Published online Mar 15, 2015. doi: 10.4239/wjd.v6.i2.234
Peer-review started: August 31, 2014
First decision: November 3, 2014
Revised: November 17, 2014
Accepted: December 16, 2014
Article in press: December 17, 2014
Published online: March 15, 2015
Processing time: 203 Days and 22.5 Hours
A previous diagnosis of gestational diabetes (GDM) carries a lifetime risk of progression to type 2 diabetes of up to 60%. Identification of those women at higher risk of progression to diabetes allows the timely introduction of measures to delay or prevent diabetes onset. However, there is a large degree of variability in the literature with regard to the proportion of women with a history of GDM who go on to develop diabetes. Heterogeneity between cohorts with regard to diagnostic criteria used, duration of follow-up, and the characteristics of the study population limit the ability to make meaningful comparisons across studies. As the new International Association for Diabetes in Pregnancy Study Group criteria are increasingly adopted worldwide, the prevalence of GDM is set to increase by two-to three-fold. Here, we review the literature to examine the evolution of diagnostic criteria for GDM, the implications of changing criteria on the proportion of women with previous GDM progressing to diabetes, and how the use of different diagnostic criteria may influence the development of appropriate follow-up strategies.
Core tip: Gestational diabetes (GDM) is associated with a greatly increased future risk of type 2 diabetes, but there are many different GDM diagnostic criteria in clinical use. Criteria with lower glucose thresholds increase GDM prevalence, and therefore the number of women requiring follow-up to detect progression to diabetes. However, lower diagnostic thresholds are also likely to decrease the proportion that progress to diabetes. Heterogeneity across studies with regard to diagnostic criteria, demographics, and duration of follow-up, limit direct comparison. As the International Association of Diabetes in Pregnancy Study Groups criteria enter widespread use, follow-up of these women will be an important issue.
- Citation: Noctor E, Dunne FP. Type 2 diabetes after gestational diabetes: The influence of changing diagnostic criteria. World J Diabetes 2015; 6(2): 234-244
- URL: https://www.wjgnet.com/1948-9358/full/v6/i2/234.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i2.234
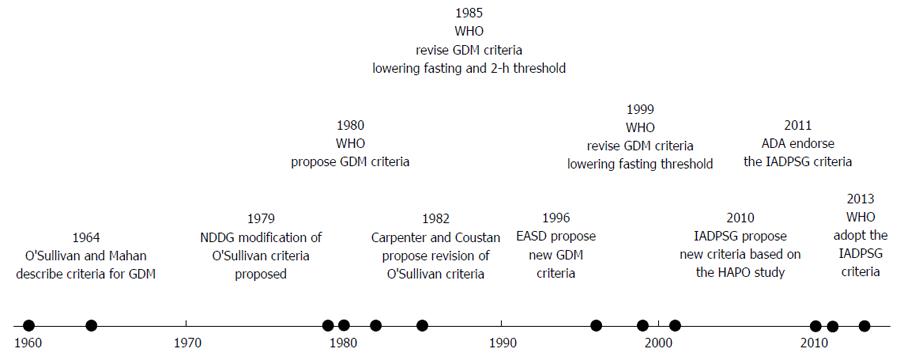
Gestational diabetes (GDM) has long been recognised clinically. First described in pregnancy in 1824 in Germany[1], Joslin[2] described in 1916 a case of diabetes which presented in pregnancy, resolved with delivery, and recurred later in life. In the 1940s and 1950s, Hoet et al[3] recognised the association of this type of diabetes with adverse perinatal outcome, and characterised the relationship between glucose tolerance during pregnancy, and in the post-partum period. However, despite the long-recognised association, no standardised criteria for diagnosis were devised until 1964. In Boston City Hospital, O’Sullivan et al[4] carried out 3-h 100 g oral glucose tolerance tests on 752 patients at different stages of pregnancy. Women with 2 out of 4 values that were greater than 2 standard deviations (rounded to the nearest 5 mg/dL) above the mean glucose levels determined in this cohort were classified as having GDM. These criteria (with some modification) have continued in clinical use over the following four decades.
The major feature of these criteria was that they defined a cohort of women with a greatly increased future risk of progression to type 2 diabetes, demonstrating a lifetime risk of up to 60%[5]. The National Diabetes Data Group (NDDG) criteria, proposed in 1979[6] (Table 1), converted the O’Sullivan/Mahan criteria from whole blood to plasma values (see Figure 1 for timeline). The Carpenter-Coustan criteria[7], proposed in 1982, also converted the O’Sullivan/Mahan criteria to plasma values, but in addition, took a change in enzymatic methods into account. They soon entered widespread clinical use, and were subsequently validated for prediction of adverse perinatal outcome[8-12]. Essentially, therefore, all 3 sets of criteria were intended to define a similar population.
| Criteria | Glucose load | Fasting glucose mmol/L | 1-h glucose mmol/L | 2-h glucose mmol/L | 3-h glucose mmol/L | No. of criteria |
| (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | required | ||
| O’Sullivan et al[4] | 100 g | 5 (90) | 9.2 (165) | 8.1 (145) | 6.9 (125) | ≥ 2 |
| NDDG | 100 g | 5.8 (105) | 10.6 (190) | 9.2 (165) | 8.1 (145) | ≥ 2 |
| WHO 1980 | 75 g | 8 (144) | N/A | 8 (144) | N/A | ≥ 1 |
| Carpenter and Coustan | 100 g | 5.3 (95) | 10 (180) | 8.6 (155) | 7.8 (140) | ≥ 2 |
| ADA | 75 g or 100 g | 5.3 (95) | 10 (180) | 8.6 (155) | 7.8 (140) | ≥ 2 |
| WHO 1985 | 75 g | 7.8 (140) | N/A | 7.8 (140) | N/A | ≥ 1 |
| EASD | 75 g | 6 (108) | N/A | 9 (162) | N/A | ≥ 1 |
| WHO 1999 | 75 g | 7 (126) | N/A | 7.8 (140) | N/A | ≥ 1 |
| IADPSG GDM | 75 g | 5.1 (92) | 10 (180) | 8.5 (153) | N/A | ≥ 1 |
| IADPSG overt diabetes | aNone/75 g | 7 (126) | N/A | 11.1 (200) | N/A | ≥ 1 |
Studies directly comparing the prevalence of GDM by either NDDG or Carpenter-Coustan criteria show, however, significant differences, with an approximately 50% relative increase in GDM prevalence if the Carpenter-Coustan criteria are used[9,11-13]. In addition, in 2001, the American Diabetes Association (ADA), having previously endorsed the Carpenter-Coustan criteria, also allowed for the use of a 75 g, 2-h oral glucose tolerance test (OGTT) to make a diagnosis of GDM, using the same one- and two-hour cut-offs as the three-hour 100 g OGTT. The post-load glucose levels are estimated as being 0.9 mmol/L lower at one hour, and 0.5 mmol/L lower at two hours with the lower glucose load[14], therefore these criteria will identify a different group of women. Indeed, only weak diagnostic agreement has been noted between the two glucose loads[15] (Cohen kappa index 0.18; although some this difference may also be attributable to day-to-day glycaemic variability).
The World Health Organisation (WHO) also recommended alternative criteria for the diagnosis of gestational diabetes beginning in 1980 (the 1965 WHO report did not comment on this issue). These thresholds were the same as those for non-pregnant adults. Initially, the WHO recommended a fasting glucose threshold of 8 mmol/L (see Table 1). These recommendations were revised again in 1985[16] (fasting glucose threshold lowered to 7.8 mmol/L, recommendation to treat impaired glucose tolerance added) and 1999[17] (fasting glucose threshold reduced to 7.0 mmol/L) (see Table 1). Although these thresholds were not chosen on the basis of predicting adverse pregnancy outcome, a subsequent large (n = 4998) prospective cohort study did show that these thresholds predicted increased risk for macrosomia (RR = 1.45, 95%CI: 1.06-1.95) and preeclampsia (1.94, 95%CI: 1.22-3.03), even when women with values diagnostic of diabetes in the nonpregnant adult[18].
The European Association for the Study of Diabetes also proposed new GDM criteria in 1996[19], using a fasting value of 6.0 mmol/L and a two-hour post 75 g glucose load value of 9.0 mmol/L, based on the distribution of glucose values on 75 g OGTTs on over 1000 European women. A subsequent retrospective cohort study supported this 2-h value in prediction of adverse perinatal outcome[20]. However, subsequent analysis of women in this cohort, with 2-h values below the 2-h threshold of 9.0 mmol/L (not treated for GDM), demonstrated a linear relationship between 2-h glucose and pregnancy outcome, with no clear threshold value[21].
In addition to these major criteria, multiple different diagnostic criteria are in use worldwide, some related to older criteria, some derived on the basis of local data. Therefore, the situation still exists where different centres in the same country, or even the same region, may employ different criteria for GDM diagnosis.
However, none of the available criteria had been designed specifically to predict adverse pregnancy outcome. To look at this issue, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) convened a consensus conference in 2008 to review the Hyperglycaemia and Adverse Pregnancy Outcomes (HAPO) study findings (published and unpublished), along with other relevant studies. This consensus conference had two major outcomes[22]. Firstly, women meeting the cut-off values for diagnosis of diabetes in the non-pregnant adult (Table 1) would now fall into the new category of “overt diabetes” rather than GDM. The rationale for this was that this group were felt to be distinct clinically and biochemically from women with milder degrees of hyperglycaemia. Secondly, the data from the 2008 HAPO study[23] was reviewed. This large (over 25000 participants screened), multicentre study showed that glucose levels at all time points on the 2-h 75 g OGTT were associated with adverse pregnancy outcomes (large for gestational age, macrosomia, cord c-peptide concentration greater than the 90th centile). In the absence of a clear threshold effect, and having considered various cutpoints, the IADPSG consensus committee ultimately decided to set new values for GDM diagnosis at the mean glucose values for which the odds ratio for adverse pregnancy outcome was 1.75. This lowered the fasting and 1-h values compared to previous values, while raising the 2-h value slightly. However, the major change was allowing a diagnosis to be made on just a single abnormal value, a change likely to greatly increase the prevalence of gestational diabetes. On applying these criteria retrospectively to the HAPO cohort, 17.8% (range 9.3%-25.5%) met the criteria for diagnosis[24].
These consensus criteria were published in March 2010, and began to enter clinical use shortly afterwards. At the time of writing, in addition to the IADPSG endorsing the criteria, the ADA[25] and WHO[26] have also endorsed the criteria. However, the American College of Obstetricians and Gynaecologists (ACOG) have not adopted the new criteria, and still recommend a 100 g OGTT using the Carpenter-Coustan criteria, for diagnosis, a position endorsed by a National Institute of Health Consensus Conference in March 2013[27].
With this in mind, we will review the impact of changing criteria for GDM diagnosis with regard to the prevalence/cumulative incidence of abnormal glucose tolerance/diabetes post GDM, risk factors for progression to diabetes, and follow-up of women with previous GDM. This is a clinically relevant problem for 2 major reasons - firstly, prevention or delay of type 2 diabetes in women with previous GDM is a possibility, as demonstrated by a subgroup analysis of the diabetes prevention program[28], and the Troglitazone In the Prevention Of Diabetes[29] and Pioglitazone In the Prevention Of Diabetes[30] studies. Secondly, undetected type 2 diabetes developing prior to a subsequent pregnancy carries the risk of congenital malformation and an increased risk of pregnancy complications.
Many studies have assessed the risk of progression to type 2 diabetes post gestational diabetes.
A major issue with all studies in this area however, is their marked heterogeneity. This is seen in several ways: (1) As discussed, the diagnostic criteria in clinical use for GDM diagnosis over the last four decades are numerous. This leads to the identification of cohorts who may not be directly comparable in terms of the severity of glucose intolerance; (2) Both the criteria and method used to diagnose diabetes and/or abnormal glucose tolerance in women who have previously had GDM varies significantly; (3) The ethnic mix of the cohorts is extremely heterogeneous with some composed entirely of a single ethnicity, and others showing very mixed composition; and (4) Duration of follow-up varies between studies, from 6 wk to almost 30 years[31].
In summary, meaningful comparison of the actual cumulative incidence or prevalence across studies is not possible. It is clear, however, that regardless of the criteria used, GDM signifies a high risk of future progression to type 2 diabetes.
Despite the heterogeneity of the cohorts, many studies identify similar factors predicting progression to diabetes/abnormal glucose tolerance. We will consider the most commonly associated risk factors here.
Given that most studies identify women with GDM at the time of diagnosis, most studies assess pre-pregnancy risk factors retrospectively. Therefore, information on this is limited. The exception to retrospective recall of pre-pregnancy factors is the large long-term longitudinal cohort population-based studies, such as the Nurse Health Study[32], which have detailed information preceding the index pregnancy. However, these also use self-reported GDM as an outcome measure. Although the diagnosis has been validated in a subset by medical record review, the precise criteria used by the healthcare provider are uncertain, and therefore lie outside the scope of this review. Of pre-pregnancy variables assessed, weight or BMI is the most common measure, and is commonly associated with increased risk of progression to abnormal glucose tolerance or diabetes[33-38], although the relationship is not particularly strong. Polycystic ovary syndrome has also been reported in a one retrospective study to be associated with later progression to abnormal glucose tolerance[39] on multivariable analysis, although this study used two different sets of criteria to diagnose GDM.
Pregnancy glucose values: Higher glucose values during pregnancy, as reflected by the index pregnancy OGTT, are consistently associated with increased later progression to diabetes. This is measured in various ways (number of abnormal values, area under the curve), but most commonly the values for plasma glucose at fasting, one hour, two hours (and three hours if applicable) are used. Fasting glucose shows the strongest association, being the most commonly identified risk factor associated with later abnormal glucose tolerance and diabetes[31,40-45]. Studies that have not identified fasting glucose as a factor associated with later progression to abnormal glucose tolerance tend to have either not measured it[46], not included it in the statistical models[47], or have excluded women with the highest fasting glucose levels from follow-up[48,49]. One large Australian study found fasting glucose was not associated with later abnormal glucose tolerance and diabetes despite its inclusion in the model[50]. One-hour[48,50,51] and two-hour glucose levels[37,40,51,52] are also associated with later glucose abnormalities, although less consistently, and to varying degrees. Also, higher haemoglobin (HbA1c) during pregnancy, although much less frequently studied, has been found to be associated with future risk of progression to diabetes[52,53].
More detailed characterisation of glycaemic response to a glucose load such as measures of insulin secretion[43], when undertaken, are also associated with later progression to abnormal glucose tolerance and diabetes. These measures, of course, are generally not available routinely clinically. Insulin use during pregnancy has also frequently been shown to be associated with increased risk of future progression to diabetes/abnormal glucose tolerance[36,46,54-56], presumably as a marker of higher glucose levels in pregnancy, even taking into account likely differences in prescribing practice between centres.
Body weight/body mass index: Body weight [or body mass index (BMI)] during the index pregnancy is commonly reported in studies of GDM cohorts, occasionally with waist circumference or body fat measurements. Studies are inconsistent as to whether weight or BMI persist as a risk factor when adjusted for other risk factors using multivariate analysis. Studies that have not found an association between pregnancy weight and BMI tend to examine women who have progressed to abnormal glucose tolerance in the early post-partum period. BMI during pregnancy may be associated with abnormal glucose tolerance at this stage, but is not independently associated when antepartum glucose levels (indicating severity of hyperglycaemia) are included in the model[41]. Most studies that do show an association between pregnancy BMI and later abnormal glucose tolerance, independent of antepartum glucose measurements, involve longer-term follow-up post delivery[43,57,58], although this is not a universal finding[59].
Gestational age at diagnosis: Gestational age at diagnosis is another commonly reported association[37,38,41,42,44,60,61]. However, many of the studies also specify a screening protocol that involves screening higher-risk women in early pregnancy, causing a significant bias. Women diagnosed with GDM in early pregnancy, before insulin resistance begins to rise[62,63], are likely to have a greater degree of hyperglycaemia, and therefore an increased likelihood of progression to abnormal glucose tolerance/diabetes. However, gestational age at diagnosis remains a risk factor, even when measures of glycaemia from the index pregnancy are included in the model, in many of these studies[41,42,44,60,61].
Ethnicity: There are few studies specifically examining the effects of ethnicity, although these that do have generally found an increased prevalence among those women of ethnicity other than white European origin[47,64-68]. Other studies have found no association[40,69]. The reasons for this are unclear. However, many studies have examined ethnically homogenous cohorts, who are often already at high risk of GDM. The prevalence of GDM is higher among ethnic groups who are not of white European origin, while the prevalence of GDM increases at a lower BMI[70] in the Asian populations studied. In addition, adoption of the IADPSG criteria may cause a disproportionate rise in GDM prevalence among Asian populations[71], which will be of relevance when determining the future risk of abnormal glucose tolerance or diabetes in these populations. In addition to the studies outlined above examining this question, comparison between studies does suggest a higher proportion of women of non-white European ethnicity progress to abnormal glucose tolerance[68]. However, meaningful comparison between studies is generally not possible due to the heterogeneity of the studies on the points listed above.
Family history of diabetes: This is uncommonly associated with progression to abnormal glucose tolerance/diabetes among women with GDM after measures of glycaemia are taken into account. Several studies examining family history have found no effect[49,72,73]. Although some studies have shown an independent effect[39,47,59,74], it appears to be small, and the association is often not seen when analysed as part of a multivariate model[38,58,75,76]. Therefore, family history does not appear to play a major independent role in predicting future risk of diabetes or abnormal glucose tolerance.
Other factors: Age at diagnosis of GDM[44,52,54,76] has been associated with future abnormal glucose tolerance or diabetes also, but is inconsistent, with other studies showing no association[57,77,78], and again, is rarely significant[54] when other variables are taken into account. Parity, most commonly classified as a binary variable (multiparous or nulliparous) has been identified[53,55,79] as potentially associated with higher risk of progression later, but this finding is inconsistent[41,78]. Potential gene associations have also been identified, but currently appear to add little to clinically assessing individual risk[80]. Autoantibody testing also been examined[81], and appears to be associated with risk of progression to type 1 rather than type 2 diabetes.
Breastfeeding: Breastfeeding among women with GDM is associated with improved glycaemic indices in the early post-partum period[47,82]. Its role in prevention of later progression to abnormal glucose tolerance is at present unclear, although long-term follow-up of the Study of Women, Infant Feeding and type 2 diabetes mellitus after GDM (SWIFT) pregnancy cohort will address this issue.
Body weight/BMI: Weight (or associated measures) after the index pregnancy has been shown to be correlated in a number of studies[33,56,59,83-86] with progression to diabetes or abnormal glucose tolerance. This correlation appears more robust than that seen with pregnancy weight/BMI, which often loses significance in multivariable models (see above). Also, weight gain since the index pregnancy has been associated with metabolic deterioration[43]. Studies not demonstrating BMI as a predictive factor may take high-risk cohorts, for example, entirely composed of participants with postpartum impaired glucose tolerance[87], or are carried out in the early post-partum period[41,69,88]. Interestingly, Wang et al[84] showed that both waist circumference and body fat performed better than BMI in predicting type 2 diabetes in a Chinese cohort, while Jang demonstrated that waist circumference showed a stronger association than BMI in a Korean cohort[37]. This may help to explain why some Asian cohorts[38,87] have not demonstrated an association between BMI and future abnormal glucose tolerance or diabetes, despite longer-term follow-up.
Others: The type of contraceptive - specifically the progesterone-only oral contraceptive - is thought to confer a higher risk[89]. Subsequent GDM is also associated with greater risk of progression to diabetes/abnormal glucose tolerance[83]. Age at follow-up is commonly reported. Although an association with later abnormal glucose tolerance has been noted[40,44,90-92], and despite the increasing prevalence of type 2 diabetes with advancing age in the general population, this is not a universal finding[38,93], particularly in multivariate analysis[94]. This may be due to the relatively small difference in ages within the cohorts of women involved in these studies, compared to the population as a whole.
Despite the heterogeneity of the studies for the reasons above, including diagnostic criteria used, there is consistency among most studies in the factors associated with a greater risk of diabetes after the index pregnancy in women with GDM. As can be seen, measures of glycaemia during the index pregnancy are not only the strongest predictor, but also frequently attenuate or remove the predictive ability of other traditional risk factors for type 2 diabetes. Thus, the most important risk factor for future abnormal glucose tolerance or diabetes in these women is simply a previous diagnosis of GDM, taking into account the degree of hyperglycaemia at diagnosis.
The prevalence of progression from GDM to abnormal glucose or type 2 diabetes varies greatly. The lifetime cumulative incidence of diabetes among women with GDM is frequently cited at up to 60%, but this summary figure does not illustrate the many underlying different factors (e.g., time since delivery, cohort demographics, and criteria for diagnosis of GDM and postpartum diabetes).
With regard to timing, many studies have documented short-term follow-up only (i.e., to the first post-partum test). Prevalence rates for diabetes at this time point differ, and are generally less than 10%, but depending on the cohort studied, and criteria used, may be significantly higher - Metzger et al[40] showed a prevalence of 38% up to one year post-partum in women meeting NDDG criteria[40]. These women are likely to be different from those developing diabetes at a later post-partum interval, and are more likely to have had pre-existing type 2 diabetes. It is therefore unlikely that any of the criteria in use for GDM diagnosis would fail to detect these women.
Beyond the post-partum period, prevalence or cumulative incidence figures continue to show great variation. Figures may be as low as 3% (up to 3 years post-partum from a Swedish cohort, using area under the glucose curve measures from the OGTT for diagnosis[85]), and as high as 62% (at up to 6.5 years in a cohort from Trinidad meeting the 1980 WHO criteria[64]). Follow-up of O’Sullivan’s original cohort at 16 years showed a cumulative incidence by life-table analysis of 60%[85]. A systematic review from 2002[31] attempted to control for the marked heterogeneity in time among studies, by plotting actuarial projections of cumulative incidence of cohorts at up to 28 years follow-up, and concluded that most cohorts progressed to diabetes at a similar rate in the first 5 years post index pregnancy, and then levelled off by 10 years with few cases after this (however, this calculation included NDDG-diagnosed women only).
Cohort selection also plays a vital role in determining later progression to abnormal glucose tolerance/diabetes, and makes comparison difficult. Selection of women who are known to have normal glucose tolerance in the early post-partum period[48], or restricting follow-up to those who did not require insulin for glycaemic control in pregnancy[34], would be expected to reduce the proportion progressing to abnormal glucose tolerance or diabetes, removing those women with the highest glucose levels during pregnancy. Ethnicity, as outlined above, appears also to be a risk factor for progression, with non-white populations demonstrating increased risk, although comparison across studies is difficult.
There is little evidence to directly compare future progression to diabetes or abnormal glucose tolerance among the different criteria in use. Studies directly comparing progression in women meeting the NDDG vs Carpenter-Coustan criteria[78] showed little difference in prevalence of diabetes at a median of 6 years post-partum (25.5% vs 25.3%) or at 3 mo post-partum (4.0% vs 3.2%)[95].
However, the WHO criteria (Table 1) would be expected to show a smaller proportion of women progressing to diabetes/abnormal glucose tolerance, given the increased number of women identified with GDM compared to the NDDG and Carpenter-Coustan criteria. However, again, direct comparison across studies is difficult. In any given population, therefore, lower diagnostic thresholds will lead to a greater prevalence of GDM. Conversely, criteria using higher thresholds to define GDM will identify fewer women with GDM, but these women will, on average, have higher glucose levels. Therefore, the proportion progressing to abnormal glucose tolerance/diabetes will be higher, despite the lower GDM prevalence.
Also, the criteria used to diagnose type 2 diabetes and abnormal glucose tolerance postpartum may differ - older cohorts in particular, using the NDDG or older WHO criteria would be expected to show a lower prevalence at follow-up due to higher thresholds for diagnosis of diabetes in the nonpregnant adult.
The new IADPSG criteria pose an important clinical question with regard to intensity of follow-up. With potentially up to one in four pregnancies in some centres meeting the new criteria for GDM diagnosis[24], lifelong follow-up of these women will have important clinical and resource implications. However, the optimal mode and timing of a follow-up strategy remains unclear. More women with milder degrees of hyperglycaemia are now classified as GDM. Accordingly, the proportion progressing to abnormal glucose tolerance should decrease. There are as of yet no prospective figures on progression to type 2 diabetes or abnormal glucose tolerance post-partum in women with IADPSG-defined GDM. The ATLANTIC-DIP study retrospectively classified women using IADPSG criteria after a universal screening programme, and found that 19% had abnormal glucose tolerance at early post-partum follow-up[47]. Capula et al[39] looked at a mixed (approximately 60% diagnosed by IADPSG criteria) cohort, and found 4% had diabetes, and a further 32% abnormal glucose tolerance at 6-12 wk post-partum, although conclusions on the relative contribution of each set of criteria are not possible. Overall, it appears certain that more women will need to be tested to identify those women progressing to abnormal glucose tolerance and diabetes.
Some clues as to how women diagnosed with GDM by IADPSG criteria may behave on follow-up may be seen in several papers which follow women meeting just a single abnormal value on the pregnancy OGTT using the older criteria. Retnakaran et al[95,96], using NDDG criteria for GDM diagnosis, examined early post-partum outcomes among women along the spectrum of glucose tolerance: from normal glucose tolerance, to abnormal glucose challenge test (GCT) with normal OGTT, a single abnormal value on OGTT, and GDM. This demonstrated a graded relationship in abnormal glucose tolerance; from 3.2% in the normal glucose tolerance (NGT) group, 10.2% in the GCT abnormal, OGTT normal group, 16.5% in the GCT abnormal, single abnormal value on OGTT group, to 32.8% in the GDM group. Indeed, detailed characterisation of these groups[97] demonstrates the similarity between women with a single abnormal value at 1-h post glucose load (as opposed to later abnormal values) and women with GDM, as measured by AUC curve on OGTT and beta-cell dysfunction at 3 mo postpartum.
Thus we can see that a cohort of women with a single abnormal value only, albeit using higher cut-offs than the new IADPSG values, still have a clinically important increased risk of abnormal glucose tolerance. Other prospective studies examining similar cohorts, although at a longer follow-up interval, have drawn similar conclusions; Stuebe et al[98], using the stricter Carpenter-Coustan criteria, showed a higher HbA1c in women with a single abnormal value at 3-year follow-up, vs both women with GDM, and those with NGT in pregnancy. Vambergue et al[76] (using Carpenter-Coustan criteria) showed a similar graded relationship for progression to type 2 diabetes at almost 7 years follow-up, with 6% of women with a single abnormal value progressing to diabetes, as compared with 18% in the those meeting GDM criteria (less than 1% of those with no abnormal values had progressed to diabetes). Carr et al[99] (using Carpenter-Coustan criteria), in a large retrospective cohort study, found a HR of 2.0 for diabetes diagnosis among women with a single abnormal value on OGTT vs those who did not.
Therefore, all degrees of glucose abnormalities in pregnancy, even those not meeting older GDM criteria, are associated with an increased risk of later glucose abnormalities. This may have important implications for those women with lesser degrees of hyperglycaemia who will now be classified as having GDM by IADPSG criteria.
Women meeting criteria for diabetes diagnosis in the non-pregnant adult are now classified as separate category by the IADPSG criteria and represent the highest-risk GDM cohort, having an increased risk of congenital abnormalities and diabetes complications, and are likely to have had undiagnosed type 2 diabetes preceding the index pregnancy[22]. The future risk of these women is unclear at present. A retrospective audit of 254 women meeting criteria for overt diabetes demonstrated that 41% had normal glucose tolerance at 6-8 wk postpartum (although testing was carried out at 24-28 wk rather than at the booking visit, and diagnoses based on a 2-h value of ≥ 11.1 mmol/L were not confirmed with HbA1c or FPG measurements)[100]. Further prospective follow-up comparing women meeting both sets of IADPSG criteria will therefore be useful in further refining risk in this population.
Current recommendations for follow-up of women with gestational diabetes vary from region to region. The ADA recommend an early post-partum OGTT (in line with ACOG guidelines) and follow-up with HbA1c, fasting plasma glucose (FPG) or 75 g OGTT thereafter, on a 1-3 yearly basis[101]. The International Diabetes Federation[102] recommend an early post-partum OGTT, and thereafter vary recommendations on whether a further pregnancy is planned, (OGTT prior to conception) and whether the woman is high-risk (annual OGTT) or low-risk (FPG every 2-3 years), the criteria for which are not defined. The British National Institute for Health and Care Excellence guidelines[103] recommend FPG alone in the early post-partum period, and an OGTT at follow-up only if a further pregnancy is planned. Several studies have examined the use of HbA1c and FPG[104-107] for both early and medium term follow-up in women with previous GDM, in order to avoid the inconvenience associated with the OGTT. Sensitivity for the detection of abnormal glucose tolerance after delivery varies widely according to the thresholds chosen, ranging from 23%-65% (specificity 68%-96%) for HbA1c values, increasing to a sensitivity of 82%-93% (specificity 84%-92%)when combined with FPG values. Further prospective study will be needed to examine the potential use of these approaches. This will be particularly important if IADPSG criteria are used, as the optimum frequency and mode of testing for such a large cohort of women with previous GDM is unknown.
Marked heterogeneity across studies of women with previous GDM with regard to the diagnostic criteria used, duration of follow-up, and cohort demographics limits the ability to compare findings across studies. However, regardless of which criteria are used, a history of GDM confers a large excess risk of progression to type 2 diabetes in later life, and the risk factors predicting progression remain similar across cohorts. The new IADPSG criteria increase the prevalence of GDM by 2-3 fold, and lifelong follow-up of these women has significant clinical and resource implications. Therefore, further prospective studies are necessary to determine the longer-term risk of progression to diabetes in those diagnosed using the new criteria, and also to determine the optimal method and frequency follow-up needed.
| 1. | Hadden DR, Hillebrand B. The first recorded case of diabetic pregnancy (Bennewitz HG, 1824, University of Berlin). Diabetologia. 1989;32:625. [PubMed] |
| 2. | Joslin EP. The Treatment of Diabetes Mellitus. Can Med Assoc J. 1916;6:673-684. [PubMed] |
| 4. | O’sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278-285. [PubMed] |
| 5. | O’Sullivan JB. Establishing criteria for gestational diabetes. Diabetes Care. 1980;3:437-439. [PubMed] |
| 6. | Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. [PubMed] |
| 7. | Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. [PubMed] |
| 8. | Gokcel A, Bagis T, Killicadag EB, Tarim E, Guvener N. Comparison of the criteria forgestational diabetes mellitus by NDDG and Carpenter and Coustan, and the outcomes of pregnancy. J Endocrinol Invest. 2002;25:357-361. [PubMed] |
| 9. | Cheng YW, Block-Kurbisch I, Caughey AB. Carpenter-Coustan criteria compared with the national diabetes data group thresholds for gestational diabetes mellitus. Obstet Gynecol. 2009;114:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Berggren EK, Boggess KA, Stuebe AM, Jonsson Funk M. National Diabetes Data Group vs Carpenter-Coustan criteria to diagnose gestational diabetes. Am J Obstet Gynecol. 2011;205:253.e1-253.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Magee MS, Walden CE, Benedetti TJ, Knopp RH. Influence of diagnostic criteria on the incidence of gestational diabetes and perinatal morbidity. JAMA. 1993;269:609-615. [PubMed] |
| 12. | Naylor CD, Sermer M, Chen E, Sykora K. Cesarean delivery in relation to birth weight and gestational glucose tolerance: pathophysiology or practice style? Toronto Trihospital Gestational Diabetes Investigators. JAMA. 1996;275:1165-1170. [PubMed] |
| 13. | Ferrara A, Hedderson MM, Quesenberry CP, Selby JV. Prevalence of gestational diabetes mellitus detected by the national diabetes data group or the carpenter and coustan plasma glucose thresholds. Diabetes Care. 2002;25:1625-1630. [PubMed] |
| 14. | Weiss PA, Haeusler M, Kainer F, Pürstner P, Haas J. Toward universal criteria for gestational diabetes: relationships between seventy-five and one hundred gram glucose loads and between capillary and venous glucose concentrations. Am J Obstet Gynecol. 1998;178:830-835. [PubMed] |
| 15. | Mello G, Elena P, Ognibene A, Cioni R, Tondi F, Pezzati P, Pratesi M, Scarselli G, Messeri G. Lack of concordance between the 75-g and 100-g glucose load tests for the diagnosis of gestational diabetes mellitus. Clin Chem. 2006;52:1679-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Diabetes mellitus. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1985;727:1-113. [PubMed] |
| 17. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 124] [Reference Citation Analysis (0)] |
| 18. | Schmidt MI, Duncan BB, Reichelt AJ, Branchtein L, Matos MC, Costa e Forti A, Spichler ER, Pousada JM, Teixeira MM, Yamashita T. Gestational diabetes mellitus diagnosed with a 2-h 75-g oral glucose tolerance test and adverse pregnancy outcomes. Diabetes Care. 2001;24:1151-1155. [PubMed] |
| 19. | Brown CJ, Dawson A, Dodds R, Gamsu H, Gillmer M, Hall M, Hounsome B, Knopfler A, Ostler J, Peacock I. Report of the Pregnancy and Neonatal Care Group. Diabet Med. 1996;13:S43-S53. [PubMed] |
| 20. | Jensen DM, Damm P, Sørensen B, Mølsted-Pedersen L, Westergaard JG, Korsholm L, Ovesen P, Beck-Nielsen H. Proposed diagnostic thresholds for gestational diabetes mellitus according to a 75-g oral glucose tolerance test. Maternal and perinatal outcomes in 3260 Danish women. Diabet Med. 2003;20:51-57. [PubMed] |
| 21. | Jensen DM, Korsholm L, Ovesen P, Beck-Nielsen H, Mølsted-Pedersen L, Damm P. Adverse pregnancy outcome in women with mild glucose intolerance: is there a clinically meaningful threshold value for glucose? Acta Obstet Gynecol Scand. 2008;87:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2777] [Cited by in RCA: 3340] [Article Influence: 208.8] [Reference Citation Analysis (1)] |
| 23. | Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3783] [Cited by in RCA: 3827] [Article Influence: 212.6] [Reference Citation Analysis (3)] |
| 24. | Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, Lowe LP, Coustan DR, Hod M, Oats JJ. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 2012;35:526-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 538] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 25. | American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34 Suppl 1:S11-S61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1908] [Cited by in RCA: 1933] [Article Influence: 128.9] [Reference Citation Analysis (1)] |
| 26. | World Health Organisation. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. 2013; Available from: http: //www.who.int/diabetes/publications/Hyperglycaemia_In_Pregnancy/en/. |
| 27. | National Institutes of Health consensus development conference statement: diagnosing gestational diabetes mellitus, March 4-6, 2013. Obstet Gynecol. 2013;122:358-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, Fowler S, Kahn SE. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774-4779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 609] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 29. | Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Kawakubo M, Buchanan TA. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55:517-522. [PubMed] |
| 30. | Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796-2803. [PubMed] |
| 31. | Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862-1868. [PubMed] |
| 32. | Bao W, Tobias DK, Bowers K, Chavarro J, Vaag A, Grunnet LG, Strøm M, Mills J, Liu A, Kiely M. Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: a prospective cohort study. JAMA Intern Med. 2014;174:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 33. | Pallardo LF, Herranz L, Martin-Vaquero P, Garcia-Ingelmo T, Grande C, Jañez M. Impaired fasting glucose and impaired glucose tolerance in women with prior gestational diabetes are associated with a different cardiovascular profile. Diabetes Care. 2003;26:2318-2322. [PubMed] |
| 34. | Lauenborg J, Hansen T, Jensen DM, Vestergaard H, Mølsted-Pedersen L, Hornnes P, Locht H, Pedersen O, Damm P. Increasing incidence of diabetes after gestational diabetes: a long-term follow-up in a Danish population. Diabetes Care. 2004;27:1194-1199. [PubMed] |
| 35. | Cao XP, Xiao HP, Chen SJ, Zhan YF, Xiu LL, Wang ZL. Beta-cell dysfunction is the primary contributor to the early postpartum diabetes among Chinese women with history of gestational diabetes mellitus. Chin Med J (Engl). 2008;121:696-700. [PubMed] |
| 36. | Russell C, Dodds L, Armson BA, Kephart G, Joseph KS. Diabetes mellitus following gestational diabetes: role of subsequent pregnancy. BJOG. 2008;115:253-259; discussion 260. [PubMed] |
| 37. | Jang HC. Gestational diabetes in Korea: incidence and risk factors of diabetes in women with previous gestational diabetes. Diabetes Metab J. 2011;35:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Cho NH, Lim S, Jang HC, Park HK, Metzger BE. Elevated homocysteine as a risk factor for the development of diabetes in women with a previous history of gestational diabetes mellitus: a 4-year prospective study. Diabetes Care. 2005;28:2750-2755. [PubMed] |
| 39. | Capula C, Chiefari E, Vero A, Foti DP, Brunetti A, Vero R. Prevalence and predictors of postpartum glucose intolerance in Italian women with gestational diabetes mellitus. Diabetes Res Clin Pract. 2014;105:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 40. | Metzger BE, Bybee DE, Freinkel N, Phelps RL, Radvany RM, Vaisrub N. Gestational diabetes mellitus. Correlations between the phenotypic and genotypic characteristics of the mother and abnormal glucose tolerance during the first year postpartum. Diabetes. 1985;34 Suppl 2:111-115. [PubMed] |
| 41. | Catalano PM, Vargo KM, Bernstein IM, Amini SB. Incidence and risk factors associated with abnormal postpartum glucose tolerance in women with gestational diabetes. Am J Obstet Gynecol. 1991;165:914-919. [PubMed] |
| 42. | Kjos SL, Peters RK, Xiang A, Henry OA, Montoro M, Buchanan TA. Predicting future diabetes in Latino women with gestational diabetes. Utility of early postpartum glucose tolerance testing. Diabetes. 1995;44:586-591. [PubMed] |
| 43. | Buchanan TA, Xiang A, Kjos SL, Lee WP, Trigo E, Nader I, Bergner EA, Palmer JP, Peters RK. Gestational diabetes: antepartum characteristics that predict postpartum glucose intolerance and type 2 diabetes in Latino women. Diabetes. 1998;47:1302-1310. [PubMed] |
| 44. | Dalfrà MG, Lapolla A, Masin M, Giglia G, Dalla Barba B, Toniato R, Fedele D. Antepartum and early postpartum predictors of type 2 diabetes development in women with gestational diabetes mellitus. Diabetes Metab. 2001;27:675-680. [PubMed] |
| 45. | Chew WF, Rokiah P, Chan SP, Chee WS, Lee LF, Chan YM. Prevalence of glucose intolerance, and associated antenatal and historical risk factors among Malaysian women with a history of gestational diabetes mellitus. Singapore Med J. 2012;53:814-820. [PubMed] |
| 46. | Aberg AE, Jönsson EK, Eskilsson I, Landin-Olsson M, Frid AH. Predictive factors of developing diabetes mellitus in women with gestational diabetes. Acta Obstet Gynecol Scand. 2002;81:11-16. [PubMed] |
| 47. | O’Reilly MW, Avalos G, Dennedy MC, O’Sullivan EP, Dunne F. Atlantic DIP: high prevalence of abnormal glucose tolerance post partum is reduced by breast-feeding in women with prior gestational diabetes mellitus. Eur J Endocrinol. 2011;165:953-959. [PubMed] |
| 48. | Buchanan TA, Xiang AH, Kjos SL, Trigo E, Lee WP, Peters RK. Antepartum predictors of the development of type 2 diabetes in Latino women 11-26 months after pregnancies complicated by gestational diabetes. Diabetes. 1999;48:2430-2436. [PubMed] |
| 49. | Metzger BE, Cho NH, Roston SM, Radvany R. Prepregnancy weight and antepartum insulin secretion predict glucose tolerance five years after gestational diabetes mellitus. Diabetes Care. 1993;16:1598-1605. [PubMed] |
| 50. | Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care. 2007;30:878-883. [PubMed] |
| 51. | Akinci B, Celtik A, Genc S, Yener S, Demir T, Secil M, Kebapcilar L, Yesil S. Evaluation of postpartum carbohydrate intolerance and cardiovascular risk factors in women with gestational diabetes. Gynecol Endocrinol. 2011;27:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Malinowska-Polubiec A, Sienko J, Lewandowski Z, Czajkowski K, Smolarczyk R. Risk factors of abnormal carbohydrate metabolism after pregnancy complicated by gestational diabetes mellitus. Gynecol Endocrinol. 2012;28:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Ekelund M, Shaat N, Almgren P, Groop L, Berntorp K. Prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetologia. 2010;53:452-457. [PubMed] |
| 54. | Chodick G, Elchalal U, Sella T, Heymann AD, Porath A, Kokia E, Shalev V. The risk of overt diabetes mellitus among women with gestational diabetes: a population-based study. Diabet Med. 2010;27:779-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Kerimoğlu OS, Yalvaç S, Karçaaltınçaba D, Kandemir O. Incidence of diabetes mellitus at postpartum six to twelve months following the diagnosis of gestational diabetes mellitus. J Turk Ger Gynecol Assoc. 2010;11:89-94. [PubMed] |
| 56. | Ziegler AG, Wallner M, Kaiser I, Rossbauer M, Harsunen MH, Lachmann L, Maier J, Winkler C, Hummel S. Long-term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes. 2012;61:3167-3171. [PubMed] |
| 57. | Löbner K, Knopff A, Baumgarten A, Mollenhauer U, Marienfeld S, Garrido-Franco M, Bonifacio E, Ziegler AG. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes. 2006;55:792-797. [PubMed] |
| 58. | Cheung NW, Helmink D. Gestational diabetes: the significance of persistent fasting hyperglycemia for the subsequent development of diabetes mellitus. J Diabetes Complications. 2006;20:21-25. [PubMed] |
| 59. | Kim SH, Kim MY, Yang JH, Park SY, Yim CH, Han KO, Yoon HK, Park S. Nutritional risk factors of early development of postpartum prediabetes and diabetes in women with gestational diabetes mellitus. Nutrition. 2011;27:782-788. [PubMed] |
| 60. | Albareda M, Caballero A, Badell G, Piquer S, Ortiz A, de Leiva A, Corcoy R. Diabetes and abnormal glucose tolerance in women with previous gestational diabetes. Diabetes Care. 2003;26:1199-1205. [PubMed] |
| 61. | Schaefer-Graf UM, Buchanan TA, Xiang AH, Peters RK, Kjos SL. Clinical predictors for a high risk for the development of diabetes mellitus in the early puerperium in women with recent gestational diabetes mellitus. Am J Obstet Gynecol. 2002;186:751-756. [PubMed] |
| 62. | Stanley K, Fraser R, Bruce C. Physiological changes in insulin resistance in human pregnancy: longitudinal study with the hyperinsulinaemic euglycaemic clamp technique. Br J Obstet Gynaecol. 1998;105:756-759. [PubMed] |
| 63. | Mills JL, Jovanovic L, Knopp R, Aarons J, Conley M, Park E, Lee YJ, Holmes L, Simpson JL, Metzger B. Physiological reduction in fasting plasma glucose concentration in the first trimester of normal pregnancy: the diabetes in early pregnancy study. Metabolism. 1998;47:1140-1144. [PubMed] |
| 64. | Ali Z, Alexis SD. Occurrence of diabetes mellitus after gestational diabetes mellitus in Trinidad. Diabetes Care. 1990;13:527-529. [PubMed] |
| 65. | Dornhorst A, Chan SP, Gelding SV, Nicholls JS, Baynes C, Elkeles RS, Beard RW, Anyaoku V, Johnston DG. Ethnic differences in insulin secretion in women at risk of future diabetes. Diabet Med. 1992;9:258-262. [PubMed] |
| 66. | Kousta E, Efstathiadou Z, Lawrence NJ, Jeffs JA, Godsland IF, Barrett SC, Doré CJ, Penny A, Anyaoku V, Millauer BA. The impact of ethnicity on glucose regulation and the metabolic syndrome following gestational diabetes. Diabetologia. 2006;49:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Sinha B, Brydon P, Taylor RS, Hollins A, Munro A, Jenkins D, Dunne F. Maternal ante-natal parameters as predictors of persistent postnatal glucose intolerance: a comparative study between Afro-Caribbeans, Asians and Caucasians. Diabet Med. 2003;20:382-386. [PubMed] |
| 68. | Girgis CM, Gunton JE, Cheung NW. The influence of ethnicity on the development of type 2 diabetes mellitus in women with gestational diabetes: a prospective study and review of the literature. ISRN Endocrinol. 2012;2012:341638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Dacus JV, Meyer NL, Muram D, Stilson R, Phipps P, Sibai BM. Gestational diabetes: postpartum glucose tolerance testing. Am J Obstet Gynecol. 1994;171:927-931. [PubMed] |
| 70. | Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35:1492-1498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 71. | Jenum AK, Mørkrid K, Sletner L, Vangen S, Torper JL, Nakstad B, Voldner N, Rognerud-Jensen OH, Berntsen S, Mosdøl A. Impact of ethnicity on gestational diabetes identified with the WHO and the modified International Association of Diabetes and Pregnancy Study Groups criteria: a population-based cohort study. Eur J Endocrinol. 2012;166:317-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 72. | Lam KS, Li DF, Lauder IJ, Lee CP, Kung AW, Ma JT. Prediction of persistent carbohydrate intolerance in patients with gestational diabetes. Diabetes Res Clin Pract. 1991;12:181-186. [PubMed] |
| 73. | Damm P, Kühl C, Bertelsen A, Mølsted-Pedersen L. Predictive factors for the development of diabetes in women with previous gestational diabetes mellitus. Am J Obstet Gynecol. 1992;167:607-616. [PubMed] |
| 74. | Kwak SH, Choi SH, Kim K, Jung HS, Cho YM, Lim S, Cho NH, Kim SY, Park KS, Jang HC. Prediction of type 2 diabetes in women with a history of gestational diabetes using a genetic risk score. Diabetologia. 2013;56:2556-2563. [PubMed] |
| 75. | Coustan DR, Carpenter MW, O’Sullivan PS, Carr SR. Gestational diabetes: predictors of subsequent disordered glucose metabolism. Am J Obstet Gynecol. 1993;168:1139-1144; discussion 1144-1145. [PubMed] |
| 76. | Vambergue A, Dognin C, Boulogne A, Réjou MC, Biausque S, Fontaine P. Increasing incidence of abnormal glucose tolerance in women with prior abnormal glucose tolerance during pregnancy: DIAGEST 2 study. Diabet Med. 2008;25:58-64. [PubMed] |
| 77. | Damm P, Kühl C, Hornnes P, Mølsted-Pedersen L. A longitudinal study of plasma insulin and glucagon in women with previous gestational diabetes. Diabetes Care. 1995;18:654-665. [PubMed] |
| 78. | Kaufmann RC, Schleyhahn FT, Huffman DG, Amankwah KS. Gestational diabetes diagnostic criteria: long-term maternal follow-up. Am J Obstet Gynecol. 1995;172:621-625. [PubMed] |
| 79. | Corrado F, D’Anna R, Cannata ML, Cannizzaro D, Caputo F, Raffone E, Di Benedetto A. Positive association between a single abnormal glucose tolerance test value in pregnancy and subsequent abnormal glucose tolerance. Am J Obstet Gynecol. 2007;196:339.e1-339.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Kwak SH, Choi SH, Jung HS, Cho YM, Lim S, Cho NH, Kim SY, Park KS, Jang HC. Clinical and genetic risk factors for type 2 diabetes at early or late post partum after gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98:E744-E752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 81. | de Leiva A, Mauricio D, Corcoy R. Diabetes-related autoantibodies and gestational diabetes. Diabetes Care. 2007;30 Suppl 2:S127-S133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Gunderson EP, Hedderson MM, Chiang V, Crites Y, Walton D, Azevedo RA, Fox G, Elmasian C, Young S, Salvador N. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: the SWIFT cohort. Diabetes Care. 2012;35:50-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 83. | Steinhart JR, Sugarman JR, Connell FA. Gestational diabetes is a herald of NIDDM in Navajo women. High rate of abnormal glucose tolerance after GDM. Diabetes Care. 1997;20:943-947. [PubMed] |
| 84. | Wang L, Liu H, Zhang S, Leng J, Liu G, Zhang C, Li WQ, Li N, Li W, Li Y. Obesity index and the risk of diabetes among Chinese women with prior gestational diabetes. Diabet Med. 2014;31:1368-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Persson B, Hanson U, Hartling SG, Binder C. Follow-up of women with previous GDM. Insulin, C-peptide, and proinsulin responses to oral glucose load. Diabetes. 1991;40 Suppl 2:136-141. [PubMed] |
| 86. | Dornhorst A, Bailey PC, Anyaoku V, Elkeles RS, Johnston DG, Beard RW. Abnormalities of glucose tolerance following gestational diabetes. Q J Med. 1990;77:1219-1228. [PubMed] |
| 87. | Lee KF, Mak MW, Lau KO, Chung H. Risk of development of diabetes mellitus in Chinese women with persistently impaired glucose tolerance after gestational diabetes. Hong Kong Med J. 2011;17:195-201. [PubMed] |
| 88. | Kjos SL, Buchanan TA, Greenspoon JS, Montoro M, Bernstein GS, Mestman JH. Gestational diabetes mellitus: the prevalence of glucose intolerance and diabetes mellitus in the first two months post partum. Am J Obstet Gynecol. 1990;163:93-98. [PubMed] |
| 89. | Kjos SL, Peters RK, Xiang A, Thomas D, Schaefer U, Buchanan TA. Contraception and the risk of type 2 diabetes mellitus in Latina women with prior gestational diabetes mellitus. JAMA. 1998;280:533-538. [PubMed] |
| 90. | Feig DS, Zinman B, Wang X, Hux JE. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. CMAJ. 2008;179:229-234. [PubMed] |
| 91. | Gobl CS, Bozkurt L, Prikoszovich T, Winzer C, Pacini G, Kautzky-Willer A. Early Risk Determinants for Overt Diabetes after Gestational Diabetes. Diabetes. 2011;60:A638. |
| 92. | Greenberg LR, Moore TR, Murphy H. Gestational diabetes mellitus: antenatal variables as predictors of postpartum glucose intolerance. Obstet Gynecol. 1995;86:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | Cypryk K, Loba J, Wilczyński J, Czupryniak L, Grabowska M. [Evaluation of carbohydrate metabolism in women with previous gestational diabetes mellitus]. Ginekol Pol. 1994;65:665-670. [PubMed] |
| 94. | Göbl CS, Bozkurt L, Prikoszovich T, Tura A, Pacini G, Kautzky-Willer A. Estimating the risk after gestational diabetes mellitus: can we improve the information from the postpartum OGTT? Am J Physiol Endocrinol Metab. 2013;304:E524-E530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Comparison of National Diabetes Data Group and American Diabetes Association diagnostic criteria for gestational diabetes in their identification of postpartum risk of glucose intolerance. Diabetes Res Clin Pract. 2009;85:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care. 2008;31:2026-2031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 97. | Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Isolated hyperglycemia at 1 hour on oral glucose tolerance test in pregnancy resembles gestational diabetes mellitus in predicting postpartum metabolic dysfunction. Diabetes Care. 2008;31:1275-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Stuebe AM, Mantzoros C, Kleinman K, Gillman MW, Rifas-Shiman S, Seely EW, Rich-Edwards J. Gestational glucose tolerance and maternal metabolic profile at 3 years postpartum. Obstet Gynecol. 2011;118:1065-1073. [PubMed] |
| 99. | Carr DB, Newton KM, Utzschneider KM, Tong J, Gerchman F, Kahn SE, Heckbert SR. Modestly elevated glucose levels during pregnancy are associated with a higher risk of future diabetes among women without gestational diabetes mellitus. Diabetes Care. 2008;31:1037-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 100. | Wong T, Ross GP, Jalaludin BB, Flack JR. The clinical significance of overt diabetes in pregnancy. Diabet Med. 2013;30:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 101. | American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2830] [Cited by in RCA: 3040] [Article Influence: 253.3] [Reference Citation Analysis (0)] |
| 102. | IDF Clinical Guidelines Task Force. Global Guideline on Pregnancy and Diabetes. Brussels: International Diabetes Federation 2009; Available from: http: //www.idf.org/guidelines/pregnancy-and-diabetes. |
| 103. | Walker JD. NICE guidance on diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. NICE clinical guideline 63. London, March 2008. Diabet Med. 2008;25:1025-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 104. | Kim C, Herman WH, Cheung NW, Gunderson EP, Richardson C. Comparison of hemoglobin A1c with fasting plasma glucose and 2-h postchallenge glucose for risk stratification among women with recent gestational diabetes mellitus. Diabetes Care. 2011;34:1949-1951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | Megia A, Näf S, Herranz L, Serrat N, Yañez RE, Simón I, Vendrell J. The usefulness of HbA1c in postpartum reclassification of gestational diabetes. BJOG. 2012;119:891-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Noctor E, Crowe C, Carmody LA, Avalos GM, Kirwan B, Infanti JJ, O’Dea A, Gillespie P, Newell J, McGuire B. ATLANTIC DIP: simplifying the follow-up of women with previous gestational diabetes. Eur J Endocrinol. 2013;169:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 107. | Picón MJ, Murri M, Muñoz A, Fernández-García JC, Gomez-Huelgas R, Tinahones FJ. Hemoglobin A1c versus oral glucose tolerance test in postpartum diabetes screening. Diabetes Care. 2012;35:1648-1653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
P- Reviewer: Petry CJ, Wahabi HA, Xu XH S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/