Surgery in Patients With Crohn's Disease. Dis Colon Rectum. 2021;64:697-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Wen J, Pan T, Yuan YC, Huang QS, Shen J. Nomogram to predict postoperative infectious complications after surgery for colorectal cancer: a retrospective cohort study in China. World J Surg Oncol. 2021;19:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Abe S, Kawai K, Nozawa H, Hata K, Kiyomatsu T, Tanaka T, Nishikawa T, Otani K, Sasaki K, Kaneko M, Murono K, Emoto S, Watanabe T. Use of a nomogram to predict the closure rate of diverting ileostomy after low anterior resection: A retrospective cohort study. Int J Surg. 2017;47:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Liu J, Zheng L, Ren S, Zuo S, Zhang J, Wan Y, Wang X, Tang J. Nomogram for Predicting the Probability of Permanent Stoma after Laparoscopic Intersphincteric Resection. J Gastrointest Surg. 2021;25:3218-3229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Li C, Qin X, Yang Z, Guo W, Huang R, Wang H. A nomogram to predict the incidence of permanent stoma in elderly patients with rectal cancer. Ann Transl Med. 2021;9:342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |