Published online Aug 27, 2022. doi: 10.4240/wjgs.v14.i8.754
Peer-review started: February 19, 2022
First decision: April 19, 2022
Revised: May 2, 2022
Accepted: August 1, 2022
Article in press: August 1, 2022
Published online: August 27, 2022
Processing time: 185 Days and 17.3 Hours
Three-dimensional (3D) laparoscopic technique has gradually been applied to the treatment of carcinoma in the remnant stomach (CRS), but its clinical efficacy remains controversial.
To compare the short-term and long-term results of 3D laparoscopic-assisted gastrectomy (3DLAG) with open gastrectomy (OG) for CRS.
The clinical data of patients diagnosed with CRS and admitted to the First Medical Center of Chinese PLA General Hospital from January 2016 to January 2021 were retrospectively collected. A total of 84 patients who met the inclusion and exclusion criteria were enrolled. All their clinical data were collected and a database was established. All patients were treated with 3DLAG or OG by experienced surgeons and were divided into two groups based on the different surgical methods mentioned above. By using outpatient and telephone follow-up, we were able to determine postoperative survival and tumor status. The postoperative short-term efficacy and 1-year and 3-year overall survival (OS) rates were compared between the two groups.
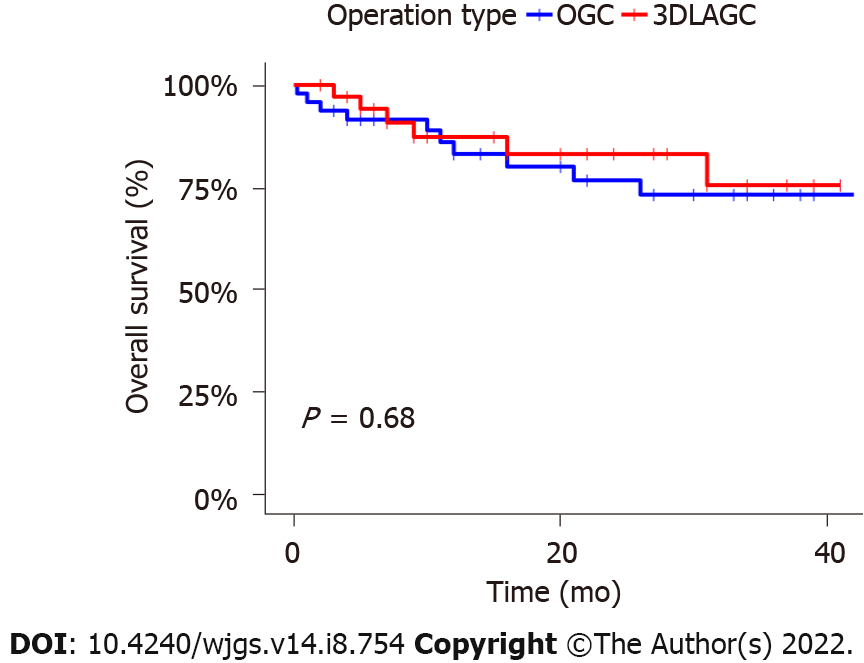
Among 84 patients with CRS, 48 were treated with OG and 36 with 3DLAG. All patients successfully completed surgery. There was no significant difference between the two groups in terms of age, gender, body mass index, ASA score, initial disease state (benign or malignant), primary surgical anastomosis method, interval time of carcinogenesis, and tumorigenesis site. Patients in the 3DLAG group experienced less intraoperative blood loss (188.33 ± 191.35 mL vs 305.83 ± 303.66 mL; P = 0.045) and smaller incision (10.86 ± 3.18 cm vs 20.06 ± 5.17 cm; P < 0.001) than those in the OG group. 3DLAGC was a more minimally invasive method. 3DLAGC retrieved significantly more lymph nodes than OG (14.0 ± 7.17 vs 10.73 ± 6.82; P = 0.036), whereas the number of positive lymph nodes did not differ between the two groups (1.56 ± 2.84 vs 2.35 ± 5.28; P = 0.413). The complication rate (8.3% vs 20.8%; P = 0.207) and intensive care unit admission rate (5.6% vs 14.5%; P = 0.372) were equivalent between the two groups. In terms of postoperative recovery, the 3DLAGC group had a lower visual analog score, shorter indwelling time of gastric and drainage tubes, shorter time of early off-bed motivation, shorter time of postoperative initial flatus and initial soft diet intake, shorter postoperative hospital stay and total hospital stay, and there were significant differences, showing better short-term efficacy. The 1-year and 3-year OS rates of OG group were 83.2% [95% confidence interval (CI): 72.4%-95.6%] and 73.3% (95%CI: 60.0%-89.5%) respectively. The 1-year and 3-year OS rates of the 3DLAG group were 87.3% (95%CI: 76.4%-99.8%) and 75.6% (95%CI: 59.0%-97.0%), respectively. However, the 1-year and 3-year OS rates were similar between the two groups, which suggested that long-term survival results were comparable between the two groups (P = 0.68).
Compared with OG, 3DLAG for CRS achieved better short-term efficacy and equivalent oncological results without increasing clinical complications. 3DLAG for CRS can be promoted safely and effectively in selected patients.
Core Tip: The application of minimally invasive surgery in carcinoma in the remnant stomach (CRS) is affected by factors such as abdominal adhesion, anatomical displacement and unclear markers caused by previous partial gastrectomy. Most previous studies were case series or small-sample studies. This study explored the therapeutic efficacy of three-dimensional (3D) laparoscopic-assisted gastrectomy (3DLAG) vs open gastrectomy for CRS. 3DLAG has shown obvious short-term advantages and equivalent long-term oncological efficacy in the treatment of CRS without increasing the incidence of complications. This study provides evidence-based medical support for the treatment of CRS by 3DLAG.
- Citation: Wu D, Song QY, Li XG, Xie TY, Lu YX, Zhang BL, Li S, Wang XX. 3D laparoscopic-assisted vs open gastrectomy for carcinoma in the remnant stomach: A retrospective cohort study. World J Gastrointest Surg 2022; 14(8): 754-764
- URL: https://www.wjgnet.com/1948-9366/full/v14/i8/754.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i8.754
Remnant gastric cancer (RGC) was initially defined as carcinoma arising in the residual stomach after gastrectomy for benign or malignant disease. The incidence of RGC is about 2%-3%, which is a relatively rare disease in the clinic[1-3]. However, as the long-term survival rate of patients with GC improves due to early detection and individual comprehensive therapy, the incidence of RGC is gradually increasing. As a unique type of GC, RGC had gained increasing attentions in recent years. The Japanese Gastric Cancer Association (JGCA) proposed the broad nomenclature of carcinoma in the remnant stomach (CRS), which contains new cancer, recurrent cancer, residual cancer, to replace the narrow definition of RGC[4].
At present, there is no consensus on the surgical and postoperative management of CRS. Completion gastrectomy of the RS combined with adequate lymph nodes dissection remains the mainstay treatment for resectable CRS[4-6]. In traditional opinion, most scholars believed that the history of upper abdominal surgery was contraindicated for laparoscopic surgery, and patients with RGC were treated with open surgery. With the development of minimally invasive techniques and equipment, three-dimensional (3D) laparoscopy is widely used in the treatment of GC, and displays advantages over two-dimensional (2D) laparoscopy and open surgery[7,8]. The emergence of 3D laparoscopy has pushed minimally invasive surgery into the stereoscopic era. 3D laparoscopy provides a sense of depth and layering that allows surgeons to obtain a field of vision similar to open surgery. At the same time, compared with open surgery, 3D laparoscopic surgery has a magnified view of the local surgical field and a better and clearer view of the anatomical structure, thus making it easier and more precise to perform the delicate procedures such as dissection, separation of tissues, stopping bleeding and ligating vessels, especially in complicated surgery. However, there are limited reports and studies about the application of 3D laparoscopic-assisted techniques in the treatment of CRS. Our study retrospectively collected the clinical data of 3D laparoscopic-assisted and open surgery in the treatment of CRS, analyzed the short-term and long-term efficacy of the two groups, and provided a reference for the minimally invasive treatment of CRS.
This retrospective cohort study was conducted in the First Medical Center of Chinese PLA General Hospital in China, and it was approved by the ethics committee of the hospital. This study set the inclusion and exclusion criteria of patients as follows.
Inclusion criteria: (1) Patients underwent function-preserving gastrectomy such as proximal or distal gastrectomy due to benign or malignant gastric lesions were diagnosed as CRS including new cancer, recurrent cancer, residual cancer, multifocal cancer by preoperative gastroscopy and biopsy pathology; (2) The surgical method was open or 3D laparoscopic-assisted total residual gastrectomy for RGC; (3) The clinical and pathological data were complete; (4) The operation was performed by experienced doctors, at least associate professor level; and (5) Patients and their relatives were fully aware of the surgical risks and signed the surgical informed consent.
Exclusion criteria: (1) Preoperative examination showed that CRS with distant metastasis such as liver, peritoneum and ovary, and other metastases could not be radically resected; (2) Patients confirmed other malignant tumors simultaneously; (3) Patients underwent palliative gastrectomy or RS-jejunal anastomosis due to acute tumor complications such as hemorrhage, obstruction and perforation; (4) Partial resection or palliative resection of the RS was performed during surgery; (5) Clinical and pathological data were missing or deficient; (6) Postoperative pathology confirmed high-grade epithelial neoplasia and other precancerous lesions; and (7) Patients received systemic chemotherapy or local radiotherapy within 1 mo before surgery.
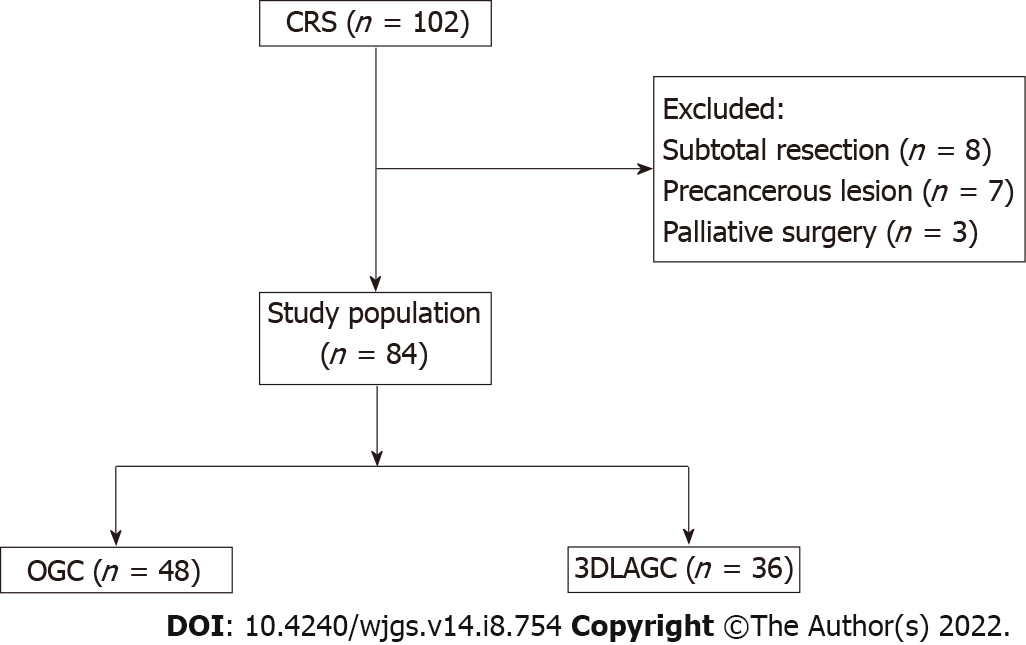
A total of 102 patients with CRS who underwent gastrectomy in the First Medical Center of Chinese PLA General Hospital from January 2016 to January 2021 were retrospectively collected. Eight patients underwent subtotal resection of the RS, seven patients were pathologically confirmed to have precancerous lesions after surgery, and three patients underwent palliative surgery due to acute complications. Thus, a total of 18 patients were excluded. Finally, a total of 84 patients with CRS were enrolled in this study and divided into two groups according to different surgical methods. Of them, 48 patients underwent open gastrectomy (OG) for CRS and 36 patients underwent 3D laparoscopic-assisted gastrectomy (3DLAG) (Figure 1).
The basic information of all patients who met the inclusion and exclusion criteria were collected based on the hospital records, including gender, age, body mass index (BMI), ASA score, initial gastric disease status (benign or malignant), operation type of initial gastrectomy, interval time from surgery to occurrence of CRS, tumor site (anastomotic or nonanastomotic), etc. The surgical information included surgical methods (3D laparoscopic-assisted or open surgery), grade of abdominal adhesions, operation time, intraoperative blood loss etc. The postoperative information included gastric tube removal time, time to first soft diet intake, time to first off-bed ambulation, time to first flatus and defecation, time to remove the drainage tube, visual analog score (VAS) of postoperative days 1, 3 and 5, intensive care unit (ICU) stay, postoperative hospital stay, and total hospital stay. Postoperative pathological information included pathological type, total number of harvested lymph nodes, number of positive lymph nodes, and TNM stage. Perioperative complications were registered and collected according to the Clavien-Dindo classification system.
Regardless of 3DLAG or OG for CRS, the common procedures of radical gastrectomy for RGC are adhesiolysis, lymph node dissection, total resection of the RS and digestive tract reconstruction. It is a major challenge for surgeons to perform adhesiolysis for CRS surgery. Severe adhesion always is a major cause of unplanned organ injury or combined resection. Laparotomy for RGC usually requires the middle incision of the upper abdomen, but it is necessary to pay attention to adhesion of the small intestine under the abdominal wall to avoid unnecessary injury. For regular LAG for GC, 1 cm below the navel is always selected for the location of the observation port. However, the location of the observation port needs to be changed according to abdominal adhesions caused by a history of upper-abdominal surgery in order to avoid unplanned intra-abdominal organ injury. The right lower-abdominal area is recommended as the optimum site for the observation port during surgery for RGC. The other trocars could be subsequently inserted carefully under visualization. Sometimes, one can also choose the left upper abdomen as the site of the observation port and then as the main operating port. When the initial operation is distal gastrectomy, lymph node dissection around the celiac axis, proximal splenic artery and paracardial nodes were routinely performed, and the left gastric artery is ligated at its base if it has been preserved. When proximal gastrectomy has been performed before, it is necessary to open the esophageal hiatus of the diaphragm and fully dissect the lower segment of the esophagus in order to obtain sufficient cutting edge and facilitate follow-up anastomosis. Meanwhile, the lymph node dissection around the celiac axis and infrapyloric and suprapyloric areas is routinely performed. Roux-en-Y anastomosis is the regular method of digestive tract reconstruction using circular stapler.
Postoperative follow-up was performed by outpatient and telephone to investigate the postoperative survival data and tumor conditions of the patients. Overall survival (OS) was defined as the time from radical operation for RGC to death due to any cause or last time of follow-up. The follow-up time was up to December 2021.
All observation indicators were included and a database of patients with CRS was established. All data were processed and analyzed using IBM SPSS Statistics 25 and R version.4.2.2. Continuous variables were analyzed using the t-test or Mann-Whitney U test; the latter was used for variables that did not meet the criteria for positivity and homogeneity. Categorical variables were compared using the2 test or Fisher’s exact probability test. OS was estimated using the Kaplan-Meier method, and curves were compared using the log-rank test. P < 0.05 was considered statistically significant.
The demographic and clinicopathological characteristics and initial gastrectomy information of the 3DLAGC group compared with those of the OG group are summarized in Table 1. In this study, there were more men than women with RGC with a male-to-female ratio of 7.4:1. Among the reasons for initial gastrectomy, patients with benign diseases accounted for 39.3%, mainly due to gastrointestinal ulcerative diseases, while patients who performed gastrectomy due to malignant tumors accounted for 60.7% in the initial surgery. Main digestive tract reconstruction methods for distal gastrectomy included Billroth-I anastomosis, Billroth-II anastomosis, and Roux-en-Y anastomosis, accounting for 33.3%, 50.0%, and 6.0%, respectively. The main anastomosis method of proximal gastrectomy was esophageal residual gastric tube-like anastomosis, accounting for 10.7%. No patient underwent proximal gastrectomy with double tract anastomosis. The interval time is generally considered to be the time from primary gastrectomy to the occurrence of adenocarcinoma in the RS. Patients with benign gastric ulcer who underwent partial gastrectomy, the interval time of CRS took longer than those with malignant gastric disease (415.64 mo vs 98.16 mo). However, there was no significant difference in the interval time between the OG and 3DLAG groups (211.56 ± 197.35 mo vs 237.97 ± 209.01 mo; P = 0.556). The incidence of CRS occurring at anastomotic stoma was higher than that at nonanastomotic stoma, and the ratio was 1.47:1. However, there were no significant differences in age, gender, BMI, disease status of the initial surgery, reconstruction method of the initial surgery, interval time from the initial surgery to the occurrence of RGC, and location of RGC between the two groups.
| OG (n = 48) | 3DLAG (n = 36) | P value | |
| Age (yr) | 60.62 (10.11) | 61.19 (9.90) | 0.797 |
| Gender (%) | 1.000 | ||
| Male | 42 (87.5) | 32 (88.9) | |
| Female | 6 (12.5) | 4 (11.1) | |
| BMI (kg/m2) | 21.65 (3.22) | 22.26 (2.59) | 0.355 |
| ASA (%) | 0.384 | ||
| 1 | 1 (2.1) | 0 (0.0) | |
| 2 | 33 (68.8) | 29 (80.6) | |
| 3 | 14 (29.2) | 7 (19.4) | |
| Previous disease (%) | 0.54 | ||
| Benign | 17 (35.4) | 16 (44.4) | |
| Malignant | 31 (64.6) | 20 (55.6) | |
| Primary reconstruction (%) | 0.617 | ||
| Billroth Ⅰ | 16 (33.3) | 12 (33.3) | |
| Billroth Ⅱ | 22 (45.8) | 20 (55.6) | |
| Roux-en-Y | 4 (8.3) | 1 (2.8) | |
| Tube-like Stomach esophagogastrostomy | 6 (12.5) | 3 (8.3) | |
| Interval time (d) | 211.56 (197.35) | 237.97 (209.01) | 0.556 |
| Site of CRS (%) | 0.352 | ||
| Non-anastomosis | 22 (45.8) | 12 (33.3) | |
| Anastomosis | 26 (54.2) | 66.7) |
Clinical data of intraoperative and postoperative recovery in patients with CRS in the 3DLAG group compared with the OG group are shown in Table 2. The initial surgical operation often causes adhesion of the RS, anastomotic stoma and surrounding tissues, thus affecting exposure of the anatomical level. One of the difficulties in the surgical resection of RGC is intra-abdominal adhesion. Abdominal adhesions grades 2 and 3 were found in most patients in both groups, with no significant difference between the groups (P = 0.098). The mean operating time was shorter in the OG group than in the 3DLAG group (215.67 min vs 243.11 min), but the difference between the wo groups was not significant (P = 0.075). The 3DLAG group had less intraoperative blood loss (188.33 ± 191.35 mL vs 305.83 ± 303.66 mL; P = 0.045), and significantly shorter surgical incision (10.86 ± 3.18 vs 20.06 ± 5.17 cm; P < 0.001), which was minimally invasive. In terms of postoperative recovery, the 3DLAG group had a lower pain score according to VAS on d 1, 3 and 5 after surgery (P < 0.001). The indwelling time of the gastric and drainage tubes, time to early off-bed motivation, time to first flatus, time to first soft diet intake, postoperative hospital stay and total hospital stay in the 3DLAG group were significantly shorter than in the OG group (P < 0.001). There was no significant difference in the incidence of complications (P = 0.372) and ICU admission rate (P = 0.207) between the two groups.
| OGC (n = 48) | 3DLAGC (n = 36) | P value | |
| Abdominal adhesion, n (%) | 0.098 | ||
| 0 | 7 (14.6) | 1 (2.8) | |
| Ⅰ | 10 (20.8) | 3 (8.3) | |
| Ⅱ | 12 (25.0) | 14 (38.9) | |
| Ⅲ | 12 (25.0) | 14 (38.9) | |
| Ⅳ | 7 (14.6) | 4 (11.1) | |
| Operation time (min) | 215.67 (73.80) | 243.11 (61.97) | 0.075 |
| Blood Loss (mL) | 305.83 (303.66) | 188.33 (191.35) | 0.045 |
| Incision size (cm) | 20.06 (5.17) | 10.86 (3.18) | < 0.001 |
| Postoperative VAS | |||
| Day 1 | 7.17 (0.88) | 6.03 (0.70) | < 0.001 |
| Day 3 | 5.52 (0.80) | 3.86 (0.68) | < 0.001 |
| Day 5 | 3.73 (1.16) | 2.06 (0.92) | < 0.001 |
| Nasogastric tube removal time (d) | 3.58 (1.93) | 1.86 (1.46) | < 0.001 |
| Abdominal drainage tube removal time (d) | 8.21 (3.14) | 5.83 (2.26) | < 0.001 |
| Time to first ambulation (d) | 2.58 (0.71) | 1.81 (0.71) | < 0.001 |
| Time to first flatus (d) | 4.00 (1.03) | 3.08 (0.55) | < 0.001 |
| Time to first soft diet (d) | 5.50 (3.58) | 3.14 (1.73) | < 0.001 |
| ICU, n (%) | 10 (20.8) | 3 (8.3) | 0.207 |
| Postoperative hospital stay (d) | 11.19 (6.34) | 7.56 (2.25) | 0.002 |
| Total hospital stay (d) | 15.75 (7.37) | 12.19 (4.02) | 0.011 |
| Complications (Grade ≥ Ⅲ), n (%) | 7 (14.5) | 2 (5.6) | 0.372 |
| Anastomosis leakage | 2 (4.2) | 1 (2.8) | |
| Cardiac failure | 1 (2.1) | 0 (0.0) | |
| Anastomosis obstruction | 2 (4.2) | 0 (0.0) | |
| Abdominal bleeding | 2 (4.2) | 1 (2.8) |
Table 3 depicts the pathological results for the 3DLAG and OGC groups. There were no significant differences between the two groups in postoperative pathological type, tumor size, tumor invasion depth or lymph node metastasis. However, the 3DLAG group exhibited a certain advantage in perigastric lymph node dissection. Total number of lymph nodes retrieved by 3DLAG was significantly higher than by OG (14.0 ± 7.17 vs 10.73 ± 6.82; P = 0.036).
| OGC (n = 48) | 3DLAGC (n = 36) | P value | |
| Pathological type, n (%) | 0.521 | ||
| Well differentiated | 24 (50.0) | 21 (58.3) | |
| Moderately differentiated | 19 (39.6) | 10 (27.8) | |
| Poorly differentiated (including signet-ring cell carcinoma) | 5 (10.4) | 5 (13.9) | |
| Tumor size (mm) | 38.67 (30.51) | 35.22 (30.93) | 0.612 |
| TNM, n (%) | 0.084 | ||
| Ⅰ | 18 (37.5) | 15 (41.7) | |
| Ⅱa | 11 (22.9) | 8 (22.2) | |
| Ⅱb | 9 (18.8) | 1 (2.8) | |
| Ⅲa | 4 (8.3) | 9 (25.0) | |
| Ⅲb | 4 (8.3) | 3 (8.3) | |
| Ⅲc | 2 (4.2) | 0 (0.0) | |
| Depth of tumor invasion, n (%) | 0.826 | ||
| T1 | 10 (20.8) | 9 (25.0) | |
| T2 | 9 (18.8) | 7 (19.4) | |
| T3 | 17 (35.4) | 13 (36.1) | |
| T4 | 10 (25.0) | 5 (19.5) | |
| Lymph nodes metastases, n (%) | 0.205 | ||
| N0 | 34 (70.8) | 20 (55.6) | |
| N1 | 6 (12.5) | 8 (22.2) | |
| N2 | 2 (4.2) | 5 (13.9) | |
| N3 | 6 (12.5) | 3 (8.3) | |
| Number of positive lymph nodes (n) | 2.35 (5.28) | 1.56 (2.84) | 0.413 |
| Total number of lymph nodes retrieved (n) | 10.73 (6.82) | 14.00 (7.17) | 0.036 |
Figure 2 depicts the survival of the two groups. The median follow-up duration of the OG group was 34 mo, compared with 27 mo for 3DLAG. The 1-year and 3-year OS rates of the OG group were 83.2% (95%CI: 72.4%-95.6%) and 73.3% (95%CI: 60.0%-89.5%), respectively. The 1-year and 3-year OS rates of the 3DLAG group were 87.3% (95%CI: 76.4%-99.8%) and 75.6% (95%CI: 59.0%-97.0%), respectively. However, these OS rates did not differ significantly between the two groups (P = 0.68).
RGC, first described by Balfour[9] in 1922, is defined as a carcinoma occurring in the RS after partial gastrectomy for peptic ulcer disease. Since then, RGC had been gradually known as a unique disease. In 1998, the concept of CRS was initially proposed and continuously used by the JGCA[10]. It was widely accepted that the adenocarcinoma occurring in the RS after gastrectomy was called CRS, regardless of whether the initial disease was benign or malignant, or the interval time.
As a subtype of GC with unique characteristics, the incidence of CRS showed a male preponderance, with a male-to-female incidence ratio of 3.1:1[11]. In our study, CRS was also more common in men, but the incidence ratio of male-to-female was 7.4:1, which was higher than the ratio reported in previous studies. Several studies clearly indicated that the RS after gastrectomy had a high risk of developing CRS, and the anastomosis had a higher prevalence to develop stump carcinomas in a shorter time interval than other site of the RS[12-14]. It has also been shown that CRS tends to arise from the sites of anastomosis in patients treated with Billroth II reconstruction, in contrast to nonanastomotic sites in patients treated with Billroth I reconstruction[5,15,16]. In our study, carcinoma in the RS at the anastomotic site accounted for about 59.5% of cases; of which, Billroth I reconstruction accounted for 32% and Billroth II for 52%, which was consistent with the epidemiological characteristics of previous studies.
Intra-abdominal adhesions and anatomical displacement presented significant challenges for surgeons in both OG and 3DLAG for RGC[17-19]. Extensive and intensive intra-abdominal adhesions due to previous surgery may significantly prolong the operation time, increase intraoperative blood loss, and lead to unplanned collateral damage to the surrounding tissues and organs. In our study, the degree of abdominal adhesions was macroscopically inspected and scored using Knightly’s grading system for assessment of the intensity and Linsky’s grading system for assessment of the extent of adhesions[20]. Almost 13.1% of patients had grade 4 abdominal adhesions, which may lead to unplanned damage to peripheral organs. While most patients with CRS, approximately 56%, had abdominal adhesion below grade 3, the abdominal adhesion mainly existed in the previous operation area. However, there was no significant difference in abdominal adhesions between the 3DLAG and OG groups (P = 0.098). The first successful application of laparoscopic surgery in the treatment of RGC was reported by Yamada et al[17] in 2005. Other reports have shown the ever-increasing feasibility and safety of LAG for RGC; in some cases, even proving superior to traditional open surgery[18,19]. However, Son et al[21] suggested that although laparoscopic total gastrectomy was technically feasible, it did not show a definite clinical advantage over laparotomy in the treatment of RGC. 3D laparoscopy in the treatment of CRS has shown many advantages in the separation of abdominal adhesions. An outstanding advantage of laparoscopic surgery is that the establishment of carbon dioxide pneumoperitoneum can make the connective tissue space appear clearly and make it possible to identify the correct dissection layer[22]. In addition, 3D laparoscopy can overcome the disadvantages of traditional laparoscopy, such as lack of sense of space and distance, presenting a stereoscopic vision closer to open surgery[23]. However, compared with open surgery, the enlarged surgical field of 3D laparoscopy shows the anatomical structure more clearly, which is more conducive to delicate operations, making it easier to find the correct anatomical level, resulting in less surgical bleeding and adverse consequences. It also avoids unnecessary damage to surrounding tissues or organs due to adhesiolysis and decreases the probability of unplanned combined devisceration.
Our study found that the 3DLAG group showed obvious advantages in short-term postoperative outcomes. We attributed those advantages to the magnification effect, 3D sense, and spatial depth of the surgical field. Because 3D laparoscopic surgery made it easier to obtain the correct anatomical landmark and dissect important tissues accurately such as blood vessels, nerves and perigastric lymph nodes[24,25]. 3DLAGC group had less intestinal traction and flipping, damage to surrounding tissues during adhesiolysis, trauma and inflammatory response. Enhanced recovery after surgery (ERAS) protocols have been effective in improving postoperative recovery after major abdominal surgeries[26,27]. All patients with CRS enrolled in this study underwent preoperative education and evaluation, intraoperative stretch socks for thrombosis prevention, intraoperative warmth, postoperative multimode analgesia, encouragement of early ambulation, and postoperative enteral and parenteral nutrition support, which were in line with ERAS protocols. Take considerations that not every patient is eligible for all items of ERAS, we hold the opinion that patients who meet a few of the items should accept the management of ERAS. However, minimally invasive surgery is the cornerstone of ERAS. Through minimally invasive surgical methods, patients can remove the gastric tube and drainage tube early after surgery, thus reducing nausea, vomiting and other gastrointestinal reactions caused by gastric tube stimulation and reduce pain and discomfort caused by the abdominal drainage tube. Early removal of the gastric tube and drainage tube is beneficial to the early off-bed activity of patients, promoting recovery of gastrointestinal function, facilitating early eating of patients and accelerating the rehabilitation process. The total number of dissected lymph nodes was significantly more in the 3DLAG than OG group, which may be related to the visual magnification and flexibility in tight spaces. While the staging system of CRS is not yet established, it generally follows the TNM staging of primary GC. The number of positive lymph nodes (pN) is key to determination of the N stage, but inadequate lymph nodes harvested in patients with CRS might influence the predictive value of pN. Some research has demonstrated that the lymph node ratio (LNR) has significant prognostic value for patients with CRS[28]. When the retrieved lymph node count is < 15, the LNR is superior to pN as an important and independent prognostic index of CRS[29]. In spite of the obvious postoperative short-term advantages shown by 3DLAG, the long-term survival results were similar between the 3DLAG and OG groups with the 1-year and 3-year OS rates comparable between the two groups.
Several limitations to our study warrant mention. Our study was a retrospective study, which had a potential for selection bias. The number of patients enrolled was small. Prospective randomized controlled trials with large samples and multiple centers are needed in the future. Despite these limitations, our study demonstrated the feasibility and efficacy of 3DLAG for CRS and showed some advantages over OG in short-term postoperative outcomes.
Nowadays, patients with GC can obtain long-term survival due to the application of comprehensive treatments, thus causing an increase in incidence of CRS. Compared with OG, 3DLAG for CRS can achieve better short-term efficacy and equivalent oncological results without increasing clinical complications. In some medical centers, 3DLAG for CRS can be applied and promoted in selected patients.
Three-dimensional (3D) laparoscopy provides a 3D sense of depth and layering that allows surgeons to obtain a field of vision similar to open surgery. 3D laparoscopic techniques are gradually being applied in the treatment of carcinoma in the remnant stomach (CRS), but their clinical efficacy remains controversial.
There are limited reports and studies about the application of 3D laparoscopic-assisted techniques in the treatment of CRS. No study has shown whether 3D laparoscopic-assisted gastrectomy (3DLAG) is superior or non-inferior to open gastrectomy (OG) for CRS.
This study retrospectively collected the clinical data of 3DLAG and OG in the treatment of CRS, analyzed the short-term and long-term efficacy of the two methods, and provided a reference for the minimally invasive treatment of CRS.
The authors retrospectively evaluated 84 patients with CRS who had undergone OG for carcinoma or 3DLAGC at the First Medical Center of Chinese PLA General Hospital from January 2016 to January 2021. The short-term and long-term outcomes were compared between the OG (n = 48) and 3DLAG (n = 36) groups.
Compared with the OG group, the 3DLAG group had less surgical trauma and faster recovery after surgery. However, the complication rate and intensive care unit admission rate were equivalent between the two groups. The 1-year overall survival (OS) and 3-year OS rates were similar between the two groups, which suggested comparable long-term survival results between the groups. Our research showed that 3DLAG for CRS can be promoted safely and effectively in selected patients.
Compared with OG, 3DLAG for CRS can achieve better short-term efficacy and equivalent oncological results without increasing clinical complications.
Prospective randomized controlled trials with large samples and multiple centers are needed in the future.
| 1. | Komatsu S, Ichikawa D, Okamoto K, Ikoma D, Tsujiura M, Nishimura Y, Murayama Y, Shiozaki A, Ikoma H, Kuriu Y, Nakanishi M, Fujiwara H, Ochiai T, Kokuba Y, Otsuji E. Progression of remnant gastric cancer is associated with duration of follow-up following distal gastrectomy. World J Gastroenterol. 2012;18:2832-2836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Nagai E, Nakata K, Ohuchida K, Miyasaka Y, Shimizu S, Tanaka M. Laparoscopic total gastrectomy for remnant gastric cancer: feasibility study. Surg Endosc. 2014;28:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Sinning C, Schaefer N, Standop J, Hirner A, Wolff M. Gastric stump carcinoma - epidemiology and current concepts in pathogenesis and treatment. Eur J Surg Oncol. 2007;33:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 4. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1414] [Article Influence: 282.8] [Reference Citation Analysis (2)] |
| 5. | Tanigawa N, Nomura E, Lee SW, Kaminishi M, Sugiyama M, Aikou T, Kitajima M; Society for the Study of Postoperative Morbidity after Gastrectomy. Current state of gastric stump carcinoma in Japan: based on the results of a nationwide survey. World J Surg. 2010;34:1540-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Sakamoto E, Dias AR, Ramos MFKP, Charruf AZ, Ribeiro-Junior U, Zilberstein B, Cecconello I. Laparoscopic Completion Total Gastrectomy for Remnant Gastric Cancer. J Laparoendosc Adv Surg Tech A. 2021;31:803-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Itatani Y, Obama K, Nishigori T, Ganeko R, Tsunoda S, Hosogi H, Hisamori S, Hashimoto K, Sakai Y. Three-dimensional Stereoscopic Visualization Shortens Operative Time in Laparoscopic Gastrectomy for Gastric Cancer. Sci Rep. 2019;9:4108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Kanaji S, Suzuki S, Harada H, Nishi M, Yamamoto M, Matsuda T, Oshikiri T, Nakamura T, Fujino Y, Tominaga M, Kakeji Y. Comparison of two- and three-dimensional display for performance of laparoscopic total gastrectomy for gastric cancer. Langenbecks Arch Surg. 2017;402:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Balfour DC. Factors influencing the life expectancy of patients operated on for gastric ulcer. Ann Surg. 1922;76:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 964] [Reference Citation Analysis (0)] |
| 11. | Yamamoto M, Yamanaka T, Baba H, Kakeji Y, Maehara Y. The postoperative recurrence and the occurrence of second primary carcinomas in patients with early gastric carcinoma. J Surg Oncol. 2008;97:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Park YE, Kim SW. Clinicopathologic features of remnant gastric cancer after curative distal gastrectomy according to previous reconstruction method: a retrospective cohort study. World J Surg Oncol. 2019;17:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Mak TK, Guan B, Peng J, Chong TH, Wang C, Huang S, Yang J. Prevalence and characteristics of gastric remnant cancer: A systematic review and meta-analysis. Asian J Surg. 2021;44:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Ohira M, Toyokawa T, Sakurai K, Kubo N, Tanaka H, Muguruma K, Yashiro M, Onoda N, Hirakawa K. Current status in remnant gastric cancer after distal gastrectomy. World J Gastroenterol. 2016;22:2424-2433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Takeno S, Hashimoto T, Maki K, Shibata R, Shiwaku H, Yamana I, Yamashita R, Yamashita Y. Gastric cancer arising from the remnant stomach after distal gastrectomy: a review. World J Gastroenterol. 2014;20:13734-13740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Ahn HS, Kim JW, Yoo MW, Park DJ, Lee HJ, Lee KU, Yang HK. Clinicopathological features and surgical outcomes of patients with remnant gastric cancer after a distal gastrectomy. Ann Surg Oncol. 2008;15:1632-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Yamada H, Kojima K, Yamashita T, Kawano T, Sugihara K, Nihei Z. Laparoscopy-assisted resection of gastric remnant cancer. Surg Laparosc Endosc Percutan Tech. 2005;15:226-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Kwon IG, Cho I, Guner A, Choi YY, Shin HB, Kim HI, An JY, Cheong JH, Noh SH, Hyung WJ. Minimally invasive surgery for remnant gastric cancer: a comparison with open surgery. Surg Endosc. 2014;28:2452-2458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Ota M, Ikebe M, Shin Y, Kagawa M, Mano Y, Nakanoko T, Nakashima Y, Uehara H, Sugiyama M, Iguchi T, Sugimachi K, Yamamoto M, Morita M, Toh Y. Laparoscopic Total Gastrectomy for Remnant Gastric Cancer: A Single-institution Experience and Systematic Literature Review. In Vivo. 2020;34:1987-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Gökçelli U, Ercan UK, İlhan E, Argon A, Çukur E, Üreyen O. Prevention of Peritoneal Adhesions by Non-Thermal Dielectric Barrier Discharge Plasma Treatment on Mouse Model: A Proof of Concept Study. J Invest Surg. 2020;33:605-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Son SY, Lee CM, Jung DH, Lee JH, Ahn SH, Park DJ, Kim HH. Laparoscopic completion total gastrectomy for remnant gastric cancer: a single-institution experience. Gastric Cancer. 2015;18:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Kitadani J, Ojima T, Nakamura M, Hayata K, Katsuda M, Takeuchi A, Tominaga S, Fukuda N, Motobayashi H, Nakai T, Yamaue H. Safety and feasibility of laparoscopic gastrectomy for remnant gastric cancer compared with open gastrectomy: Single-center experience. Medicine (Baltimore). 2021;100:e23932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Kim JK, Na W, Cho JH, Ahn EJ, Kim E, Song IG, Han EC, Lee DW, Park BK, Park YG, Kim BG. Refinement of recto-sigmoid colon vaginoplasty using a three-dimensional laparoscopic technique. Medicine (Baltimore). 2021;100:e27042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Zhao B, Lv W, Mei D, Luo R, Bao S, Huang B, Lin J. Comparison of short-term surgical outcome between 3D and 2D laparoscopy surgery for gastrointestinal cancer: a systematic review and meta-analysis. Langenbecks Arch Surg. 2020;405:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Kang SH, Won Y, Lee K, Youn SI, Min SH, Park YS, Ahn SH, Kim HH. Three-dimensional (3D) visualization provides better outcome than two-dimensional (2D) visualization in single-port laparoscopic distal gastrectomy: a propensity-matched analysis. Langenbecks Arch Surg. 2021;406:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Lee Y, Yu J, Doumouras AG, Li J, Hong D. Enhanced recovery after surgery (ERAS) versus standard recovery for elective gastric cancer surgery: A meta-analysis of randomized controlled trials. Surg Oncol. 2020;32:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Desiderio J, Trastulli S, D’Andrea V, Parisi A. Enhanced recovery after surgery for gastric cancer (ERAS-GC): optimizing patient outcome. Transl Gastroenterol Hepatol. 2020;5:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Kano K, Yamada T, Yamamoto K, Komori K, Watanabe H, Takahashi K, Maezawa Y, Fujikawa H, Numata M, Aoyama T, Tamagawa H, Cho H, Yukawa N, Yoshikawa T, Rino Y, Masuda M, Ogata T, Oshima T. Evaluation of Lymph Node Staging Systems as Independent Prognosticators in Remnant Gastric Cancer Patients with an Insufficient Number of Harvested Lymph Nodes. Ann Surg Oncol. 2021;28:2866-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Wang H, Qi H, Liu X, Gao Z, Hidasa I, Aikebaier A, Li K. Positive lymph node ratio is an index in predicting prognosis for remnant gastric cancer with insufficient retrieved lymph node in R0 resection. Sci Rep. 2021;11:2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Demirli Atici S, Turkey; Ramesh PV, India S-Editor: Ma YJ L-Editor: Kerr C P-Editor: Ma YJ