©The Author(s) 2024.
World J Gastrointest Surg. Sep 27, 2024; 16(9): 2829-2841
Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.2829
Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.2829
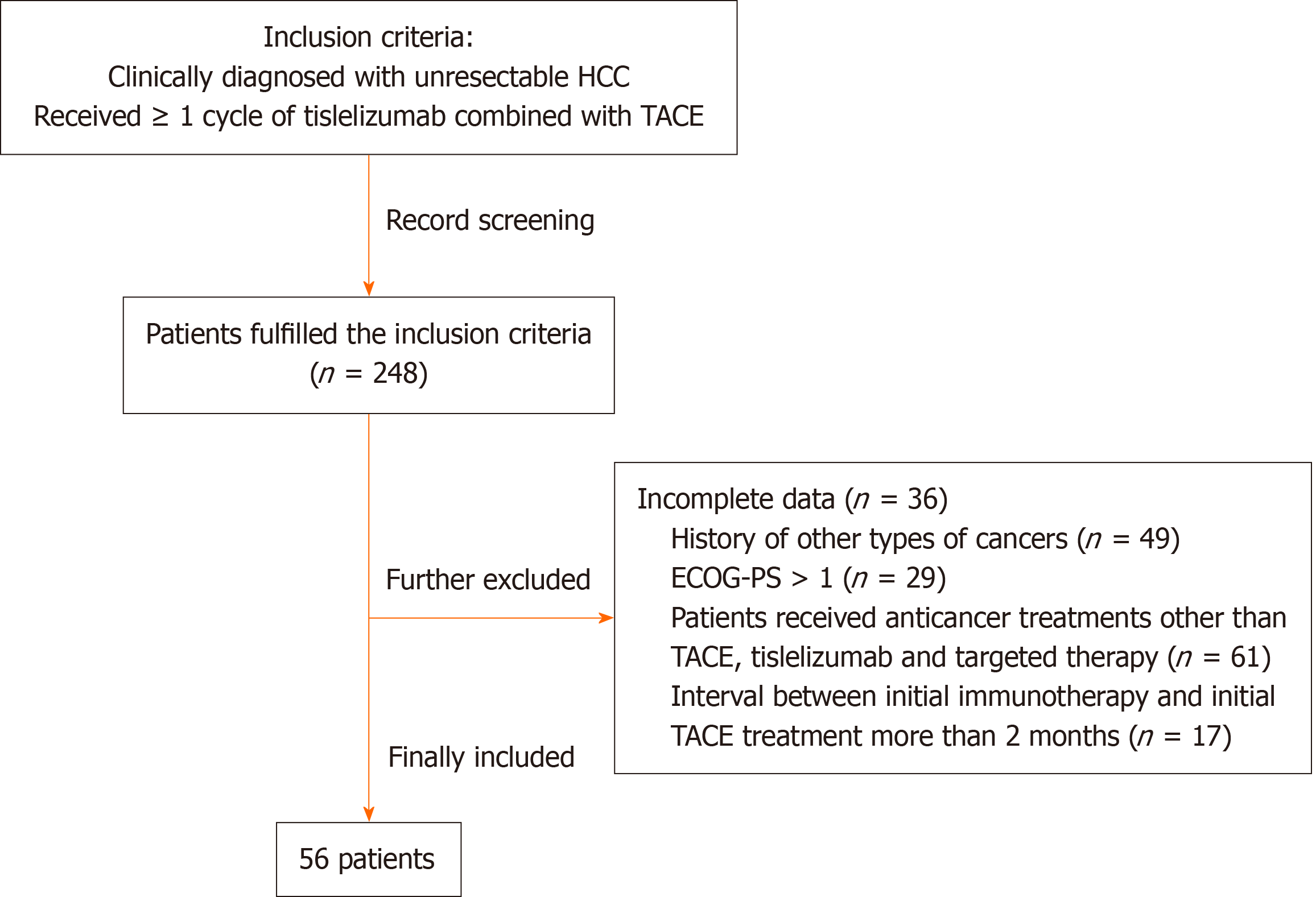
Figure 1 Flowchart showing the detailed patients screening process of this study.
HCC: Hepatocellular carcinoma; TACE: Transarterial chemoembolization; ECOG-PS: Eastern Cooperative Oncology Group Performance Status.
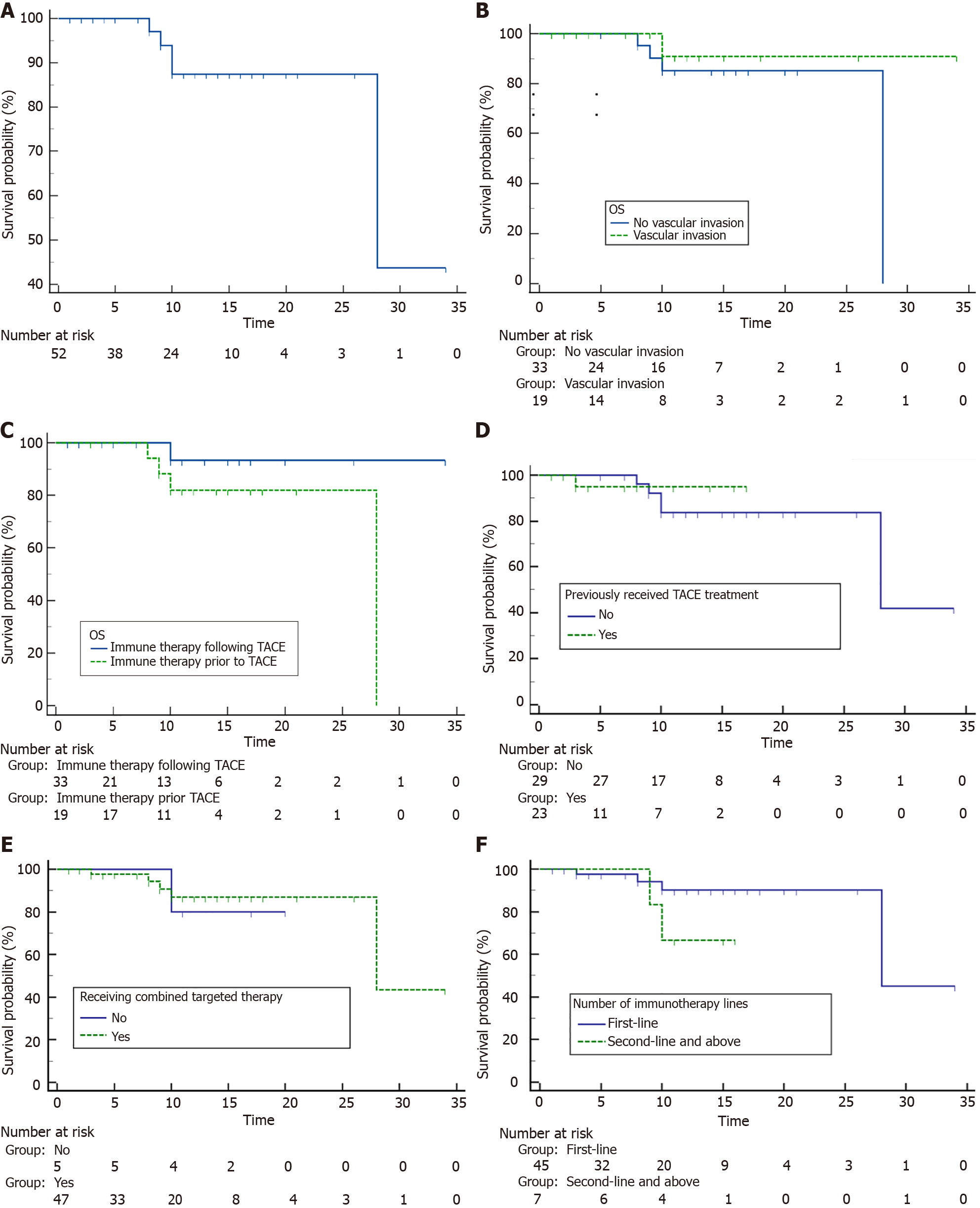
Figure 2 Patient overall survival.
A: Overall survival (OS); B-F: OS comparison by vascular invasion status (B), immunotherapy sequence (C), prior transarterial chemoembolization (D), combined targeted therapy (E) and treatment line of immunotherapy (F). OS: Overall survival; TACE: Transarterial chemoembolization.
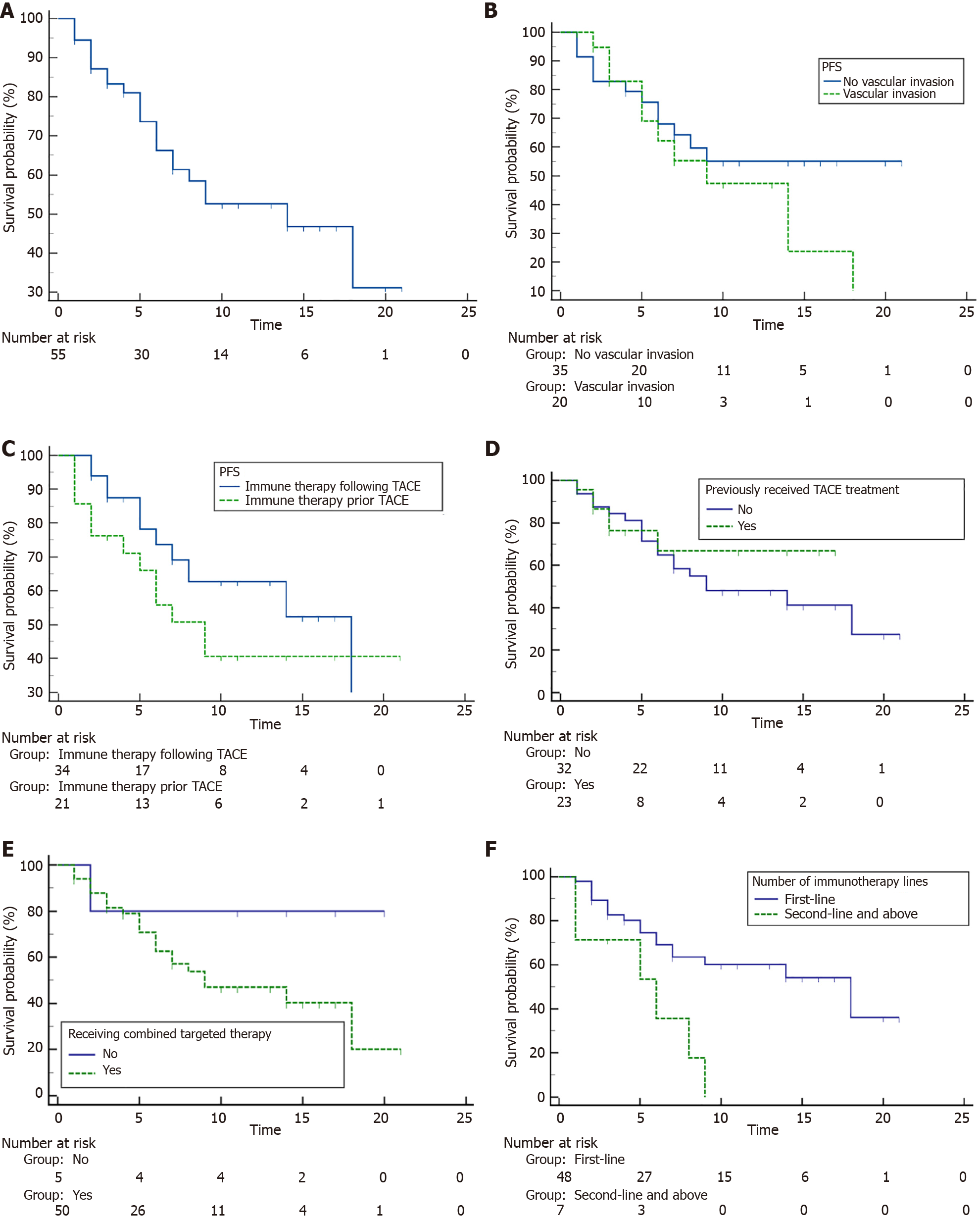
Figure 3 Patient progression-free survival.
A: Overall progression-free survival (PFS); B-F: PFS comparison by vascular invasion status (B), immunotherapy sequence (C), prior transarterial chemoembolization ((D), combined targeted therapy (E) and treatment line of immunotherapy (F). PFS: Progression-free survival; TACE: Transarterial chemoembolization.
- Citation: Tan BB, Fu Y, Shao MH, Chen HL, Liu P, Fan C, Zhang H. Combined transarterial chemoembolization and tislelizumab for patients with unresectable hepatocellular carcinoma. World J Gastrointest Surg 2024; 16(9): 2829-2841
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/2829.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.2829