Published online May 15, 2017. doi: 10.4239/wjd.v8.i5.172
Peer-review started: August 31, 2016
First decision: October 20, 2016
Revised: February 25, 2017
Accepted: March 14, 2017
Article in press: March 17, 2017
Published online: May 15, 2017
Processing time: 288 Days and 20.7 Hours
Hyperglycaemia contributes to the onset and progression of diabetic kidney disease (DKD). Observational studies have not consistently demonstrated a glucose threshold, in terms of HbA1c levels, for the onset of DKD. Tight glucose control has clearly been shown to reduce the incidence of micro- or macroalbuminuria. However, evidence is now also emerging to suggest that intensive glucose control can slow glomerular filtration rate loss and possibly progression to end stage kidney disease. Achieving tight glucose control needs to be balanced against the increasing appreciation that glucose targets for the prevention of diabetes related complications need be individualised for each patient. Recently, empagliflozin which is an oral glucose lowering agent of the sodium glucose cotransporter-2 inhibitor class has been shown to have renal protective effects. However, the magnitude of empagliflozin’s reno-protective properties are over and above that expected from its glucose lowering effects and most likely largely result from mechanisms involving alterations in intra-renal haemodynamics. Liraglutide and semaglutide, both injectable glucose lowering agents which are analogues of human glucagon like peptide-1 have also been shown to reduce progression to macroalbuminuria through mechanisms that remain to be fully elucidated. Here we review the evidence from observational and interventional studies that link good glucose control with improved renal outcomes. We also briefly review the potential reno-protective effects of newer glucose lowering agents.
Core tip: Tight glucose control has been clearly shown to reduce the incidence of micro- and macroalbuminuria. Evidence is now also emerging to suggest that intensive glucose control can slow glomerular filtration rate loss and possibly progression to end stage kidney disease. Furthermore, empagliflozin which is a glucose lowering agent of the sodium glucose like transporter-2 inhibitor class has been shown to have reno-protective effects over and above those expected from its glucose lowering effects alone. Recent clinical trials have also shown that Liraglutide and Semaglutide, injectable glucose lowering agents which are analogues of human glucagon like peptide-1, reduce progression to macroalbuminuria.
- Citation: MacIsaac RJ, Jerums G, Ekinci EI. Effects of glycaemic management on diabetic kidney disease. World J Diabetes 2017; 8(5): 172-186
- URL: https://www.wjgnet.com/1948-9358/full/v8/i5/172.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i5.172
Diabetic kidney disease (DKD) is the most common cause of end-stage kidney disease (ESKD). People with DKD are not only at significant risk of progression to ESKD but have a greater concomitant increase in the risk for cardiovascular (CV) morbidity and mortality[1]. Therefore, optimising treatments to prevent the development and progression of DKD are of the utmost importance.
DKD does not occur in the absence of hyperglycaemia and glucose control is the main determinant of the onset of nephropathy. Despite this, the role of optimising glucose control in slowing the progression of DKD still remains controversial, but evidence is now emerging to suggest that intensive glucose control can slow glomerular filtration rate (GFR) loss and possibly progression to ESKD. Indeed, there is now evidence from observational studies that that good glucose control, at least in patients with type 1 diabetes (T1DM), is associated with improved kidney health even in the setting of advanced DKD[2-4].
Some of the best evidence supporting the beneficial effects of tight glucose control on the kidneys of people with T1DM and advanced nephropathy has come from studies involving patients that have received a combined pancreatic and kidney transplant. Near complete reversal of many of the structural parameters associated with classical diabetic nephropathy has been observed in the native kidneys of these patients after 10 years of normoglycaemia[5].
Here we review the results of observational and interventional studies that have examined the effects of glycaemia on markers of kidney health in people with diabetes. We also briefly review the potential reno-protective effects of newer glucose lowering agents, especially the sodium glucose cotransporter-2 (SGLT-2) inhibitors which have recently been shown to reduce the chances of high risk vascular patients with type 2 diabetes progressing to clinically meaningful renal endpoints. A review of glycaemic management and the optimum way to assess glycaemic control in ESKD patients is beyond the scope of this review. We have also not reviewed the impact of failing kidney function on glucose and insulin metabolism.
Studies examining the effect of glycaemia on the development and progression of DKD have usually focused on three outcome parameters. One parameter is changes in albuminuria, with a transition from micro- to macroalbuminuria occurring in the majority of patients with diabetes who develop ESKD. It is now firmly established that good glucose control can prevent the onset and progression of albuminuria. However, the entities of normoalbuminuric and non-proteinuric renal insufficiency are increasingly being recognised and the specificity of microalbuminuria as a marker for progressive diabetic renal disease remains to be established. These findings call into question the reliability of albuminuria, especially within the microalbuminuric range, as a surrogate kidney health outcome[6].
The second parameter is changes in creatinine or GFR. Caution is also required in interpreting the significance of these outcomes. Under certain circumstances, a rise in serum creatinine, and hence a decrease in estimated GFR (eGFR), may represent transient changes in kidney perfusion or function which are not necessarily related to the causal pathway for the development of chronic kidney disease (CKD)[7].
Lastly, the development of “hard-renal endpoints”, death due to renal disease and/or the development of ESKD are the outcomes that ideally should be the primary endpoint of most renoprotective trials. From a practical point of view, end-points such as these are usually only worth considering in large, long-term trials in high risk patients with sufficient rates of events to allow statistical comparisons to be made.
In recent years, tight glucose control had been shown to slow GFR decline in subjects with T1DM in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes and Interventions and Complications (EDIC) study and to slow progression to ESKD in subjects with type 2 diabetes (T2DM) in the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) study and its follow up observational study, ADVANCE ON[7-9].
The cellular mechanisms responsible for hyperglycaemia mediated renal damage include accelerated generation of advanced glycation end products and activation of their receptor (RAGE), which triggers increases in protein kinase protein kinase C, nuclear factor-kappa B, transforming growth factor-β and connective tissue growth factors[10]. The resultant generation of reactive oxygen species (ROS) and a chronic subacute inflammatory process play a pivotal role in the development of DKD.
In addition, RAGE activation induces glomerular matrix production and increases oxidative stress through mitochondrial superoxide production[11]. Studies in experimental diabetes have also shown that RAGE activation promotes epithelial-mesenchymal transdifferentiation of renal tubular cells, thereby contributing to interstitial fibrosis[12]. Recent studies have also demonstrated that RAGE medicated mitochondrial dysfunction, most likely due to activation of NAD(P)H oxidase (NOX), an enzyme dedicated to the production of ROS, is an early manifestation of DKD and precedes the development of albuminuria and renal histological change[13]. Despite these promising experimental studies like those mentioned above, clinical trials to date have failed to establish that non-glucose lowering based approaches that specifically target pathways linked to oxidative stress or inflammation are renoprotective. Therefore, in recent years there has been renewed interest in robustly establishing the beneficial effects of tight glucose control for reducing the development and progression of DKD.
Even before the glucose thresholds for the diagnosis of diabetes are reached, elevated glucose levels have been associated with an increasing prevalence of CKD. A systematic review and meta-analysis has recently examined the relationship of prediabetes (impaired fasting glucose and/or impaired glucose tolerance) and the incidence of CKD. In an analysis involving eight studies with samples sizes ranging between 2398 and 118924, prediabetes was found to be modestly associated with an increased risk for CKD compared to normal glucose tolerance after adjustment for established risk factors with a RR of 1.11(95%CI: 1.02-1.21)[14]. Higher HbA1c levels have been shown to be associated with lower eGFR values in 24594 South Korean Adults without a history of diabetes and with all subjects used in the analysis having a HbA1c level < 6.5% (48 mmol/mol). The association between HbA1c levels and CKD in this study was evident in subjects with and without the metabolic syndrome[15].
The DCCT in T1DM and the United Kingdom Prospective Diabetes Study (UKPDS) in T2DM have both demonstrated a strong relationship between glucose control and the risk of the development of diabetic microvascular complications without a clear-cut HbA1c threshold[16,17]. The rate of progression of albuminuria in patients (both with T1DM and T2DM) who developed persistent microalbuminuria has also been shown to correlate with the degree of long-term glucose control over approximately 12 years of follow-up[18].
However, a sophisticated observational analysis from the ADVANCE study, involving subjects with T2DM, suggests that within the range of HbA1c studied [5.5%-10.5% (37-91 mol/mol)], there was evidence of a glucose threshold such that below HbA1c levels of 6.5% (48 mmol/mol) there was no significant change in the risks for the development of eye or kidney complications including macroalbuminuria, doubling of serum creatinine levels, need for renal-replacement therapy, or death due to renal disease[19].
In contrast, an observational sub-study of the Outcome Reduction with Initial Glargine Intervention trial that involved 12537 people with T2DM or prediabetes followed for 6.2 years has suggested that the risk for adverse renal outcomes rises progressively from the lowest [< 5.7% (33.8 mmol/mol)] to the highest quintile [7.4% (57.4 mmol/mol)] of HbA1c levels. In this study, the HR for renal failure was 1.54 (95%CI: 1.24-1.91, P = 0.001) per 1% higher baseline HbA1c[20]. In the Atherosclerosis Risk in Communities study involving 1871 adults with diabetes (presumed T2DM in the vast majority) followed for 11 years, there was a graded relationship between higher HbA1c levels and incident CKD (defined as an eGFR < 60 mL/min per 1.73 m2) that was independent of traditional risk factors and present even in the absence of albuminuria and retinopathy. However, a significant increase in risk for the development of CKD was only evident for HbA1c values greater that 7% (53 mmol/mol)[21].
A large population-based cohort study has also shown an increased risk of ESKD with higher HbA1c levels. In this study, 23296 patients with stage 3 or 4 CKD were identified and then followed for approximately 4 years using laboratory data, hospitalisation and insurance claims. Patients were then stratified by glucose control based on the first HbA1c measured during the study period. The median baseline HbA1c was 6.9% (52 mmol/mol) and 11% had levels > 9% (75 mmol/mol). During the study, 16% patients died, 16% had a cardiovascular event and 6% progressed to ESKD. In an analysis adjusted for age, sex, GFR, socio-economic factors and co-morbidities, an increased risk of ESKD was associated with higher HbA1c levels but this relationship was attenuated in patients with a lower eGFR[22].
Among patients with stage 3 CKD (eGFR 30-59 mL/min per 1.73 m2), the risk for ESKD was increased by 22% for those with HbA1c levels between 7 and 9% (53 and 75 mmol/mol) and by 152% for those with levels > 9% (75 mmol/mol), compared to those with a HbA1c < 7% (53 mmol/mol). In contrast, for patients with stage 4 CKD (eGFR 15-29 mL/min per 1.73 m2) at baseline, the corresponding increases in risk for ESKD were only 3% and 13%, respectively, compared with patients with a HbA1c < 7% (53 mmol/mol). Interestingly, the severity of CKD was not a significant modifier of the relationship between glucose control and a doubling of serum creatinine. However, it is worth noting that the use of doubling of serum creatinine in isolation as a renal endpoint has been questioned recently. It was suggested by the authors that the relationship between HbA1c and ESKD was stronger in patients with milder CKD as there may be a threshold of kidney function below which better glucose control alone in not enough to prevent progressive kidney loss.
Interestingly, in the above study, a U-shaped relationship was found between glucose control and total mortality, with increases in the risk of mortality apparent at HbA1c levels < 6.5% (48 mmol/mol) and greater than 8.0% (64 mmol/mol). The nature of the relationship between HbA1c and mortality was different from that between HbA1c and other outcomes such as ESRD, hospitalization, and cardiovascular events. Furthermore, the relationship between HbA1c and outcomes, apart from that of ESKD, was not modified by initial GFR level or stage of CKD. This result together those of the already mentioned observational study from ADVANCE suggest that ideally a HbA1c threshold of 6.5% (48 mmol/mol) should be targeted as a means of preventing the development and progression of DKD[19]. However, the importance of individualising glycaemic targets according to a patient’s age, co-morbidities and type of glucose lowering therapies prescribed is appreciated.
Two observational studies from the Joslin Diabetes Clinic, Boston, United States, have also highlighted the importance of the relationship between glucose control and progression of kidney disease in people with diabetes. In the first study, patients with T1DM that experienced an early progressive decline in GFR, defined as a GFR loss over and above that expected with aging alone that starts before a GFR threshold of 60 mL/min per 1.73 m2 is reached, were identified from a cohort with microalbuminuria[23]. For the 301 patients studied, 207 had stable renal function and 94 had declining renal function over 8 to 12 years of observation. Patients with a baseline HbA1c ≥ 9% (75 mmol/mol) had an odds ratio of 2.5 (95%CI: 1.2-5.4) for having an early progressive decline in GFR compared with patients with an initial HbA1c < 9.0% (75 mmol/mol). This early decline in GFR was believed to be distinct from resolution of hyperfiltration, because it was progressive, occurred in patients with relatively long duration of diabetes (approximately 18 years), and because improved, and not worsening glucose control, has been associated with resolution of hyperfiltration.
The second study from the Joslin Diabetes Clinic suggests that sustained improvement in glucose control in T1DM patients with overt proteinuria can reduce the long-term risk of ESKD. A 1% improvement in HbA1c over a follow-up period of approximately 3.5 years was associated with a HR for ESKD of 0.72 (95%CI: 0.61-0.89) which was independent of other covariates[24].
Some of the most impressive information supporting the beneficial effects of tight glucose control on the kidneys of people with T1DM has come from a study involving subjects with DKD who received a combined pancreatic and kidney transplant. After 5 years of normoglycaemia, achieved by pancreatic transplantation, biopsy specimens of the transplant recipient’s native kidneys showed no change in kidney structure. However, after 10 years of normoglycaemia, there was remarkable remodelling of kidney structure, with near complete reversal of many of the structural parameters associated with classical diabetic nephropathy[25].
There is no convincing evidence that short term (within-day) variability in glucose levels influences the risk for the development of DKD. A substudy from the DCCT showed that within-day glucose variability, as assessed by seven-point laboratory measured glucose levels, did not influence the risk for the development of nephropathy over and above that conferred by mean glucose levels alone[26].
In contrast, numerous studies have demonstrated that long-term glucose variability as assessed by HbA1c measurements is associated with the risk for the development and progression of DKD. In patients with T1DM followed for approximately 6 years, the standard deviation of serial HbA1c measurements has been shown to be associated with the risk of progressive renal disease (HR = 1.94, 95%CI: 1.49-2.47) as assessed by progression of albuminuria or the development of ESKD, even after adjustment for mean HbA1c and traditional risk factors[27].
In patients with T2DM, variability of HbA1c measurements over 2 years period before enrollment in the Renal Insufficiency and Cardiovascular Events Italian Multicentre study was found to be independently related to the prevalence of albuminuria and reduced GFR assessed during the subsequent 2 years of the study. The variably in HbA1c measurements in the preceding 2 year period was found to be more strongly associated with the prevalence of DKD than average HbA1c levels. In contrast, average values but not variability in HbA1c was associated with the prevalence of retinopathy[28]. The above results suggest that variability as opposed to mean HbA1c levels may be associated with the development and progression of diabetic microvascular complications via different but as yet, unexplained mechanisms.
In the DCCT, subjects with T1DM were randomised to intensive glucose control [HbA1c 7.3% (56 mmol/mol), n = 711] or conventional glucose control [HbA1c 9.1% (76 mmol/mol), n = 730] and studied for 6.5 years. At the end of the intervention, intensive therapy was associated with a significant reduction in the number of subjects that developed microalbuminuria (10.2 vs 17.7%, respectively, P < 0.01) or macroalbuminuria (1.4 vs 3.2, respectively, P < 0.05). Estimated GFR decreased in the intensive glucose control group (from 126 to 121.8 mL/min per 1.73 m2) during the first year of the trial, a decrease that was 1.4 mL/min per 1.73 m2 (P < 0.001) greater than that in the conventional glucose control group. Thereafter GFR declined in parallel for the two treatment groups. This initial drop in estimated GFR in the intensive glucose control group most likely presented a resolution of hyperfiltration. Due a low event rate it was not possible to assess the effects of good glucose control on ESKD rates in the DCCT[16].
In the UKPDS, subjects with T2DM were randomised to intensive glucose control [HbA1c 7.0% (53 mmol/mol), n = 2408] or conventional glucose control [HbA1c 7.9% (63 mmol/mol), n = 994] and studied for 10 years. Nine years into the intervention, intensive therapy was associated with a significant reduction in the number of subjects that developed microalbuminuria (19% vs 25%, respectively, P < 0.001) or macroalbuminuria (4.4% vs 6.5%, respectively, P = 0.026). No differences in ESKD rates were reported in the trial but the number of patients experiencing a doubling of serum creatinine 9 years were lower in the intensive therapy vs the conventional therapy group, although it should be noted that event rates in both groups were very low (0.71% vs 1.76%, P = 0.027)[29].
In the ADVANCE study, 11140 subjects with T2DM were randomised to intensive glucose control or conventional glucose control and followed for a median of 5 years. The time weighted average HbA1c difference was 0.67% for intensively [mean HbA1c 6.5% (48 mmol/mol) at the end of the study] and conventionally treated subjects [HbA1c 7.3% (56 mmol/mol) at the end of the study]. The majority of subjects in the study had normal kidney function but, 19% had a GFR < 60 mL/min per 1.73 m2, 27% had microalbuminuria and 4% had macroalbuminuria. The ADVANCE study originally reported that intensive glucose control reduced the risk of the composite endpoint of new or worsening nephropathy, comprising the composites of new-onset macroalbuminuria, ESKD, renal death or doubling of serum creatinine to > 200 μmmol/L) by 21% (HR = 0.79, 95%CI: 0.66-0.93, P = 0.006) compared to conventional glucose control[30].
Due to concerns about the reliability of changes in albuminuria and creatinine as surrogate endpoint of kidney health a new analysis of the ADVANCE data was performed to specifically examine the effects of intensive glucose control on clinical kidney health outcomes such as the development of ESKD. In this study, the risk of ESKD was 65% lower in subjects randomised to intensive compared with conventional glucose control (HR = 0.35, 95%CI: 0.15-0.83, P = 0.01). This result was still seen after considering the confounding influences of renal or all-cause death. Fewer subjects randomised to intensive glucose control had a sustained doubling of serum creatinine compared to conventional glucose control but this outcome failed to reach statistical significance (HR = 0.87, 95%CI: 0.54-1.27). Subjects with a sustained doubling of serum creatinine were restricted to those with a final creatinine measurement that was still above the doubling threshold[7].
However, it should be noted that two other contemporary intensive glucose control trials in subjects with T2DM have failed to demonstrate that tight glucose control significantly attenuates a decline in GFR and the development of ESKD. In the Action to Control Cardiovascular Risks in Diabetes (ACCORD) study the risk for ESKD was 5% lower with intensive glucose control (HR = 0.95, 95%CI: 0.73-1.24, NS) but doubling of serum creatinine or a 20 mL/min per 1.73 m2 decrease in estimated GFR risk was 7% higher with intensive glucose control (HR = 1.07, 95%CI: 1.01-1.13, P = 0.016)[31]. The limitations of using single creatinine measurements as an outcome parameter have been already discussed. In the Veterans Affairs Diabetes Trial (VADT), rates of decline in GFR and the achievement of an estimated GFR < 15 mL/min per 1.73 m2 were not different with intensive or conventional glucose control. Although intensive glucose control had no significant effect on estimated GFR decline in the whole VADT cohort, it did slow estimated GFR decline in subjects with high baseline levels of albuminuria (OR = 0.61, 95%CI: 0.37-1.00, P = 0.04)[32]. The effects of intensive glucose control on renal outcomes in trials involving participants with T2DM are summarised in Table 1.
| UKPDS[29] | ACCORD[30,31] | ADVANCE[7,30] | VADT[32] | |
| Baseline characteristics | ||||
| No. of subjects | 3867 | 10251 | 11140 | 1791 |
| Age (yr) | 53 | 62 | 66 | 60 |
| Duration of diabetes (yr) | 0 | 10 | 8 | 11.5 |
| History of CV disease (%) | NR | 35 | 32 | 40 |
| Median HbA1c at baseline (%) | 7.0 | 8.1 | 7.2 | 9.4 |
| Duration of follow-up (yr) | 10.0 | 3.5 | 5.0 | 5.6 |
| Achieved median HbA1c for I vs S (%) (mmol/mol) | 7.0 vs 7.9 (53 vs 63) | 6.4 vs 7.5 (46 vs 59) | 6.3 vs 7.0 (45 vs 53) | 6.9 vs 8.5 (52 vs 69) |
| Microalbuminuria (%) | 11 | 25 | 26 | N/A |
| Macroalbuminuria (%) | 2 | 7 | 4 | N/A |
| Renal outcomes | ||||
| Microalbuminuria (HR or RR) | 0.67a (0.53-0.86) | 0.79a (0.69-0.90) | 0.91a (0.85-0.98) | 0.85 (0.59-1.23) |
| Macroalbuminuria (HR or RR) | 0.66 (0.39-1.10) | 0.68a (0.58-0.86) | 0.70a (0.57-0.85) | 0.56a (0.33-0.96) |
| Worsening albuminuria (HR or RR) | N/A | N/A | N/A | 0.72a (0.53-0.97) |
| Doubling of creatinine (HR or RR) | 0.26a (0.39-1.10) | 1.07 (1.01-1.13) | 0.83 (0.54-1.27) | 1.0 (0.74-1.35) |
| Decline in eGFR (HR or RR) | N/A | N/A | N/A | 0.61 (0.37-1.00) |
| ESKD (HR or RR) | N/A | 0.95 (0.73-1.24) | 0.35a (0.15-0.83) | 0.63 (0.25-1.6) |
A Cochrane review punished in 2014 examined the potential benefits of intensive vs conventional glucose control in T1DM. Whist intensive glucose control was found to reduce the risk for the development of microalbuminuria for 1475 participants in 3 trials (RR = 0.56, 95%CI: 0.46-0.68, P < 0.00001). There was no significant reduction in progression to macroalbuminuria (RR = 0.79, 95%CI: 0.37-1.70). Insufficient trial participants reached ESKD for any meaningful analysis of the benefits of intensive glucose control on this outcome[33].
In contrast, a very recent systemic review and meta-analysis of 5 trials that included information on 1635 participants with T1DM has shown that nephropathy (defined as albumin excretion rate > 300 mg/24 h or using an outcome of “clinical nephropathy”) is reduced with intensive glucose control compared with conventional glucose control (RR = 0.37, 95%CI: 0.27 to 0.50; P < 0.00001). Not unexpectedly, due to low event rates, the effect of intensive glucose targets on ESKD rates was not statistically significant (RR = 0.96, 95%CI: 0.13-7.05, 3 trials, 124 participants)[34].
A meta-analysis of intensive glucose control studies in T2DM by Coca et al[35], that included results from the UKPDS, ADVANCE and ACCORD trials and evaluated outcomes in 28065 patients with type 2 diabetes over 2 to 15 years, has shown that compared to conventional control [median HbA1c values 7.3%-9.4% (56-79 mmol/mol)], intensive glucose control [median HbA1c 6.4%-7.4% (46-57 mmol/mol)] reduced the risk for development of microalbuminuria (RR = 0.86, 95%CI: 0.76-0.96) and macroalbuminuria (RR = 0.74, 95%CI: 0.65-0.85). Although the risk for the development of ESKD was estimated to be reduced by 31% (RR = 0.69, 95%CI: 0.45-1.05) by this analysis, this reduction with intensive glucose lowering was not significantly different compared with conventional glucose control. This result was possibly related to the relatively low incidence of ESKD (< 1.5%) compared with that of microalbuminuria (23%) and macroalbuminuria (5%).
Another Cochrane review published in 2012 also examined the potential benefits of intensive vs conventional glucose control in T2DM and reported that the risk of progressive nephropathy (defined as the development of macroalbuminuria, renal failure, doubling of creatinine or reduced GFR in 27929 participants in 9 trials) was reduced by intensive glucose control (RR = 0.78, 95%CI: 0.61-0.99, P < 0.04). As above, intensive glucose control was associated with a non-significant reduction in the risk for ESKD for 28075 participants in 7 trials (RR = 0.87, 95%CI: 0.72-1.07)[36].
Although there is good evidence to suggest that elevated glucose levels are an important initiator and promoter of early DKD and interventions to improve glycaemia can slow the progression of early DKD, as discussed above, there has been only limited evidence to suggest that improving glycaemia slows the rate of GFR decline and retards progression to ESKD. However, evidence from very recent studies suggests that improving glucose levels may ameliorate a decline in GFR and progression to ESKD (Table 2).
A study from the DCCT/EDIC Research group has shown that the long term risk for the development of an impaired GFR (< 60 mL/min per 1.73 m2) was significantly lower for subjects with T1DM who were initially treated to achieve tight glucose control[8]. In this study, participants were followed for 6.5 years during randomisation to intensive or conventional glucose control arms of the DCCT and then followed up for a further 16 years in the EDIC study. Of note, the approximate 2% difference in HbA1c levels in the intensive compared to conventional glucose control arms of DCCT was no longer apparent at the end of the EDIC study with Hb1Ac levels being approximately 8.0% (64 mmol/mol) for all subjects.
At the end of a further 16 year observational study, less subjects who were originally randomised to intensive glucose control developed macroalbuminuria (3.2 vs 7.3, P < 0.01). However, there was no difference in the rate of development of microalbuminuria[8,16]. The risk for developing a sustained impairment of GFR was approximately 50% lower in subjects originally randomised to intensive glucose control in the DCCT compared to conventional glucose control. However, this effect was not evident until 10 years after the completion of randomisation to vs conventional intensive glucose control. In this study, the outcome of a sustained impairment of GFR was based on the finding of two consecutive annual estimates of GFR. ESKD developed in only 8 subjects randomised to intensive glucose control compared with 16 subjects randomised to conventional glucose control. However, due to the low number of subjects that developed ESKD the difference in this event rate was not statistically significant. In contrast, initial intensive glucose control reduced the risk of impaired GFR or death by 37% (95%CI: 10-55, P = 0.01).
After the end of the randomised component of the UKPDS, participants were followed up for a further 10 years to assess the development of microvascular complications. Any difference in HbA1c levels between the intensive and conventional glucose control groups was lost after one year of follow-up. Despite this, the significant 24% reduction in the risk for microvascular complications at the end of the intervention trial was sustained during the post-trial observational period. However, during the 10-year observational period, subjects originally randomised to intensive or conventional glucose control had similar levels of albuminuria and serum creatinine levels, suggesting that the reduction in microvascular complications was almost entirely accounted for by lower rates of eye related events[37].
In a similar fashion to the follow-up that occurred in the UKPDS, after the completion of the randomisation component of the ADVANCE trial, patients were followed for a further 5.4 years. Any difference in HbA1c levels disappeared by the first post-trial visit and values remained at around 7.3% (56 mmol/mol) during the observational component of the study. During the total follow period of 9.9 years, the significant reduction in ESKD during the in-trial period (7 vs 20 events, HR = 0.35, 95%CI: 0.15-0.83, P = 0.02) persisted, but the HR was attenuated (29 vs 53 events, HR = 0.54, 95%CI: 0.34-0.85, P < 0.01). It was suggested that over the 9.9 years of the study, 194 participants would need to be treated with intensive glucose control to revent one ESKD event.
The renal results from ADVANCE-ON suggest that intensive glucose control may only be of benefit before the onset of clinically relevant CKD. Subgroup analysis demostrated a significant heterogenisity for the risk of ESKD with intensive glucose control according to baseline CKD stage: No CKD (HR = 0.16, 95%CI: 0.04-0.74), CKD stages 1 and 2 (HR = 0.34, 95%CI: 0.12-0.95) and CKD stges ≥ 3 (HR = 0.89, 95%CI: 0.47-1.67). Furthermore, for participants with an eGFR greater than or equal to 60 mL/min per 1.73 m2 compared to those with estimated GFR levels below this value, numbers needed to treat to prevent one ESKD event were reported to be 109 and 393, respectively[9]. The above result was highlihed by the authors of the ADVANCE-ON study to indicate the importance of commencing intensive glucose control in patients with type 2 diabetes before the development of established DKD.
Increased intra-glomerular pressure as a result of increased plasma flow and/or vasodilatation of the afferent glomerular arterioles and/or constriction of the efferent arterioles, resulting in the state of hyperfiltration is a hallmark of early DKD. One of the most important determinants of hyperfiltration is hyperglycaemia. Indeed hyperfiltration can even be induced by a state of acute hyperglycaemia, for example the elevation in glucose levels induced by a hyperglycaemic clamp. A recent study of 17 normo-filtering T1DM subjects demonstrated that GFR (measured by inulin clearance) increased from 118 mL/min per 1.73 m2 when measured directly after an 8 h euglycaemic clamp (blood glucose 4-6 mmol/L) to 137 mL/min per 1.73 m2 when measured after a hyperglycaemic clamp (blood glucose 9-11 mmol/L)[38].
Early hyperfiltration, occurring in the first months of T1DM has been shown to reverse with insulin therapy[39]. By contrast, late or persistent hyperfiltration may persist for years and may not be associated with glucose control when assessed by HbA1c measurements several years after the onset of diabetes. In a study involving 12 patients with T1DM and an increased GFR for a year after they were randomly assigned either to continuous subcutaneous insulin pump therapy or to unchanged conventional therapy, the glomerular filtration rate fell significantly in the pump group and became normal in four of the six patients although the kidneys remained enlarged. GFR did not change in the conventional-treatment group[40]. Therefore, these results support the theory that strict glucose control normalizes GFR, at least in the first years after the development of diabetes. The effects of improving glucose control on hyperfiltration are less well documented in patients with long standing diabetes.
The mechanism linking hyperglycaemia with the onset of hyperfiltration most likely involves an increase in sodium reabsorption via the SGLT-2 receptor in the proximal tubule which ultimately results in tubulo-glomerular feedback modulating blood flow in the glomerular afferent arteriole[41]. Evidence exists in diabetic rats and humans for a primary increase in proximal tubular sodium and glucose reabsorption in the setting of hyperglycaemia. This occurs due to augmented sodium-glucose co-transport that subsequently results in a reduced sodium chloride concentration being delivered to the macula densa. This reduction in sodium chloride concentration is interpreted by the juxtaglomerular apparatus to represent a decline in circulating volume and renal perfusion. To maintain GFR, dilatation of the afferent glomerular arterioles occurs, possibly through an adenosine mediated process, which ultimately results in a state of hyperfiltration. As discussed below the renal protective effects of the SGLT-2 receptor inhibitors may be partly related to their ability to reduce intra-glomerular pressure by increasing sodium chloride delivery to the macular densa with tubulo-glomerular feedback then resulting in a constriction of the afferent arteriole[42,43].
The relationship between SGLT-2 inhibition and the renin-angiotensin system (RAS) is complex and as yet not fully elucidated. The decrease in intraglomerular pressure and the modest volume depletion volume depletion seen with SGLT-inhibition has the potential to result in RAS activation. In contrast, the increased delivery of sodium to the macular densa may result in a reduction in RAS activation. Despite the above, SGLT-2 inhibition appears to result in an increase in RAS activity[42]. It has been suggested that increased RAS activity may in fact play an important role in maintain adequate glomerular filtration in the setting of SGLT-2 inhibition[44]. In any event, the cardiac and renal protective effects of empagliflozin appear to be consistent in patients treated or not treated with agents that block RAS activity[45,46].
One major concern regarding the application of intensive glucose control is the potential risk of adverse outcomes, especially patients with diabetes and CKD. As mentioned previously, randomisation to intensive glucose control in the ACCORD trial was associated with higher CV and all-cause mortality rates compared to standard glucose control. A follow-up study from ACCORD has suggested that the excess mortality associated with randomisation to tight glucose control was predominantly accounted for by participants with prevalent CKD (Stages I-III). The risk for the primary outcome (the first occurrence of nonfatal myocardial infarction or stroke, or cardiovascular death) for the study was 87% higher in participants with CKD compared to those without CKD (HR = 1.87, 95%CI: 1.65-2.11)[47]. In the ACCORD study rates of hypoglycaemia were also approximately twice as great in patients with CKD compared to those without CKD.
In contrast to the above, the ADVANCE-ON study results have reiterated the in trial results from ADVANCE and shown that intensive glucose control had no clear effect on all-cause mortality or cardiovascular events or death from CV disease. Importantly the results from ADVANCE-ON showed that there was no evidence that baseline CKD status (stages I, II and ≥ III) had any impact on the rates of the above outcomes with intensive glucose control (Table 3).
| ACCORD | ADVANCE | |||
| Total mortality | CV mortality | Total mortality | CV mortality | |
| Overall study results (HR or RR) | 1.22a (1.01-1.46) | 1.35a (1.04-1.76) | 0.93 (0.83-1.06) | 0.88 (0.74-1.04) |
| Non-CKD participants (HR or RR) | 1.08 (0.87-1.34) | 1.14 (0.82-1.58) | 0.74 (0.76-1.15) | 0.78 (0.58-1.07) |
| CKD participants (HR or RR) | 1.31a (1.07-1.60) | 1.41a (1.05-1.89) | 0.91 (0.72-1.14) | 0.90 (0.67-1.22) |
Furthermore, as discussed in more detail later, it has recently been shown that the glucose lowering medications liraglutide and empagliflozin reduce CV events in patients with reduced GFR. Therefore, in contrast to the ACCORD study, the results of the ADVANCE-ON study and the CV safety trials involving empagliflozin and liraglutide suggest that it is possible to aim for tighter glucose control in patients with T2DM and CKD without exaggerating their risk for all cause and cardiovascular mortality[9].
No large clinical studies have specifically examined the renal protective effects of insulin, sulphoylureas or metformin apart from the possible effects derived from improved glucose control.
The thiazolidinediones (TZDs) activate the peroxisome proliferator-activated receptor-Gamma and improve glucose control by improving insulin sensitivity. This class of medication has also been reported to have anti-inflammatory and antithrombotic effects but they also cause weight gain and fluid retention in some patients. Animal studies have shown that TZDs reduce albumin excretion, prevent glomerulosclerosis, tubulo-interstitial fibrosis and maintain podocyte structure and function. Although a meta-analysis of 15 studies involving 2860 patients has shown that TZDs can significantly decrease urinary albumin excretion it should be noted that not all studies have found a favourable effect of TZDs on urinary albumin excretion[48]. For example, in the Diabetes Reduction Assessment With Ramipril and Rosiglitazone Medication trial, rosiglitazone reduced a combined renal endpoint by 20% in people with prediabetes (HR = 0.80, 95%CI: 0.68-0.93, P = 0.005) over the 4 years of the trial. Prevention of diabetes was also independently associated with prevention of the renal endpoint of the trial (P < 0.001). In contrast, in the bypass angioplasty revascularisation investigation 2 Diabetes trial, participants who were treated with insulin sensitising medications (the majority taking TZDs in combination with metformin), as compared to those treated with insulin-provision therapy (insulin plus sulphonylureas), had greater progression of urinary albumin excretion despite having lower HbA1c values. Rates of decline in estimated GFR were reported to be similar in both treatment groups over 5 years[49].
There is an emerging signal to suggest that the incretin based therapies for type 2 diabetes such as the dipeptidyl peptidase-4 (DPP-4) inhibitors and the glucagon-like peptide-1 (GLP-1) receptors may have specific renal protective properties. DPP-4 inhibitors and GLP-1 receptor agonists target the “incretin effect” which involves the modulation of peptide hormones that normally regulate glucose levels when nutrients are given orally. The incretins are a family of gut hormones that lower blood glucose levels via the so called “incretin effect”. This phenomenon accounts for the two-to-three fold increase in plasma insulin concentrations observed after the oral ingestion compared to the intravenous administration of an equivalent glucose load. The principal incretin peptide hormone that has been targeted for thermotherapy is GLP-1 secreted by intestinal L cells[50].
Normally GLP-1 has a very short half-life and is quickly degraded by the DPP-4 enzyme. Two pharmacological approaches have been taken to target the “incretin-system” to develop new glucose lowering medications. One approach has been to develop GLP-1 receptor analogues that are resistant to degradation by the DPP-4 enzyme, hence enhancing their half-life. The other approach has been to develop inhibitors of the DPP-4 enzyme, with the aim of increasing plasma levels of native GLP-1 by inhibiting its degradation[51].
GLP-1 and DPP-4 inhibitors have been shown to inhibit the sodium-hydrogen ion exchanger in the proximal tubule which increases sodium excretion and triggers tubulo-glomerular feedback to constrict the afferent glomerular arteriole and hence reduce intra-glomerular pressure as described previously. The potential renal protective effects of these medications likely involves deceases in oxidative stress, inflammation and glomerulosclerosis[52].
Experimental studies involving GLP-1 receptor knockout mice with diabetes have shown that they exhibit higher urinary albumin levels and more advanced mesangial expansion than wild-type mice with diabetes, despite comparable levels of hyperglycaemia. Increased glomerular superoxide and upregulated renal NOX were also seen in the GLP-1 receptor knockout mice. Furthermore, treatment with the GLP-1 receptor agonist liraglutide suppressed the progression of nephropathy in mice with diabetes, as demonstrated by reduced albuminuria and mesangial expansion, decreased levels of glomerular superoxide and renal NOX. These results suggest that GLP-1 normally plays a crucial role to maintain kidney health by protecting against increased renal oxidative stress induced by chronic hyperglycemia[53].
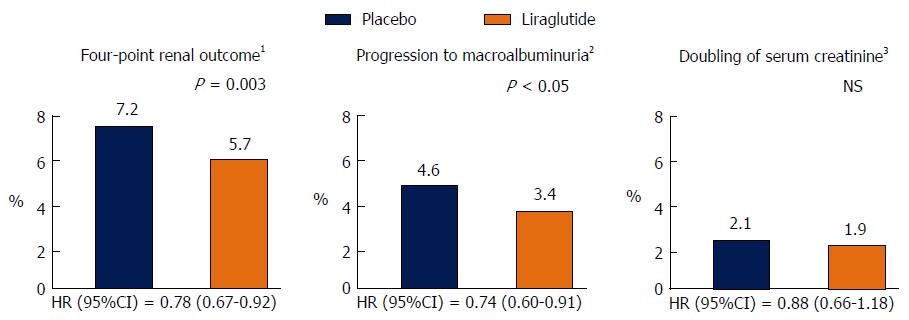
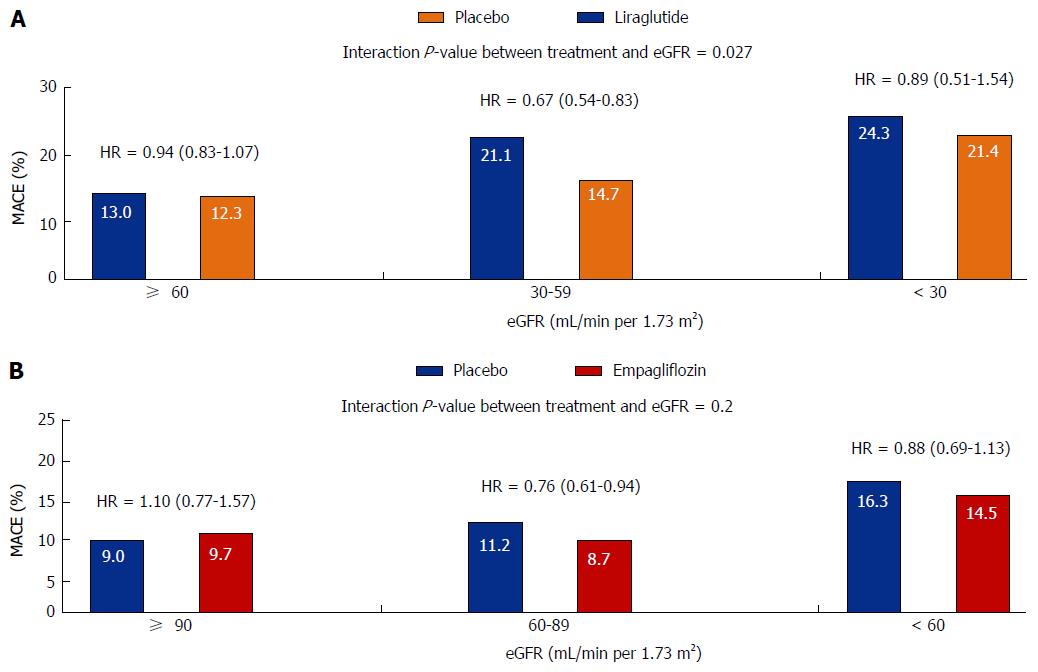
Information regarding the microvascular benefits of the GLP-1 receptor agonist Liraglutide has also just been released. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome (LEADER) trial examined the effects of a daily injection of liraglutide vs placebo in 9340 high risk vascular patients over a median follow-up of 3.8 years. Approximately, 23% of the trial participants had an eGFR < 60 mL/min per 1.73 m2 whist 21% had microalbuminuria and 6% had macroalbuminuria. The use of liraglutide was associated with a reduction in the primary end point of the trial which was a composite CV end point of death due to CV disease, non-fatal myocardial infarction and non-fatal stroke (HR = 0.87, 95%CI: 0.78-0.97, P = 0.01). There was also a 22% significant reduction in the time to the first primary renal endpoint of the trial, defined as a composite of the development of macroalbuminuria, doubling of serum creatinine and eGFR < 45 mL/min per 1.73 m2, the need for renal replacement therapy and death from renal causes (HR = 0.78, 95%CI: 0.67-0.92, P = 0.03). The renal benefit of liraglutide was mainly derived from a 26% significant reduction in new onset macroalbuminuria (HR = 0.74, 95%CI: 0.60-0.91) without any significant changes in eGFR. Lower, but non-significant rates of doubling of serum creatinine and the need for the initiation of renal replacement therapy were also seen in liraglutide treated patients (Figure 1). Furthermore, most of the CV benefits observed with liraglutide were seen in patients with eGFR levels < 60 mL/min per 1.73 m2 (Figure 2A)[54].
The mechanisms whereby liraglutide reduces progression to macroalbuminuria remain unknown but may be in part related to improved metabolic control and modulation of inflammatory pathways. In the LEADER study, the use of liraglutide vs placebo was associated with decreases in HbA1c, weight and systolic blood pressure of 0.4%, 2.3 kg and 1.2 mmHg, respectively. Of relevance to the reno-protective effects of the SGLT-2 inhibitors discussed below, GLP-1 receptor agonists do not appear to have any direct effect on renal haemodynamics. A recent study has shown that short term infusion of the GLP-1 receptor agonist exenatide had no effect on directly measured GFR (inulin clearance) and renal blood flow (para-aminohippurate clearance), but did induce a natriuresis[55].
After this manuscript was submitted for review the results of the Trial to Evaluate Cardiovascular and other long-term outcomes with semaglutide (SUSTAIN-6) have been released. This trial showed that a once weekly injection of semaglutide significantly reduced CV endpoints in high risk vascular patients with type 2 diabetes. In a similar fashion to liraglutide it also reduces progression to macroalbuminuria (HR = 0.54, 95%CI: 0.37-0.77, P < 0.001). It should also be noted that currently, liragludite and semaglutide have only been shown to reduce progression to macroalbuminuria and that their ability to reduce progression to ESKD remains to be proven[54,56].
The results of large clinical studies investigating the potential reno-protective effects of DPP-4 inhibitors have not been published to date. However, the renal outcomes for a pooled analysis of clinical trial data involving 3505 participants treated with the DPP-4 inhibitor linagliptin and 1961 placebo treated participants with a median treatment exposure of 171 d (range, 1-531) for linagliptin and 172 d (range, 1-531) for placebo have been published. The primary renal outcome was defined as first occurrence during the study of 6 predefined end points: New onset of microalbuminuria, new onset of macroalbuminuria, reduction in kidney function (serum creatinine increase to ≥ 250 μmol/L), halving of estimated glomerular filtration rate (loss of baseline eGFR > 50%), acute renal failure (ascertained from diagnostic codes), or death from any cause. The primary composite outcome occurred in 448 (12.8%) and 306 (15.6%) participants in the linagliptin and placebo groups, respectively. Linagliptin treatment significantly reduced the hazard of kidney disease events by 16% compared with placebo (HR = 0.84; 95%CI: 0.72-0.97, P = 0.02). A sensitivity analysis showed that adjustment for kidney function at baseline did not influence the association between reduced renal risk and linagliptin treatment (HR = 0.83; 95%CI: 0.72-0.97, P = 0.02). Moreover, the observed risk reduction for kidney disease end points with linagliptin was consistent across examined subgroups, including those using renin-angiotensin system (RAS) inhibitors.
Overall medications that target the incretin effect are generally well tolerated. The DPP-4 inhibitors are virtually free from side-effects and usually can be used at any level of renal function with an appropriate dose reduction. It should however, be noted that Linagliptin is not renally excreted and therefore does require a dose reduction at lower GFR levels. The side-effects of GLP-1 receptor agonists are mainly related to the gastrointestinal tract and include nausea, vomiting and gallstone disease. Concerns that medications targeting the incretin effect may promote the development of pancreatitis and pancreatic cancer have been raised but have not been supported by the results of large randomised clinical trials. A possible link between the use of liraglutide and semiglutide and the promotion of diabetic retinopathy has also been raised. The significance of these findings remains to be fully established. An important point practice point to highlight for patients with CKD is that the GLP-1 receptor agonists are currently not recommended for used in patients with an eGFR < 30 mL/min per 1.73 m2.
The SGLT2 receptor mediates high-capacity glucose uptake in the early proximal tubule, and SGLT2 inhibitors, via their ability to promote glycosuria, have been developed as glucose lowering medications. There is an emerging body of evidence suggesting that this class of medication may have an important role in reducing intra-glomerular pressure which is then in part translated into a reno-protective effect.
In hyperfiltering patients with T1DM, the SGLT-2 inhibitor empagliflozin has been shown to reduce GFR and resolve hyperfiltration in short term studies by counteracting the tubulo-glomerular feedback mechanism. Specifically, SGLT-2 inhibitors constrict the afferent glomerular arteriole in response to an increase in tubular sodium sensed by the macular densa. This increase in tubular sodium results from less sodium being reabsorbed in the proximal tubule due to inhibition of sodium-glucose transport in the proximal tubule.
In a recent euglycaemic clamp study involving 13 normofiltering and 27 hyperfiltering T1DM subjects, eight weeks of treatment with empagliflozin resulted in a reduction in GFR of 33 mL/min per 1.73 m2 in the hyperfiltration group without any change in GFR in the normofiltering group[42]. A similar degree of GFR reduction associated with RAS inhibitors was observed in previous studies of hyperfiltration in young patients with uncomplicated T1DM[57]. Apart from the above, SGLT-2 inhibitors may possibly have additional renal protective effects as they improve glycaemic control, lower blood pressure and promote weight loss[58].
In the landmark CV safety trial of empagliflozin (EMPA-REG), 7020 patients T2DM patients at high risk for CV events who received empagliflozin (with no differences seen for 10 or 25 mg doses) had reduced rates of CV and all-cause mortality when compared with placebo when studied for 3.1 years. Reductions in death from CV causes (HR = 0.62, 95%CI: 0.49-0.77, P < 0.001), hospitalization for heart failure (HR = 0.65, 95%CI: 0.5-0.85, P = 0.002) and death from any cause (HR = 0.68, 95%CI: 0.57-0.82, P < 0.001) where seen in empagliflozin compared with placebo treated participants, respectively[45].
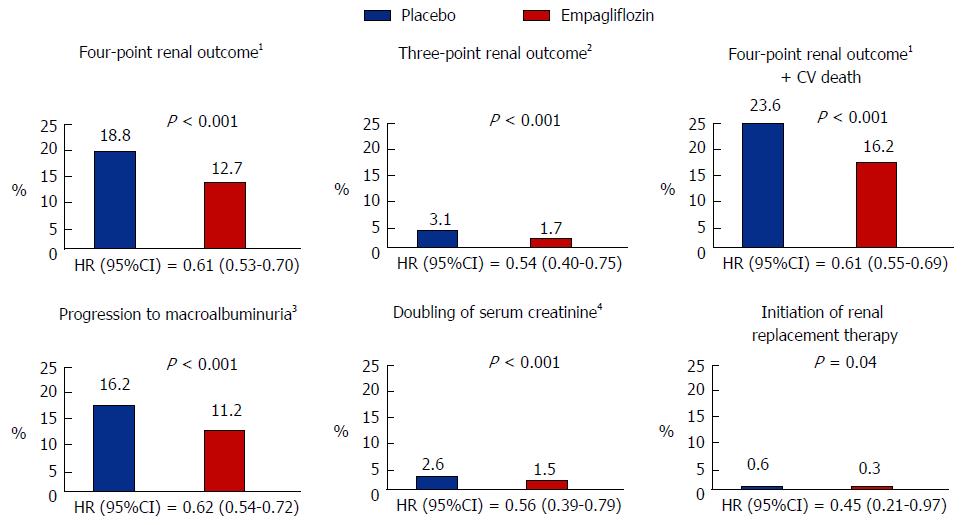
Furthermore, a recent follow-up study from the EMPA-REG OUTCOME study has shown that empagliflozin reduces clinically important renal outcomes. All patients in the study had an eGFR > 30 mL/min per 1.73 m2 and approximately 25% had eGFR < 60 mL/min per 1.73 m2, 11% had macroalbuminuria, 29% had microalbuminuria and 80% were on RAS blockers. The primary renal end point of the trial was a four point composite of new onset or worsening of nephropathy (progression to macroalbuminuria, doubling of serum creatinine level associated with an eGFR < 45 mL/min per 1.73 m2, initiation of renal replacement therapy and death from renal disease). This endpoint occurred in 18.8% of placebo and 12.7% of empaglifozin treated patients which resulted in a risk reduction of 39% in patients that received empaglifozin (HR = 0.61, 95%CI: 0.53-0.7, P < 0.001). In a similar fashion to what seen for CV outcomes in the EMPA-REG study, there were no differences in the renal outcomes for patients receiving the 10 or 25 mg doses of empagliflozin. Importantly, the impact of empagliflozin on the primary CV (Figure 2B) and renal end-points was not diminished in patients with CKD compared to those without CKD[46].
The main driver for a reduction in the primary renal endpoint in empaglifozin treated patients was a slowing in progression to macroalbuminuria, 16.2% vs 11.2% in placebo and empaglifozin treated patients, respectively (HR = 0.62, 95%CI: 0.54-0.72, P < 0.001). Several sensitivity analyses were therefore performed to test the strength of the relationship between empagliflozin treatment and the reduction in clinically meaningful renal endpoints. This analysis showed a consistent empagliflozin effect on indices of renal health as summarised in Figure 3. Of note, empaglifozin vs placebo treatment resulted in a 46% risk reduction in the traditional clinically meaningful composite endpoint of doubling of serum creatinine levels (plus in this study achieving an eGFR < 45 mL/min per 1.73 m2), initiation of renal replacement therapy and death from renal disease (HR = 0.54, 95%CI: 0.40-0.75, P < 0.001).
In empagliflozin treated patients there was an initial drop in eGFR of approximately 4 mL/min per 1.73 m2 but at the end of the 5 years of the trial eGFR values were 4.7 mL/min per 1.73 m2 higher in patients that received empagliflozin. This difference in eGFR values appears to be accounted for by a progressive drop in eGFR values in the placebo treated patients, which could be related to the expected decline in GFR that is associated with aging, and a stabilisation of GFR values in empaglifloszin treated patients after the initial drop in eGFR described above. This initial drop in eGFR and rapid increase in eGFR values that was seen after discontinuation of empagliflozin are consistent with a direct effect of the medication on renal haemodynamics, as discussed above.
The above findings also suggests that a reduction in intraglomerular pressure ultimately results in a preservation of eGFR and is the main mechanism responsible for lower rates of progression to clinically meaningful renal end points for empagliflozin treated patients in EMPA-REG. The rapid divergence in the rates of development of the primary renal endpoint, within 3 mo, in the EMPA-REG study over time also suggests that direct haemodynamic effects rather than improvements in metabolic parameters were primarily responsible for the improvement in renal outcomes seen in the trial. A recent study has also shown that empagliflozin can reduce albuminuria in micro- and macroalbuminuric patients and that this effect is mainly independent of the metabolic and known systemic haemodynamic effects of SGLT-2 inhibitors[59]. The above finding also supports a direct renal effect of SGLT-2 inhibitors. However, contributions from decreases in HbA1c, weight, blood pressure and uric acid levels that are observed in SGLT-2 inhibitor treated patients should also be considered. Furthermore, it has recently been suggested that the use of empagliflozin results in a shift in renal and cardiac fuel metabolism from fat and glucose oxidation to ketone bodies and that this metabolic substrate shift improves the function of these organs[60,61].
Potential side-effects or concerns related to the use of SGLT-2 inhibitors include increased rates of urinary tract infections, genital tract infections, postural hypotension, diabetic ketoacidosis, acute kidney injury and possible increased rates of fractures[58,62-64]. Furthermore, the main disadvantage of the mode of action of the SGLT-2 inhibitors is that their effectiveness for lowering blood glucose levels is dependent on renal function. Hence they are not recommended as glycaemic management agents in patients with significantly impaired renal function.
In summary, the HbA1c threshold for the development of kidney dysfunction remains to be clearly defined but is possibly around 6.5%. Achieving this threshold should be balanced against the increasing appreciation that glucose targets for the prevention of diabetes related complications need be individualised for each patient. Tight glucose control has clearly been shown to reduce the incidence of micro- or macroalbuminuria. However, it is only in very recent times that some evidence has emerged to suggest that intensive glucose control can slow GFR loss and possibly progression to ESKD. The concept of “metabolic memory” is again highlighted in the DCCT/ECIC study of changes in GFR. This type of study emphasises the importance of early intensive glucose control in delaying the development of subsequent diabetes related complications.
Furthermore, recent large randomised clinical trials have shown that the glucose lowering medications liraglutide, semaglutide and empagliflozin have renal protective effects. The mechanisms whereby there medications improve renal outcomes remain to be fully elucidated but are almost certainly over and above those expected from their glucose lowering effects alone with a decrease in intra-glomerular pressure appearing to be a likely mechanism that links the use of empagliflozin with lower rates of progression to ESKD.
The authors would like to thank Varuni Obeyesekere for her assistance in the preparation of this manuscript.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cheng JT, Futrakul P, Zhao JB S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | ADA Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care. 2016;39 Suppl 1:S4-S5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Cooper ME. Diabetes: treating diabetic nephropathy-still an unresolved issue. Nat Rev Endocrinol. 2012;8:515-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Shurraw S, Tonelli M. Intensive glycemic control in type 2 diabetics at high cardiovascular risk: do the benefits justify the risks? Kidney Int. 2013;83:346-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | de Borst MH, Navis G. Diabetes: Risks of strict glycaemic control in diabetic nephropathy. Nat Rev Nephrol. 2015;11:5-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Fioretto P, Barzon I, Mauer M. Is diabetic nephropathy reversible? Diabetes Res Clin Pract. 2014;104:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | MacIsaac RJ, Ekinci EI, Jerums G. ‘Progressive diabetic nephropathy. How useful is microalbuminuria?: contra’. Kidney Int. 2014;86:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, Li Q, MacMahon S, Cooper ME, Hamet P, Marre M. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 8. | de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 423] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 9. | Wong MG, Perkovic V, Chalmers J, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, Heller S, MacMahon S. Long-term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: ADVANCE-ON. Diabetes Care. 2016;39:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Jerums G, Ekinc EI, Premaratne E, Baker ST, Panagiotopolous S, MacIsaac RJ. Diabetic Nephropathy. 4th ed. In: deFronzo R, Ferrannini E, Zimmet P, Alberti G. Edited by deFronzo R, Ferrannini E, Zimmet P, Alberti G. 4th ed: Wiley-Blackwell, 2015: 911-925. . [DOI] [Full Text] |
| 11. | Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 12. | Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J Clin Invest. 2001;108:1853-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 326] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 13. | Coughlan MT, Nguyen TV, Penfold SA, Higgins GC, Thallas-Bonke V, Tan SM, Van Bergen NJ, Sourris KC, Harcourt BE, Thorburn DR. Mapping time-course mitochondrial adaptations in the kidney in experimental diabetes. Clin Sci (Lond). 2016;130:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 14. | Echouffo-Tcheugui JB, Narayan KM, Weisman D, Golden SH, Jaar BG. Association between prediabetes and risk of chronic kidney disease: a systematic review and meta-analysis. Diabet Med. 2016;33:1615-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Kang SH, Jung da J, Choi EW, Cho KH, Park JW, Do JY. HbA1c Levels Are Associated with Chronic Kidney Disease in a Non-Diabetic Adult Population: A Nationwide Survey (KNHANES 2011-2013). PLoS One. 2015;10:e0145827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16426] [Article Influence: 497.8] [Reference Citation Analysis (4)] |
| 17. | Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5819] [Cited by in RCA: 6080] [Article Influence: 233.8] [Reference Citation Analysis (0)] |
| 18. | Gilbert RE, Tsalamandris C, Bach LA, Panagiotopoulos S, O’Brien RC, Allen TJ, Goodall I, Young V, Seeman E, Murray RM. Long-term glycemic control and the rate of progression of early diabetic kidney disease. Kidney Int. 1993;44:855-859. [PubMed] |
| 19. | Zoungas S, Chalmers J, Ninomiya T, Li Q, Cooper ME, Colagiuri S, Fulcher G, de Galan BE, Harrap S, Hamet P. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012;55:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Gilbert RE, Mann JF, Hanefeld M, Spinas G, Bosch J, Yusuf S, Gerstein HC. Basal insulin glargine and microvascular outcomes in dysglycaemic individuals: results of the Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial. Diabetologia. 2014;57:1325-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Bash LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Arch Intern Med. 2008;168:2440-2447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Shurraw S, Hemmelgarn B, Lin M, Majumdar SR, Klarenbach S, Manns B, Bello A, James M, Turin TC, Tonelli M. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med. 2011;171:1920-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 23. | Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18:1353-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 289] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 24. | Skupien J, Warram JH, Smiles A, Galecki A, Stanton RC, Krolewski AS. Improved glycemic control and risk of ESRD in patients with type 1 diabetes and proteinuria. J Am Soc Nephrol. 2014;25:2916-2925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 752] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 26. | Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2006;29:1486-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 252] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 27. | Wadén J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009;58:2649-2655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Morano S, Cavalot F, Lamacchia O, Laviola L. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care. 2013;36:2301-2310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 12797] [Article Influence: 457.0] [Reference Citation Analysis (0)] |
| 30. | Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6292] [Cited by in RCA: 5676] [Article Influence: 315.3] [Reference Citation Analysis (0)] |
| 31. | Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1099] [Cited by in RCA: 1008] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 32. | Agrawal L, Azad N, Emanuele NV, Bahn GD, Kaufman DG, Moritz TE, Duckworth WC, Abraira C. Observation on renal outcomes in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:2090-2094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014;2:CD009122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Kähler P, Grevstad B, Almdal T, Gluud C, Wetterslev J, Lund SS, Vaag A, Hemmingsen B. Targeting intensive versus conventional glycaemic control for type 1 diabetes mellitus: a systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ Open. 2014;4:e004806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012;172:761-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 36. | Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, Wetterslev J. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;CD008143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5314] [Cited by in RCA: 5374] [Article Influence: 298.6] [Reference Citation Analysis (1)] |
| 38. | Cherney DZ, Sochett EB, Dekker MG, Perkins BA. Ability of cystatin C to detect acute changes in glomerular filtration rate provoked by hyperglycaemia in uncomplicated Type 1 diabetes. Diabet Med. 2010;27:1358-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Mogensen CE, Andersen MJ. Increased kidney size and glomerular filtration rate in untreated juvenile diabetes: normalization by insulin-treatment. Diabetologia. 1975;11:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 150] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Wiseman MJ, Saunders AJ, Keen H, Viberti G. Effect of blood glucose control on increased glomerular filtration rate and kidney size in insulin-dependent diabetes. N Engl J Med. 1985;312:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 168] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Vallon V, Blantz RC, Thomson S. Glomerular hyperfiltration and the salt paradox in early [corrected] type 1 diabetes mellitus: a tubulo-centric view. J Am Soc Nephrol. 2003;14:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 1017] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 43. | Premaratne E, Verma S, Ekinci EI, Theverkalam G, Jerums G, MacIsaac RJ. The impact of hyperfiltration on the diabetic kidney. Diabetes Metab. 2015;41:5-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Musetti C, Quaglia M, Stratta P. The authors reply. Kidney Int. 2014;86:1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8687] [Article Influence: 789.7] [Reference Citation Analysis (1)] |
| 46. | Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2303] [Cited by in RCA: 2633] [Article Influence: 263.3] [Reference Citation Analysis (0)] |
| 47. | Papademetriou V, Lovato L, Doumas M, Nylen E, Mottl A, Cohen RM, Applegate WB, Puntakee Z, Yale JF, Cushman WC. Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 48. | De Cosmo S, Prudente S, Lamacchia O, Lapice E, Morini E, Di Paola R, Copetti M, Ruggenenti P, Remuzzi G, Vaccaro O. PPARγ2 P12A polymorphism and albuminuria in patients with type 2 diabetes: a meta-analysis of case-control studies. Nephrol Dial Transplant. 2011;26:4011-4016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | August P, Hardison RM, Hage FG, Marroquin OC, McGill JB, Rosenberg Y, Steffes M, Wall BM, Molitch M. Change in albuminuria and eGFR following insulin sensitization therapy versus insulin provision therapy in the BARI 2D study. Clin J Am Soc Nephrol. 2014;9:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2787] [Cited by in RCA: 2888] [Article Influence: 144.4] [Reference Citation Analysis (1)] |
| 51. | Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 521] [Article Influence: 30.6] [Reference Citation Analysis (6)] |
| 52. | Lytvyn Y, Bjornstad P, Pun N, Cherney DZ. New and old agents in the management of diabetic nephropathy. Curr Opin Nephrol Hypertens. 2016;25:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, Tsukiyama K, Narita T, Takahashi T, Drucker DJ. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 54. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4164] [Cited by in RCA: 5249] [Article Influence: 524.9] [Reference Citation Analysis (0)] |
| 55. | Tonneijck L, Smits MM, Muskiet MH, Hoekstra T, Kramer MH, Danser AH, Diamant M, Joles JA, van Raalte DH. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia. 2016;59:1412-1421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 56. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5185] [Cited by in RCA: 4486] [Article Influence: 448.6] [Reference Citation Analysis (1)] |
| 57. | Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol. 2006;17:1703-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 58. | Thomas MC, Jandeleit-Dahm K, Bonnet F. Beyond Glycosuria: Exploring the intrarenal effects of SGLT-2 inhibition in diabetes. Diabetes Metab. 2014;40:S17-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Cherney D, Lund SS, Perkins BA, Groop PH, Cooper ME, Kaspers S, Pfarr E, Woerle HJ, von Eynatten M. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59:1860-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 60. | Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPA-REG OUTCOME Trial: A “Thrifty Substrate” Hypothesis. Diabetes Care. 2016;39:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 770] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 61. | Mudaliar S, Alloju S, Henry RR. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care. 2016;39:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 471] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 62. | Vlotides G, Mertens PR. Sodium-glucose cotransport inhibitors: mechanisms, metabolic effects and implications for the treatment of diabetic patients with chronic kidney disease. Nephrol Dial Transplant. 2015;30:1272-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, Neal B. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016;4:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 362] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 64. | Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation. 2016;134:752-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 1042] [Article Influence: 104.2] [Reference Citation Analysis (0)] |