Published online May 15, 2015. doi: 10.4239/wjd.v6.i4.626
Peer-review started: September 4, 2014
First decision: November 14, 2014
Revised: January 30, 2015
Accepted: February 9, 2015
Article in press: February 11, 2015
Published online: May 15, 2015
Processing time: 253 Days and 20.1 Hours
Under normal metabolic conditions insulin stimulates microvascular perfusion (capillary recruitment) of skeletal muscle and subcutaneous adipose tissue and thus increases blood flow mainly after meal ingestion or physical exercise. This helps the delivery of insulin itself but also that of substrates and of other signalling molecules to multiple tissues beds and facilitates glucose disposal and lipid kinetics. This effect is impaired in insulin resistance and type 2 diabetes early in the development of metabolic dysregulation and reflects early-onset endothelial dysfunction. Failure of insulin to increase muscle and adipose tissue blood flow results in decreased glucose handling. In fat depots, a blunted postprandial blood flow response will result in an insufficient suppression of lipolysis and an increased spill over of fatty acids in the circulation, leading to a more pronounced insulin resistant state in skeletal muscle. This defect in blood flow response is apparent even in the prediabetic state, implying that it is a facet of insulin resistance and exists long before overt hyperglycaemia develops. The following review intends to summarize the contribution of blood flow impairment to the development of the atherogenic dysglycemia and dyslipidaemia.
Core tip: Insulin resistance and type 2 diabetes present with diminished glucose transport and disposal in muscles and fat and inadequate inhibition of lipolysis after meal ingestion or during physical exercise. This defect lies mainly in the cellular and subcellular level of insulin action. However, the resistance in the haemodynamic properties of insulin is another facet of type 2 diabetes and the metabolic syndrome. In this review, we intend to summarize the contribution of this impairment to the development of the atherogenic dysglycemia and dyslipidaemia.
- Citation: Lambadiari V, Triantafyllou K, Dimitriadis GD. Insulin action in muscle and adipose tissue in type 2 diabetes: The significance of blood flow. World J Diabetes 2015; 6(4): 626-633
- URL: https://www.wjgnet.com/1948-9358/full/v6/i4/626.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i4.626
The role of insulin in regulating glucose disposal in peripheral tissues, such as skeletal muscle and adipose tissue is well established. In the 50s, when it was discovered that insulin stimulated glucose uptake and disposal into the muscles, this effect was thought to be the most important way by which insulin regulated glycaemia in vivo. When the glucose/fatty acid regulatory cycle was discovered in the 60s a new insight of the way that insulin regulates glucose metabolism was introduced[1]. However, apart from its direct action on cells, insulin is also a vasoactive hormone, and it is now recognized that its vascular and metabolic actions are closely linked. Baron et al[2-4] originally introduced the concept that insulin might control its own access and that of other substrates - like glucose, lipids and several signalling molecules- to peripheral tissues, by increasing blood flow, and that this effect is compromised in states of insulin resistance.
In states of metabolic dysregulation, as in diabetes and obesity, there is deterioration in the cellular effects of insulin in peripheral tissues, which leads to a reduced ability of the latter to stimulate glucose uptake from the skeletal muscle and adipose tissue, as well as to inhibit lipolysis in fat depots[5]. Apart from the defects at the cellular level, the metabolic derangement could also be a result of the inability of insulin to cause vasodilatation and delivery of substrates to peripheral tissues especially in the postprandial period. This could contribute to the progression to type 2 diabetes as well as to the development of atherosclerosis, which is often evident even before overt hyperglycaemia develops[6].
In this review we summarize the current understanding of insulin action on peripheral blood flow and its implications on metabolic impairment both under fasting and postprandial conditions in type 2 diabetes.
In skeletal muscle, insulin promotes the rate of glucose transport and the activities of hexokinase and 6-phosphofructokinase and subsequently the rate of glycolysis. In terms of protein metabolism, insulin increases synthesis and decreases degradation of proteins, in favour of an anabolic process[1]. Insulin also enhances vasodilatation and capillary recruitment, consequently increasing the flow of nutrients in peripheral tissues and especially in skeletal muscle[7]. It acts through traditional insulin receptors on the vascular endothelium to stimulate production of nitric oxide and induce vasodilatation[8]. The endothelial insulin response is mediated through a PI3-kinase pathway, which after several intermediate steps ends up activating endothelial nitric oxide synthase (eNOS)[6].
Blood flow is highly important for the metabolic function of skeletal muscle and under normal conditions increases after meal ingestion and during exercise and a correlation between the rate of insulin stimulated glucose uptake and the extent of vasodilatation seems to exist[9].
Insulin stimulates skeletal muscle glucose disposal and total muscle blood flow in a time- and dose-dependent fashion. In vivo, it enhances nitric oxide synthase-dependent vascular actions, in order to increase total skeletal muscle blood flow and to recruit muscle capillaries (by relaxing resistance and terminal arterioles, respectively). It is speculated that enhancing blood flow in this way on resistance vessels may induce the delivery of glucose and insulin to peripheral tissues and thus contribute to overall glucose disposal.
Capillary blood volume increases when precapillary arterioles dilate, thus increasing the flow to previously unperfused or underperfused areas, and total blood flow to skeletal muscle increases when larger resistance vessels relax[10].
Insulin increases tissue perfusion by augmenting microvasculature and, at normal concentrations, the rise in total muscle blood flow follows 60-90 min later[11,12].
Both haemodynamic effects of insulin, muscle blood flow increase and capillary recruitment seem to be independent of each other. Capillary recruitment occurs earlier in vivo, and at lower doses of insulin[13].
Insulin resistance may correlate to endothelial dysfunction in many ways, including dysregulation of sub-cellular signalling pathways that influence both insulin action and nitric oxide production[14,15].
Subcutaneous adipose tissue represents about 85% of whole body fat stores in subjects with various degrees of adiposity. Its main metabolic role is the storage of triglycerides which derive from energy overflow, and the release of stored lipids when other tissues are in need. Adipose tissue metabolism is under distinct control: usually, when a person consumes a meal, within the first hour postprandially, fat catabolism converts to fat storage, while the opposite happens in the case of physical activity. Adipose tissue interacts with the circulation by providing or drawing triglycerides and non-esterified fatty acids depending on metabolic needs. There are two kinds of triglyceride-rich lipoproteins: (1) chylomicrons, the largest particles, that carry the fat from absorbed nutrients within the intestine; and (2) very-low-density lipoproteins, that carry “endogenous” triglycerides and are released by the liver. Chylomicron- triglycerides are preferably stored within adipose tissue, and the fatty acid composition of adipose tissue (i.e., the kind of fatty acids that form its triglycerides) usually represents the composition of a person’s dietary fat intake, suggesting that adipose tissue triglycerides derive mainly from the ingested fat through diet. However, a proportion of plasma triglycerides are endogenously produced from non-lipid substrates (de novo lipogenesis) in adipose tissue[16-22].
In terms of metabolic regulation, adipose tissue can be divided into central (abdominal) and peripheral (lower body) depots. An unfavourable metabolic profile has been related to central fat accumulation (visceral and subcutaneous, each with distinct metabolic, endocrine and paracrine characteristics and blood flow rates)[23,24].
Adipose tissue regulates its metabolism, at least in part, by increasing its blood flow rate mainly in the early postprandial period[16]. Capillary perfusion is essential for that function. In the case of increased energy demands, as in physical activity, blood flow increases to facilitate the delivery of lipolytic products to peripheral tissues. Furthermore, after meal consumption, it helps the delivery of ingested substrates to fat depots for storage[16]. Adipose tissue blood flow responses are subject to adrenergic stimulation or inhibition. Adrenaline administration stimulates postprandial increases whereas beta-blockers inhibit the latter. Genetic studies in subcutaneous adipose tissue biopsies have identified expression of the type A receptor of A natriuretic peptide and of the synthase of nitric oxide, and have found an association of those with post-challenge blood flow responses[16,25-28].
Lipid kinetics and subcutaneous adipose tissue blood flow alterations are closely linked. More specifically, blood flow rises in response to an increased demand for lipolytic products as energy, or that for cleavage of free fatty acids from the circulation. In euglycaemic subjects with normal weight blood flow peaks within the first hour after a glucose load or a mixed meal. This facilitates the postprandial delivery of energy substrates and insulin to the fat depot, leading to adipose tissue lipoprotein lipase stimulation which stores circulating triglycerides and the suppression of hormone-sensitive lipase, which results in the inhibition of endogenous lipolysis[16,29-31].
On the other hand, visceral adiposity exerts even more unfavourable metabolic actions. Increased visceral fat has been associated with atherogenic dyslipidaemia and the development of atherosclerosis, even in non-diabetic individuals[32]. Although increased abdominal fat is in general positively associated with markers of inflammation and atherosclerosis, visceral fat is more strongly correlated with C-reactive protein, monocyte chemoattractant protein-1, interleukin-6 and isoprostanes independently of total adiposity, indicating a major role in systemic inflammation[33]. Furthermore, visceral fat has been more strongly related to hypertension both in men and women, and provides information towards the latter above BMI and waist circumference. However, subcutaneous adipose tissue is also contributing to vascular dysfunction, possibly through the actions of leptin apart from the presence of insulin resistance[34]. Both adipose tissue beds’ size has been found correlated to adipose tissue blood flow, independently of BMI, leptin or adiponectin concentrations[35].
Insulin provokes microvascular recruitment in skeletal muscle[10]. Impaired muscle blood flow as a facet of insulin resistance in subjects with either dysglycaemia or diabetes is well recognized in the literature. In the early 90s Steinberg et al[36] have shown that obese insulin resistant subjects present with an endothelial dysfunction and during a euglycaemic hyperinsulinaemic clamp they fail to increase endothelium-dependent vasodilation. In these trials, catheterizations of the femoral artery was used to measure the response to an intra-arterial vasodilator stimulus, comparing control to euglycemic-hyperinsulinemic clamp conditions[36].
Thereafter, it was suggested that since insulin exerts its vasodilatory effects through endothelial nitric oxide release, in vivo stopping nitric oxide production could inhibit insulin’s vasoactive actions in skeletal muscle and consequently reduce glucose uptake[37]. Moreover in obese insulin resistant subjects, insulin resistance in skeletal muscle was promoted by the increased endogenous endothelin action[38].
A rat model of insulin resistance has shown that endothelial-dependent vasodilation is blunted, in part due to an unresponsive nitric oxide synthase to insulin, leading to decreased nitric oxide levels in the endothelial cells[39,40].
In type 2 diabetes and other insulin-resistant states, impaired suppression of adipose tissue lipolysis and postprandial hyperglycemia favour non-esterified fatty acid utilization and oxidation and increase glucose uptake from insulin independent tissues (like liver). Dyslipidaemia, usually related to lack of insulin sensitivity, enhances atherosclerosis and triggers inflammation in endothelial cells[41].
In insulin-resistant patients basal blood flow is generally not altered[42-44]. Laakso et al[45], demonstrated that insulin cannot effectively increase muscle blood flow in type 2 diabetic patients, using the combined euglycemic clamp and leg balance techniques during different insulin infusions. They also concluded that impaired insulin-dependent rise in skeletal muscle blood flow can be attributed to the diabetic milieu and not to obesity, in a study of obese diabetic patients[45].
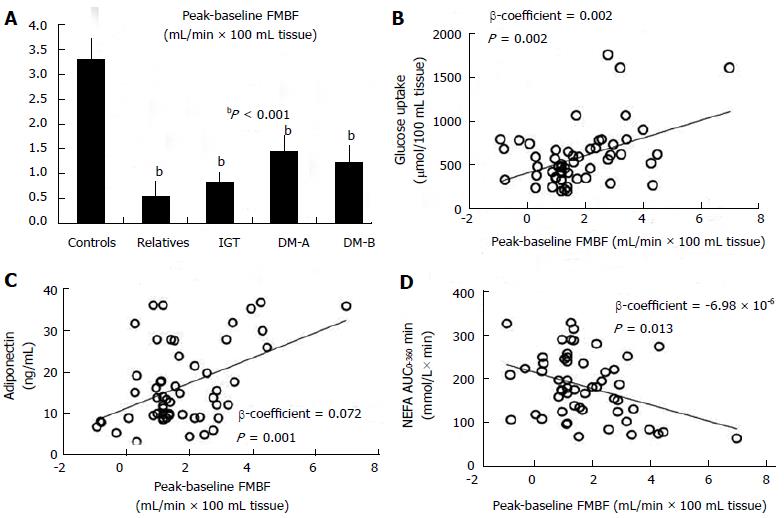
Lambadiari et al[46] studied simultaneously lean subjects with insulin sensitivity varying from normoglycaemic insulin-resistant first-degree relatives of diabetic subjects to prediabetic and diabetic patients with either isolated postprandial hyperglycaemia or overt diabetes[46]. They demonstrated that using a physiological mixed meal as a stimulus, the postprandial augmentation in forearm muscle blood flow is blunted throughout all stages of metabolic impairment compared to controls; this occurs even before overt hyperglycaemia develops. The latter affected glucose disposal in muscle, which was also unresponsive after meal delivery and was also positively correlated to the post-load muscle blood flow differences. Lipid substrates affected blood flow peak as well. Triglyceride levels had a negative impact on blood flow responsiveness in the fed as well as in the fasting period. Post-challenge non-esterified fatty acids levels exhibited a negative effect on blood flow responsiveness, suggesting a possible mechanism for the decrease in muscle glucose clearance after the meal. A lower serum adiponectin level was also seen in the diabetic and the prediabetic insulin-resistant subjects, with the latter being positively related to the decreased postload blood flow rise[46] (Figure 1).
In subjects with morbid obesity postprandial muscle blood flow was also blunted in a study by the same group and this contributed to the decrease in muscle glucose uptake postprandially[47]. The same was observed by the same group in another insulin resistant state, such as hypothyroidism, in which a decreased postprandial blood flow response was coupled with an impairment in muscle glucose uptake[48]. In a study by Magalhães et al[49] administration of metformin to non-obese type 2 diabetic patients increased post-load forearm muscle blood flow and lowered free fatty acids, thus improving glucose oxidation and insulin sensitivity in the muscle bed.
However, there is not universal agreement with the above mentioned results, since numerous studies have failed to reveal a defect in insulin-mediated blood flow in type 2 diabetic patients[42,50]. There is a certain discrepancy since the literature either confirms or not a substantial[45,51], or an unimportant correlation between insulin kinetics, muscle blood flow and glucose disposal[52,53].
Some of these discrepancies may at least partially explained by the different studies populations and by the experimental protocol used. The commonly used clamp technique is not physiological, because these exceptionally high insulin concentrations are not normally present for long after meal consumption. Hence, one could question the physiological significance of such an increase in blood flow rates. A normal stimulus, such as a mixed meal, can provide evidence of a real life metabolic state[46,54]. However, not only the type of meal but the method for the detection of blood flow is important in this evaluation.
In lean insulin-sensitive subjects, abdominal adipose tissue blood flow increases by two- to four-times in response to feeding. The same seems to be true for blood flow in lower body fat depots (thigh) and forearm tissues. Physiologically, adipose tissue blood flow peaks within half to one hour after nutrient ingestion. This rise coincides with plasma insulin peak and the inhibition of lipolysis[23].
By studying obese or diabetic individuals in the 90s, Jansson et al[55,56] detected impairment in adipose tissue blood flow response as a facet of insulin resistance coupled with hypertension and elevated lipolysis products.
Since then, numerous studies have shown that in states of decreased insulin sensitivity, as in “diabesity’’, the postprandial increase in adipose tissue blood flow is reduced[57-60]. Karpe et al[61] showed that the postprandial blood flow rise is associated with insulin sensitivity independently of weight. Moreover, they showed that hyperinsulinaemia affects adipose tissue blood flow indirectly by stimulation of sympathetic activity[61].
Previous reports in healthy subjects by the same research group have demonstrated that nitric oxide determines the actual rate of adipose tissue blood flow, whereas postprandial augmentation of it is mainly under adrenergic regulation in vivo, and that blood flow regulation and lipolysis are co-regulated[25].
Dimitriadis et al[62] showed an altered fasting and postprandial adipose tissue blood flow in all stages of metabolic regulation, from the prediabetic state to clinical diabetes, even in lean first-degree relatives of diabetic patients. This study, using a mixed meal as a stimulus, showed significant association of postprandial adipose tissue blood flow with insulin sensitivity. Basal and post-challenge triglycerides were negatively correlated to the responsiveness of adipose tissue blood flow; the same was true for postprandial non-esterified fatty acids but not for fasting values[62] (Figure 2).
Fatty acid overflow (mainly palmitic acid), a well recognized factor to interfere with insulin sensitivity, causes both cellular and vascular insulin dysfunction[63]. The increased rate of lipolysis in diabetes may result in increased lipid oxidation and a decreased glucose oxidation rate[61,64].
Impairment in blood flow response of adipose tissue has been found in other insulin resistance states. Mitrou et al[47] study in morbidly obese subjects, shows a drop in postprandial adipose tissue blood flow response and in glucose disposal per 100 mL fat tissue. However, glucose fractional extraction from subcutaneous fat depot was unaltered and glucose uptake per total fat mass was increased. Thus, it seems that although an expanded adipose tissue causes insulin resistance, total fat mass provides a buffer for glucose overflow and compensates for insulin resistance.
Diabetic subjects fail to increase adipose tissue blood flow during prolonged exercise of moderate intensity, in combination to the inability to regulate non-esterified fatty acid mobilization and adipose tissue glucose clearance[65]. Exercise augments adipose tissue lipolysis in diabetic patients, but due to an impaired blood flow response, a high proportion of free fatty acids that come from lipolysis cannot be released into the circulation. Visceral glucose release is lower than whole-body glucose utilisation during exercise and post-exercise recovery[66].
The cause of the impairment in postprandial adipose tissue blood flow reactivity in insulin resistance is still obscure. One potential explanation is the downregulation of the adrenergic receptor during chronic sympathetic stimulation in a milieu of long-standing hyperinsulinaemia. Sympathetic nervous system overactivity induces oxidative stress. Increased levels of circulating free oxygen radicals consumes nitric oxide, and inhibits physiological insulin-dependent vasodilatation[23]. Interestingly, the transcription of eNOS and natriuretic peptide receptor-A, which are expressed in adipose tissue and interfere with vasoactive actions, was associated with adipose tissue blood flow responsiveness to feeding. This finding suggests that part of blood flow regulation is at a transcriptional level and it is independent of adiposity[28].
At the bottom line, adipose tissue is an important buffer against the postprandial spill-over of nonesterified fatty acids in the circulation, thus protecting other peripheral tissues. This buffering effect is dysregulated in states of an over-expanded inflammatory, hypoxic adipose tissue, where the postprandial blood flow response is minimized, potentially leading to atherogenic dyslipidaemia[67].
Resistance in the haemodynamic actions of insulin is essential for the development of type 2 diabetes and insulin resistant states as well as their complications, namely cardiovascular disease, the development of which often precedes overt hyperglycaemia and which is the primary cause of mortality within the diabetic population.
Insulin normally stimulates microvascular perfusion (capillary recruitment) of skeletal muscle and subcutaneous adipose tissue and thus increases blood flow mainly after meal ingestion or physical exercise. This effect is impaired in insulin resistance and type 2 diabetes early during metabolic dysregulation development and reflects early-onset vascular dysfunction. Failure of insulin to increase muscle blood flow results in the inability to regulate its own delivery and that of other substrates and hormones and consequently to a decrease in glucose disposal. In fat depots blood flow is closely related to triglyceride clearance and non-esterified fatty acid kinetics. Therefore, we may speculate that dysregulation of post-challenge blood flow responsiveness in skeletal muscle and adipose tissue may together underlie some of the detrimental aspects of insulin resistance.
| 1. | Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 2011;93 Suppl 1:S52-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 2. | Baron AD. Hemodynamic actions of insulin. Am J Physiol. 1994;267:E187-E202. [PubMed] |
| 3. | Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. J Clin Endocrinol Metab. 1991;73:637-643. [PubMed] |
| 4. | Baron AD, Steinberg H, Brechtel G, Johnson A. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol. 1994;266:E248-E253. [PubMed] |
| 5. | Baron AD. Insulin resistance and vascular function. J Diabetes Complications. 2002;16:92-102. [PubMed] |
| 6. | Mather KJ. The vascular endothelium in diabetes--a therapeutic target? Rev Endocr Metab Disord. 2013;14:87-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Newman JM, Ross RM, Richards SM, Clark MG, Rattigan S. Insulin and contraction increase nutritive blood flow in rat muscle in vivo determined by microdialysis of L-[14C]glucose. J Physiol. 2007;585:217-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep. 2003;3:279-288. [PubMed] |
| 9. | Baron AD, Brechtel-Hook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol. 1996;271:E1067-E1072. [PubMed] |
| 10. | Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 320] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Vincent MA, Dawson D, Clark AD, Lindner J-, Rattigan S, Clark MG, Barrett EJ. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes. 2002;51:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Eggleston EM, Jahn LA, Barrett EJ. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes. 2007;56:2958-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Rattigan S, Zhang L, Mahajan H, Kolka CM, Richards SM, Clark MG. Factors influencing the hemodynamic and metabolic effects of insulin in muscle. Curr Diabetes Rev. 2006;2:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2168] [Cited by in RCA: 2305] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 15. | Frayn KN. Fat as a fuel: emerging understanding of the adipose tissue-skeletal muscle axis. Acta Physiol (Oxf). 2010;199:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Frayn KN, Karpe F. Regulation of human subcutaneous adipose tissue blood flow. Int J Obes (Lond). 2014;38:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Bickerton AS, Roberts R, Fielding BA, Hodson L, Blaak EE, Wagenmakers AJ, Gilbert M, Karpe F, Frayn KN. Preferential uptake of dietary Fatty acids in adipose tissue and muscle in the postprandial period. Diabetes. 2007;56:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 1045] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 19. | Strawford A, Antelo F, Christiansen M, Hellerstein MK. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am J Physiol Endocrinol Metab. 2004;286:E577-E588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 244] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | Pinnick KE, Neville MJ, Fielding BA, Frayn KN, Karpe F, Hodson L. Gluteofemoral adipose tissue plays a major role in production of the lipokine palmitoleate in humans. Diabetes. 2012;61:1399-1403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Collins JM, Neville MJ, Pinnick KE, Hodson L, Ruyter B, van Dijk TH, Reijngoud DJ, Fielding MD, Frayn KN. De novo lipogenesis in the differentiating human adipocyte can provide all fatty acids necessary for maturation. J Lipid Res. 2011;52:1683-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Frayn KN, Tan GD, Karpe F. Adipose tissue: a key target for diabetes pathophysiology and treatment? Horm Metab Res. 2007;39:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Sotornik R, Brassard P, Martin E, Yale P, Carpentier AC, Ardilouze JL. Update on adipose tissue blood flow regulation. Am J Physiol Endocrinol Metab. 2012;302:E1157-E1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941-E948. [PubMed] |
| 25. | Ardilouze JL, Fielding BA, Currie JM, Frayn KN, Karpe F. Nitric oxide and beta-adrenergic stimulation are major regulators of preprandial and postprandial subcutaneous adipose tissue blood flow in humans. Circulation. 2004;109:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Hjemdahl P, Linde B. Influence of circulating NE and Epi on adipose tissue vascular resistance and lipolysis in humans. Am J Physiol. 1983;245:H447-H452. [PubMed] |
| 27. | Patel JN, Eisenhofer G, Coppack SW, Miles JM. Norepinephrine spillover in forearm and subcutaneous adipose tissue before and after eating. J Clin Endocrinol Metab. 1999;84:2815-2819. [PubMed] |
| 28. | Perez-Matute P, Neville MJ, Tan GD, Frayn KN, Karpe F. Transcriptional control of human adipose tissue blood flow. Obesity (Silver Spring). 2009;17:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Coppack SW, Evans RD, Fisher RM, Frayn KN, Gibbons GF, Humphreys SM, Kirk ML, Potts JL, Hockaday TD. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism. 1992;41:264-272. [PubMed] |
| 30. | Summers LK, Samra JS, Humphreys SM, Morris RJ, Frayn KN. Subcutaneous abdominal adipose tissue blood flow: variation within and between subjects and relationship to obesity. Clin Sci (Lond). 1996;91:679-683. [PubMed] |
| 31. | Karpe F, Olivecrona T, Olivecrona G, Samra JS, Summers LK, Humphreys SM, Frayn KN. Lipoprotein lipase transport in plasma: role of muscle and adipose tissues in regulation of plasma lipoprotein lipase concentrations. J Lipid Res. 1998;39:2387-2393. [PubMed] |
| 32. | Wang Y, Ma X, Zhou M, Zong W, Zhang L, Hao Y, Zhu J, Xiao Y, Li D, Bao Y. Contribution of visceral fat accumulation to carotid intima-media thickness in a Chinese population. Int J Obes (Lond). 2012;36:1203-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Meigs JB, Lipinska I, Kathiresan S. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234-1241. [PubMed] |
| 34. | Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39-48. [PubMed] |
| 35. | Andersson J, Karpe F, Sjöström LG, Riklund K, Söderberg S, Olsson T. Association of adipose tissue blood flow with fat depot sizes and adipokines in women. Int J Obes (Lond). 2012;36:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1214] [Article Influence: 40.5] [Reference Citation Analysis (11)] |
| 37. | Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Lteif A, Vaishnava P, Baron AD, Mather KJ. Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes. 2007;56:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123-E129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 240] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 40. | Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Invest. 2013;123:1003-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev. 2006;22:423-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 335] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 42. | Dela F, Larsen JJ, Mikines KJ, Galbo H. Normal effect of insulin to stimulate leg blood flow in NIDDM. Diabetes. 1995;44:221-226. [PubMed] |
| 43. | Dela F, Larsen JJ, Mikines KJ, Ploug T, Petersen LN, Galbo H. Insulin-stimulated muscle glucose clearance in patients with NIDDM. Effects of one-legged physical training. Diabetes. 1995;44:1010-1020. [PubMed] |
| 44. | Francesconi M, Koizar C, Wascher TC. Postprandial impairment of resistance vessel function in insulin treated patients with diabetes mellitus type-2. Clin Physiol. 2001;21:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes. 1992;41:1076-1083. [PubMed] |
| 46. | Lambadiari V, Mitrou P, Maratou E, Raptis A, Raptis SA, Dimitriadis G. Increases in muscle blood flow after a mixed meal are impaired at all stages of type 2 diabetes. Clin Endocrinol (Oxf). 2012;76:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Mitrou P, Boutati E, Lambadiari V, Maratou E, Papakonstantinou A, Komesidou V, Sidossis L, Tountas N, Katsilambros N, Economopoulos T. Rates of glucose uptake in adipose tissue and muscle in vivo after a mixed meal in women with morbid obesity. J Clin Endocrinol Metab. 2009;94:2958-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Dimitriadis G, Mitrou P, Lambadiari V, Boutati E, Maratou E, Panagiotakos DB, Koukkou E, Tzanela M, Thalassinos N, Raptis SA. Insulin action in adipose tissue and muscle in hypothyroidism. J Clin Endocrinol Metab. 2006;91:4930-4937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 49. | Magalhães FO, Gouveia LM, Torquato MT, Paccola GM, Piccinato CE, Foss MC. Metformin increases blood flow and forearm glucose uptake in a group of non-obese type 2 diabetes patients. Horm Metab Res. 2006;38:513-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Tack CJ, Smits P, Willemsen JJ, Lenders JW, Thien T, Lutterman JA. Effects of insulin on vascular tone and sympathetic nervous system in NIDDM. Diabetes. 1996;45:15-22. [PubMed] |
| 51. | Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990;85:1844-1852. [PubMed] |
| 52. | Bonadonna RC, Saccomani MP, Del Prato S, Bonora E, DeFronzo RA, Cobelli C. Role of tissue-specific blood flow and tissue recruitment in insulin-mediated glucose uptake of human skeletal muscle. Circulation. 1998;98:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Ferrannini E, Taddei S, Santoro D, Natali A, Boni C, Del Chiaro D, Buzzigoli G. Independent stimulation of glucose metabolism and Na+-K+ exchange by insulin in the human forearm. Am J Physiol. 1988;255:E953-E958. [PubMed] |
| 54. | Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab. 2003;284:E241-E258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 249] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 55. | Jansson PA, Larsson A, Lönnroth PN. Relationship between blood pressure, metabolic variables and blood flow in obese subjects with or without non-insulin-dependent diabetes mellitus. Eur J Clin Invest. 1998;28:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Jansson PA, Larsson A, Smith U, Lönnroth P. Glycerol production in subcutaneous adipose tissue in lean and obese humans. J Clin Invest. 1992;89:1610-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 140] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Coppack SW, Fisher RM, Humphreys SM, Clark ML, Pointon JJ, Frayn KN. Carbohydrate metabolism in insulin resistance: glucose uptake and lactate production by adipose and forearm tissues in vivo before and after a mixed meal. Clin Sci (Lond). 1996;90:409-415. [PubMed] |
| 58. | Dimitriadis G, Boutati E, Lambadiari V, Mitrou P, Maratou E, Brunel P, Raptis SA. Restoration of early insulin secretion after a meal in type 2 diabetes: effects on lipid and glucose metabolism. Eur J Clin Invest. 2004;34:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Tobin L, Simonsen L, Bülow J. The dynamics of the microcirculation in the subcutaneous adipose tissue is impaired in the postprandial state in type 2 diabetes. Clin Physiol Funct Imaging. 2011;31:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Ardilouze JL, Sotorník R, Dennis LA, Fielding BA, Frayn KN, Karpe F. Failure to increase postprandial blood flow in subcutaneous adipose tissue is associated with tissue resistance to adrenergic stimulation. Diabetes Metab. 2012;38:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Karpe F, Fielding BA, Ilic V, Macdonald IA, Summers LK, Frayn KN. Impaired postprandial adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes. 2002;51:2467-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 62. | Dimitriadis G, Lambadiari V, Mitrou P, Maratou E, Boutati E, Panagiotakos DB, Economopoulos T, Raptis SA. Impaired postprandial blood flow in adipose tissue may be an early marker of insulin resistance in type 2 diabetes. Diabetes Care. 2007;30:3128-3130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 280] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 64. | Summers LK, Samra JS, Frayn KN. Impaired postprandial tissue regulation of blood flow in insulin resistance: a determinant of cardiovascular risk? Atherosclerosis. 1999;147:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Tobin L, Simonsen L, Galbo H, Bülow J. Vascular and metabolic effects of adrenaline in adipose tissue in type 2 diabetes. Nutr Diabetes. 2012;2:e46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Simonsen L, Henriksen O, Enevoldsen LH, Bülow J. The effect of exercise on regional adipose tissue and splanchnic lipid metabolism in overweight type 2 diabetic subjects. Diabetologia. 2004;47:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Summers LK. Adipose tissue metabolism, diabetes and vascular disease--lessons from in vivo studies. Diab Vasc Dis Res. 2006;3:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Magalhaes FO S- Editor: Ji FF L- Editor: A E- Editor: Wu HL