Published online Feb 15, 2015. doi: 10.4239/wjd.v6.i1.92
Peer-review started: July 9, 2014
First decision: September 23, 2014
Revised: November 22, 2014
Accepted: December 3, 2014
Article in press: December 10, 2014
Published online: February 15, 2015
Processing time: 206 Days and 10.3 Hours
Diabetes mellitus (DM) is a important health problem that induces ernestful complications and it causes significant morbidity owing to specific microvascular complications such as, retinopathy, nephropathy and neuropathy, and macrovascular complications such as, ischaemic heart disease, and peripheral vasculopathy. It can affect children, young people and adults and is becoming more common. Ocular complications associated with DM are progressive and rapidly becoming the world’s most significant cause of morbidity and are preventable with early detection and timely treatment. This review provides an overview of five main ocular complications associated with DM, diabetic retinopathy and papillopathy, cataract, glaucoma, and ocular surface diseases.
Core tip: Ocular complications associated with diabetes mellitus (DM) are progressive and rapidly becoming the world’s most significant cause of morbidity and are preventable with early detection and timely treatment. This review provides an overview of five main ocular complications associated with DM, diabetic retinopathy and papillopathy, cataract, glaucoma, and ocular surface diseases.
- Citation: Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World J Diabetes 2015; 6(1): 92-108
- URL: https://www.wjgnet.com/1948-9358/full/v6/i1/92.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i1.92
Complications of diabetes mellitus (DM) are progressive and almost resulting by chronic exposure to high blood levels of glucose caused by impairments in insulin metabolism and biological macromolecules such as carbohydrates, lipids, proteins and nucleic acids[1]. DM and its complications are rapidly becoming the world’s most significant cause of morbidity and mortality[2,3]. The DM pandemic has expanded speedily in the developed and developing countries. It is expected that DM will reach epidemic proportions within the near future[4]. DM affects more than 240 million people worldwide, and this number is expected to reach roughly 370 million by 2030[5,6]. DM can lead to several ocular complications such as diabetic retinopathy, diabetic papillopathy, glaucoma, cataract, and ocular surface diseases[7]. Diabetes related ocular complications are general public health problem, so we purpose of putting emphasis on the frequencies, pathogenesis, and management of these ocular com-plications.
Diabetic retinopathy (DR), a microangiopathy affecting all of the small retinal vessels, such as arterioles, capillaries and venules, is characterized by increased vascular permeability, ocular haemorrhages, lipid exudate, by vascular closure mediated by the development of new vessels on the retina and the posterior vitreous surface[8]. DR, the most common microvascular complication of DM, is predicted to be the principal reason of new blindness among working population[9,10]. DR is the major reason of blindness in adults 20-74 years of age in the United States of America[11]. In patients with type 1 and type 2 diabetics with disease duration of over twenty years, the prevalences of DR are 95% and 60%, respectively[12]. Roughly 25% of type 1 diabetic patients have been reported to be influenced with DR, with the frequency increasing to about 80% after 15 years of anguish[13]. The type 2 DM is responsible for a higher percentage of patients with visual loss[13]. The incidence of DR is related primarily to duration and control of diabetes and is related to hyperglycemia, hypertension, hyperlipidemia, pregnancy, nephropathy, and anemia[14-16]. According to reports published by Wisconsin epidemiologic study of diabetic retinopathy (WESDR)[17], the general 10-year incidence of DR was 74%. Moreover in 64% of people with baseline DR developed more severe DR and 17% of those advanced to occur proliferative DR[18].
There is a very strong relationship between chronic hyper-glycemia and the development of DR[19,20]. Hyperglycemia triggers a sequence of events causing vascular endothelial dysfunction. Many interdependent metabolic pathways have been put forward as important connections between hyperglycemia and DR. These implicated metabolic pathways include increased polyol[21] and protein kinase C (PKC) pathway[22] activity, upregulation of growth factors of which vascular endothelial growth factor (VEGF)[22], generation of advanced glycation endproducts (AGEs)[23,24], chronic oxidative damage[25], increased activation of the renin angiotensin system (RAS)[26], chronic inflammation and abnormal clumping of leukocytes (leukostasis)[26].
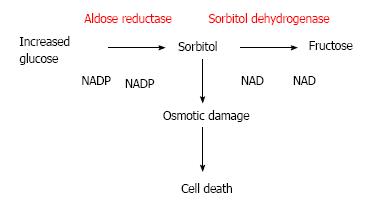
When excessive amounts of glucose increase the polyol way is activated to reduce glucose into sorbitol. The aldose reductase enzyme and nicotinamide adenine dinucleotide phosphate are involved in this biochemical reaction. Sorbitol is further metabolized to fructose by sorbitol dehydrogenase. Since sorbitol movement is severely restricted by cellular membrane, excessive accumulation of sorbitol in the cell occurs[27,28]. The increased sorbitol has potential osmotic damage in retinal cells[29] (Figure 1).
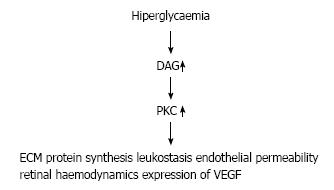
Chronic hyperglycemia increases quantity of diacy-lglycerol (DAG), which is leading to activate protein kinase C[30]. This activation leads to increase vascular permeability and upregulation of VEGF in the retinal structure. However, this abnormal pathway may lead to increase the activation of leukostasis[31-33] and significant changes in extracellular matrix (ECM) protein synthesis (Figure 2). Eventually, DAG and PKC pathway adversely affect inflammation, neovascularization, and retinal haemodynamics, which redounds to progression of DR[26].
VEGF is a crucial mediator in microvascular comp-lications of DM. Normally, numerous retinal cells such as, retinal pigment epithelial (RPE) cells, Mueller cells, and pericytes, produce VEGF[31-33]. When a hypoxia occurs VEGF is secreted much more than normal production by hypoxic retinal tissues[31]. Clinical studies have rep-orted that there is a strong correlation between DR and intraocular VEGF concentrations. Intravitreal and intracameral VEGF levels were prominently increased in patients with proliferative diabetic retinopathy (PDR)[34]. Additionally, VEGF has a crucial role in the pathogenesis of diabetic macular edema (DME) by increasing vascular permeability[35,36].
AGEs have been implicated in several diabetic complications, such as DR, and DME. Under chronic hyperglycemic circumstances, proteins are nonenzymatically glycated and the excessive amount of AGEs alter structures and functions of ECM, basement membranes, and vessel wall.
Oxidative stress is also a serious condition that may result in microvascular complications[37,38]. Severe prod-uction of reactive oxygen radicals may increase the oxidative stress and reduce antioxidant capacity[39].
RAAS is the endocrine system that takes an essential role to regulate vascular blood pressure, electrolyte, and fluid balance and shows an aberration in patients with DM[40], although the accurate process of RAAS leads to DR is not well clarified.
Inflammation is a prominent part of the pathogenesis of DR[41,42]. In response to hyperglycemic stress, AGE formation, and hypertension, a sequence of inflammatory mediators are increased in DM. Retinal subclinical inflammation contributes to elevated intraocular perfusion pressure by means of endothelial nitric oxide synthase (eNOS), the development of neovascularization (NV) due to hypoxia and VEGF. Although there are no strong association between systemic inflammation and development of DR[43,44], leukostasis is a likely to be a significant local factor in DR pathogenesis, causing capillary occlusion.
Previously, DR was classified into three forms, such as, background, pre-proliferative, and proliferative DR. The current classification is based on the location, extent, and degree of various clinically significant features, such as microaneurysms, intraretinal hemorrhages, venous abnormaities such as beading, intraretinal microvascular abnormalities (IRMA), and NV. Recently, DR is classified as either nonproliferative or proliferative.
Nonproliferative diabetic retinopathy: (1) Mild non-proliferative diabetic retinopathy (NPDR): There are a few microaneurysms; (2) Moderate NPDR: In this form, there are less than 20 microaneurysms. Hard yellow exudates, cotton wool spots, and venous beading are present also in only one quadrant; (3) Severe NPDR: It is identified as any of following clinic features; Microaneurysms in all 4 quadrants; Venous beading in 2 or more quadrants; IRMA in 1 or more quadrant; and (4) Very severe NPDR: This form includes 2 or more of the criteria for severe NPDR.
PDR: As a response to ischemia, NV grows at the optic nerve (NVD) and elsewhere in the retina except the optic disc (NVE). In general, NV grows at the border zone of perfused and non-perfused retina. These new vessels are permeable, and the leakage of plasma contents probably causes a structural change in the adjacent vitreous. Also, NV may cause preretinal and subhyaloid vitreous hemorrhages and can become membrane formations on the posterior hyaloid surface.
Macular edema is defined as retinal thickening or the existence of hard exudates at 2 disk diameter of the macula. Diabetic macular edema (DME) is the most common cause of moderate or severe visual loss in diabetic patients. DME occurs apart from the stage of DR, so it should be evaluated independently. In diabetic eyes, central macular thickness does not correlate directly with visual acuity, but there is a vigorous link between the unity of the photoreceptor inner/outer segment junction and visual acuity[45].
The Early Treatment Diabetic Retinopathy Study (ETDRS) described the clinically significant macular edema (CSME) as the following conditions: (1) Retinal thickening within 500 microns of the center of the fovea; (2) Hard yellow exudate within 500 microns of the center of the fovea with adjacent retinal thickening; and (3) Retinal thickening 1 disc area or larger, any part of which is within 1 disc diameter of the center of the fovea.
The ETDRS indicated that the presence of CMSE guide ophthalmologyst for the focal laser treatment.
Optical coherence tomography (OCT) shows four diff-erent types of DME: Sponge like retinal swelling, cystoid macular edema (CME), macular edema with serous retinal detachment (SRD) and tractional macular edema (TDME)[46-48].
Sponge like retinal swelling: There is an increased diffuse retinal thickness with reduced intraretinal reflectivity. This type of retinal swelling has a better visual outcome than the CME, SRD and TRD types after laser treatment[49].
CME: In this type, there is diffuse or focal retinal thick-ening with intraretinal cystic spaces.
SRD: There is an accumulation of subretinal fluid below reflective elevation. It is possible to confirm the presence of SRD only by OCT.
TDME: TDME is identified by a hyperreflective mem-brane on OCT with loss of foveal depression and macular edema.
The WESDR study represented that, for type 1 diabetic patients, the frequency of NPDR at less than 5 years was 17% and the frequency of PDR was nearly 0%[50]. These frequencies were nearly 99%, and 50% after 20 years later, respectively. So, the first eye exam should be performed almost 4 years after diagnosis with annual follow-up exams.
The same study indicated that, for type 2 diabetic patients, the frequency of NPDR at 5 years was nearly 30% and the frequency of PDR was nearly 2%[51]. These frequencies were nearly 80%, and 15% after 15 years later, respectively. So, the first eye exam should be examined at diagnosis with annual follow-up exams.
Mild NPDR can be followed with dilated fundus exams every 12 mo. If DME that is not CSME is present, follow-up every 3 mo is advised. If CSME is present, treatment is advised promptly. Severe NPDR should be followed up every 2 mo. If very severe NPDR is present, patients should be followed more closely. After treatment of PDR, they should be observed every 3 mo not to overlook complications, such as TRD and CSME.
The treatment of DR includes increased metabolic control, laser treatment, intravitreal medication, and surgery.
Poor metabolic control is a good marker for development and progression of DR. So, related risk factor such as, hyperglycemia, hypertension, and hyperlipidemia should be controlled. It reduces the risk of retinopathy occurrence and progression[52].
The trial research group[53] showed that, for type 1 diabetic patients, a 10% reduction in the hemoglobin A1c (HbA1c) was associated with a 43% and 45% diminution in improvement of DR in the rigorous and traditional treatment group, respectively[53]. The another trial group[54] found that, for type 2 diabetic patients, tighter blood glucose control had been found to correlate most closely with a lower rate of DR[54]. However, very strict control of blood glucose may lead to cause worsening of DR due to up regulation of insulin-like growth factor-1 (IGF-1)[52,55,56].
Hypertension is more common in type 2 diabetic patients rather than patients with type 1 DM. Approximately 40%-60% of patients with hypertension are over the age range of 45 to 75[57]. Although the relationship between hypertension and progression of retinopathy is not certain, good blood pressure control pulls down the risk of DR. An another study[58] reported that strict control of blood pressure reduces the risk of diabetic ocular complications[58].
There is a positive correlations between the severity of DR and plasma lipid levels, particularly LDL-HDL cholesterol ratio[59]. Hard yellow exudates, which are lipid rich, have been found to correlate with plasma protein levels. Dietary and medicine therapy may reduce hard exudates[60,61]. Systemic lipid-lowering drugs such as, fenofibrate reduced the need for focal laser treatment of CSME in type 2 diabetic patients[62].
Laser treatment has been considered the evidence-based treatment for DME and PDR for a long time. Randomized studies have demonstrated the efficacy of laser photocoagulation to prevent vision loss from DME[63,64]. In eyes observed with CSME, prompt photocoagulation is highly recommended. Treatment is performed at areas of focal leaking microaneurysms by using focal laser photocoagulation or at areas of diffuse leakage by using grid laser photocoagulation. Laser spot size should not be greater than 100 μm for focal laser treatment. Grid laser treatment is characterized by mild RPE whitening spots as far as 2 optic disks diameters from the center of the fovea[65]. Combination treatment is applied in most patients, which involves focal and grid laser treatment.
Patients are reevaluated for retreatment at 3 mo intervals. For each retreatment, clinicians repeat the flu-orescein angiogram to determine sites of persistent dye leakage. If patients have focal leakage with a circinate lipid ring, it may not be necessary to repeat angiogram before the treatment because the leaking focal lesions are in the lipid ring.
Panretinal laser photocoagulation (PRP) treatment became a standard of care for DR when the results of the Diabetic Retinopathy Study (DRS) were published[66,67]. DRS showed that PRP enormously reduced the risk of severe vision loss from 16% to 6.4% in patient with PDR. The goal of PRP is not to improve visual acuity. It is applied to regress of the NVD or NVE and to prevent the blinding complications of DRP. Generally, laser treatment should be performed over a period of 4-6 wk by applying 1.500-2.000 burns, with a size of 500 μm, spacing spots 0.5 burn widths from each other with a 0.1-0.2 s duration[65].
The results of several investigations showed that these different intravitreal agents are effective not only in the prevention of visual loss, but also allowed a regain of visual acuity. The two main categories of intravitreal drugs recently used in the management of DME and PDR are steroids and anti-VEGF agents.
The use of intravitreal steroids are preferred to manage the DME. They have antiinflammatory and antiangiogenic effects that stabilize of the inner blood-retina barrier. Intraocular steroid injections have bene-ficial effects in PDR, by inhibiting production of the VEGF[68,69]. Many various studies reported the benefits of injections of triamcinolone acetonide (IVTA) to reduce DME and increase visual acuity[70-74].
The effects of intravitreal steroids are temporary and last for about 3 mo. In this cases, intravitreal steroids may be repeated. But complications such as elevated intraocular pressure and infection may occur. However, IVTA is more likely to be associated with cataract progression. Combination of IVTA and laser treatment has more beneficial effects in pseudophakic eyes than laser alone[74].
Recently, a novel, biodegradable, slow-release de-xamethasone implant (DEX implant, Ozurdex) was developed to gradually release 0.7 mg of preservative-free dexamethasone in the vitreous cavity after a small incision[75]. DEX implant have the advantage of a lower incidence of cataract and glaucoma than IVTA[76]. The maximum effects of the DEX implant occur at 3 mo and gradually diminish from month 4 to 6[77].
Anti-VEGF agents (pegaptanib, bavacizumab, ranibizumab, aflibercept) have been investigated as a treatment for DME and for PDR. Also, anti-VEGF injections might be useful adjuncts to facilitate effective fibrovascular membrane dissection in eyes with active vascularity components[78]. TRD occur or progress within 1-4 wk of anti-VEGF injection, so, in general, these cases should be scheduled in a timely manner after the injection[79].
Nowadays, clinicians have the option of four anti VEGF agents: Pegaptanib (Macugen), Bevacizumab (Avastin), Ranibizumab (Lucentis), Aflibercept (Eylea).
Pegaptanib is a selective VEGF antagonist that binds to the VEGF165 isoform. Intravitreal pegaptanib is currently an approved treatment in neovascular choroidal membrane, but several trials addressed the efficacy and safety of intravitreal pegaptanib injections in the treatment of PDR and DME[80-82].
Bevacizumab[83] is a full-size humanized antibody that binds to all VEGF-A isoform. Intravitreal bevacizumab is currently used beneficially in the off-label treatment of DR. There have been many studies with intravitreal bevacizumab injections and DME. The results of these retrospective or prospective trials showed an improvement in visual acuity and OCT outcomes. However, bevacizumab injections were also associated with short-term efficacy and a high recurrence rate[83-88].
Ranibizumab is a high affinity anti-VEGF Fab specifically designed for ophthalmic use. It binds to all isoforms of VEGF-A and related degradation products and neutralizes their biological activity. Several studies confirmed its efficacy in treating DME[89-94].
Aflibercept[95] is an intravitreally administered fusion protein that is designed to bind both the VEGF-A and the placental growth factor with higher affinity in comparison to other anti- VEGF agents[95]. Aflibercept has a longer duration of action in the eye after intraocular injection. This new agent has been recently investigated in the treatment of DME[96,97].
Pars plana vitrectomy (PPV) is considered an option for patients not responding to combined anti-VEGF- laser and/or steroid-laser theraphy in DME[98]. PPV, including posterior hyaloid, internal limiting membrane (ILM) and epiretinal membrane (ERM) removal, might achieve DME resolution. However, the removal of the vitreous gel might improve inner retina oxygenation and thus promote the resolution of DME[98-101].
PPV was introduced in the early 1970 as a promising treatment for the severe late complications of PDR, including vitreous hemorrhage, TRD, and fibrovascular proliferation[102]. The proper timing for PPV in PDR was under discussion for a long time. The Diabetic Retinopathy Vitrectomy Study (DRVS) considered the early PPV effects compared to deferral PPV in patients with severe vitreous hemorrhage (VH)[103]. The DRVS showed that at 2-year follow up, early PPV for nonclearing VH primarily increased the chance for retaining vision ≥ 20/40. Today, PPV can be performed as early as it is needed by the patients. The aim of PPV in PDR includes removal of opacity from the vitreous space, and the removal of tractional membrane from the retinal surface. Anti-VEGF injections might be useful adjuncts to ease effective fibrovascular membrane dissection in eyes with active vascularity components[78].
Finally, enzymatic vitrectomy performed by the intravitreal injection of autologous plasmin enzyme might be effective and could be considered as an alternative for diabetic patients before performing other treatments, such as intravitreal injections of anti-VEGF or steroids, surgical vitrectomy or laser. Several investigations on enzymatic vitreolysis, such as microplasmin, showed that many agents might achieve vitreous dissolution, PVD, or VH clearance[104,105].
Indications for PPV in PDR: Severe nonclearing vitreous hemorrhage; Nonclearing vitreous hemorrhage; Premacular subhyaloid hemorrhage; TRD involving the fovea; Tractional and rhegmatogenous retinal detach-ment; Macular edema due to vitreomacular traction; Nontractional macular edema that is refractory to pharmacotherapy and laser therapy.
Diabetic papillopathy (DP) is an uncommon ocular manifestation of DM identified by unilateral or bilateral disk swelling associated with minimal or no optic nerve dysfunction[106-108]. DP, which is self-limited disease, was repoted in 1971 in T1DM patients for the first time[109]. So, it is very difficult to predict the exact incidence of DP. The prevalence of DP in both types of DM is about 0.5%, regardless of glycemic control and seriousness of DRP[106-108]. The percentage of patients with DP presenting a NPDRP is higher than in the PDRP.
The pathophysiology is not fully understood and several theories have been suggested. There are no links between DP and either DRP or metabolic control. Some researchers suggest that DP is a subtype of non-arteritic anterior ischemic optic neuropathy (NAION), but there are some differential features between NAION and DP, for insance, DP is an asymptomatic optic disc edema, whereas NAION is an acute optic disc infarction[110,111]. However, the most plausible mechanism responsible for DP is a limited impairment to the peripapillary vascular network, and superficial capillary network endothelial cells[111,112].
The other causes of disk swelling, and PDRP with NV on the disc have been ruled out to verify the diagnosis of DP[113]. DP, which occurs generally in patients with uncontrolled diabetes, has following features: painless visual loss, macular edema, disk hyperfluorescence on fluorescein angiography, and significant visual improvement after the treatment[106].
However, several diseases can imitate DP, such as infection, inflammation, metastatic infiltration, hyper-tension, and papilledema[106,108,114]. Pseudopapilloedema, that is seen in patients with disc drusen[113], can be confused with DP.
In order to reach differential diagnosis, investigations are required, such as fluorescein angiography, orbital magnetic resonance imaging, blood tests including serum angiotensin-converting enzyme, anti nuclear antibody, vitamin B12, folate, erythrocyte sedimentation rate, C reactive protein, and fluorescent treponemal antibody test.
So far, definitive treatment has not been found to change its native progression, as in most cases the disc edema resolves within a few months with no visual impairment. Intravitreal anti-VEGF injection increased visual acuity and decreased disk edema in patients with DP[114-117]. At the same time, it is unknown that how anti-VEGF agents affect to the patients with DP. Another study showed that periocular corticosteroids stabilize the blood-ocular barrier at the disc and the macula and causes resolution of the disc and macular edema[118]. Some degree of optic atrophy is seldom present after treatment. Tight control of blood pressure optimises the visual outcome.
Association of DM and glaucoma has been investigated much in the literature. DM is the major etiologic factor for neovascular glaucoma (NVG)[119]. However, the association of DM with other types of glaucoma such as open angle glaucoma (OAG) and angle closure glaucoma (ACG) is controversial. Since glaucoma is a type of optic neuropathy and DM alone could cause optic neuropathy, a complex relation may occur between DM and glaucomatous optic neuropathy. On the other hand, central corneal thickness (CCT) is found to be thicker in patients with DM that could cause higher intraocular pressure (IOP) readings[120]. Since the mechanisms of glaucoma subtypes are different from each other; it would be more logical to investigate the association of glaucoma subtypes individually with DM.
OAG is one of the most common causes of vision loss worldwide. In several studies, DM was reported as a risk factor for OAG, along with other risk factors such as elevated IOP, older age, family history of glaucoma and black race[121-123]. It was found that as the duration of type 2 DM increases, risk of having OAG also increases[123]. On the other hand, an association of having a history of DM and risk of OAG was not found in several studies[124,125]. It is possible that diabetic patients are more likely to have an ocular examination than the general population and are thus more likely to be diagnosed with OAG[122]. Small vascular abnormalities including optic nerve vessels and oxidative damage are some of the possible mechanisms by which DM might increase risk of OAG[122]. In the aspect of treatment, OAG patients with DM undergoing trabeculectomy do not have the same long-term IOP control and surgical survival rate when compared with patients without DM[126]. Medical treatment, laser trabeculoplasty, and surgery (filtering surgery, aqueous drainage devices, etc.) are the treatment options.
The association between DM and ACG is not very clear. But several studies showed that DM might be considered as a risk factor for ACG[127,128]. Saw and colleagues[127] reported that diabetic patients have shallower anterior chambers than individuals without DM, irrespective of age, gender, and socioeconomic factors. Senthil et al[128] found that DM is associated with ACG, possibly because of the thicker lenses of diabetic patients. Weinreb et al[129] reported that pseudophakic pupillary block with ACG might occur in patients with DM. Also, treatment of DR with argon laser panretinal photocoagulation could cause ACG soon after the laser[130]. Medical treatment (topical, oral, and intravenous agents) and laser iridotomy are the treatment options.
NVG is a severe and intractable glaucoma type. DR is one of the most common etiologic factors for NVG. NVG might occur in cases with no retinal or optic disc neovascularization, but it is more likely seen in PDR[131]. The association of iris and angle NV with DM mostly increase with the duration of the disease and blood sugar control[132]. Although iris and angle NVs are common in DM, they do not always progress to NVG; but NVs always develop prior to IOP increase[132]. This is due to a fibrovascular membrane that occurs on the anterior surface of the iris and iridocorneal angle. This membrane then causes anterior synechiae, angle closure, and rise of IOP[131,132].
NVG may develop in diabetic patients after cataract surgery, laser posterior capsulotomy and pars plana vitrectomy[132]. NVG following these operations probably results from a combination of surgical inflammation and disruption of a barrier preventing diffusion of angiogenesis factors to the anterior segment[132]. Prompt diagnosis and treatment are very important to prevent blindness due to NVG. Panretinal photocoagulation is the key treatment method for prevention of NVG in DRP[131]. Panretinal photocoagulation laser therapy in the early stages may be efficacious in inhibiting and even reversing new vessel proliferation in the anterior segment of the eye. Medical treatment, cyclophotocoagulation, cryotherapy, and surgery (trabeculectomy with antimetabolites and valve implantation) are the other therapeutic options.
Pseudoexfoliation (Psx) has been supposed to be a generalized or systemic disorder of the extracellular matrix[133]. Psx increases the risk of glaucoma develo-pment[133]. It was reported that there is not a significant relationship between DM and psx[134]. Also, HbA1c levels do not vary among patients with DM based on psx status[134]. Ellis et al[135] found that DM is not associated with ocular hypertension. On the other hand, it was revealed that DM is significantly associated with bilateral eye involvement in normotension glaucoma, maybe due to several impaired neurovascular autoregulation processes related to DM[136].
Retinal ganglion cell death is the major cause of blindness in glaucoma. DM may increase susceptibility of retinal ganglion cells to apoptosis when there is a co-morbidity with elevated IOP in glaucoma[137]. DM disrupts vascular tissues, compromises neuro-glial functions, and thus may take a role in the pathogenesis of optic neuropathy related with glaucoma[138]. In the literature, it was shown that DM may accelerate apoptosis of retinal inner neurons, alter metabolism of astrocytes and Müller cells, and impair microglial function[138]. All of these factors contribute to visual acuity, contrast sensitivity and color vision loss in comorbidity of DM and glaucoma[138].
DM is associated with increased corneal stiffness, and corneal hysteresis which have been shown to have an effect on glaucoma risk[125,139]. IOP may increase in patients with DM due to aqueous outflow resistance in trabecular meshwork, because of glycation and crosslinking of meshwork glycoproteins[140].
Since DM is frequently found with other systemic disorders, such as hypertension, this comorbid condition may also affect glaucoma risk. Shoshani et al[141] reported that DM may interfere with normal vascular regulation and contribute to glaucoma progression. Moïse et al[142] suggested that blindness due to glaucoma may be prevented by using a regular Mediterranean diet and maintaining regular intake of vegetables in patients with DM.
Cataract, the commonest cause of curable blindness worldwide, is the opacification of the crystalline lens[143,144]. Diabetic cataract is considered a complication of DM, which can affect individuals at younger ages[145]. Cataract formation in diabetics seems to be related to the hyperglicemia or to hastened senile lens opacity. A snowflake like cataract is occured commonly in patients with insulin-dependent diabetes and more prones to progress than others.
Diabetic patients are 2-5 times more at risk for cataract formation and and are more likely to get it at an earlier age[146,147]. Although cataract frequency varies based on ethnic populations and geographic locations (ranges from 35% to 48%), it is higher in diabetics when compared to non-diabetics[148-152]. In a study by Raman et al[153], it has been indicated that the mixed cataract was more common than mono type cataract (42% vs 19%, respectively). A combination of cortical, nuclear, and posterior subcapsular cataract was the most common form of the mixed types (20%), followed by the combined posterior subcapsular cataract and cortical (16%). Among the monotype cataracts, rate of cortical cataract was the highest (15%), followed by nuclear cataract (5%) and posterior subcapsular cataract (1%)[153]. On the other hand, cataract frequency varies from 1% to 27% in patients with type 1 diabetes[154].
Several different pathogenetic mechanisms that may precipitate formation of diabetic cataracts have been proposed: increased osmotic stress caused by activation of the polyol pathway[155], non-enzymatic glycation of lens proteins[156-159], and increased oxidative stress[160-164].
In cases of high blood glucose levels in diabetic patients, the crystalline lens is exposed to a hyperosmotic aqueous humour and its glucose concentration progressively increases. During hyperglycemic conditions excess glucose to sorbitol. Sorbitol is further metabolized to fructose. In diabetic patients, the excessive accumulation of sorbitol in the crystalline lens produces a high osmotic gradient that leads to a fluid infusion to equilibrate the osmotic gradient. The accumulation of sorbitol in lens cell causes a collapse and liquefaction of lens fibers, which eventually results in the cataract formation[165,166]. Moreover, increased osmotic stress in the crystalline lens produced by excess accumulation of sorbitol initiates apoptotic process in epithelial cells which contributes to the cataractogenesis[155,167,168].
Advanced glycation occurs during normal aging but to a greater degree in diabetic patients in which it contributes the formation of lens opacity[156]. Advanced glycation produced by a nonenzymatically reaction between the piece of the excess glucose and proteins, which may leads to production of superoxide radicals and AGE formation[169]. Excessive accumulation of AGEs in the crystalline lens of diabetic patients plays an essential role in cataractogenesis[157-161].
It is well known that chronic hyperglycemia may increase the oxidant load[162] and facilitate the onset of senile cataract[163]. In diabetic eyes, antioxidant capacity is reduced free radical load is increased, which increases the susceptibility of crystalline lens to oxidative damage. The decrease in antioxidant capacity is facilitated by advanced glycation and defects of antioxidant enzyme activity [164].
DM can cause anterior segment changes as well as posterior segment; therefore, a comprehensive oph-thalmologic examination including visual acuity measurement, evaluation of relative afferent pupil defect, slit-lamb biomicroscopy, gonioscopy, intraocular pressure measurement, and dilated fundus examination are mandatory. In selected cases, ancillary tests such as fundus angiography and OCT may also be useful.
The level of cataract should correspond to patient’s
visual complaints including decreased visual acuity, decreased contrast sensitivity, and glare. If the biomicro-scopic examination shows mild cataract but the patient reports severe visual dysfunction, other ocular diabetic complications such as DR should be investigated. Recently, there has been a shift in emphasis towards early cataract removal in diabetics to enable adequate identification for examination of posterior segment, and facilitate panretinal photocoagulation and treatment of underlying macular edema[170]. Pre-existing PDR and macular edema may exacerbate after cataract surgery[171] which contributes to the poor visual outcomes[172]. Therefore if posterior segment is visualized, diabetic patients with pre-existing retinopathy should be preoperatively treated.
First of all, good blood glucose control is main goal to prevention of diabetic cataract. It has however been suggested that cataractogenesis can be prevented through nutrition and supplementation, including high content of nutritional antioxidants[173], lower dietary carbohydrate[174] and linolenic acid intake[175], and aldose reductase inhibitors[144,176].
Currently, the main treatment for the diabetic cataract is surgery. Phacoemulsification results in better visual results, less intraocular inflammation and less capsular opacification as compared to extracapsular surgery[177]. Femtosecond assisted cataract surgery may be a better option for diabetics; however, there has been no com-parative study comparing the results of femtosecond assisted to conventional cataract surgery in diabetics. It is advisable to perform a large capsulorrhexis with a large diameter IOLs, thus allowing better visualization of the posterior segment for examination and further treatment of DR.
After cataract surgery, using topical anti-inflammatory drugs such as steroids and nonsteroidal anti-inflammatory drops may be useful to control inflammation and macular edema. Despite an uneventfully performed cataract surgery, DR and macular edema can become exacerbated after surgery, hence patients should be followed closely with fundus examinations and ancillary tests.
Ocular surface diseases, such as dry eye is frequently present in diabetic patients. Ocular surface diseases related with DM are developed in many mechanisms including abnormal ocular surface sensitivity[178,179], decreased tear production[179-181], and delayed corneal re-epithelialization[181].
Dry eye is a condition which is a complex disease of tear film and anterior surface of the cornea. The resulting changes in the ocular surface may lead to ocular discomfort, and visual disturbance. Tear osmolarity, and ocular surface inflammation[182] are also increased in diabetic patients causing dry eye disease. Burning, foreign body sensation, photophobia, blurred vision[183], and blurred vision are present in patients with dry eye. Both dry eye disease and DM increase the risk of corneal infections and scarring, in advanced disease, corneal perforation and irreversible tissue damages[184] may occur. Patients with dry eye have serious corneal complications such as, superficial punctuate keratitis, neurotrophic keratopathy, and persistent epithelial defect[185]. Dry eye syndrome (DES) is more like to occur in the industrial country. Studies showed that approximately 1.68 million men and 3.2 million women[186] aged 50 and older are affected with DES in the United States[187]. DES, one of the most common diagnosis for diabetic patients[188], is a condition in which abnormal tear film and an changed anterior surface of the cornea is present. Studies show at least 50% of DM patients have either symptomatic or asymptomatic DES. 92 patients with diabetes types I and II have been evaluated by Seifart[189]. The patients were aged from 7 to 69 years old as well as normal healthy controls comparable in number, age and sex. The study demonstrated that 52.8 of all diabetic patients complained about eye dry symptoms, whereas 9.3% of the healthy controls complained about dry eye symptoms.
DM can lead to DES through a variety of mech-anisms[190-192], but the association between DM and DES is unclear[193]. The most possible mechanism responsible for dry eye in DM is extensive hyperglycemia bring about corneal neuropathy. Corneal neuropathy leads to tear film instability and lower tear break up time (TBUT) values due to conjunctival goblet cell loss. Mucin, which covers the villus surface of the corneal epithelium and reduce evaporative tear loss[181] is produced by conjunctival goblet cells.
The other suggested mechanisms for disruption of corneal integrity include AGE accumulation[194,195] and polyol pathway[196,197] bi-product accumulation within the corneal layers. It is believed that DM affects tear production and quality by compromising the functional integrity of the lacrimal gland. Corneal sensitivity is also reduced in DM, which affects the stimulation of basal tear production. Both lacrimal gland integrity[180] and corneal sensitivity are shown to be affected by diabetic neuropathy[180,198]. These proposed mechanisms imply that DM affects both tear production and corneal integrity, suggesting disruption to one or both may cause and lead to the exacerbation of DES.
During routine eye examination clinicians should be aware of dry eye in diabetic patients[199]. Dry eye index scores can be used for uncovering the presence of dry eye and for evaluating the response to therapeutic treatment. Several questionnaires are available, with the most common being the Ocular Surface Disease Index (OSDI)[200]. However, there is still no standardized dry eye disease questionnaire that is universally accepted.
The most common test for determining tear film quality in use today is the TBUT which shows the tear film stability. The TBUT value is the time from the last complete blink to the appearance of dry spot. The Schirmer test is used for measuring the aqueous tear manufacture. Normally, the Schirmer filter paper gets wet 10 mm for 5 min. A result yielding less than 5mm shows aqueous tear deficiency. Fluorescein is useful in assessing dry eye where its application can detect the epithelial defects due to dry eye disease.
Risk factors for DES include duration of DM and higher HbA1c levels[188,201]. So, strict blood glucose control and close follow-up reduce the risk of DES[188].
DES may cause loss of vision, scarring, perforation, and corneal infection. If patients with dry eye are treated in time, there will be no complications of DES[185]. The patients should be treated with tear supplements called “artificial tears” which contains surfactans, different viscosity agents, and electrolytes[202].
Dry eye disease is the outcome of many factors resulting in inflammation of the cornea and conjunctiva. Artificial tears can reduce blurred vision, and the symptoms of dry eye, temporarily. These agents do not contain the cytokines and growth factors which are comprised in normal tears and do not have direct anti-inflammatory effect[203,204]. Anti-inflammatory drugs are widely used for the treatment of DES. The most widely used anti-inflammatory agents are topical corticosteroids, NSAID, and cyclosporine A[203-205].
Corticosteroids can reduce the symptoms and signs of dry eye[206] to control inflammatory process. On the other hand, after long-term use, steroids produce severe side effects such as bacterial, viral, and fungal infection, elevated IOP, and cataract formation. NSAIDs are increasingly used as dry eye treatment instead of steroids because of their non-severe side effects. Topical cyclosporine A are used to increase tear production[207] and the number of goblet cells decreased by chronic inflammation due to dry eye disease[207].
DM can trigger acceleration of ocular surface abnor-malities which have been termed diabetic keratopathy[208]. In contrast to healthy persons, patients with diabetes have corneal epithelial erosions that may recur and be associated with unresponsiveness to conventional treatment regimens[209-211]. This clinical condition is known as diabetic keratopathy[212-214]. Diabetic keratopathy includes various symptomatic corneal conditions, such as, punctate keratopathy and persistent corneal epithelial defect[208].
Diabetic keratopathy is a common complication of patients with evidence of DR. A study reported that several symptomatic corneal epithelial lesions have been occured in diabetic patients at the rate of 47% to 64%[208]. In another study, authors showed that the incidence of diabetic keratopathy in diabetic patients with DR was 2 times greater than that of patients without DR[215]. Several studies reported that the incidence of diabetic keratopathy increased following pars plana vitrectomy[216,217], penetrating keratoplasty[218], laser iridectomy[219], and refractive surgery[220] in diabetic patients.
Several pathophysiological abnormalities have been shown in diabetic keratopathy, including, an abnormally thickened and discontinuous basement membrane, ab-normal adhesion between the stroma and basement membrane[219-223], increased epithelial fragility[206], de-creased epithelial healing rates, increased sorbitol con-centrations[224], decreased oxygen consumption and up-take[225], increase in the polyol metabolism[196], decreased or alter epithelial hemidesmosomes, and increased glycosy-ltransferas activity[214,226].
Recently, studies have demonstrated[194,195,227] that there is a relationship between AGE and development of diabetic keratopathy. Increased AGE in the laminin of the corneal epithelial basement membrane causes abnormal weak attachment between the basal cells and basement membrane of the cornea in diabetics[194]. Also, the loss of the corneal sensation and neural stimulus have been regarded as the reason of the development of diabetic keratopathy[228]. Axonal degeneration of corneal unmyelinated nerves occurs under chronic hyperglycemic conditions.
Diabetic keratopathy is a condition that can result in blindness and should be closely monitored. Early diagnosis and treatment of diabetic keratopathy, particularly, before corneal complications occur, is very crucial. If the diagnosis is late, patients will become resistance to the routine treatment of corneal defects. Nonhealing corneal epithelial erosion may also occur after pars plana vitrectomy for advanced PDR[208,211]. If corneal epithelium is removed manually for clarity by surgeons, this conditions may accelerate dramatically. So, when diabetic patients are examined after vitrectomy their corneas should be examined carefully.
Keratopathy is generally treated with artificial tears, and antibiotics. Additionally, bandage contact lens, and tarsorrhaphy can be used for re-epithelialization. In selected cases new treatments modalities will be used such as, topical administration of naltrexone, nicergoline[229], aldose reductase inhibitor[194,214,230], and some growth hormones[231] to accelerate re-epithelialization. All of these drugs were associated with a high corneal epithelial wound healing rate.
Recently, new topical drugs such as substance P and IGF-1 were tested on diabetic animals to accelerate re-epithelialization. Successful outcomes were obtained with these new drugs[231]. Corneal epithelial barrier function was improved by topical aldose reductase inhibitors, but superficial punctate keratopathy could not be prevented by these topical drugs. Aminoguanidine had beneficial effects in corneal epithelial defects, by improving attach-ment between the epithelial cells and basement membrane of the cornea[185,194]. The in vivo beneficial effect of amino-guanidine were unknown[194]. In additional to these new drugs, amniotic membrane transplantation is used to treat persistent corneal epithelial defects[232].
DM and its ocular complications remain a major cause of blindness despite increased understanding of these ocular conditions and identification of successful treatments. All of diabetic ocular complications can be prevented by early diagnosis and theraphy. Therefore, periodic eye examinations are required for the reduction of diabetes-related vision loss. Good blood glucose control and other systemic risk factors such as hypertension, and hyperlipidemia are main goal to prevention of ocular complications of DM.
| 1. | Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 431] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 2. | Forbes JM, Soldatos G, Thomas MC. Below the radar: advanced glycation end products that detour “around the side”. Is HbA1c not an accurate enough predictor of long term progression and glycaemic control in diabetes? Clin Biochem Rev. 2005;26:123-134. [PubMed] |
| 3. | Jang C, Lim JH, Park CW, Cho YJ. Regulator of Calcineurin 1 Isoform 4 (RCAN1.4) Is Overexpressed in the Glomeruli of Diabetic Mice. Korean J Physiol Pharmacol. 2011;15:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2650] [Cited by in RCA: 2700] [Article Influence: 180.0] [Reference Citation Analysis (3)] |
| 5. | International Diabetes Federation. The Diabetes Atlas 2006. 3rd ed. Available from: http: //www.idf.org/sites/default/files/Diabetes-Atlas-3rd-edition.pdf. |
| 6. | International Diabetes Federation. The Diabetes Atlas 2011. 5th ed. Available from: http: //www.drsharma.ca/world-diabetes-atlas-5th-edition.html. |
| 7. | Threatt J, Williamson JF, Huynh K, Davis RM. Ocular disease, knowledge and technology applications in patients with diabetes. Am J Med Sci. 2013;345:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Singh PP, Mahadi F, Roy A, Sharma P. Reactive oxygen species, reactive nitrogen species and antioxidants in etiopathogenesis of diabetes mellitus type-2. Indian J Clin Biochem. 2009;24:324-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 9. | Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 371] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Aiello LM. Perspectives on diabetic retinopathy. Am J Ophthalmol. 2003;136:122-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Klein R, Klein B. National Diabetes Data Group. Diabetes in America. 2. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Vision disorders in diabetes. USA: Bethesda, MD 1995; 293-337. |
| 12. | Garg S, Davis RM. Diabetic Retinopathy Screening Update. Clinical Diabetes Fall. 2009;4:140-145. [DOI] [Full Text] |
| 13. | Kumari S, Panda S, Mangaraj M, Mandal MK, Mahapatra PC. Plasma MDA and antioxidant vitamins in diabetic retinopathy. Indian J Clin Biochem. 2008;23:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1088] [Cited by in RCA: 1041] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 15. | Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 674] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 16. | Kaštelan S, Tomić M, Pavan J, Orešković S. Maternal immune system adaptation to pregnancy--a potential influence on the course of diabetic retinopathy. Reprod Biol Endocrinol. 2010;8:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Varma R. From a population to patients: the Wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology. 2008;115:1857-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 18. | Klein R. Epidemiology of Diabetic Retinopathy. Diabetic Retinopathy. Totowa: Humana Press 2008; . |
| 19. | Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004;122:1631-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | White NH, Cleary PA, Dahms W, Goldstein D, Malone J, Tamborlane WV. Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr. 2001;139:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 317] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Naruse K, Nakamura J, Hamada Y, Nakayama M, Chaya S, Komori T, Kato K, Kasuya Y, Miwa K, Hotta N. Aldose reductase inhibition prevents glucose-induced apoptosis in cultured bovine retinal microvascular pericytes. Exp Eye Res. 2000;71:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Kowluru RA. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxid Redox Signal. 2005;7:1581-1587. [PubMed] |
| 23. | Stitt AW. The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp Mol Pathol. 2003;75:95-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Chu J, Ali Y. Diabetic Retinopathy: A Review. Drug Dev Res. 2008;69:1-14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 307] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 286] [Article Influence: 22.0] [Reference Citation Analysis (1)] |
| 27. | Gabbay KH. Hyperglycemia, polyol metabolism, and complications of diabetes mellitus. Annu Rev Med. 1975;26:521-536. [PubMed] |
| 28. | Kinoshita JH. A thirty year journey in the polyol pathway. Exp Eye Res. 1990;50:567-573. [PubMed] |
| 29. | Gabbay KH. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973;288:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 530] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 30. | Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006;27:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 264] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 31. | Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995;113:1538-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 364] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 32. | Simorre-Pinatel V, Guerrin M, Chollet P, Penary M, Clamens S, Malecaze F, Plouet J. Vasculotropin-VEGF stimulates retinal capillary endothelial cells through an autocrine pathway. Invest Ophthalmol Vis Sci. 1994;35:3393-3400. [PubMed] |
| 33. | Adamis AP, Shima DT, Yeo KT, Yeo TK, Brown LF, Berse B, D’Amore PA, Folkman J. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993;193:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 258] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2594] [Cited by in RCA: 2655] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 35. | Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 360] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 36. | Murata T, Ishibashi T, Khalil A, Hata Y, Yoshikawa H, Inomata H. Vascular endothelial growth factor plays a role in hyperpermeability of diabetic retinal vessels. Ophthalmic Res. 1995;27:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Zong H, Ward M, Stitt AW. AGEs, RAGE, and diabetic retinopathy. Curr Diab Rep. 2011;11:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 38. | Cui Y, Xu X, Bi H, Zhu Q, Wu J, Xia X, Qiushi Ren PC. Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Exp Eye Res. 2006;83:807-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1773] [Cited by in RCA: 1836] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 40. | Wilkinson-Berka JL. Angiotensin and diabetic retinopathy. Int J Biochem Cell Biol. 2006;38:752-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 527] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 42. | Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 516] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 43. | Klein BE, Knudtson MD, Tsai MY, Klein R. The relation of markers of inflammation and endothelial dysfunction to the prevalence and progression of diabetic retinopathy: Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol. 2009;127:1175-1182. [PubMed] |
| 44. | Nguyen TT, Alibrahim E, Islam FM, Klein R, Klein BE, Cotch MF, Shea S, Wong TY. Inflammatory, hemostatic, and other novel biomarkers for diabetic retinopathy: the multi-ethnic study of atherosclerosis. Diabetes Care. 2009;32:1704-1709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol. 2010;150:63-67.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (2)] |
| 46. | Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999;127:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 438] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 47. | Kim NR, Kim YJ, Chin HS, Moon YS. Optical coherence tomographic patterns in diabetic macular oedema: prediction of visual outcome after focal laser photocoagulation. Br J Ophthalmol. 2009;93:901-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Yamamoto S, Yamamoto T, Hayashi M, Takeuchi S. Morphological and functional analyses of diabetic macular edema by optical coherence tomography and multifocal electroretinograms. Graefes Arch Clin Exp Ophthalmol. 2001;239:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Otani T, Kishi S. Tomographic assessment of vitreous surgery for diabetic macular edema. Am J Ophthalmol. 2000;129:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1043] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 51. | Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 946] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 52. | Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1998;116:874-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 364] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 53. | The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 742] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 54. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 12805] [Article Influence: 457.3] [Reference Citation Analysis (0)] |
| 55. | Chantelau E. Evidence that upregulation of serum IGF-1 concentration can trigger acceleration of diabetic retinopathy. Br J Ophthalmol. 1998;82:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Chantelau E, Meyer-Schwickerath R. Reversion of ‘early worsening’ of diabetic retinopathy by deliberate restoration of poor metabolic control. Ophthalmologica. 2003;217:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | RR Associates. Blood pressure and diabetes: everyone’s concern. London: British Diabetic Association 1994; . |
| 58. | Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4930] [Cited by in RCA: 4304] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 59. | Kissebah AH, Kohner EM, Lewis B, Siddiq YK, Lowy C, Fraser TR. Plasma-lipids and glucose/insulin relationship in non-insulin-requiring diabetics with and without retinopathy. Lancet. 1975;1:1104-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Duncan LJ, Cullen JF, Ireland JT, Nolan J, Clarke BF, Oliver MF. A three-year trial of atromid therapy in exudative diabetic retinopathy. Diabetes. 1968;17:458-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Houtsmuller AJ, Zahn KJ, Henkes HE. Unsaturated fats and progression of diabetic retinopathy. Doc Ophthalmol. 1980;48:363-371. [PubMed] |
| 62. | Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 773] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 63. | Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2067] [Cited by in RCA: 2034] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 64. | Olk RJ. Modified grid argon (blue-green) laser photocoagulation for diffuse diabetic macular edema. Ophthalmology. 1986;93:938-950. [PubMed] |
| 65. | Treatment techniques and clinical guidelines for photoco-agulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1987;94:761-774. [PubMed] |
| 66. | Preliminary report on effects of photocoagulation therapy. The Diabetic Retinopathy Study Research Group. Am J Ophthalmol. 1976;81:383-396. [PubMed] |
| 67. | Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology. 1978;85:82-106. [PubMed] |
| 68. | Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005;80:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 69. | Brooks HL, Caballero S, Newell CK, Steinmetz RL, Watson D, Segal MS, Harrison JK, Scott EW, Grant MB. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004;122:1801-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 70. | Audren F, Erginay A, Haouchine B, Benosman R, Conrath J, Bergmann JF, Gaudric A, Massin P. Intravitreal triamcinolone acetonide for diffuse diabetic macular oedema: 6-month results of a prospective controlled trial. Acta Ophthalmol Scand. 2006;84:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 270] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 72. | Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R, Caulin C, Gaudric A. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218-224; discussion 224-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 286] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 73. | Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E, Baumal C. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 660] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 74. | Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL, Friedman SM, Glassman AR, Miller KM. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064-1077.e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1061] [Cited by in RCA: 1043] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 75. | Haller JA, Dugel P, Weinberg DV, Chou C, Whitcup SM. Evaluation of the safety and performance of an applicator for a novel intravitreal dexamethasone drug delivery system for the treatment of macular edema. Retina. 2009;29:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 76. | Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA, Weinberg DV, Chou C, Whitcup SM. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 77. | Zucchiatti I, Lattanzio R, Querques G, Querques L, Del Turco C, Cascavilla ML, Bandello F. Intravitreal dexamethasone implant in patients with persistent diabetic macular edema. Ophthalmologica. 2012;228:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R, Patel A. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695.e1-1695.15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (1)] |
| 79. | Arevalo JF, Maia M, Flynn HW, Saravia M, Avery RL, Wu L, Eid Farah M, Pieramici DJ, Berrocal MH, Sanchez JG. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:213-216. [PubMed] |
| 80. | Gragoudas ES, Adamis AP, Cunningham ET, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1598] [Cited by in RCA: 1695] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 81. | Cunningham ET, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D’Amico DJ, Goldbaum M, Guyer DR, Katz B, Patel M. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112:1747-1757. [PubMed] |
| 82. | Loftus JV, Sultan MB, Pleil AM. Changes in vision- and health-related quality of life in patients with diabetic macular edema treated with pegaptanib sodium or sham. Invest Ophthalmol Vis Sci. 2011;52:7498-7505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, Friedman SM, Greven CM, Maturi RK, Pieramici DJ. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860-1867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 316] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 84. | Lam DS, Lai TY, Lee VY, Chan CK, Liu DT, Mohamed S, Li CL. Efficacy of 1.25 MG versus 2.5 MG intravitreal bevacizumab for diabetic macular edema: six-month results of a randomized controlled trial. Retina. 2009;29:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 85. | Arevalo JF, Sanchez JG, Wu L, Maia M, Alezzandrini AA, Brito M, Bonafonte S, Lujan S, Diaz-Llopis M, Restrepo N. Primary intravitreal bevacizumab for diffuse diabetic macular edema: the Pan-American Collaborative Retina Study Group at 24 months. Ophthalmology. 2009;116:1488-1497, 1497.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 86. | Kook D, Wolf A, Kreutzer T, Neubauer A, Strauss R, Ulbig M, Kampik A, Haritoglou C. Long-term effect of intravitreal bevacizumab (avastin) in patients with chronic diffuse diabetic macular edema. Retina. 2008;28:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 87. | Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, Boos CJ, Xing W, Egan C, Peto T. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117:1078-1086.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 375] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 88. | Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, Peto T, Egan C, Bunce C, Leslie RD. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 89. | Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399-2405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 537] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 90. | Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1269] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 91. | Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, Abraham P, Campochiaro PA. Primary End Point (Six Months) Results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2009;116:2175-2181.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 92. | Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, Boyer D, Heier JS, Abraham P, Thach AB. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 389] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 93. | Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 1009] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 94. | Filho JA, Messias A, Almeida FP, Ribeiro JA, Costa RA, Scott IU, Jorge R. Panretinal photocoagulation (PRP) versus PRP plus intravitreal ranibizumab for high-risk proliferative diabetic retinopathy. Acta Ophthalmol. 2011;89:e567-e572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 95. | Economides AN, Carpenter LR, Rudge JS, Wong V, Koehler-Stec EM, Hartnett C, Pyles EA, Xu X, Daly TJ, Young MR. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med. 2003;9:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 96. | Do DV, Schmidt-Erfurth U, Gonzalez VH, Gordon CM, Tolentino M, Berliner AJ, Vitti R, Rückert R, Sandbrink R, Stein D. The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011;118:1819-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 97. | Do DV, Nguyen QD, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R, Berliner AJ, Gao B, Zeitz O, Ruckert R. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119:1658-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 98. | Stefánsson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006;51:364-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 99. | Stefánsson E, Hatchell DL, Fisher BL, Sutherland FS, Machemer R. Panretinal photocoagulation and retinal oxygenation in normal and diabetic cats. Am J Ophthalmol. 1986;101:657-664. [PubMed] |
| 100. | Stefansson E, Landers MB, Wolbarsht ML. Increased retinal oxygen supply following pan-retinal photocoagulation and vitrectomy and lensectomy. Trans Am Ophthalmol Soc. 1981;79:307-334. [PubMed] |
| 101. | Yamamoto T, Akabane N, Takeuchi S. Vitrectomy for diabetic macular edema: the role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol. 2001;132:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 102. | Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:813-820. [PubMed] |
| 103. | Diabetic Retinopathy Vitrectomy Study Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Four-year results of a randomized trial: Diabetic Retinopathy Vitrectomy Study Report 5. Arch Ophthalmol. 1990;108:958-964. [PubMed] |
| 104. | Lopez-Lopez F, Rodriguez-Blanco M, Gómez-Ulla F, Marticorena J. Enzymatic vitreolysis. Curr Diabetes Rev. 2009;5:57-62. [PubMed] |
| 105. | Benz MS, Packo KH, Gonzalez V, Pakola S, Bezner D, Haller JA, Schwartz SD. A placebo-controlled trial of microplasmin intravitreous injection to facilitate posterior vitreous detachment before vitrectomy. Ophthalmology. 2010;117:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 106. | Regillo CD, Brown GC, Savino PJ, Byrnes GA, Benson WE, Tasman WS, Sergott RC. Diabetic papillopathy. Patient characteristics and fundus findings. Arch Ophthalmol. 1995;113:889-895. [PubMed] |
| 107. | Friedrich Y, Feiner M, Gawi H, Friedman Z. Diabetic papillopathy with macular star mimicking clinically significant diabetic macular edema. Retina. 2001;21:80-82. [PubMed] |
| 108. | Bayraktar Z, Alacali N, Bayraktar S. Diabetic papillopathy in type II diabetic patients. Retina. 2002;22:752-758. [PubMed] |
| 109. | Lubow M, Makley TA. Pseudopapilledema of juvenile diabetes mellitus. Arch Ophthalmol. 1971;85:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 110. | Hayreh SS, Zimmerman MB. Nonarteritic anterior ischemic optic neuropathy: clinical characteristics in diabetic patients versus nondiabetic patients. Ophthalmology. 2008;115:1818-1825. [PubMed] |
| 111. | Slagle WS, Musick AN, Eckermann DR. Diabetic papillopathy and its relation to optic nerve ischemia. Optom Vis Sci. 2009;86:e395-e403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Wise GN, Dollery CT, Henkind P. The Retinal Circulation. New York: Harper & Row 1971; . |
| 113. | Zachariah S, Sharfi O, Burton B, Nussey SS, Bano G. Diabetic papillopathy diagnosed on retinal screening in an asymptomatic patient. BJDVD. 2007;7:140. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 114. | Kim M, Lee JH, Lee SJ. Diabetic papillopathy with macular edema treated with intravitreal ranibizumab. Clin Ophthalmol. 2013;7:2257-2260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 115. | Al-Hinai AS, Al-Abri MS, Al-Hajri RH. Diabetic papillopathy with macular edema treated with intravitreal bevacizumab. Oman J Ophthalmol. 2011;4:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 116. | Al-Dhibi H, Khan AO. Response of diabetic papillopathy to intravitreal bevacizumab. Middle East Afr J Ophthalmol. 2011;18:243-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 117. | Willerslev A, Munch IC, Larsen M. Resolution of diabetic papillopathy after a single intravitreal injection of ranibizumab. Acta Ophthalmol. 2012;90:e407-e409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 118. | Mansour AM, El-Dairi MA, Shehab MA, Shahin HK, Shaaban JA, Antonios SR. Periocular corticosteroids in diabetic papillopathy. Eye (Lond). 2005;19:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 119. | Al-Shamsi HN, Dueker DK, Nowilaty SR, Al-Shahwan SA. Neovascular glaucoma at king khaled eye specialist hospital - etiologic considerations. Middle East Afr J Ophthalmol. 2009;16:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 120. | Ozdamar Y, Cankaya B, Ozalp S, Acaroglu G, Karakaya J, Ozkan SS. Is there a correlation between diabetes mellitus and central corneal thickness? J Glaucoma. 2010;19:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 121. | Bonovas S, Peponis V, Filioussi K. Diabetes mellitus as a risk factor for primary open-angle glaucoma: a meta-analysis. Diabet Med. 2004;21:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 122. | Newman-Casey PA, Talwar N, Nan B, Musch DC, Stein JD. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology. 2011;118:1318-1326. [PubMed] [DOI] [Full Text] |
| 123. | Chopra V, Varma R, Francis BA, Wu J, Torres M, Azen SP. Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:227-232.e1. [PubMed] |
| 124. | de Voogd S, Ikram MK, Wolfs RC, Jansonius NM, Witteman JC, Hofman A, de Jong PT. Is diabetes mellitus a risk factor for open-angle glaucoma? The Rotterdam Study. Ophthalmology. 2006;113:1827-1831. [PubMed] |
| 125. | Tan GS, Wong TY, Fong CW, Aung T. Diabetes, meta-bolic abnormalities, and glaucoma. Arch Ophthalmol. 2009;127:1354-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 126. | Law SK, Hosseini H, Saidi E, Nassiri N, Neelakanta G, Giaconi JA, Caprioli J. Long-term outcomes of primary trabeculectomy in diabetic patients with primary open angle glaucoma. Br J Ophthalmol. 2013;97:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 127. | Saw SM, Wong TY, Ting S, Foong AW, Foster PJ. The relationship between anterior chamber depth and the presence of diabetes in the Tanjong Pagar Survey. Am J Ophthalmol. 2007;144:325-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 128. | Senthil S, Garudadri C, Khanna RC, Sannapaneni K. Angle closure in the Andhra Pradesh Eye Disease Study. Ophthalmology. 2010;117:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 129. | Weinreb RN, Wasserstrom JP, Forman JS, Ritch R. Pseudo-phakic pupillary block with angle-closure glaucoma in diabetic patients. Am J Ophthalmol. 1986;102:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 130. | Blondeau P, Pavan PR, Phelps CD. Acute pressure elevation following panretinal photocoagulation. Arch Ophthalmol. 1981;99:1239-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 131. | Hayreh SS. Neovascular glaucoma. Prog Retin Eye Res. 2007;26:470-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 132. | Morrison JC, Pollack IP. Neovascular glaucoma (Chapter 21). Glaucoma Science and Practice, 1st ed. New York: Thieme Medical Publishers 2003; 226-236. |
| 133. | Miyazaki M, Kubota T, Kubo M, Kiyohara Y, Iida M, Nose Y, Ishibashi T. The prevalence of pseudoexfoliation syndrome in a Japanese population: the Hisayama study. J Glaucoma. 2005;14:482-484. [PubMed] [DOI] [Full Text] |
| 134. | Wood SD, Asefzadeh B, Fisch B, Jiwani A, Lee RK, Conlin PR, Pasquale LR. The relationship between diabetes mellitus and exfoliation syndrome in a United States Veterans Affairs population: a case-control study. J Glaucoma. 2011;20:278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 135. | Ellis JD, Evans JM, Ruta DA, Baines PS, Leese G, MacDonald TM, Morris AD. Glaucoma incidence in an unselected cohort of diabetic patients: is diabetes mellitus a risk factor for glaucoma? DARTS/MEMO collaboration. Diabetes Audit and Research in Tayside Study. Medicines Monitoring Unit. Br J Ophthalmol. 2000;84:1218-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 136. | Kim C, Kim TW. Comparison of risk factors for bilateral and unilateral eye involvement in normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2009;50:1215-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 137. | Kanamori A, Nakamura M, Mukuno H, Maeda H, Negi A. Diabetes has an additive effect on neural apoptosis in rat retina with chronically elevated intraocular pressure. Curr Eye Res. 2004;28:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 138. | Nakamura M, Kanamori A, Negi A. Diabetes mellitus as a risk factor for glaucomatous optic neuropathy. Ophthalmologica. 2005;219:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 139. | Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006;141:868-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 363] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 140. | Chihara E. Myopia and diabetes mellitus as modificatory factors of glaucomatous optic neuropathy. Jpn J Ophthalmol. 2014;58:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 141. | Shoshani Y, Harris A, Shoja MM, Arieli Y, Ehrlich R, Primus S, Ciulla T, Cantor A, Wirostko B, Siesky BA. Impaired ocular blood flow regulation in patients with open-angle glaucoma and diabetes. Clin Experiment Ophthalmol. 2012;40:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 142. | Moïse MM, Benjamin LM, Doris TM, Dalida KN, Augustin NO. Role of Mediterranean diet, tropical vegetables rich in antioxidants, and sunlight exposure in blindness, cataract and glaucoma among African type 2 diabetics. Int J Ophthalmol. 2012;5:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 143. | Kothadia AD, Shenoy AM, Shabaraya AR, Rajan MS, Viradia UM, Patel NH. Evaluation of cataract preventive action of phycocyanin. Int J Pharm Sci Drug Res. 2011;3:42-44. [DOI] [Full Text] |
| 144. | Kato A, Yasuko H, Goto H, Hollinshead J, Nash RJ, Adachi I. Inhibitory effect of rhetsinine isolated from Evodia rutaecarpa on aldose reductase activity. Phytomedicine. 2009;16:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 145. | Falck A, Laatikainen L. Diabetic cataract in children. Acta Ophthalmol Scand. 1998;76:238-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 146. | Klein BE, Klein R, Moss SE. Incidence of cataract surgery in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Am J Ophthalmol. 1995;119:295-300. [PubMed] |
| 147. | Klein BE, Klein R, Wang Q, Moss SE. Older-onset diabetes and lens opacities. The Beaver Dam Eye Study. Ophthalmic Epidemiol. 1995;2:49-55. [PubMed] |
| 148. | Foster PJ, Wong TY, Machin D, Johnson GJ, Seah SK. Risk factors for nuclear, cortical and posterior subcapsular cataracts in the Chinese population of Singapore: the Tanjong Pagar Survey. Br J Ophthalmol. 2003;87:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 149. | Nirmalan PK, Robin AL, Katz J, Tielsch JM, Thulasiraj RD, Krishnadas R, Ramakrishnan R. Risk factors for age related cataract in a rural population of southern India: the Aravind Comprehensive Eye Study. Br J Ophthalmol. 2004;88:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 150. | Husain R, Tong L, Fong A, Cheng JF, How A, Chua WH, Lee L, Gazzard G, Tan DT, Koh D. Prevalence of cataract in rural Indonesia. Ophthalmology. 2005;112:1255-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 151. | Dandona L, Dandona R, Naduvilath TJ, McCarty CA, Mandal P, Srinivas M, Nanda A, Rao GN. Population-based assessment of the outcome of cataract surgery in an urban population in southern India. Am J Ophthalmol. 1999;127:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 152. | Chen SJ, Liu JH, Shih HC, Chou P, Tsai CY, Tung TH. Prevalence and associated factors of lens opacities among Chinese type 2 diabetics in Kinmen, Taiwan. Acta Diabetol. 2008;45:7-13. [PubMed] |
| 153. | Raman R, Pal SS, Adams JS, Rani PK, Vaitheeswaran K, Sharma T. Prevalence and risk factors for cataract in diabetes: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study, report no. 17. Invest Ophthalmol Vis Sci. 2010;51:6253-6261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 154. | Bron AJ, Cheng H. Cataract and retinopathy: screening for treatable retinopathy. Clin Endocrinol Metab. 1986;15:971-999. [PubMed] |
| 155. | Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 385] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 156. | Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1029] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 157. | Araki N, Ueno N, Chakrabarti B, Morino Y, Horiuchi S. Immunochemical evidence for the presence of advanced glycation end products in human lens proteins and its positive correlation with aging. J Biol Chem. 1992;267:10211-10214. [PubMed] |
| 158. | Duhaiman AS. Glycation of human lens proteins from diabetic and (nondiabetic) senile cataract patients. Glycoconj J. 1995;12:618-621. [PubMed] |
| 159. | Lyons TJ, Silvestri G, Dunn JA, Dyer DG, Baynes JW. Role of glycation in modification of lens crystallins in diabetic and nondiabetic senile cataracts. Diabetes. 1991;40:1010-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 160. | Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ, Monnier VM. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc Natl Acad Sci USA. 1991;88:10257-10261. [PubMed] |
| 161. | Shamsi FA, Sharkey E, Creighton D, Nagaraj RH. Maillard reactions in lens proteins: methylglyoxal-mediated modifications in the rat lens. Exp Eye Res. 2000;70:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 162. | Agte VV, Tarwadi KV. Combination of diabetes and cataract worsens the oxidative stress and micronutrient status in Indians. Nutrition. 2008;24:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 163. | Jeganathan VS, Wang JJ, Wong TY. Ocular associations of diabetes other than diabetic retinopathy. Diabetes Care. 2008;31:1905-1912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 164. | Ookawara T, Kawamura N, Kitagawa Y, Taniguchi N. Site-specific and random fragmentation of Cu,Zn-superoxide dismutase by glycation reaction. Implication of reactive oxygen species. J Biol Chem. 1992;267:18505-18510. [PubMed] |
| 165. | Kinoshita JH. Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 1974;13:713-724. [PubMed] |
| 166. | Kinoshita JH. Cataracts in galactosemia. The Jonas S. Friedenwald Memorial Lecture. Invest Ophthalmol. 1965;4:786-799. [PubMed] |
| 167. | Takamura Y, Sugimoto Y, Kubo E, Takahashi Y, Akagi Y. Immunohistochemical study of apoptosis of lens epithelial cells in human and diabetic rat cataracts. Jpn J Ophthalmol. 2001;45:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 168. | Li WC, Kuszak JR, Dunn K, Wang RR, Ma W, Wang GM, Spector A, Leib M, Cotliar AM, Weiss M. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol. 1995;130:169-181. [PubMed] |
| 169. | Stitt AW. The maillard reaction in eye diseases. Ann N Y Acad Sci. 2005;1043:582-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 170. | Chew EY, Benson WE, Remaley NA, Lindley AA, Burton TC, Csaky K, Williams GA, Ferris FL. Results after lens extraction in patients with diabetic retinopathy: early treatment diabetic retinopathy study report number 25. Arch Ophthalmol. 1999;117:1600-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 171. | Pollack A, Dotan S, Oliver M. Course of diabetic retinopathy following cataract surgery. Br J Ophthalmol. 1991;75:2-8. [PubMed] |
| 172. | Squirrell D, Bhola R, Bush J, Winder S, Talbot JF. A prospective, case controlled study of the natural history of diabetic retinopathy and maculopathy after uncomplicated phacoemulsification cataract surgery in patients with type 2 diabetes. Br J Ophthalmol. 2002;86:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 173. | Pollreisz A, Schmidt-Erfurth U. Diabetic cataract-pathogenesis, epidemiology and treatment. J Ophthalmol. 2010;2010:608751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 256] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 174. | Chiu CJ, Morris MS, Rogers G, Jacques PF, Chylack LT, Tung W, Hankinson SE, Willett WC, Taylor A. Carbohydrate intake and glycemic index in relation to the odds of early cortical and nuclear lens opacities. Am J Clin Nutr. 2005;81:1411-1416. [PubMed] |
| 175. | Lu M, Taylor A, Chylack LT, Rogers G, Hankinson SE, Willett WC, Jacques PF. Dietary linolenic acid intake is positively associated with five-year change in eye lens nuclear density. J Am Coll Nutr. 2007;26:133-140. [PubMed] |
| 176. | Drel VR, Pacher P, Ali TK, Shin J, Julius U, El-Remessy AB, Obrosova IG. Aldose reductase inhibitor fidarestat counteracts diabetes-associated cataract formation, retinal oxidative-nitrosative stress, glial activation, and apoptosis. Int J Mol Med. 2008;21:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 177. | Dowler JG, Hykin PG, Hamilton AM. Phacoemulsification versus extracapsular cataract extraction in patients with diabetes. Ophthalmology. 2000;107:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 178. | Arthur SN, Peng Q, Apple DJ, Escobar-Gomez M, Bianchi R, Pandey SK, Werner L. Effect of heparin surface modification in reducing silicone oil adherence to various intraocular lenses. J Cataract Refract Surg. 2001;27:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 179. | Rosenberg ME, Tervo TM, Immonen IJ, Müller LJ, Grönhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41:2915-2921. [PubMed] |
| 180. | Cousen P, Cackett P, Bennett H, Swa K, Dhillon B. Tear production and corneal sensitivity in diabetes. J Diabetes Complications. 2007;21:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 181. | Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001;108:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 229] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 182. | The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75-92. [PubMed] |
| 183. | Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221-232. [PubMed] |
| 184. | Lubniewski AJ, Houchin KW, Holland EJ, Weeks DA, Wessels IF, McNeill JI, Cameron JD. Posterior infectious crystalline keratopathy with Staphylococcus epidermidis. Ophthalmology. 1990;97:1454-1459. [PubMed] |
| 185. | Riordan-Eva , Asbury T, Whitcher JP. Vaughan and Asbury’s General Ophthalmology. USA: McGraw-Hill Medical 2003; 308-310. |
| 186. | Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 838] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 187. | Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127:763-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 419] [Cited by in RCA: 447] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 188. | Seifart U, Strempel I. [The dry eye and diabetes mellitus]. Ophthalmologe. 1994;91:235-239. [PubMed] |
| 189. | Kaiserman I, Kaiserman N, Nakar S, Vinker S. Dry eye in diabetic patients. Am J Ophthalmol. 2005;139:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 190. | Alves Mde C, Carvalheira JB, Módulo CM, Rocha EM. Tear film and ocular surface changes in diabetes mellitus. Arq Bras Oftalmol. 2005;71:96-103. [PubMed] |
| 191. | Goebbels M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol. 2000;84:19-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 192. | Figueroa-Ortiz LC, Jiménez Rodríguez E, García-Ben A, García-Campos J. [Study of tear function and the conjunctival surface in diabetic patients]. Arch Soc Esp Oftalmol. 2011;86:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 193. | Hom M, De Land P. Self-reported dry eyes and diabetic history. Optometry. 2006;77:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 194. | Kaji Y, Usui T, Oshika T, Matsubara M, Yamashita H, Araie M, Murata T, Ishibashi T, Nagai R, Horiuchi S. Advanced glycation end products in diabetic corneas. Invest Ophthalmol Vis Sci. 2000;41:362-368. [PubMed] |
| 195. | Sato E, Mori F, Igarashi S, Abiko T, Takeda M, Ishiko S, Yoshida A. Corneal advanced glycation end products increase in patients with proliferative diabetic retinopathy. Diabetes Care. 2001;24:479-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 196. | Kinoshita JH, Fukushi S, Kador P, Merola LO. Aldose reductase in diabetic complications of the eye. Metabolism. 1979;28:462-469. [PubMed] |
| 197. | Kador PF, Kinoshita JH. Role of aldose reductase in the development of diabetes-associated complications. Am J Med. 1985;79:8-12. [PubMed] |
| 198. | Inoue K, Kato S, Ohara C, Numaga J, Amano S, Oshika T. Ocular and systemic factors relevant to diabetic keratoepitheliopathy. Cornea. 2001;20:798-801. [PubMed] |
| 199. | Jin J, Chen LH, Liu XL, Jin GS, Lou SX, Fang FN. [Tear film function in non-insulin dependent diabetics]. Zhonghua YanKe ZaZhi. 2003;39:10-13. [PubMed] |
| 200. | Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1621] [Cited by in RCA: 2160] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 201. | Akinci A, Cetinkaya E, Aycan Z. Dry eye syndrome in diabetic children. Eur J Ophthalmol. 2007;17:873-878. [PubMed] |
| 202. | Albietz JM, Bruce AS. The conjunctival epithelium in dry eye subtypes: effect of preserved and non-preserved topical treatments. Curr Eye Res. 2001;22:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 203. | Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:163-178. [PubMed] |
| 204. | Jackson WB. Management of dysfunctional tear syndrome: a Canadian consensus. Can J Ophthalmol. 2009;44:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 205. | Pflugfelder SC. Anti-inflammatory therapy of dry eye. Ocul Surf. 2003;1:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 206. | Yang CQ, Sun W, Gu YS. A clinical study of the efficacy of topical corticosteroids on dry eye. J Zhejiang Univ Sci B. 2006;7:675-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 207. | Zhou XQ, Wei RL. Topical cyclosporine A in the treatment of dry eye: a systematic review and meta-analysis. Cornea. 2014;33:760-767. [PubMed] |
| 208. | Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Trans Am Ophthalmol Soc. 1981;79:180-199. [PubMed] |
| 209. | Friend J, Thoft RA. The diabetic cornea. Int Ophthalmol Clin. 1984;24:111-123. [PubMed] |
| 210. | Datiles MB, Kador PF, Fukui HN, Hu TS, Kinoshita JH. Corneal re-epithelialization in galactosemic rats. Invest Ophthalmol Vis Sci. 1983;24:563-569. [PubMed] |
| 211. | Perry HD, Foulks GN, Thoft RA, Tolentino FI. Corneal complications after closed vitrectomy through the pars plana. Arch Ophthalmol. 1978;96:1401-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 212. | Cisarik-Fredenburg P. Discoveries in research on diabetic keratopathy. Optometry. 2001;72:691-704. [PubMed] |
| 213. | Kaji Y. Prevention of diabetic keratopathy. Br J Ophthalmol. 2005;89:254-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 214. | Sánchez-Thorin JC. The cornea in diabetes mellitus. Int Ophthalmol Clin. 1998;38:19-36. [PubMed] |
| 215. | Saini JS, Khandalavla B. Corneal epithelial fragility in diabetes mellitus. Can J Ophthalmol. 1995;30:142-146. [PubMed] |
| 216. | Chung H, Tolentino FI, Cajita VN, Acosta J, Refojo MF. Reevaluation of corneal complications after closed vitrectomy. Arch Ophthalmol. 1988;106:916-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 217. | Foulks GN, Thoft RA, Perry HD, Tolentino FI. Factors related to corneal epithelial complications after closed vitrectomy in diabetics. Arch Ophthalmol. 1979;97:1076-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 129] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 218. | Chou L, Cohen EJ, Laibson PR, Rapuano CJ. Factors associated with epithelial defects after penetrating keratoplasty. Ophthalmic Surg. 1994;25:700-703. [PubMed] |
| 219. | Jeng S, Lee JS, Huang SC. Corneal decompensation after argon laser iridectomy--a delayed complication. Ophthalmic Surg. 1991;22:565-569. [PubMed] |
| 220. | Simpson RG, Moshirfar M, Edmonds JN, Christiansen SM. Laser in-situ keratomileusis in patients with diabetes mellitus: a review of the literature. Clin Ophthalmol. 2012;6:1665-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 221. | Gipson IK, Grill SM, Spurr SJ, Brennan SJ. Hemidesmosome formation in vitro. J Cell Biol. 1983;97:849-857. [PubMed] |
| 222. | Gipson IK, Spurr-Michaud SJ, Tisdale AS. Anchoring fibrils form a complex network in human and rabbit cornea. Invest Ophthalmol Vis Sci. 1987;28:212-220. [PubMed] |
| 223. | McDermott AM, Xiao TL, Kern TS, Murphy CJ. Non-enzymatic glycation in corneas from normal and diabetic donors and its effects on epithelial cell attachment in vitro. Optometry. 2003;74:443-452. [PubMed] |
| 224. | Yue DK, Hanwell MA, Satchell PM, Turtle JR. The effect of aldose reductase inhibition on motor nerve conduction velocity in diabetic rats. Diabetes. 1982;31:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 225. | Graham CR, Richard RD, Varma SD. Oxygen consumption by normal and diabetic rat and human corneas. Ophthalmol Res. 1981;13:65–71. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 226. | Akimoto Y, Kawakami H, Yamamoto K, Munetomo E, Hida T, Hirano H. Elevated expression of O-GlcNAc-modified proteins and O-GlcNAc transferase in corneas of diabetic Goto-Kakizaki rats. Invest Ophthalmol Vis Sci. 2003;44:3802-3809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 227. | Kaji Y, Amano S, Usui T, Oshika T, Yamashiro K, Ishida S, Suzuki K, Tanaka S, Adamis AP, Nagai R. Expression and function of receptors for advanced glycation end products in bovine corneal endothelial cells. Invest Ophthalmol Vis Sci. 2003;44:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 228. | Saito J, Enoki M, Hara M, Morishige N, Chikama T, Nishida T. Correlation of corneal sensation, but not of basal or reflex tear secretion, with the stage of diabetic retinopathy. Cornea. 2003;22:15-18. [PubMed] |
| 229. | Awata T, Sogo S, Yamagami Y, Yamamoto Y. Effect of an aldose reductase inhibitor, CT-112, on healing of the corneal epithelium in galactose-fed rats. J Ocul Pharmacol. 1988;4:195-201. [PubMed] |
| 230. | Nakamura M, Kawahara M, Morishige N, Chikama T, Nakata K, Nishida T. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance P-derived peptide (FGLM-NH2) and insulin-like growth factor-1. Diabetologia. 2003;46:839-842. [PubMed] |
| 231. | Abdelkader H, Patel DV, McGhee CNj, Alany RG. New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin Experiment Ophthalmol. 2011;39:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 232. | Kheirkhah A, Casas V, Raju VK, Tseng SC. Sutureless amniotic membrane transplantation for partial limbal stem cell deficiency. Am J Ophthalmol. 2008;145:787-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
P- Reviewer: Hong YJ, Irons BK S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/