INTRODUCTION
The notion that diabetes mellitus (DM) impacts brain function and structure is not new. The theory arose for the first time in 1922[1], and since then the idea has intrigued many investigators, especially in regard to its effect on quality of life in young children and adolescents.
Although it is well-known that type 1 DM (T1DM) is associated with neuro-cognitive impairments, there are still some open debates regarding which abilities are impaired, their appearance according to disease acquisition, and their underling mechanisms. Understanding the full impact of T1DM on the brain, and glycemic control in particular, is critical[2], especially in children and adolescents, a key period for development of brain matter as well as cognitive functions[3,4]. One should remember that this is the most challenging period of T1DM management due to the demanding and exhausting therapeutic self-care guidelines of this chronic disorder, especially in this pediatric and youth age group[5].
The current review aims to explore the reported neuro-behavioral alterations in children and adolescences from a novel point of view. The review focuses on cognitive and behavioral impairments according to duration of T1DM and its timeline, and according to modifiable disease related risk parameters. We do not present data regarding the physiological and tissue or cell related pathogenic processes, which are available elsewhere. Our aim is to summarize for clinicians the historical and novel data available, as compared to normal cognitive and behavioral age related development, in order to direct, modify and individualize the specific clinical goals in order to encourage both better outcomes in day-to-day life, as well as to increase long-term outcome, with a focus on age and developmental stage.
We wish to clarify: what is known about the disorder, what is its association to modifiable diabetes related metabolic aspects, what clinicians should pay attention to and prevent by clinical care and what is needed in future research.
BEHAVIORAL ALTERATIONS
Adolescence is a crucial time for the formation of healthy and responsible habits, yet it is an age characterized by an increasing tendency for risk-taking behavior. This is especially notable for adolescents with T1DM, who may require long-term healthcare support in creating healthy and responsible habits[3].
Studies show that mastery over one’s environment, an important component of resiliency, is related to life satisfaction, quality of life and improved daily functioning, including school functioning[6,7]. Better glycemic control is related to resiliency and quality of life, which are both associated with better school functioning in youth with T1DM[6].
Importantly, children and adolescents with T1DM show lower levels of life satisfaction than control populations[8]. Life satisfaction is often discussed in the context of quality of life, which in children and adolescents with T1DM is associated with better glycemic control parameters, assessed as lower HbA1c (Glycated hemoglobin) levels[9].
Unfortunately, poor health behaviors, such as drinking alcohol and smoking, are equally high among youth with T1DM as compared to their healthy peer counterparts, despite the increased risk associated with such behaviors in T1DM[8].
Self-care
Older studies found that young adults with T1DM have a higher tendency to live at home and study at local colleges[10,11]; however, more recent research no longer shows such differences between those with T1DM and the typical population[12]. This lack of a difference in more recent research may be explained by the progress in T1DM education, self-care empowerment and technological progress in T1DM management and glycemic control. These advances enable youth with T1DM to more flexible self-care and better glycemic control outcomes[13].
School
Teachers often have limited knowledge about diabetes, leading to confusion in many areas associated with the disorder, particularly in regard to physical education. This lack of knowledge may have great implications on the child and their disorder. Teachers may wrongly exclude diabetic children from activities in which they are actually able to participate, and likewise children may get away with using their illness to avoid participation in activities that are in fact not harmful, and are possibly even favorable to their health. This may lead to skipping physical activity, which can in turn cause isolation among children with T1DM[14]. Further, increased absences from school or missing class time in order to check glucose levels[9,13,15] can contribute to lower academic achievement among students with T1DM[7,13,15,16]. Importantly, overall it was found that children with fair to good glycemic control earn better grades in school as compared to children with poor glycemic control[17]. The mechanism involved is not yet discerned. Both direct effects of glycemic control on the ability to learn may operate here, in addition to indirect paths, such as by affecting sleep-wake rhythms. School grades and school absences of youth with T1DM were found to be related to sleep duration and those, in turn, were found to be related to increased burden associated with diabetes management[7]. Hence, a special focus on sleep quality is warranted for more optimal care of T1MD.
Physical activity
Results are inconsistent in regard to physical activity levels in those with and without T1DM. Few studies found that both groups showed similar levels of self-reported physical activity, while others found lower physical activity among youth with T1DM as compared to controls[18], and one research reported higher activity among diabetic youth[19]. It should be emphasized that physical activity has many health benefits for T1DM patients, in that it is known to improve their physical fitness, strength and overall well-being[18,20-22], while decreasing long-term health deficiencies, such as vascular complications, for which T1DM patients are particularly vulnerable[23].
Sleep
There are several reports regarding associations between sleep patterns and T1DM. Restricted sleep may contribute to reduced peripheral insulin sensitivity in T1DM[24,25]. Children with T1DM experience ventilatory dysfunction during sleep, which is related to diabetes duration and to glycemic control during sleep[26]. Adolescences with T1DM have more awakenings due to glucose fluctuations[27] and they do not sleep as deeply as healthy population[7,28]. Further, there may be increased sleep disturbances among adolescent males with T1DM as compared to females and healthy peers[8]. In addition, children with T1DM are known to experience longer and more frequent apnea (sleep disordered breathing) events, increased awakening from night sleep, more daytime sleepiness, decrease in total sleep time, decreased sleep efficiency and increased sleep latency, as compared to healthy children[27]. Those with T1DM spend slightly less time in slow-wave sleep during the first half of the night and report less restorative sleep than their healthy peers as well[25]. Based on the reported influence that T1DM may have on sleep, glycemic variation and poor glycemic control were associated with sleep-wake cycles in T1DM patients[29], and associations between sleep loss and high-caloric intake as seen in Western lifestyles may reinforce this circle given that glycemic alterations during the night may affect the waking phase of the sleep-wake cycle[29]. Youth with T1DM who spend less time in slow-wave sleep have higher average daily glucose values, higher HbA1c levels and more frequent hyperglycemia occurrences. More time in fast-wave sleep was associated with parental reporting of sleepiness, depressive moods, emotional and behavioral difficulties, lower grades in school and lower overall quality of life[28].
Healthier life styles, which include proper quality and duration of sleep, may improve metabolic control, which in turn will further improve sleep quality. This suggests that proper diagnosis and treatment for sleep disorders among the T1DM population is paramount to improving outcome for the disorder.
Mood
During adolescents, particularly in patients who are challenged by school’s academic and social demands symptoms of depression arise more frequently, these symptoms may lead to yet poorer glycemic control, and thus increase complications associated with diabetes[30]. Youth with T1DM report more mental health symptoms as compared to healthy peers[6]. This includes increased depression and more anxiety as compared to controls, although the differences seem to be smaller in more recent studies[31]. Hanna et al[32] reported that depressive symptoms among adolescents with T1DM are associated with diabetes related weight control behavior. Depressive symptoms were found to be a predictor for poorer diabetic management and glycemic control among adolescences with T1DM, along with increased age, longer durations of diabetes, insulin via injections and diabetic specific family conflict[33]. Further research found that depression and anxiety are associated with poorer glycemic control and more long term complications, which can apparently be explained by the negative impact these symptoms have on the capacity to follow diabetes treatment routines[31,34,35].
Importantly, better parental relationships are associated with fewer depressive symptoms in emerging adulthood in this population[36], and increased family support among youth with diabetes is related to better glycemic control[37]. Mood and parental relationships then play an important role in good glycemic control, which can predict less diabetes related long term damages. This underscores the importance of addressing family relations early during T1DM diagnosis and in management education.
Disturbed eating behavior
In considering family relations for glycemic control in T1DM, familial eating habits and familial management of gender specific maturational issues should be addressed. Importantly in this regard are the findings that adolescent females with T1DM tend to develop eating disorders twice as often as those without the disorder, and eating disorders are associated with poorer glycemic control[38]. Therefore, disturbed eating behavior is another relevant aspect that has strong implications for diabetes related complications in T1DM[39].
COGNITIVE ALTERATIONS
Taking together the physiological, psychological and academic issues reviewed above in T1DM, it is important to explore the cognitive alterations that may account for some of these symptoms. Many studies have reported cognitive impairments in people with T1DM, including children and adolescents. In adults, deficits in cognitive abilities are seen mostly in the domains of general intelligence, psychomotor speed and mental flexibility. In children, lower overall IQ scores were found in those with T1DM as compared to controls, and more noticeable difference were found in children with early onset diabetes (usually under 7 years old)[40]. In addition, multiple studies have reported that children with T1DM show difficulties in response time, abstract reasoning, cognitive flexibility and verbal memory, as compared to controls; however, the differences reported are fairly small and inconsistent with borderline significance[4,13,41-50].
The progression of these deficits when no other microvascular complications are present is generally present yet slow[51]. Cognitive impairments may be seen within two years of diagnosis[52], while after six years after diabetes onset, measures of intelligence, attention, processing speed, long term memory, executive function and self-monitoring were found to be lower than those of healthy peers, with stronger effects in attention, processing speed and executive function in children who were younger than 4 at disease onset[53].
Twelve years after diagnosis, youth with T1DM showed impaired performance on working memory, compared to healthy controls. Early diabetes onset was associated with poorer attention, learning and mental efficiency. This has been thought to be related to both hypoglycemic and hyperglycemic events: Hypoglycemic events were associated with impaired learning and memory and slower processing speed, and hyperglycemia was associated with impaired working memory[54]. Yet results are not consistent: In another study on preschoolers, T1DM patients showed no difference in neurocognitive performance as compared to healthy controls, however, poor glycemic control was associated with lower cognitive abilities, slower fine motor speed and lower receptive language scores[55]. Poor glycemic control in children 4-10 years old was associated with lower verbal comprehension scores, and a history of hypoglycemic seizures was associated with lower processing speed, lower full scale IQ score, impaired working memory and perceptual reasoning[56]. A recent study on T1DM children from the same age group assessed the influence of hypoglycemic as well as hyperglycemic excursions on cognitive functioning, demonstrating a tendency for lower general IQ scores and executive functions compared to healthy peers[57]. It may be the case that the presence of several risk factors rather than one or none is associated with poorer cognitive performance that may compromise learning and memory and executive functions in T1DM[54].
Learning and memory
Diabetes effect on memory and learning was first raised in 1922 by Miles et al[1], who reported that people with diabetes complained of memory loss and attention difficulties. Later research found that early onset of T1DM was associated with verbal and visual learning and memory skills[46], verbal and nonverbal intelligence, attention and psychomotor skills[58]. In a recent study chronic hyperglycemia was associated with learning and memory in children[57]. Further, one research found that in boys with T1DM, lower verbal intelligence was associated with poor glycemic control; however, most studies found no gender differences in neurocognitive abilities[59].
Memory, especially working memory, is influenced by multiple risk factors[60,61] including glycemic excursions, but by itself this was not proven to cause long term damage in T1DM, yet early diabetes onset may be a relevant additional risk factor in this respect[45,62].
Executive functions
Early onset T1DM is associated with deficits in executive functioning[46,58], which include the ability to initiate, plan, consolidate and sustain problem solving in working memory, to control emotions and behavior and to modify a cognitive set through proper inhibition control[63]. The integrity of executive functions may be of particular importance for management of T1DM: People with T1DM have many daily tasks that must be organized, including timely insulin administration, blood glucose monitoring and regulating dietary intake[64]. This suggests that executive functions are critically important in adolescents with T1DM during a time in which diabetes self-management must be learned and maintained. In fact, a relation was found between executive functioning and adherence to a self-management schedule in adolescents with T1DM[64,65]. A mild trend was recently noticed in young children with T1DM, such that lower executive function was associated with hyperglycemia, a deficit that becomes more apparent and more debilitating in older children or in those with longer durations of the disorder[57]. Further, impaired decision making was found to be associated with T1DM in several studies, and was associated with comorbid depression, cognitive deficits and hypoglycemia unawareness[59,66,67]. Impaired decision making may be related to white-matter microstructural deficits that were reported on neuroimaging studies in youth with T1DM[66]. This cognitive disadvantage may cause poorer glycemic control, and thus elicit more brain alterations.
BRAIN STRUCTURE ALTERATIONS
Neuroplasticity is the ability of the brain to change its structure and function due to environmental changes. Deficiencies in brain trajectories involved in neuroplasticity were reported among T1DM, including hyperglycemia and hyper insulinemia[68]. Yet, the findings in this regard do not yet offer a coherent framework.
Consistent findings in neuroimaging research show structural changes, especially in cortical grey matter[69]; however, very few studies have investigated participants younger than 20 years old[70]. The few that exist show that brain volume alterations are detectable already in childhood[70-72] and that these alterations have long term influences into adulthood[51]. On the other hand, there is a consensus that some reported atrophic changes are short term and may be related to glucose excursion[70]. For example, early results from a magnetic resonance imaging (MRI) study found that higher rates of ventricular atrophy and hippocampal white matter lesions were correlated with early diabetes onset[62], and larger hippocampal volumes were associated with recurrent severe hypoglycemia[73]. Future studies may explore a potential vicious circle between poor glycemic control in this population, learning and memory deficits and these brain structural changes. In this regard, it is important to consider both grey and white matter volume changes.
Grey matter volume
Smaller grey matter volume in the left superior temporal region and in the thalamus were reported among youth with T1DM, as well as among children ages 4-10, as compared to healthy controls[56,74,75]. Those findings in both reports were associated with a history of severe hypoglycemic events. Smaller grey matter volume in the right cuneus and precuneus was also reported in T1DM patients, but associated with greater exposure to hyperglycemia[74]. The decreased grey matter in those areas was also associated with high rest activity and dramatic decrease in brain activity during goal-oriented tasks[70]. No other findings dealing with children and adolescents with T1DM support these associations to grey matter volume, however studies in adults showed similar findings[70]. Smaller grey matter volume in bilateral temporal-occipital and cerebellar regions, and larger grey matter volume in left inferior prefrontal, insula and temporal pole regions, associated with hyperglycemia, were seen in young children (mean age 7 years old) with early onset of T1DM[71]. Similarly, higher HbA1c was associated overall decrease in grey matter volume[76].
White matter volume
Perantie et al[74] reported that smaller white matter volume in the right posterior parietal region is associated with greater exposure to hyperglycemia in T1DM patients, and that distinct decrease in white mater volume, especially in the occipito-parietal cortex, is associated with sever hypoglycemia[76]. Compared to healthy controls, young children between 4-10 years of age with T1DM who experienced hypoglycemic seizures showed significantly altered age-related white matter development (amygdala and hippocampus as well) and smaller white matter volumes[56].
It is obvious that findings regarding T1DM, glycemic control and glucose excursions and their association to white and grey brain matter regions, are inconsistent and rely on few reports. In order to clear up these inconsistencies, further studies are required regarding the clinical and neurocognitive impact of such findings, and their cause. Whether there is any long term effect of severe hypoglycemia or of poor glycemic control on the developing brain, and whether the effect is subtle, as suggested by Arbelaez et al[70], should be clarified with further research. These different findings raise new questions regarding the possible explanations of increase or decrease in brain volume, the processes they may reflect as a function of age, gender, and severity, and the impact they may have on the lives and well-being of young patients with diabetes, in order to improve management at the various age groups[77]. Some of these disparities concerning structural changes at different ages and glycemic control may be resolved in the future by adding brain properties into the model.
BRAIN PROPERTIES
White matter integrity
Barnea-Goraly et al[78] recently suggested that differences in fibers integrity and radial diffusivity are negatively associated with age of T1DM onset. Yet little is still known with regard to young patients.
Middle-aged adults with T1DM showed a decrease in white matter integrity of posterior parietal region[51]. In children with T1DM, microstructural white matter integrity changes were seen in the thalamus, hippocampus and superior parietal regions[72]. White matter microstructural changes were more apparent in children with higher HbA1c. White matter microstructural changes manifested especially in the frontal and temporal regions, including lower axial diffusivity values in diabetic children compared to controls, and in higher radial diffusivity among those who had higher HbA1c[79]. These finding support the association between poor glycemic control and demyelination and gliosis in frontal and temporal lobes[80]. Lower axial diffusivities involving many white matter trajectories in all cortical lobes were observed in T1DM children as compared to controls. These white matter integrity alterations were associated with hyperglycemia and with cognitive impairments. White matter integrity alterations in the superior parietal and particularly in the precuneus and cuneus region, and decreased density in the hippocampus were associated with hyperglycemia[72].
Connectivity
Alterations in neural networks were correlated with cognitive functions, such as with attention and memory[81] and with mental illness, both of which are known to be associated with T1DM[68]. In adults, abnormalities in functional magnetic fields and in the brain neural connectivity were detected in individuals with T1DM using magnetic encephalography (MEG)[69]. Several studies employing various modalities and methodologies [electroencephalography (EEG), functional MRI (fMRI), MEG] agree on the overall influence that T1DM has on the brain’s functional connectivity[68,82-84]. These researches support the hypothesis that brain connectivity is altered due to diabetes, but with respect to affected brain regions, the results vary substantially[51]. Additionally, these findings have not yet been replicated on children[70]. The influence of hypoglycemia, glucose excursions or overall glycemic control on those findings requires further research.
BRAIN ACTIVITY ALTERATIONS
EEG differences have been seen between individuals with T1DM and healthy controls, including loss of high frequency activity in temporal, frontal and occipital regions and lower frequencies overall. These changes were attributed to the metabolic disturbances caused by a history of prolonged diabetes and past severe hypoglycemic events[85-89]. However, most studies did not assess simultaneous glucose concentration, thus relation to hyperglycemic excursions could not be delineated, as well as duration of the disorder. Transient changes in electrical activity in various brain regions were reported during hyperglycemia during wakefulness and sleep among youth with T1DM. Glucose excursions above 280 mg/dL during wakefulness was associated with a decrease in the power of high frequency bands (α, β, and γ), and increase in the power of low frequency bands (δ and θ) in the EEG from central and occipital regions. Glucose concentration > 200 mg/dL during sleep was associated with increased power in high frequency bands in the EEG in frontal and central areas and more low frequencies generally. However, the clinical neurocognitive relevance of those findings is still not clear[90].
T1DM RELATED RISK FACTORS
Glycemic control
Daily hypoglycemic and hyperglycemic excursions are frequent among T1DM patients despite efforts to keep glucose concentration within a narrow range, especially in the pediatric and adolescent population. Despite efforts to maintain strict intensive management, including dietary restriction, multiple daily glucose measurements and insulin injections in order to prevent glucose excursions in daily life, glucose concentration in children and young adolescents is more frequently in the hyperglycemic range[91]. Possible effects of those excursions and their length on cognitive functions, brain activity and brain structure have mostly been examined in adults; yet, their effects on young children and adolescent brains may be critical. This age is a common period for marked developmental changes in the brain and in the maturation of cognitive functions, making the brain more susceptible to an array of pathogeneses, including metabolic instability[3,4].
Hypoglycemic events
The anxiety associated with severe hypoglycemia is a major barrier in optimizing glycemic control, yet, a recent ten-year longitudinal study testing 1770 participants found that poor glycemic control was not associated with an increased risk for severe hypoglycemia[92]. This is an important factor, since frequent mild hypoglycemic events are related to impaired cognitive functions, including abstract reasoning, motor responses, processing speeds, selective attention and behavioral inhibition[40] all critical for problem solving, academic and social achievements and to well-being. Early studies reported that severe hypoglycemia is a primary cause of neurocognitive impairments[42,93,94], but this was not supported by two meta-analyses that investigated poor glycemic control as a potential risk factor[16,46]. Unfortunately, hyperglycemic and hypoglycemic events may be convolutedly intertwined, which makes it complicated to rule out severe hypoglycemia’s role in cognitive impairments[74].
Hyperglycemic excursions
Poor glycemic control, defined by high values of HbA1c, is often referred to as chronic hyperglycemia[70]. Chronic hyperglycemia is associated with negative effects on memory[95-97], and with lower estimated verbal intelligence[49] in children with T1DM. The effects of chronic hyperglycemia on young children include lower cognitive abilities, slower fine motor speed and lower receptive language scores[55]. Hence, hyperglycemia is a major risk factor for cognitive decrements.
Age of diabetes onset
A diagnosis of T1DM in the first 4-7 years of life appears to be a major risk factor for significant clinical neurocognitive deficiencies[62]. Both severe hypoglycemia and chronic hyperglycemia may impair cognitive abilities at this age range[98].
Specifically, early onset diabetes, particularly before the age of 5, is associated with a significant reduction in IQ scores, slower motor speed, visuospatial processing deficits, selective attention, verbal memory deficits and executive function deficits. Not all domains of functioning were impaired in children with early onset diabetes, and different studies showed contradicting outcomes regarding the impact of early diabetes onset[49,94]. From a neuro-structural perspective early onset (5-9 years old) was associated with bilateral grey matter decrease in the cerebellum and occipital regions and with grey matter increase in left insula, inferior frontal and temporal poles[71]. The meaning of these findings has yet to be investigated in particularly with regard to implications on cognitive function and on management.
DISCUSSION
Neuro-behavioral alterations and cognitive deficits associated with T1DM may be found in children and in adolescence soon after diabetes onset, as well as after long standing disease duration. However, its reported frequency and severity differ with age, T1DM duration and reported timeline according to medical therapeutic development. These clinical alterations seem to be correlated with brain changes (i.e., structural and white matter integrity findings), and were found to be associated with diabetes metabolic consequences. By reviewing the recent literature we can emphasize some ideas and insights to help us understand the known findings that may be associated with T1DM, and to suggest future research goals.
Looking at the history of the disorder, less pervasive and behavioral findings are reported in children and adolescents with T1DM in more recent research. This is highly promising given the rise in affected children, and may be a result of better care, better education, and better health technologies that gradually became available, together with improved glycemic control management plans.
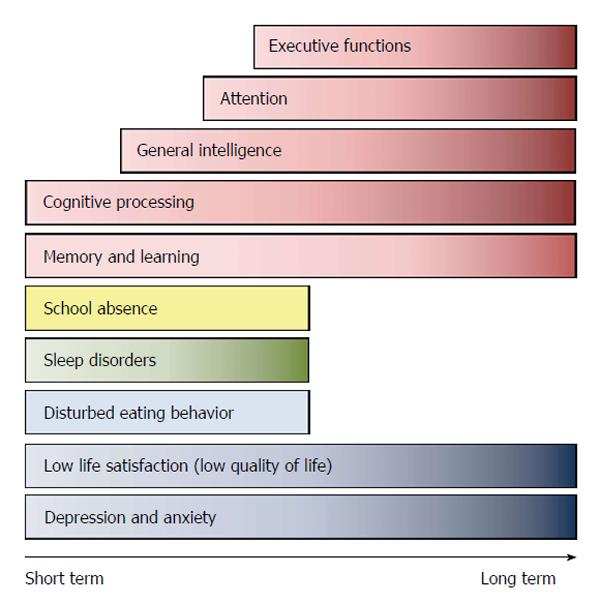
Overall, findings point to the notion that T1DM is associated with interconnected behavioral alterations and glycemic control pathologies, a timeline summarizing core finding is presented in Figure 1.
Figure 1 Short and long term behavioral and cognitive reported alterations following type 1 diabetes mellitus onset in children and adolescents according to type 1 diabetes mellitus duration.
Figure is general and based on data retrieved from published reports as detailed in text.
In the cognitive realm T1DM seems to be associated with impaired memory and learning, slow cognitive processing, reduced general intelligence and impaired attention and executive functions.
As for the psychological - emotional domain, T1DM seems to be tied to depression, anxiety and low life satisfaction, which may contribute to poorer glycemic control, but may also be exacerbated by it. Glucose concentration excursions may impair the length and efficiency of night sleep, affecting school achievements and physical activity, which are also in turn associated with yet poor glycemic control. Poor glycemic control may also affect mood, sleep, physical activity and grades in school, which once again circularly lead to poorer glycemic control, more depression and anxiety, cognitive impairments and eventually diabetes complications. The good news is that good glycemic control may balance self-control, mood and physical shape and moderate this escalating deterioration to some degree.
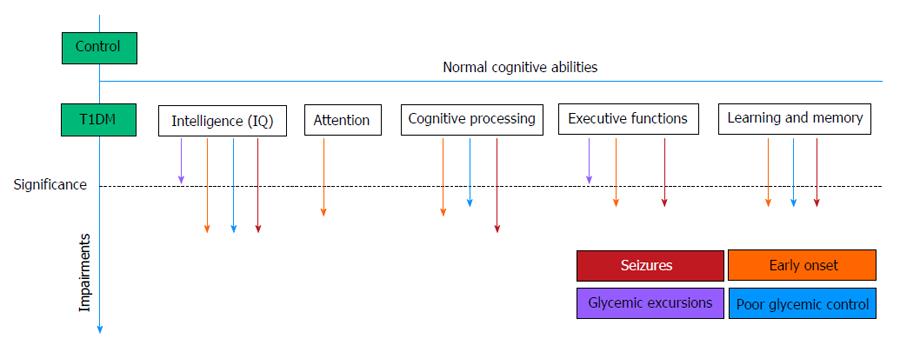
In recent studies, only mild cognitive deficits were observed as compared to healthy controls, which may become a marked deficit in the presences of additional risk factors. Combinations of risk factors are known to increase the probability of significant impairments in T1DM. Among these, we reviewed early onset diabetes from the ages 4-7 years of age, severe hypoglycemic seizures, hypoglycemia, hyperglycemia and poor glycemic control. Early diabetes onset, together with severe hypoglycemic seizures, may cause more significant impairments in some cognitive domains. Figure 2 summarizes the associations between cognitive domains and diabetes risk factor.
Figure 2 Impaired cognitive domains in children and adolescents with type 1 diabetes mellitus.
Mild impairments were found compared to healthy controls, but several risk factors (marked by arrows) increase potential deficits. This figure is generalized, qualitative and not quantitative, based on reported studies. T1DM: Type 1 diabetes mellitus.
Overall, it seems that early achievement of glycemic control (i.e., age dependent goal of HbA1c), may reduce the risk of cognitive impairments. Fear of hypoglycemia is considered to be a barrier to reaching good glycemic control, but recent studies support the understanding that though recurrent episodes of moderate hypoglycemia may compromise memory and executive function, in manners that are corroborated by alterations in the hippocampus, in the short term; implications for affects on long term cognitive abilities have not been proven. On the other hand, poor glycemic control and recurrent hyperglycemia seems to have long term effects on cognitive abilities.
Poor glycemic control is also associated with changes in brain structure, brain integrity and brain activity. Studies focusing only on the adult population demonstrated differences in connectivity underscoring the importance of extending this research line to younger patients in whom, plasticity is expected to be marked both as a function of poor glycemic control and by affecting cognitive competence.
PERSPECTIVES
Despite a wide range of previous research dealing with adults, this line of study in the pediatric population is still in its beginning phases for several reasons. First, glycemic control is accompanied with glycemic excursions, transient hypoglycemia and transient hyperglycemia, and their impact on brain functionality and on cognitive and behavioral domains has yet to be proven. Second, new studies with novel brain imaging data analysis methods have uncovered associations and impacts that diabetes has on brain properties and on brain function. Third, technological development introduced new modalities of brain study as well as ability to assess glucose excursions longitudinally and glycemic control parameters more accurately. Importantly, due to a lack of research dealing with developing age groups whose neurobehavior performances are essential to understanding the impact that T1DM, the brain the review revealed open questions regarding sensitivity and specificity of brain-behavior relations in typical and in atypical development in children and adolescents. Some reported findings with T1DM patients actually be part of normal variability of brain development, others may be a general response to stress that is not necessarily specific to T1DM.
Further research is required in a longitudinal prospective manner, beginning with T1DM diagnosis and continuing throughout the years of management. This research should include simultaneous objective assessments of neurocognitive function, behavioral environmental objective questionnaire based assessment and brain function analysis. Brain function analysis may include novel modalities of fMRI, infusibility and connectivity assessment, and electroencephalographic assessment. Analysis should be performed in an attempt to assess simultaneous glucose excursions in a wide range of glucose concentrations, including the hypoglycemic and hyperglycemic ranges.
Studies should be performed in large populations, but within specific age groups to enable comparison with normal brain development and to avoid statements of possible abnormalities that may actually be part of normal brain function and development variability and not related to T1DM. On the other hand, this will elucidate the modifiable parameters of disease management.
In summary, review of neurobehavioral findings with pediatric populations with T1DM indicates that neurocognitive and behavioral function varies with age and depends on multiple endogenous and exogenous factors that are relevant to care methodologies. These functions are dependent on brain structure, integrity, connectivity, metabolic immediate and long term changes and vary constantly. Since understanding of brain maturation with age is an emerging field of research, delineation of T1DM impact on these processes is challenging but possible with current advances.
Importantly the available studies presented hereby already indicate the need for a change in the care of pediatric population with T1DM. Periodic psychological and neurological ongoing evaluation of children and youth with T1DM, including cognition specific questionnaires and direct testing, should be performed as part of clinical care, especially while taking into account patients’ daily and nocturnal glucose variability. Clear goals of glycemic control criteria should be stated to families, including explanation of possible immediate cognitive impact in order to improve compliance. Gaining a deeper understanding of the effects of T1DM on cognitive functions, brain activity and brain connectivity may deepen the understanding of the aftermaths of diabetes and eventually lead to better individual titration of management of young patients with T1DM in order to prevent long term and short term neurocognitive complications.
ACKNOWLEDGMENTS
We acknowledge the contribution of Ms. Jessica Schreiber of Bar-Ilan University in reviewing the manuscript and editing it for grammatical clarity and appropriate vocabulary.
P- Reviewer: Schuurman HJ S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/