Published online Mar 25, 2016. doi: 10.4239/wjd.v7.i6.112
Peer-review started: November 3, 2015
First decision: November 30, 2015
Revised: December 13, 2015
Accepted: January 27, 2016
Article in press: January 29, 2016
Published online: March 25, 2016
Processing time: 147 Days and 0.3 Hours
For the last decade, low serum amylase (hypoamylasemia) has been reported in certain common cardiometabolic conditions such as obesity, diabetes (regardless of type), and metabolic syndrome, all of which appear to have a common etiology of insufficient insulin action due to insulin resistance and/or diminished insulin secretion. Some clinical studies have shown that salivary amylase may be preferentially decreased in obese individuals, whereas others have revealed that pancreatic amylase may be preferentially decreased in diabetic subjects with insulin dependence. Despite this accumulated evidence, the clinical relevance of serum, salivary, and pancreatic amylase and the underlying mechanisms have not been fully elucidated. In recent years, copy number variations (CNVs) in the salivary amylase gene (AMY1), which range more broadly than the pancreatic amylase gene (AMY2A and AMY2B), have been shown to be well correlated with salivary and serum amylase levels. In addition, low CNV of AMY1, indicating low salivary amylase, was associated with insulin resistance, obesity, low taste perception/satiety, and postprandial hyperglycemia through impaired insulin secretion at early cephalic phase. In most populations, insulin-dependent diabetes is less prevalent (minor contribution) compared with insulin-independent diabetes, and obesity is highly prevalent compared with low body weight. Therefore, obesity as a condition that elicits cardiometabolic diseases relating to insulin resistance (major contribution) may be a common determinant for low serum amylase in a general population. In this review, the novel interpretation of low serum, salivary, and pancreas amylase is discussed in terms of major contributions of obesity, diabetes, and metabolic syndrome.
Core tip: Low serum amylase was believed to occur in uncommon conditions such as type 1 diabetes, advanced chronic pancreatitis, and cystic fibrosis. However, in the last decade, low serum amylase has been observed in more common conditions related with insulin resistance than was previously believed. In this review, a novel interpretation for low serum, salivary, and pancreatic amylase is discussed, particularly in terms of the cardiometabolic conditions of obesity, diabetes, and metabolic syndrome.
- Citation: Nakajima K. Low serum amylase and obesity, diabetes and metabolic syndrome: A novel interpretation. World J Diabetes 2016; 7(6): 112-121
- URL: https://www.wjgnet.com/1948-9358/full/v7/i6/112.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i6.112
Traditionally, the level of serum amylase has been commonly measured to determine the presence of acute pancreatitis and biliary tract disease in primary clinical settings[1-3]. In contrast, most physicians seldom measure it to determine the degree of advanced chronic pancreatitis, which eventually results in secondary diabetes concomitant with weight loss, lipid diarrhea, and malnutrition[4-6]. The former condition predisposes to higher serum amylase (but not necessarily), whereas the latter condition lowers serum amylase. While some cancers, such as lung, ovarian, and colon cancer and myeloma, often produce amylase and increase serum amylase[7-9], such increased serum amylase may be exceptional in a general population. By contrast, low serum amylase has been empirically known in diabetic patients particularly with insulin-dependent diabetes (primarily type 1 diabetes), although the clinical relevance and precise underlying mechanism are not fully understood[10-15].
In earlier clinical studies, conflicting results were reported regarding the level of serum amylase in diabetic patients, possibly because a precise measurement for serum amylase had not then been established. In recent decades, however, the measurement of serum and its isoforms has been stably performed in clinical laboratories with several methods including electrophoresis, inhibitor method and antibody method[16]. Because serum amylase generally consists of salivary and pancreatic amylase at almost equal proportion, i.e., 1:1[17], abnormal levels of both or one of the two isoforms affect the level of total serum amylase.
Low serum amylase was believed to occur in uncommon conditions such as type 1 diabetes, advanced chronic pancreatitis, and cystic fibrosis[1-3,10-15,18,19] (minor contribution) (Table 1). However, in the past decade, low serum amylase has been observed in more common conditions (major contribution) than was previously believed. In this review, a novel interpretation for low serum, salivary, and pancreatic amylase is discussed, particularly in terms of the cardiometabolic conditions of obesity, diabetes, and metabolic syndrome (MetS).
| Ref. | |
| Traditional (minor contributions) | |
| Type 1 diabetes (juvenile diabetes) | [1,2,10-15] |
| Advanced chronic pancreatitis | [3-6] |
| Type 2 diabetes with insulin dependence | [14] |
| Cystic fibrosis | [18,19] |
| Novel (major contributions) | |
| Obesity | [35-38] |
| Insulin resistance (high HOMA-R) | [44] |
| Metabolic Syndrome | [38,41,42] |
| Type 2 diabetes with insufficient insulin action | [38,42] |
| Diabetic ketoacidosis | [13,23] |
| Non-alcoholic fatty liver disease | [40,43] |
| Smoking | [38,68-70] |
| Heavy alcohol drinking | [46,63] |
| Low CNVs of AMY1 | [54,60,61] |
In the general population, the cause of acute pancreatitis is mainly high alcohol intake and/or high serum triglycerides, both of which injure the pancreas[1-6]. Consequently, transient and acute increases (from several to ten times beyond the upper normal level) in serum amylase concentration may occur as a result of destruction of acinar cells in the pancreas. However, repeated acute pancreatitis eventually results in exhausted acinar cells and restricted flow of enzymes from pancreas parenchyma into the circulation[4-6], in turn leading to low serum amylase due to low pancreatic amylase. Secondary diabetes also develops because of the destruction of β-cells in the course of chronic pancreatitis. In addition, low serum amylase has been observed in patients with cystic fibrosis concomitant with pancreatic insufficiency[18,19].
Around 20% of patients with diabetic ketoacidosis develop hyperamylasemia[20-22]. In this author’s opinion, however, because diabetic ketoacidosis is accompanied by severely insufficient insulin action, theoretically serum amylase should be decreased in such conditions, at least before insulin therapy is initiated.
Consistently with this, Yokoyama et al[13] and Somogyi et al[23] found that serum amylase activity was reduced at onset of the disease before treatment with insulin. The author of the current review and collaborators, have frequently observed low serum amylase in diabetic ketoacidosis before treatment with insulin in clinical settings (unpublished data). The unexpectedly high serum amylase in previous studies[20-22] may be explained by the fact that diabetic ketoacidosis involves numerous etiologies that can contribute to high serum amylase: Acute pancreatitis (mild to moderate grades)[22] including hypertriglyceridemic pancreatitis[24], renal dysfunction, and dehydration, all of which increase serum amylase.
Meanwhile, low serum amylase has been empirically observed in clinical settings in patients with type 1 diabetes, type 2 diabetes with insulin dependence, or advanced overt pancreatitis[10-15]. The action of insulin is critical for the production of pancreatic amylase[25,26]. A common etiology in these conditions may be depleted secretion of insulin from the pancreas. However, patients with these specific conditions are minor populations compared with diabetic patients with insulin independence.
The clinical relevance of salivary amylase has been focused on diseases of the salivary glands, sympathetic nerve system, and oral health. Under physiological conditions, secretion and activation of salivary amylase, i.e., hyperamylasemia, is reportedly stimulated by psychosocial stress[27,28]. Many α-amylase inhibitors, which are often extracted from plants, have also been intensively investigated in terms of carbohydrate digestion and diabetic treatment[29,30]. Unfortunately, however, few clinical studies have reported low salivary amylase.
Dentists and investigators who work with, or are interested in, oral care have addressed the etiology of low salivary amylase[31,32]. Although insulin may also exert its action on the production of salivary amylase in the salivary glands[33,34], physicians have paid little attention to the issue. Therefore, the clinical relevance of low salivary amylase has not been elucidated, particularly in cardiometabolic conditions.
An early animal study by Schneeman et al[35] showed that pancreatic amylase activity was reduced in obese rats but remained elevated in lean rats. An early clinical study of healthy young men aged 19 to 22 years by Kondo et al[36] showed that serum pancreatic amylase and trypsin, but not lipase, were reduced in obese subjects (n = 85) compared with lean subjects (n = 75). The reduction in serum pancreatic amylase was significantly improved by a weight loss program over 6 mo, and remained improved for a further 10 mo. No such trend was observed in pancreatic trypsin. To the best of this author’s knowledge, these are the first studies to show an inverse relationship between obesity and serum pancreatic amylase. However, in the clinical study[36], the sample size was relatively small and relevant confounding factors including smoking, alcohol intake, exercise, and kidney function were not adjusted for in the analysis. Another small clinical study in children (n = 58) showed that obese boys (n = 29) presented a significantly lower salivary amylase concentration than control boys[37]. Except for these early studies[35-37], no clinical studies have investigated the relationship between serum amylase and obesity and obesity-related conditions.
In our previous cross-sectional (n = 2425) and longitudinal (n = 571) studies during the last decade[38], low serum (total) amylase (≤ 57 IU/L) was significantly associated with MetS, diabetes (mostly type 2 diabetes), and remained significant even after adjustment for relevant confounding factors including age, sex, smoking, alcohol drinking, and regular exercise, pharmacotherapies, and kidney function assessed by estimated glomerular filtration rate (eGFR). In this study, body mass index (BMI) was the factor most associated with serum amylase[38]. Furthermore, low serum amylase was associated with non-alcoholic fatty liver disease (NAFLD), a hepatic manifestation of MetS and insulin resistance[39], in asymptomatic adults independently of relevant confounding factors[40]. The results of these epidemiological studies[38,40] were subsequently confirmed in other large Asian populations[41-43]. Furthermore, in our previous study of asymptomatic subjects not being treated for diabetes[44], a homeostasis model assessment of insulin resistance, plasma insulin levels at fasting and at 60 min in the 75 g oral glucose tolerance test were significantly associated with low serum amylase (< 60 IU/L) after adjustment for relevant confounding factors including BMI, although the sample size was small (n = 54).
These results suggest that low serum amylase is observed in not only rare conditions of insulin depletion (minor contribution) but also in common cardiometabolic conditions such as MetS, type 2 diabetes, or NAFLD (major contribution). Obesity, as a condition associated with various cardiometabolic diseases concomitant with insulin resistance, may be a major determinant for low serum amylase in the general population (a novel interpretation). A clinical study in hospitalized patients by Curd et al[45] showed that hypoamylasemia was associated with cystic fibrosis, hypertriglyceridemia and use of the antibiotic gentamicin, besides diabetes mellitus. Although cystic fibrosis and use of gentamicin may be uncommon, hypertriglyceridemia is rather common.
Williams et al[46] mentioned in an early review article that insulin is necessary for normal acinar function and that endogenous insulin potentiates zymogen release. However, exogenous insulin supplementation can improve low serum amylase in type 1 diabetes[13]. Schneeman et al[35] proposed in an animal study that insulin resistance may prevent the potentiating effect of insulin on amylase synthesis, leading to lower amylase levels. Early clinical studies have also shown that serum pancreatic amylase was closely related to C-peptide concentration and pancreatic β-cell function[13,14]. One would therefore expect serum amylase to be reduced in obese and diabetic subjects.
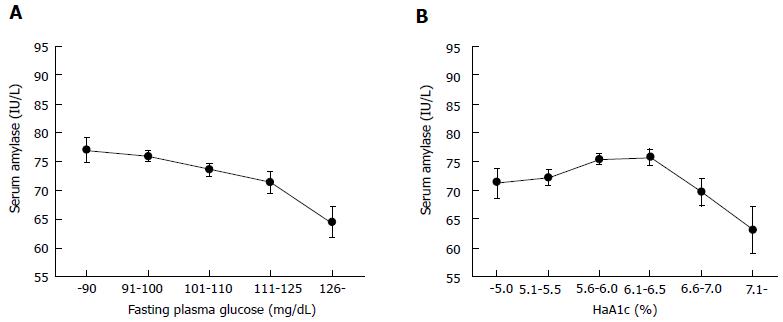
Regarding the relationship between diabetes and serum amylase, it is noteworthy that serum amylase levels are not linearly correlated with HbA1c values in the general population including healthy individuals and diabetic patients, although fasting plasma glucose was negatively and linearly correlated with serum amylase[47]. In this study, serum amylase showed an inverse U-shaped relationship with HbA1c categories (Figure 1). Unexpectedly, serum amylase level was highest in subjects with HbA1c of 5.6%-6.5%. We experienced a similar result in an entirely different Japanese population (unpublished data). These findings may be consistent with the results of an early study by Dandona et al[14], which showed no significant correlation between HbA1c and pancreatic amylase activity. The discrepancy between HbA1c and fasting plasma glucose may be owing to the presence of postprandial hyperglycemia, i.e., impaired glucose tolerance, a common finding in obese individuals[48,49], primarily related to HbA1c only. Additionally, hyperinsulinemia induced by insulin resistance for the maintenance of euglycemia, a common finding in early type 2 diabetes, may increase pancreatic amylase production.
After progression to overt diabetes with HbA1c over 6.5%, insulin resistance is not managed by hyperinsulinemia and insulin secretion begins to decline, resulting in a corresponding decrease in pancreatic and/or salivary amylase. Therfore, a linear relationship between HbA1c and serum amylase was observed in the data when only overt diabetic patients were studied[13,47].
Taken together, these results suggest that the relationship between serum amylase and diabetes and obesity is an exocrine-endocrine interrelationship, which in turn may contribute to the feedback system in energy homeostasis.
While vertebrate animals express amylase in the pancreas, its expression in the salivary gland is limited to some primates and other herbivores and omnivores[50]. Carnivores (domesticated dogs and cats) do not have salivary amylase, whereas many herbivores (including goats, cows, horse, koala, rabbit, and elephant) do. Conversely, most omnivores, including humans, have considerable amounts of salivary amylase. Salivary amylase is higher in humans compared with many other animals including ape species, suggesting a dietary shift in the direction of high starch content during evolution[50,51].
Salivary amylase can affect an individual’s oral sensory properties, in turn altering the threshold of satiety and appetite. While amylase expression particularly in salivary glands may be roughly determined by genetic regulation, high amylase levels can be induced by carbohydrate-rich diets passed on over generations. This hypothesis warrants further study.
Postprandial plasma glucose concentrations after ingestion of a 50 g starch solution were significantly higher in healthy nonobese adults with low salivary amylase than in those with high salivary amylase[52]. High salivary amylase activity is associated with a rapid insulin response accompanied by a swift reduction in blood glucose levels following starch ingestion. A plausible explanation is the response of insulin secretion at the early cephalic phase.
Genetic regulation is likely to play a key role in the primary determination of salivary amylase[53,54]. In newborns the predominant amylase isozymes seen in the urine are of salivary origin and later both salivary and pancreas, which increases during development. AMY1 is expressed as early as 18 wk of gestation and salivary amylase gradually increases during development, as the total amylase activity approaches adult values.
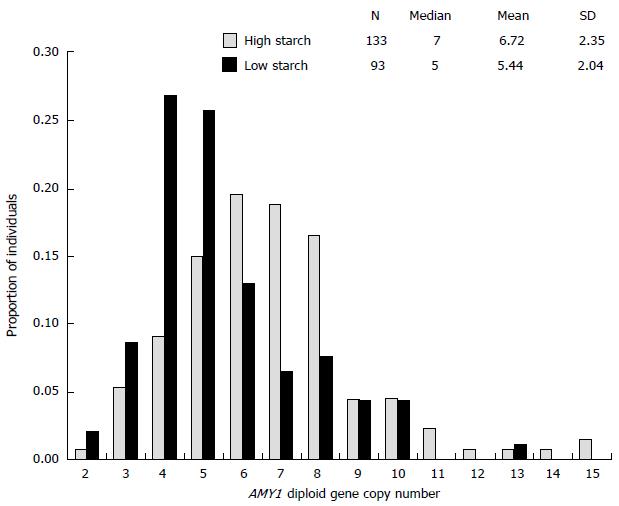
In recent years, several studies have reported that serum and salivary amylase was significantly correlated with copy number variations (CNVs) of salivary amylase gene (AMY1). Moreover, CNV of AMY1 was inversely associated with BMI, insulin resistance, and glucose tolerance[52,55-57]. CNVs seem to be higher in humans, particularly in American Europeans and Japanese, who relied on a starch-rich diet in the remote past[58] (Figure 2). According to Falchi et al[55], CNV of AMY1 had a stronger association with BMI than polymorphisms in FTO, although conflicting results exist[59]. These findings suggest a genetic link between carbohydrate metabolism and obesity, possibly also involving gut microbiota[60].
A clinical study in Mexico suggests putative benefits of a high number of AMY1 copies (and related production of salivary amylase) on obesity and energy metabolism in children[61]. Furthermore, a clinical study in Finland showed that low CNV of AMY1 was associated with early-onset female obesity[62]. In this context, it is possible that individualized carbohydrate diets according to CNV of AMY1 may help prevent obesity and type 2 diabetes.
Compared with AMY1, the relation of AMY2 with cardiometabolic conditions is equivocal. In humans, the variation of CNVs of AMY1 was wider than those of AMY2[57,59]. Furthermore, CNV of AMY1 was independent of those of AMY2, which showed no association with BMI[59]. Meanwhile, in domesticated dogs for instance, pancreatic amylase not salivary amylase likely contributes to total amylase[50,63]. However, even in such animals, the variation of CNV of AMY2B was estimated to explain only 14.8% of the variance in amylase activity, indicating that additional factors may explain the majority of the variation[63].
Other common conditions besides obesity-related conditions have been reported (Table 1). Alcohol consumption may affect the serum amylase level independently of BMI[47,64,65]. This may occur via damage of pancreatic tissue, i.e., chronic pancreatitis, and reduced salivary amylase[66]. However, the underlying mechanism may be complicated because the effect of alcohol on glucose homeostasis can differ according to the quantity consumed[67], age, and lifestyle[68]. Low serum amylase was also observed in smokers compared with nonsmokers[69-71]. In contrast, high serum amylase has been observed in individuals who exercise regularly[72] and in high-performance long distance runners[73]. Because both smoking and fitness have a substantial impact on insulin action, these results may be explained from the view of insulin sensitivity[74]. Furthermore, low serum pancreatic enzyme levels predict mortality and are associated with malnutrition-inflammation[75], although the underlying mechanism remains unknown.
A recent study by Shimizu et al[76] showed that circulating pancreatic amylase was higher in female subjects with O blood type than those with A blood type (lower pancreatic amylase in A blood type). Serum total amylase was also higher (but not statistically significant) in O blood type in both sexes. Coincidentally, we confirmed a similar finding of lower serum amylase in A blood type relative to O blood type in a general Japanese adult population (n = 1185), although no ABO blood types were associated with hyperglycemia (HbA1c ≥ 5.7% and/or pharmacotherapy for diabetes)[77]. Meanwhile, some clinical studies[78,79] have shown that people with the O blood type had a lower risk of developing type 2 diabetes compared with other blood types. Therefore, it is possible that unknown and unquantified factors including CNV of AMY1 and prevalence of Rhesus factor in the study may also contribute to the relationship among ABO blood types, serum amylase, and impaired glucose metabolism. Together with the putative lower prevalence of pancreatic cancers in individuals with O blood type[80-82], this indicates a possible relationship between ABO blood type, which is under strict genetic regulation, and susceptibility to pancreatic disease.
Intriguingly, elevated serum amylase has been sporadically observed in a series of eating disorders. Anorexia nervosa (AN) and bulimia nervosa (BN) are two major eating disorders with a complex relationship with abnormal physical conditions such as severe weight loss, binge eating, frequent vomiting or endocrine disorders[83,84]. Several studies have shown that serum amylase levels were significantly elevated in patients with BN[85-87]. It is likely that vomiting, rather than binge behavior, increases amylase in BN patients[87]. Frequent vomiting may also be associated with enlarged submandibular and parotid glands. It was also confirmed that hyperamylasemia in patients with AN or BN was caused by increased salivary-type amylase activity[85]. Consequently, these results suggest that the direct effect on elevated serum amylase may be primarily enlarged salivary glands. Conversely, it is also possible that concomitant low body weight or depleted energy storage, common findings in AN, cause the increased serum amylase. This needs to be studied further.
Psychosocial stress contributes to elevated salivary amylase even in a healthy population[27,28], which likely leads to elevated total serum amylase. However, whether psychosocial stress has a long-term effect on serum amylase has not been confirmed in clinical studies. Investigators should pay attention to the mental and physical conditions in their patients when measuring salivary and serum amylase levels. Kidney function is also a crucial modifier that affects the clearance of circulating amylase in the blood[38,88]. Serum amylase is expected to be elevated in patients with renal dysfunction, and is a permanent phenomenon. Marked serum amylase elevation is observed in patients with only chronic kidney disease (CKD)[89]. While renal dysfunction should be kept in mind when high amylase levels are detected, the conditions of low serum amylase can be hidden and thus overlooked in patients with CKD and renal dysfunction, because lower amylase can be converted to normal amylase as a result of diminished clearance. GFR, for instance by the use of inulin, is not measured in usual clinical settings, so eGFR should be at least considered as a relevant confounding factor in the analysis of serum amylase. Nevertheless, it is unknown whether hyperfiltration, which is often observed in early diabetes, lowers serum amylase.
Some pharmacotherapies, particularly against diabetes, for instance dipeptidyl peptidase-4 inhibitors and glucagon-like peptide 1 receptor agonists, reportedly increase serum amylase. Several investigators[90-92] have recommended caution when starting incretin therapy to avoid pancreatitis. However, theoretically, a mild increase in serum amylase levels within the normal range or from low to normal levels can represent a potential beneficial effect of incretin therapy on glucose homeostasis especially in individuals with appreciable weight loss. Physicians may withdraw incretin therapy in patients with a slight increase in serum amylase of 10-20 IU/mL, for example, interpreting this as a sign of pancreatitis. Increase in serum amylase, particularly from low to normal levels, may reflect improved glucose homeostasis rather than acute pancreatitis. However, it remains pivotal to accumulate further data to investigate the clinical relevance of increases in pancreatic enzymes during incretin therapy.
For several decades, some clinical studies have revealed that α-amylase inhibitors, most of which are extracted from plants (clinically unavailable), can improve postprandial hyperglycemia[93] and obesity[94,95]. It is unclear whether α-amylase inhibitors would be truly effective for preventing diabetes and obesity if clinically available. Furthermore, while acarbose, an α-glucosidase inhibitor that also inhibits α-amylase, is available, such agents may be ineffective in obese subjects who have already low serum amylase.
Collectively, low serum amylase may reflect a manifestation of insufficient insulin action regardless of cause including insufficient pancreatic insulin secretion and/or systemic insulin resistance. Unfortunately, the cut-off point for low serum amylase has not been defined, primarily because of the lack of a concept for low serum amylase and the differences in assay methods. Unlike minor contributions, major contributions for low serum amylase include common cardiometabolic conditions such as obesity, MetS, and type 2 diabetes, which are all increasing in incidence worldwide. Although genetic regulation may have a substantial impact on primary salivary amylase, whether epigenetic background and individual diet can alter salivary amylase and thus affect serum amylase is unclear, and requires further investigation.
P- Reviewer: Das UN, Lyerly Jr GW, Liu SH S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Kameya A, Hayakawa T, Noda A, Kondo T. Differential determination of serum isoamylase using an amylase inhibitor and its clinical application. Am J Gastroenterol. 1985;80:54-59. [PubMed] |
| 2. | Garrison R. Amylase. Emerg Med Clin North Am. 1986;4:315-327. [PubMed] |
| 3. | Pieper-Bigelow C, Strocchi A, Levitt MD. Where does serum amylase come from and where does it go. Gastroenterol Clin North Am. 1990;19:793-810. [PubMed] |
| 4. | Worning H. Chronic pancreatitis: pathogenesis, natural history and conservative treatment. Clin Gastroenterol. 1984;13:871-894. [PubMed] |
| 5. | Giger U, Stanga Z, DeLegge MH. Management of chronic pancreatitis. Nutr Clin Pract. 2004;19:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Waljee AK, Dimagno MJ, Wu BU, Schoenfeld PS, Conwell DL. Systematic review: pancreatic enzyme treatment of malabsorption associated with chronic pancreatitis. Aliment Pharmacol Ther. 2009;29:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Flood JG, Schuerch C, Dorazio RC, Bowers GN. Marked hyperamylasemia associated with carcinoma of the lung. Clin Chem. 1978;24:1207-1212. [PubMed] |
| 8. | Ebisawa S, Yamazaki S, Yasuo M, Urushihata K, Tsushima K, Hanaoka M, Koizumi T, Fujimoto K, Kubo K. [Multiple hepatic metastases due to germ cell tumor on initial clinical presentation]. Nihon Kokyuki Gakkai Zasshi. 2007;45:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Sosnoff DR, Friend RB, Berkovic M, Rasansky RJ, Hoffman SM. Salivary amylase-producing multiple myeloma: case report and review of the current literature. J Clin Oncol. 2013;31:e309-e311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Domschke W, Tympner F, Domschke S, Demling L. Exocrine pancreatic function in juvenile diabetics. Am J Dig Dis. 1975;20:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Frier BM, Saunders JH, Wormsley KG, Bouchier IA. Exocrine pancreatic function in juvenile-onset diabetes mellitus. Gut. 1976;17:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Frier BM, Faber OK, Binder C, Elliot HL. The effect of residual insulin secretion on exocrine pancreatic function in juvenile-onset diabetes mellitus. Diabetologia. 1978;14:301-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Yokoyama J, Tajima N, Ikeda Y, Ohno M, Saito S, Sakamoto Y, Tanese T, Abe M. The amylase activity and its isoenzyme analysis in juvenile-onset diabetes mellitus. Tonyobyo. 1980;23:607-617. [DOI] [Full Text] |
| 14. | Dandona P, Freedman DB, Foo Y, Perkins J, Katrak A, Mikhailidis DP, Rosalki SB, Beckett AG. Exocrine pancreatic function in diabetes mellitus. J Clin Pathol. 1984;37:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Swislocki A, Noth R, Hallstone A, Kyger E, Triadafilopoulos G. Secretin-stimulated amylase release into blood is impaired in type 1 diabetes mellitus. Horm Metab Res. 2005;37:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Ogawa Z, Hasegawa A. Amylase. Rinsho Byori. 2001;Suppl 116:36-44. [PubMed] |
| 17. | Skrha J, Stĕpán J. Clinical significance of amylase isoenzyme determination. Acta Univ Carol Med Monogr. 1987;120:1-81. [PubMed] |
| 18. | Gillard BK, Cox KL, Pollack PA, Geffner ME. Cystic fibrosis serum pancreatic amylase. Useful discriminator of exocrine function. Am J Dis Child. 1984;138:577-580. [PubMed] |
| 19. | Wolf RO, Hubbard VS, Gillard BK, Kingman A. Three methods compared for determination of pancreatic and salivary amylase activity in serum of cystic fibrosis patients. Clin Chem. 1986;32:296-300. [PubMed] |
| 20. | Knight AH, Williams DN, Ellis G, Goldberg DM. Significance of hyperamylasaemia and abdominal pain in diabetic ketoacidosis. Br Med J. 1973;3:128-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Yadav D, Nair S, Norkus EP, Pitchumoni CS. Nonspecific hyperamylasemia and hyperlipasemia in diabetic ketoacidosis: incidence and correlation with biochemical abnormalities. Am J Gastroenterol. 2000;95:3123-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Rizvi AA. Serum amylase and lipase in diabetic ketoacidosis. Diabetes Care. 2003;26:3193-3194. [PubMed] |
| 23. | Somogyi M. Blood diastase in health and diabetes. J Biol Chem. 1940;134:315-318. |
| 24. | Quintanilla-Flores DL, Rendón-Ramírez EJ, Colunga-Pedraza PR, Gallardo-Escamilla J, Corral-Benavides SA, González-González JG, Tamez-Pérez HE. Clinical course of diabetic ketoacidosis in hypertriglyceridemic pancreatitis. Pancreas. 2015;44:615-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Korc M, Owerbach D, Quinto C, Rutter WJ. Pancreatic islet-acinar cell interaction: amylase messenger RNA levels ar determined by insulin. Science. 1981;213:351-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 202] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Mössner J, Logsdon CD, Williams JA, Goldfine ID. Insulin, via its own receptor, regulates growth and amylase synthesis in pancreatic acinar AR42J cells. Diabetes. 1985;34:891-897. [PubMed] |
| 27. | Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, Ehlert U. Stress-induced changes in human salivary alpha-amylase activity -- associations with adrenergic activity. Psychoneuroendocrinology. 2006;31:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 28. | Schumacher S, Kirschbaum C, Fydrich T, Ströhle A. Is salivary alpha-amylase an indicator of autonomic nervous system dysregulations in mental disorders--a review of preliminary findings and the interactions with cortisol. Psychoneuroendocrinology. 2013;38:729-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Etxeberria U, de la Garza AL, Campión J, Martínez JA, Milagro FI. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin Ther Targets. 2012;16:269-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 30. | Barrett ML, Udani JK. A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): a review of clinical studies on weight loss and glycemic control. Nutr J. 2011;10:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 31. | Scannapieco FA, Torres G, Levine MJ. Salivary alpha-amylase: role in dental plaque and caries formation. Crit Rev Oral Biol Med. 1993;4:301-307. [PubMed] |
| 32. | Sánchez GA, Miozza VA, Delgado A, Busch L. Relationship between salivary mucin or amylase and the periodontal status. Oral Dis. 2013;19:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Carter DA, Wobken JD, Dixit PK, Bauer GE. Immunoreactive insulin in rat salivary glands and its dependence on age and serum insulin levels. Proc Soc Exp Biol Med. 1995;209:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Rocha EM, Carvalho CR, Saad MJ, Velloso LA. The influence of ageing on the insulin signalling system in rat lacrimal and salivary glands. Acta Ophthalmol Scand. 2003;81:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Schneeman BO, Inman MD, Stern JS. Pancreatic enzyme activity in obese and lean Zucker rats: a developmental study. J Nutr. 1983;113:921-925. [PubMed] |
| 36. | Kondo T, Hayakawa T, Shibata T, Sato Y, Toda Y. Serum levels of pancreatic enzymes in lean and obese subjects. Int J Pancreatol. 1988;3:241-248. [PubMed] |
| 37. | de Oliveira CG, Collares EF, Barbieri MA, Fernandes MI. Production and concentration of saliva and salivary amylase in obese children. Arq Gastroenterol. 1997;34:105-111. [PubMed] |
| 38. | Nakajima K, Nemoto T, Muneyuki T, Kakei M, Fuchigami H, Munakata H. Low serum amylase in association with metabolic syndrome and diabetes: A community-based study. Cardiovasc Diabetol. 2011;10:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Tarantino G, Finelli C. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome. World J Gastroenterol. 2013;19:3375-3384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 40. | Nakajima K, Oshida H, Muneyuki T, Saito M, Hori Y, Fuchigami H, Kakei M, Munakata H. Independent association between low serum amylase and non-alcoholic fatty liver disease in asymptomatic adults: a cross-sectional observational study. BMJ Open. 2013;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Lee JG, Park SW, Cho BM, Lee S, Kim YJ, Jeong DW, Yi YH, Cho YH. Serum amylase and risk of the metabolic syndrome in Korean adults. Clin Chim Acta. 2011;412:1848-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Zhao Y, Zhang J, Zhang J, Wu J, Chen Y. Metabolic syndrome and diabetes are associated with low serum amylase in a Chinese asymptomatic population. Scand J Clin Lab Invest. 2014;74:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Yao J, Zhao Y, Zhang J, Hong Y, Lu H, Wu J. Serum amylase levels are decreased in Chinese non-alcoholic fatty liver disease patients. Lipids Health Dis. 2014;13:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Muneyuki T, Nakajima K, Aoki A, Yoshida M, Fuchigami H, Munakata H, Ishikawa SE, Sugawara H, Kawakami M, Momomura S. Latent associations of low serum amylase with decreased plasma insulin levels and insulin resistance in asymptomatic middle-aged adults. Cardiovasc Diabetol. 2012;11:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Curd R, Crook MA. Causes of hypoamylasaemia in a hospital population. Scand J Clin Lab Invest. 2015;75:585-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Williams JA, Goldfine ID. The insulin-pancreatic acinar axis. Diabetes. 1985;34:980-986. [PubMed] |
| 47. | Nakajima K, Muneyuki T, Munakata H, Kakei M. Revisiting the cardiometabolic relevance of serum amylase. BMC Res Notes. 2011;4:419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Blaak EE, Antoine JM, Benton D, Björck I, Bozzetto L, Brouns F, Diamant M, Dye L, Hulshof T, Holst JJ. Impact of postprandial glycaemia on health and prevention of disease. Obes Rev. 2012;13:923-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 331] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 49. | Sandqvist M, Strindberg L, Schmelz M, Lönnroth P, Jansson PA. Impaired delivery of insulin to adipose tissue and skeletal muscle in obese women with postprandial hyperglycemia. J Clin Endocrinol Metab. 2011;96:E1320-E1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Boehlke C, Zierau O, Hannig C. Salivary amylase - The enzyme of unspecialized euryphagous animals. Arch Oral Biol. 2015;60:1162-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Mandel AL, Peyrot des Gachons C, Plank KL, Alarcon S, Breslin PA. Individual differences in AMY1 gene copy number, salivary α-amylase levels, and the perception of oral starch. PLoS One. 2010;5:e13352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 52. | Mandel AL, Breslin PA. High endogenous salivary amylase activity is associated with improved glycemic homeostasis following starch ingestion in adults. J Nutr. 2012;142:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 53. | Tye JG, Karn RC, Merritt AD. Differential expression of salivary (Amy1) and pancreatic (Amy2) human amylase loci in prenatal and postnatal development. J Med Genet. 1976;13:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Davis MM, Hodes ME, Munsick RA, Ulbright TM, Goldstein DJ. Pancreatic amylase expression in human pancreatic development. Hybridoma. 1986;5:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Falchi M, El-Sayed Moustafa JS, Takousis P, Pesce F, Bonnefond A, Andersson-Assarsson JC, Sudmant PH, Dorajoo R, Al-Shafai MN, Bottolo L. Low copy number of the salivary amylase gene predisposes to obesity. Nat Genet. 2014;46:492-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 56. | Choi YJ, Nam YS, Yun JM, Park JH, Cho BL, Son HY, Kim JI, Yun JW. Association between salivary amylase (AMY1) gene copy numbers and insulin resistance in asymptomatic Korean men. Diabet Med. 2015;32:1588-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Carpenter D, Dhar S, Mitchell LM, Fu B, Tyson J, Shwan NA, Yang F, Thomas MG, Armour JA. Obesity, starch digestion and amylase: association between copy number variants at human salivary (AMY1) and pancreatic (AMY2) amylase genes. Hum Mol Genet. 2015;24:3472-3480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 58. | Perry GH, Dominy NJ, Claw KG, Lee AS, Fiegler H, Redon R, Werner J, Villanea FA, Mountain JL, Misra R. Diet and the evolution of human amylase gene copy number variation. Nat Genet. 2007;39:1256-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1120] [Cited by in RCA: 945] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 59. | Usher CL, Handsaker RE, Esko T, Tuke MA, Weedon MN, Hastie AR, Cao H, Moon JE, Kashin S, Fuchsberger C. Structural forms of the human amylase locus and their relationships to SNPs, haplotypes and obesity. Nat Genet. 2015;47:921-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 60. | Greenhill C. Obesity. Copy number variants in AMY1 connected with obesity via carbohydrate metabolism. Nat Rev Endocrinol. 2014;10:312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Mejía-Benítez MA, Bonnefond A, Yengo L, Huyvaert M, Dechaume A, Peralta-Romero J, Klünder-Klünder M, García Mena J, El-Sayed Moustafa JS, Falchi M. Beneficial effect of a high number of copies of salivary amylase AMY1 gene on obesity risk in Mexican children. Diabetologia. 2015;58:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 62. | Viljakainen H, Andersson-Assarsson JC, Armenio M, Pekkinen M, Pettersson M, Valta H, Lipsanen-Nyman M, Mäkitie O, Lindstrand A. Low Copy Number of the AMY1 Locus Is Associated with Early-Onset Female Obesity in Finland. PLoS One. 2015;10:e0131883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 63. | Arendt M, Fall T, Lindblad-Toh K, Axelsson E. Amylase activity is associated with AMY2B copy numbers in dog: implications for dog domestication, diet and diabetes. Anim Genet. 2014;45:716-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 64. | Maruyama K, Takahashi H, Okuyama K, Yokoyama A, Nakamura Y, Kobayashi Y, Ishii H. Low serum amylase levels in drinking alcoholics. Alcohol Clin Exp Res. 2003;27:16S-21S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 65. | Li J, Zhou C, Wang R, Liu R, Huang Z, Tang C. Irreversible exocrine pancreatic insufficiency in alcoholic rats without chronic pancreatitis after alcohol withdrawal. Alcohol Clin Exp Res. 2010;34:1843-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Enberg N, Alho H, Loimaranta V, Lenander-Lumikari M. Saliva flow rate, amylase activity, and protein and electrolyte concentrations in saliva after acute alcohol consumption. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Schrieks IC, Heil AL, Hendriks HF, Mukamal KJ, Beulens JW. The effect of alcohol consumption on insulin sensitivity and glycemic status: a systematic review and meta-analysis of intervention studies. Diabetes Care. 2015;38:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 68. | Poli A, Marangoni F, Avogaro A, Barba G, Bellentani S, Bucci M, Cambieri R, Catapano AL, Costanzo S, Cricelli C. Moderate alcohol use and health: a consensus document. Nutr Metab Cardiovasc Dis. 2013;23:487-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 69. | Callegari C, Lami F. Cigarette smoking and salivary amylase activity. Gut. 1984;25:909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Nourane YA, Alaa D. Effect of Smoking on Serum Amylase and Lipase Enzymes. J Am Sci. 2012;8:406-410. |
| 71. | Oshida H, Kutsuma A, Nakajima K. Associations of eating a late-evening meal before bedtime with low serum amylase and unhealthy conditions. J Diabetes Metab Disord. 2013;12:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 72. | Koibuchi E, Suzuki Y. Exercise upregulates salivary amylase in humans (Review). Exp Ther Med. 2014;7:773-777. [PubMed] |
| 73. | Lippi G, Salvagno GL, Danese E, Tarperi C, La Torre A, Guidi GC, Schena F. The baseline serum value of α-amylase is a significant predictor of distance running performance. Clin Chem Lab Med. 2015;53:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Nakajima K. High serum amylase levels may reflect a wide spectrum of health benefits. Clin Chem Lab Med. 2015;53:e67-e68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Ozkok A, Elcioglu OC, Cukadar T, Bakan A, Sasak G, Atilgan KG, Alisir S, Kanbay M, Covic A, Odabas AR. Low serum pancreatic enzyme levels predict mortality and are associated with malnutrition-inflammation-atherosclerosis syndrome in patients with chronic kidney disease. Int Urol Nephrol. 2013;45:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Shimizu Y, Ichihara K. Sources of variation analysis and derivation of reference intervals for ALP, LDH, and amylase isozymes using sera from the Asian multicenter study on reference values. Clin Chim Acta. 2015;446:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Nakajima K, Oda E. Lower serum amylase in A blood type relative to O blood type in a general Japanese adult population. Clin Chim Acta. 2015;450:181-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 78. | Qureshi MA, Bhatti R. Frequency of ABO blood groups among the diabetes mellitus type 2 patients. J Coll Physicians Surg Pak. 2003;13:453-455. [PubMed] |
| 79. | Fagherazzi G, Gusto G, Clavel-Chapelon F, Balkau B, Bonnet F. ABO and Rhesus blood groups and risk of type 2 diabetes: evidence from the large E3N cohort study. Diabetologia. 2015;58:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 80. | Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Jacobs EJ. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41:986-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 532] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 81. | Iodice S, Maisonneuve P, Botteri E, Sandri MT, Lowenfels AB. ABO blood group and cancer. Eur J Cancer. 2010;46:3345-3350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 82. | Nakao M, Matsuo K, Hosono S, Ogata S, Ito H, Watanabe M, Mizuno N, Iida S, Sato S, Yatabe Y. ABO blood group alleles and the risk of pancreatic cancer in a Japanese population. Cancer Sci. 2011;102:1076-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Kaye WH, Gendall K, Kye C. The role of the central nervous system in the psychoneuroendocrine disturbances of anorexia and bulimia nervosa. Psychiatr Clin North Am. 1998;21:381-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Mehler PS, Krantz MJ, Sachs KV. Treatments of medical complications of anorexia nervosa and bulimia nervosa. J Eat Disord. 2015;3:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Humphries LL, Adams LJ, Eckfeldt JH, Levitt MD, McClain CJ. Hyperamylasemia in patients with eating disorders. Ann Intern Med. 1987;106:50-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Robertson C, Millar H. Hyperamylasemia in bulimia nervosa and hyperemesis gravidarum. Int J Eat Disord. 1999;26:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 87. | Wolfe BE, Jimerson DC, Smith A, Keel PK. Serum amylase in bulimia nervosa and purging disorder: differentiating the association with binge eating versus purging behavior. Physiol Behav. 2011;104:684-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Collen MJ, Ansher AF, Chapman AB, Mackow RC, Lewis JH. Serum amylase in patients with renal insufficiency and renal failure. Am J Gastroenterol. 1990;85:1377-1380. [PubMed] |
| 89. | Kurt Ö, Demirci H, Ozturk K, Kantarcioglu M, Uygun A. Severe serum amylase elevation, with only chronic kidney disease. Ren Fail. 2015;37:915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 90. | Lando HM, Alattar M, Dua AP. Elevated amylase and lipase levels in patients using glucagonlike peptide-1 receptor agonists or dipeptidyl-peptidase-4 inhibitors in the outpatient setting. Endocr Pract. 2012;18:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 91. | Tokuyama H, Kawamura H, Fujimoto M, Kobayashi K, Nieda M, Okazawa T, Takemoto M, Shimada F. A low-grade increase of serum pancreatic exocrine enzyme levels by dipeptidyl peptidase-4 inhibitor in patients with type 2 diabetes. Diabetes Res Clin Pract. 2013;100:e66-e69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Lengyel Z. Report all increases in serum amylase in patients starting incretins. BMJ. 2013;347:f5333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 93. | Layer P, Rizza RA, Zinsmeister AR, Carlson GL, DiMagno EP. Effect of a purified amylase inhibitor on carbohydrate tolerance in normal subjects and patients with diabetes mellitus. Mayo Clin Proc. 1986;61:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Van Gaal L, Mertens I, Ballaux D, Verkade HJ. Modern, new pharmacotherapy for obesity. A gastrointestinal approach. Best Pract Res Clin Gastroenterol. 2004;18:1049-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 95. | Tucci SA, Boyland EJ, Halford JC. The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: a review of current and emerging therapeutic agents. Diabetes Metab Syndr Obes. 2010;3:125-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |