©The Author(s) 2025.
World J Gastrointest Surg. Nov 27, 2025; 17(11): 112557
Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.112557
Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.112557
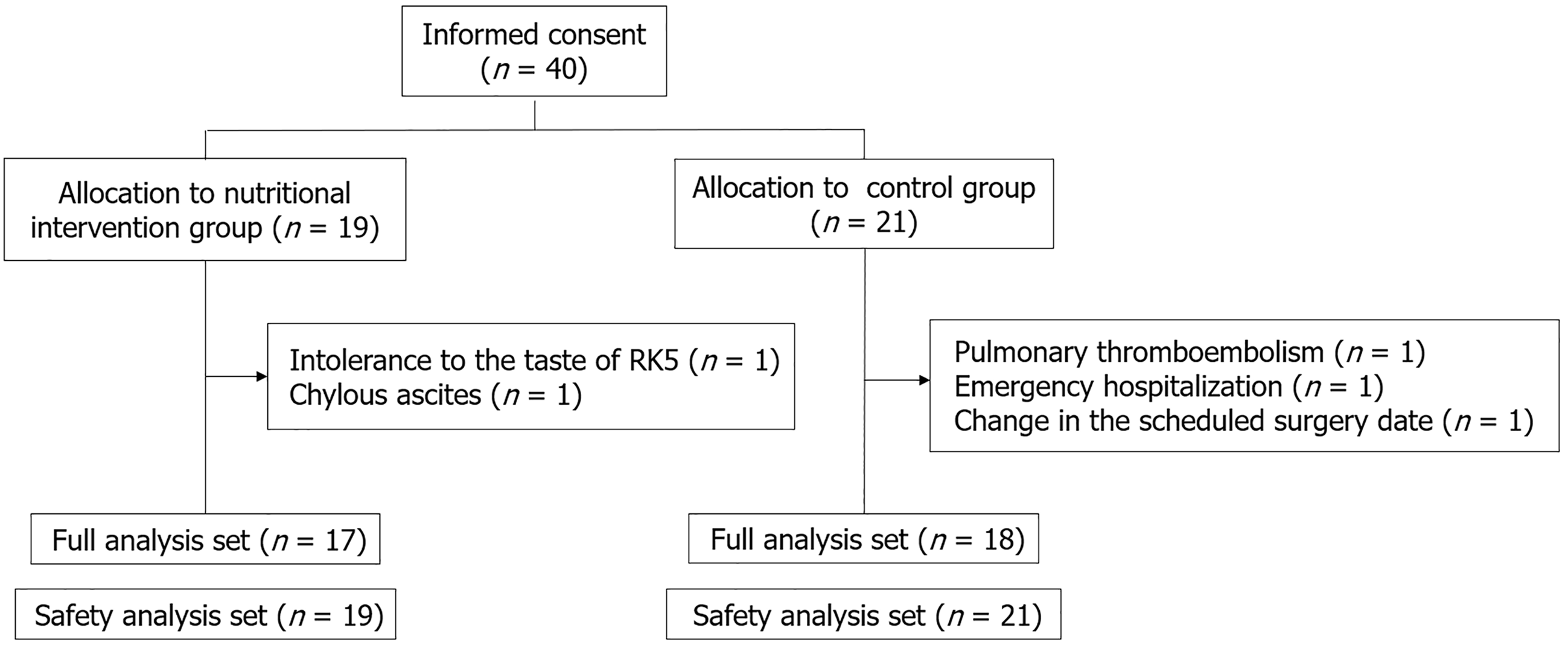
Figure 1 Patient disposition.
Of 40 enrolled patients, 2 in the intervention group (taste intolerance, n = 1; chylous ascites, n = 1) and 3 in the control group (pulmonary thromboembolism, n = 1; emergency hospitalization, n = 1; surgery rescheduling, n = 1) withdrew. The full analysis set comprised 17 intervention and 18 control patients; the safety set included 19 and 21 patients, respectively. RK5: Recovery K5.
- Citation: Shinji S, Yamada T, Matsuda A, Uehara K, Yokoyama Y, Takahashi G, Iwai T, Miyasaka T, Kanaka S, Hayashi K, Yoshida H. Clinical utility of a novel concentrated enteral formula in patients undergoing colorectal cancer surgery: A randomized controlled trial. World J Gastrointest Surg 2025; 17(11): 112557
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/112557.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.112557