Published online Feb 15, 2015. doi: 10.4239/wjd.v6.i1.37
Peer-review started: July 3, 2014
First decision: July 29, 2014
Revised: November 18, 2014
Accepted: December 16, 2014
Article in press: December 17, 2014
Published online: February 15, 2015
Processing time: 211 Days and 20.7 Hours
Diabetic foot ulcer (DFU) is the most costly and devastating complication of diabetes mellitus, which affect 15% of diabetic patients during their lifetime. Based on National Institute for Health and Clinical Excellence strategies, early effective management of DFU can reduce the severity of complications such as preventable amputations and possible mortality, and also can improve overall quality of life. The management of DFU should be optimized by using a multidisciplinary team, due to a holistic approach to wound management is required. Based on studies, blood sugar control, wound debridement, advanced dressings and offloading modalities should always be a part of DFU management. Furthermore, surgery to heal chronic ulcer and prevent recurrence should be considered as an essential component of management in some cases. Also, hyperbaric oxygen therapy, electrical stimulation, negative pressure wound therapy, bio-engineered skin and growth factors could be used as adjunct therapies for rapid healing of DFU. So, it’s suggested that with appropriate patient education encourages them to regular foot care in order to prevent DFU and its complications.
Core tip: Diabetic foot ulcer (DFU) is the most common complication of diabetes mellitus that usually fail to heal, and leading to lower limb amputation. Early effective management of DFU as follows: education, blood sugar control, wound debridement, advanced dressing, offloading, advance therapies and in some cases surgery, can reduce the severity of complications, and also can improve overall quality of life of patients especially by using a multidisciplinary team approach.
- Citation: Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes 2015; 6(1): 37-53
- URL: https://www.wjgnet.com/1948-9358/full/v6/i1/37.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i1.37
Diabetes mellitus (DM) is one of the main problems in health systems and a global public health threat that has increased dramatically over the past 2 decades[1,2]. According to epidemiological studies, the number of patients with DM increased from about 30 million cases in 1985, 177 million in 2000, 285 million in 2010, and estimated if the situation continues, more than 360 million people by 2030 will have DM[3,4].
Patients with DM are prone to multiple complications such as diabetic foot ulcer (DFU). DFU is a common complication of DM that has shown an increasing trend over previous decades[5-7]. In total, it is estimated that 15% of patients with diabetes will suffer from DFU during their lifetime[8]. Although accurate figures are difficult to obtain for the prevalence of DFU, the prevalence of this complication ranges from 4%-27%[9-11].
To date, DFU is considered as a major source of morbidity and a leading cause of hospitalization in patients with diabetes[1,5,12,13]. It is estimated that approximately 20% of hospital admissions among patients with DM are the result of DFU[14]. Indeed, DFU can lead to infection, gangrene, amputation, and even death if necessary care is not provided[14]. On the other hand, once DFU has developed, there is an increased risk of ulcer progression that may ultimately lead to amputation. Overall, the rate of lower limb amputation in patients with DM is 15 times higher than patients without diabetes[8]. It is estimated that approximately 50%-70% of all lower limb amputations are due to DFU[8]. In addition, it is reported that every 30 s one leg is amputated due to DFU in worldwide[9]. Furthermore, DFU is responsible for substantial emotional and physical distress as well as productivity and financial losses that lower the quality of life[15]. The previous literature indicates that healing of a single ulcer costs approximately $$17500 (1998 United States Dollars). In cases where lower extremity amputation is required, health care is even more expensive at $30000-33500[16]. These costs do not represent the total economic burden, because indirect costs related to losses of productivity, preventive efforts, rehabilitation, and home care should be considered. When all this is considered, 7%-20% of the total expenditure on diabetes in North America and Europe might be attributable to DFU[17].
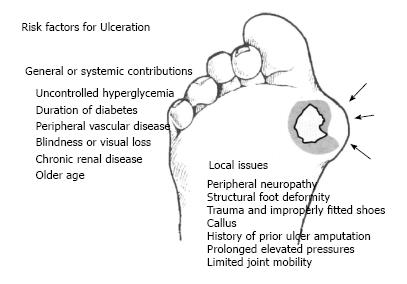
Recent studies have indicated multiple risk factors associated with the development of DFU[18-21]. These risk factors are as follows: gender (male), duration of diabetes longer than 10 years, advanced age of patients, high Body Mass Index, and other comorbidities such as retinopathy, diabetic peripheral neuropathy, peripheral vascular disease, glycated hemoglobin level (HbA1C), foot deformity, high plantar pressure, infections, and inappropriate foot self-care habits]1,12,20-22] (Figure 1).
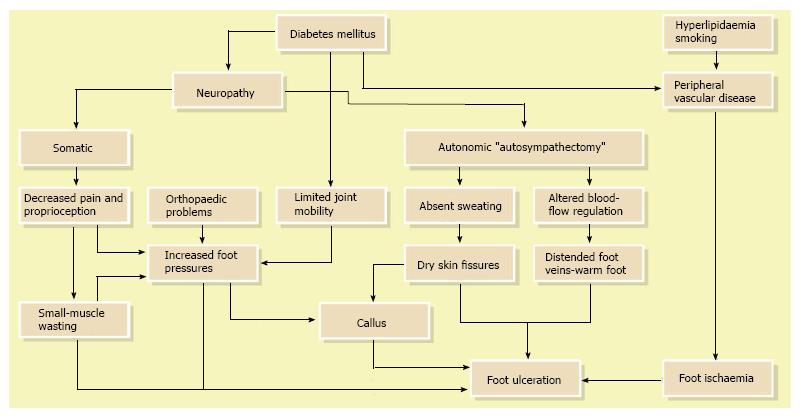
Although the literature has identified a number of diabetes related risk factors that contribute to lower-extremity ulceration and amputation, to date most DFU has been caused by ischemic, neuropathic or combined neuroischemic abnormalities[6,17] (Figure 2). Pure ischemic ulcers probably represent only 10% of DFU and 90% are caused by neuropathy, alone or with ischemia. In recent years, the incidence of neuroischemic problems has increased and neuroischemic ulcers are the most common ulcers seen in most United Kingdom diabetic foot clinics now[23].
In total, the most common pathway to develop foot problems in patients with diabetes is peripheral sensori-motor and autonomic neuropathy that leads to high foot pressure, foot deformities, and gait instability, which increases the risks of developing ulcers[24-26]. Today, numerous investigations have shown that elevated plantar pressures are associated with foot ulceration[27-29]. Additionally, it has been demonstrated that foot defor-mities and gait instability increases plantar pressure, which can result in foot ulceration[24,30].
Unfortunately, often patients are in denial of their disease and fail to take ownership of their illness along with the necessary steps to prevent complication and to deal with the many challenges associated with the management of DFU. However, numerous studies have shown that proper management of DFU can greatly reduce, delay, or prevent complications such as infection, gangrene, amputation, and even death[6,31,32].
The primary management goals for DFU are to obtain wound closure as expeditiously as possible[33,34]. As diabetes is a multi-organ systemic disease, all comor-bidities that affect wound healing must be managed by a multidisciplinary team for optimal outcomes with DFU[35-38]. Based on National Institute for Health and Clinical Excellence strategies, the management of DFU should be done immediately with a multidisciplinary team that consists of a general practitioner, a nurse, an educator, an orthotic specialist, a podiatrist, and consultations with other specialists such as vascular surgeons, infectious disease specialists, dermatologists, endocrinologists, dieticians, and orthopedic specialists[39]. Today, numerous studies have shown that a multi-disciplinary team can reduce amputation rates, lower costs, and leads to better quality of life for patients with DFU[39-41]. The American Diabetes Association has concluded that a preventive care team, defined as a multidisciplinary team, can decrease the risks associated with DFU and amputation by 50%-85%[42]. It’s suggested that with applying this approach take appropriate strategies for management of DFU to consequently reduce the severity of complications, improve overall quality of life, and increase the life expectancy of patients[36]. In this article, we review available evidence on the management of DFU as follows: education, blood sugar control, wound debridement, advanced dressing, offloading, surgery, and advanced therapies that are used clinically.
In this review article, we searched for articles published between March 1, 1980 and July 28, 2014 in the following five electronic databases: PubMed, Science Direct, Embase, Web of Science, and Scopus, for both English and non-English language articles with the following keywords: “diabetic foot ulcer”, “amputations”, “wound management”, “debridement”, “advanced dressings”, “offloading modalities”, “hyperbaric oxygen therapy”, “electrical stimulation”, “negative pressure wound therapy”, “bio-engineered skin“, “growth factors”, and “foot care” as the medical subject heading (MeSH). Study designs that were included were randomized controlled trials (RCTs), case-control studies, cohort studies, prospective and retrospective uncontrolled studies, cross-sectional studies, and review studies. Case reports and case series were excluded. We searched bibliographies for all retrieved and relevant publications to identify other studies.
It has been shown that up to 50% of DFU cases can be prevented by effective education. In fact, educating patients on foot self-management is considered the cornerstone to prevent DFU[12,43-45].
Patient education programs need to emphasize patient responsibility for their own health and well-being. The ultimate aim of foot care education for people with diabetes is to prevent foot ulcers and amputation. Currently, a wide range and combinations of patient educational interventions have been evaluated for the prevention of DFU that vary from brief education to intensive education including demonstration and hands-on teaching[46]. Patients with DFU should be educated about risk factors and the importance of foot care, including the need for self-inspection, monitoring foot temperature, appropriate daily foot hygiene, use of proper footwear, and blood sugar control[47]. However, education is better when combined with other care strategies, because previous reviews on patient education has suggested that when these methods were combined with a comprehensive approach, these methods can reduce the frequency and morbidity of the limb threatening complications caused by DFU[48].
In patients with DFU, glucose control is the most important metabolic factor. In fact, it is reported inadequate control of blood sugar is the primary cause of DFU[6,49,50].
The best indicator of glucose control over a period of time is HbA1C level. This test measures the average blood sugar concentration over a 90-d span of the average red blood cell in peripheral circulation. The higher the HbA1C level, the more glycosylation of hemoglobin in red blood cells will occur. Studies have shown that blood glucose levels > 11.1 mmol/L (equivalent to > 310 mg/mL or an HbA1C level of > 12) is associated with decreased neutrophil function, including leukocyte chemotaxis[50]. Indeed, a greater elevation of blood glucose level has been associated with a higher potential for suppressing inflammatory responses and decreasing host response to an infection[6]. Pomposelli et al[51] has indicated that a single blood glucose level > 220 mg/dL on the first postoperative day was a sensitive (87.5%) predictor of postoperative infection. Furthermore, the authors found that patients with blood glucose values > 220 mg/dL had infection rates that were 2.7 times higher than for patients with lower blood glucose values (31.3% vs 11.5%, respectively)[51]. In addition, it’s indicated that a 1% mean reduction in HbA1C was associated with a 25% reduction in micro vascular complications, including neuropathy[47]. Investigations have found that poor glucose control accelerated the manifestation of Peripheral Arterial Disease (PAD). It has been shown that for every 1% increase in HbA1C, there is an increase of 25%-28% in the relative risk of PAD, which is a primary cause of DFU[52]. However, to date, no RCT has been performed to determine whether improved glucose control has benefits after a foot ulcer has developed.
Debridement is the removal of necrotic and senescent tissues as well as foreign and infected materials from a wound, which is considered as the first and the most important therapeutic step leading to wound closure and a decrease in the possibility of limb amputation in patients with DFU[53-56]. Debridement seems to decrease bacterial counts and stimulates production of local growth factors. This method also reduces pressure, evaluates the wound bed, and facilitates wound drainage[32,57].
There are different kinds of debridement including surgical, enzymatic, autolytic, mechanical, and biological[58] (Table 1). Among these methods, surgical debridement has been shown to be more effective in DFU healing[54,59-62]. Surgical or sharp debridement involves cutting away dead and infected tissues followed by daily application of saline moistened cotton gauze[53]. The main purpose of this type of debridement is to turn a chronic ulcer into an acute one. Surgical debridement should be repeated as often as needed if new necrotic tissue continues to form[63]. It has been reported that regular (weekly) sharp debridement is associated with the rapid healing of ulcers than for less frequent debridement[59,64-66]. In a retrospective cohort study, Wilcox et al[66] indicated that frequent debridement healed more wounds in a shorter time (P < 0.001). In fact, the more frequent the debridement, the better the healing outcome.
| Method | Explanation | Advantages | Disadvantages |
| Surgical or Sharp | Callus and all nonviable soft tissues and bone remove from the open wound with a scalpel, tissue nippers, curettes, and curved scissors. Excision of necrotic tissues should extend as deeply and proximally as necessary until healthy, bleeding soft tissues and bone are encountered[59] | Only requires sterile scissors or a scalpel, so is cost-effective[55] | Requires a certain amount of skill to prevent enlarging the wound[55] |
| Mechanical | This method includes wet to dry dressings, high pressure irrigation, pulsed lavage and hydrotherapy[76], and commonly used to clean wounds prior to surgical or sharp debridement[76] | Allows removal of hardened necrosis | It is not discriminating and may remove granulating tissue It may be painful for the patients[55] |
| Autolytic | This method occurs naturally in a healthy, moist wound environment when arterial perfusion and venous drainage are maintained[18] | It’s cost-effective[55] It is suitable for an extremely painful wound[18] | It’s time consuming and may require an equivocal time for treatment[18] |
| Enzymatic | The only formulation available in the United Kingdom contains Streptokinase and Streptodornase (Varidase Topical® Wyeth Laboratories). This enzyme aggressively digests the proteins fibrin, collagen and elastin, which are commonly found in the necrotic exudate of a wound[77,78] | They can be applied directly into the necrotic area[55] | Streptokinase can be systemically absorbed and is therefore contraindicated in patients at risk of an MI It’s expensive[55] |
| Biological | Sterile maggots of the green bottle fly (Lucilia sericata) are placed directly into the affected area and held in place by a close net dressing. The larvae have a ferocious appetite for necrotic material while actively avoiding newly formed healthy tissue[79,80] | They discriminate between the necrotic and the granulating tissue[79] | There may be a reluctance to use this treatment by patients and clinicians It’s expensive[79,80] |
The method of debridement depends on chara-cteristics, preferences, and practitioner level of expertise[54]. When surgical or sharp debridement is not indicated, then other types of debridement could be used.
An older debridement type that is categorized as biological debridement is maggot debridement therapy (MDT), which is also known as maggot therapy or larval therapy. In this method, sterile and live forms of the Lucilia sericata larvae are applied to the wound to achieve debridement, disinfection, and ultimately wound healing[67-69]. Indeed, larvae secrete a powerful autolytic enzyme that liquefies necrotic tissues, stimulates the healing processes, and destroys bacterial biofilms[70-72]. This technique is indicated for open wounds and ulcers that contain gangrenous or necrotic tissues with or without infection[72]. To date, paucity of RCTs show efficacy of this method with DFU; however, some of retrospective[71,73]; and prospective[74] studies have shown MDT as a clinically effective treatment for DFU. These studies reported that MDT can significantly diminish wound odor and bacterial count, including Methicillin-Resistant Staphylococcus Aurous, prevent hospital admission, and decrease the number of outpatient visits among patients with DFU[71,73-75].
Despite the advantages of debridement, adequate debridement must always precede the application of topical wound healing agents, dressings, or wound closure procedures, which may be expensive.
The use of offloading techniques, commonly known as pressure modulation, is considered the most important component for the management of neuropathic ulcers in patients with diabetes[81,82]. Recent studies have provided evidence indicating that proper offloading promotes DFU healing [83-85].
Although many offloading modalities are currently in use (Table 2), only a few studies describe the frequency and rate of wound healing with some of the methods frequently used clinically. The choice of these methods is determined by patient physical characteristics and abilities to comply with the treatment along with the location and severity of the ulcer[82].
| Technique | Casting techniques | Footwear related techniques | Surgical offloading techniques | Other techniques |
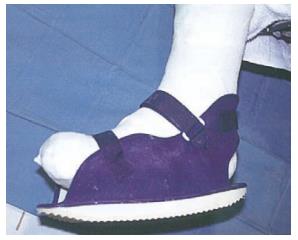
| Examples | TCC (Figure 3) | Shoes or half shoes (Figure 7) | ATL | Bed rest |
| iTCC (Figure 5) | Sandals | Liquid silicone injections/tissue augmentation | Crutches/Canes/Wheelchairs | |
| RCW (Figure 4) | Insoles | Callus debridement | Bracing (patella tendon bearing, ankle-foot orthoses) | |
| Scotch-cast boots (Figure 6) | In-shoe orthoses | Metatarsal head resection osteotomy/arthroplasty/os ectomy/ exostectomy | Walkers | |
| Windowed casts | Socks | External fixation | Offloading dressings | |
| Custom splints | Felted foam/padding | |||
| Plugs |
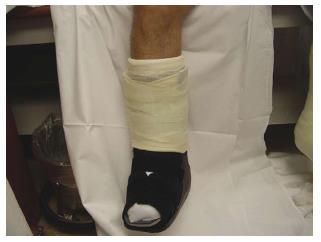
The most effective offloading technique for the treatment of neuropathic DFU is total contact casts (TCC)[82,86,87]. TCC is minimally padded and molded carefully to the shape of the foot with a heel for walking (Figure 3). The cast is designed to relieve pressure from the ulcer and distribute pressure over the entire surface of the foot; thus, protecting the site of the wound[82]. Mueller et al[87] conducted an RCT that showed TCC healed a higher percentage of plantar ulcers at a faster rate when compared with the standard treatment. In addition, a histologic examination of ulcer specimens has shown that patients treated with TCC before debridement had better healing as indicated by angiogenesis with the formation of granulation tissue than for patients treated with debridement alone as indicated by a predominance of inflammatory elements[88]. The contributory factors to the efficacy of TCC treatment are likely to be due to pressure redistribution and offloading from the ulcer area. In addition, the patient is unable to remove the cast, which thereby forces compliance, reduces activity levels, and consequently improves wound healing[84]. However, the frequency of side effects referred to in the literature and minimal patient acceptance make this approach inappropriate for wide applications[89,90]. Fife et al[91] has shown that TCC is vastly underutilized for DFU wound care in the United States. Based on this study, only 16% of patients with DFU used TCC as their offloading modalities. The main disadvantage of TCC was the need for expertise in its application. Most centers do not have a physician or cast technician available with adequate training or experience to safely apply TCC. In addition, improper cast application can cause skin irritation and in some cases even frank ulceration. Also, the expense of time and materials (the device should be replaced weekly), limitations on daily activities (e.g., bathing), and the potential of a rigid cast to injure the insensate neuropathic foot are considered other disadvantages. Furthermore, TCC does not allow daily assessment of the foot or wound, which is often contraindicative in cases of soft tissue or bone infections[36,32,83]. In some cases, it is suggested to use other kinds of offloading techniques such as a removable cast walker (RCW) or Instant TCC (iTCC).
An RCW is cast-like device that is easily removable to allow for self-inspection of the wound and application of topical therapies that require frequent administration[82,90] (Figure 4). The application of this method allows for bathing and comfortable sleep. In addition, because RCW is removable, they can be used for infected wounds as well as for superficial ulcers[82]. However, in a study that compared the effectiveness of TCC, RCW, and half-shoe, this method did not show equivalent healing time (mean healing time: 33.5, 50.4, and 61.1 d, respectively), and a significantly higher proportion of people with DFU were healed after 12 wk wearing a TCC compared with the two other widely used offloading modalities[81].
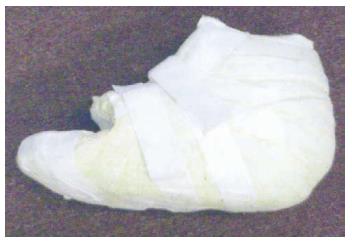
iTCC, which involves simply wrapping a RCW with a single layer of cohesive bandage, Elastoplast or casting tape (Figure 5), is another offloading technique that is shown to be more effective than TCC [92] and RCW [93]. This technique forces the patient to adhere to advice to immobilize the foot while allowing for ease of application and examination of the ulcer as needed. A preliminary randomized trial of TCC vs iTCC (Figure 6) in the management of plantar neuropathic foot ulcers has confirmed equivalent efficacy of the two devices and that iTCC is cheaper, quicker to apply, and has fewer adverse effects than traditional TCC[93]. As this device does not require a skilled technician to apply it, it could revolutionize the future management of plantar neuropathic ulcers. It has been suggested that iTCC will dramatically change the treatment of non-ischemic, neuropathic, diabetic plantar ulcers, and has the potential to replace TCC as the gold standard for offloading plantar neuropathic ulcers[92].
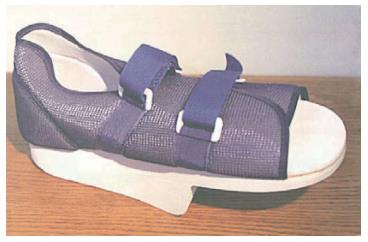
Regardless of the modality selected, patients should return to an unmodified shoe until complete healing of the ulcer has occurred (Figure 7). Furthermore, any shoe that resulted in the formation of an ulcer should not be worn again[94].
A major breakthrough for DFU management over the last decades was the demonstration of novel dressings[13,95]. Ideally, dressings should confer moisture balance, protease sequestration, growth factor stimulation, antimicrobial activity, oxygen permeability, and the capacity to promote autolytic debridement that facilitates the production of granulation tissues and the re-epithelialization process. In addition, it should have a prolonged time of action, high efficiency, and improved sustained drug release in the case of medicated therapies[95,96]. Hence, no single dressing fulfills all the requirements of a diabetic patient with a foot ulcer. The choice of dressing is largely determined by the causes of DFU, wound location, depth, amount of scar or slough, exudates, condition of wound margins, presence of infection and pain, need for adhesiveness, and conformability of the dressing[13].
Wound dressing can be categorized as passive, active, or interactive[97]. Passive dressings are used as protective functions and for acute wounds because they absorb reasonable amounts of exudates and ensure good protection. Active and interactive dressings are capable of modifying the physiology of a wound by stimulating cellular activity and growth factors release. In addition, they are normally used for chronic wounds because they adapt to wounds easily and maintain a moist environment that can stimulate the healing process[95,98]. The main categories of dressings used for DFU are as follows: films, hydrogels, hydrocolloids, alginates, foams, and silver-impregnated (Table 3).
| Type | Example | Explanation | Advantages | Disadvantages |
| Hydrocolloids | Duoderm (Convatec) Granuflex (Convatec) Comfeel (Coloplast) | These kind of dressings usually composed of a hydrocolloid matrix bonded onto a vapor permeable film or foam backing. When in contact with the wound surface this matrix forms a gel to provide a moist environment[102] | Absorbent Can be left for several days Aid autolysis[96] | Concerns about use for infected wounds May cause maceration Unpleasant odor[96] |
| Hydrogels | Aquaform (Maersk Medical) Intrasite Gel (Smith and Nephew) Aquaflo (Covidien) | These dressings consist of cross-linked insoluable polymers (i.e., starch or carboxymethylcellulose) and up to 96% water. These dressings are designed to absorb wound exudate or rehydrate a wound depending on the wound moisture levels. They are supplied in either flat sheets, an amorphous hydrogel or as beads[96] | Absorbent Donate liquid Aid autolysis[96] | Concerns about use for infected wounds May cause maceration using for highly exudative wounds[96] |
| Foams | Allevyn (Smith and Nephew) Cavicare (Smith and Nephew) Biatain (Coloplast) Tegaderm (3M) | These dressings normally contain hydrophilic polyurethane foam and are designed to absorb wound exudate and maintain a moist wound surface[103] | Highly absorbent and protective Manipulate easily[96] Can be left up to seven days Thermal insulation[96] | Occasional dermatitis with adhesive[96] Bulky[6] May macerate surrounding skin[6] |
| Films | Tegaderm (3M) Opsite (Smith and Nephew) | Film dressings often form part of the construction of other dressings such as hydrocolloids, foams, hydrogel sheets and composite dressings, which are made up of several materials with the film being used as the outer layer[107,108] | Cheap Manipulate easily Permeable to water vapor and oxygen but not to water microorganisms[95] | May need wetting before removal[96] Aren’t suitable for infected wounds[107,108] Nonabsorbent If fluid collects under film it must be drained or the film replaced[6] |
| Alginates | Calcium Alginate Dressing (Smith and Nephew Inc., Australia) Kaltostat (ConvaTec) Sorbalgon (Hartman United States, Inc.l) Medihoney (Derma Sciences Inc., Canada) | The alginate forms a gel when in contact with the wound surface which can be lifted off with dressing removal or rinsed away with sterile saline. Bonding to a secondary viscose pad increases absorbency[104] | Highly absorbent Bacteriostatic Hemostatic Useful in cavities[96] | May need wetting before removal[96] |
| Silver-impregnated | Acticoat (Smith and Nephew) Urgosorb Silver (Urgo) | These dressing used to treat infected wounds as silver ions are thought to have antimicrobial properties[109] | Antiseptic Absorbent[96] Reduce odor Improved pain-related symptoms Decrease wound exudates Have a prolonged dressing wear time[112] | High cost[96] |
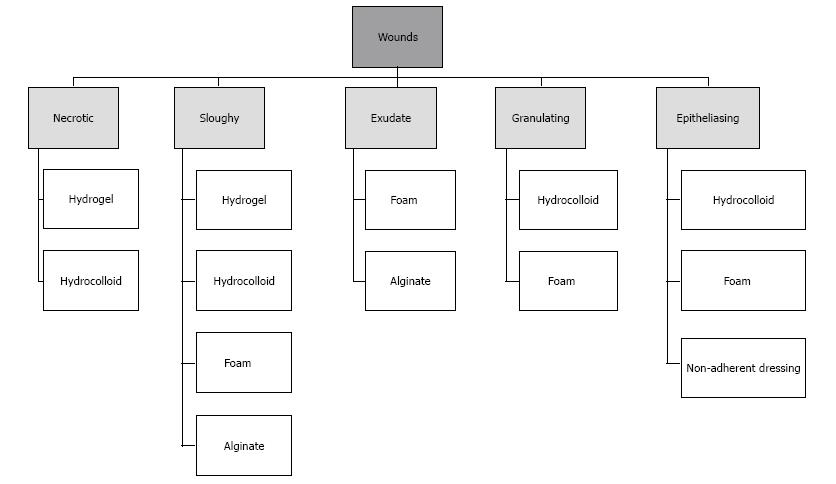
Today, all dressings are commonly used in clinical practice, while the efficacy of these products has been a challenge for researchers and clinicians, and there are controversial results regarding their use[36,99]. However, dressings are used based on DFU characteristics (Figure 8), hydrogels have been found to be the most popular choice of dressing for all DFU types[96]. Some studies dealing with the incorporation of these products show great potential in the treatment of DFU[100,101]. However, these findings do not represent a practical option since the application of these compounds is expensive and difficult to regulate[102-105]. Nevertheless, they have longer wear times, greater absorbency, may be less painful, and are typically less traumatic when removed. Moreover, in certain patients, they are cost effective because of the lowered frequency of dressing changes and not requiring extensive nursing time[106].
Diabetic foot surgery plays an essential role in the prevention and management of DFU[110], and has been on the increase over the past 2 decades[111,112]. Although surgical interventions for patients with DFU are not without risk, the selective correction of persistent foot ulcers can improve outcomes[113].
In general, surgery for DFU healing includes non-vascular foot surgery, vascular foot surgery, and in some cases amputation. Nonvascular foot surgery is divided into elective, prophylactic, curative, and emergent surgeries that aim to correct deformities that increase plantar pressure[114] (Table 4). Today, a few studies have reported long-term outcomes for diabetic foot surgery in RCTs [60,115,116]. In one study conducted by Mueller et al[115], subjects were randomized into two groups of Achilles Tendon-Lengthening (ATL) group, who received treatment of ATL and TCC, and a group who received TCC only. Their results showed that all ulcers healed in the ATL group and the risk for ulcer recurrence was 75% less at seven months and 52% less at two years than for the TCC group[115].
| Type | Explanation |
| Elective | The main goal of this surgery is to relieve the pain associated with particular deformities such as hammertoes, bunions, and bone spurs in patients without peripheral sensory neuropathy and at low risk for ulceration |
| Prophylactic | These procedures are indicated to prevent ulceration from occurring or recurring in patients with neuropathy, including those with a past history of ulceration (but without active ulceration) |
| Curative | These procedures are performed to effect healing of a non-healing ulcer or a chronically recurring ulcer when offloading and standard wound care techniques are not effective. These include multiple surgical procedures aimed at removing areas of chronically increased peak pressure as well as procedures for resecting infected bone or joints as an alternative to partial foot amputation |
| Emergent | These procedures are performed to arrest or limit progression of acute infection |
Vascular foot surgery such as bypass grafts from femoral to pedal arteries and peripheral angioplasty to improve blood flow for an ischemic foot have been recently developed[117]. While studies have shown that these procedures help to heal ischemic ulcers[118-120], no RCT has been shown to reduce DFU.
While the primary goal of DFU management focuses on limb salvage, in some cases amputation may offer a better functional outcome, although this is often not clearly defined[41]. This decision is individualized and multifactorial to match patient lifestyle, medical, physical, and psychological comorbidities[121]. In general, amputation is considered as an urgent or curative surgery and should be the last resort after all other salvage techniques have been explored, and the patient must be in agreement[122]. Indications for an amputation include the removal of infected or gangrenous tissues, control of infection, and creation of a functional foot or stump that can accommodate footwear or prosthesis[123].
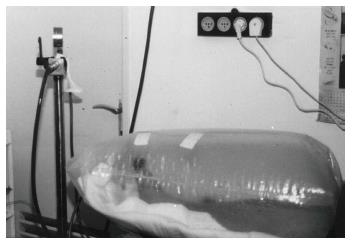
Hyperbaric oxygen therapy (HBOT) has shown promise in the treatment of serious cases of non-healing DFU, which are resistant to other therapeutic methods[124-127]. HBOT involves intermittent administration of 100% oxygen, usually in daily sessions[128]. During each session, patients breathed pure oxygen at 1.4-3.0 absolute atmospheres during 3 periods of 30 min (overall 90 min) intercalated by 5 min intervals in a hyperbaric chamber[124,129] (Figure 9).
Today, RCTs have reported beneficial effects from HBOT in numerous studies[130-134]. A recent double-blind RCT conducted by Löndahl et al[134] demonstrated a significantly improved outcome in the intervention group as the treated patients were more likely to heal within 12 mo [25.48 (52%) vs 12.42 (29%); P = 0.03]. In addition, Kranke et al[135], in a systematic review, revealed that treatment with HBOT resulted in a significantly higher proportion of healed DFU when compared with treatment without HBO (relative risk, 5.20; 95%CI: 1.25-21.66; P = 0.02). However, in another systematic review conducted by O’Reilly et al[136], no significant effects on amputation rates were found in the RCT evidence and in the high quality studies, no difference was found between HBOT group compared to standard wound care group.
The exact mechanism of HBOT remains poorly understood. Some studies have reported that HBOT improved wound tissue hypoxia, enhanced perfusion, reduced edema, down regulated inflammatory cytokines, and promoted fibroblast proliferation, collagen production, and angiogenesis[137-140]. In addition, it was demonstrated that HBOT stimulated vasculogenic stem cell mobilization from bone marrow and recruited them to the skin wound[139].
Despite reports of increased healing rates and decr-eased amputation rates with using HBOT, adjuvant use of this method in DFU remains a controversial issue. HBOT does not substitute for antibiotic therapy, local humid therapy, or surgical wound debridement. Furthermore, HBOT is available in only a minority of communities as it is expensive [a full course of treatment in the United States typically costs $50000 (Medicare) to $200000 (private pay)] and is time-consuming (an average of 60 total hours in the chamber)[5,6].
Electrical stimulation (ES) has been reported as a perfect adjunctive therapy for DFU healing in recent literature. Currently, there is a substantial body of work that supports the effectiveness of ES for DFU healing[141-144]. In a randomized, double-blind, placebo-controlled trial study conducted by Peters et al[141] on 40 patients with DFU, significant differences in number of healed ulcers (65% in treatment group vs 35% in control group) were found at 12 wk.
Based on the literature review, it is suggested that ES could improve common deficiencies that have been associated with faulty wound healing in DFU, such as poor blood flow, infection, and deficient cellular responses[141,145]. This therapy is a safe, inexpensive, and a simple intervention to improve wound healings in patients with DFU[145,146].
Negative pressure wound therapy (NPWT) is a non-invasive wound closure system that uses controlled, localized negative pressure to help heal chronic and acute wounds. This system uses latex-free and sterile polyurethane or polyvinyl alcohol foam dressing that is fitted at the bedside to the appropriate size for every wound, and then covered with an adhesive drape to create an airtight seal. Most commonly, 80-125 mmHg of negative pressure is used, either continuously or in cycles. The fluid suctioned from the wound is collected into a container in the control unit[147,148] (Figure 10).
It seems that NPWT removes edema and chronic exudate, reduces bacterial colonization, enhances formation of new blood vessels, increases cellular proliferation, and improves wound oxygenation as the result of applied mechanical force[149-151].
This method has been advocated by numerous RCTs as a safe and effective adjunctive modality in the treatment of DFU. Studies have shown that wound healing with this approach results in a higher proportion of healed wounds, faster time for wound closure, a more rapid and robust granulation tissue response, and a potential trend towards reduced risk for a second amputation than for the control treatment[148,152-156]. In addition, meta-analysis studies have indicated that NPWT significantly reduces healing times and increases the number of healed wounds[147,157,158].
While the evidence for NPWT in DFU patients is promising, this method does not replace surgical wound debridement to improve blood circulation in all DFU patients. Investigations have shown that when NPWT is initiated, there must be no significant infection or gangrene in the wound[147,158]. Also, RCTs have shown significantly higher mean material expenses for wounds treated with NPWT when compared to conventional therapy (moist gauze) in the management of full-thickness wounds requiring surgical closure[159,160].
Bio-engineered skin (BES) has been used during the last decades as a new therapeutic method to treat DFU[161-164]. This method replaces the degraded and destructive milieu of extra cellular matrix (ECM) with the introduction of a new ground substance matrix with cellular components to start a new healing trajectory[165]. Currently, three kinds of BES products approved in the United States are available to use for DFU including Derma graft (Advanced Bio healing Inc., La Jolla, CA), Apligraf (Organogenesis Inc., Canton, Mass), and, more recently, Oasis (Cook Biotech, West Lafayette, IN)[164,166]; and numerous RCT studies shown their efficacy in DFUs healing (Table 5).
| Type | Explanation | Use | RCT studies |
| Apligraf (Advanced Biohealing Inc., La Jolla, CA) | A bilayered living-skin construct containing an outer layer of live allogeneic human keratinocytes and a second layer of live allogeneic fibroblasts on type 1 collagen dispersed in a dermal layer matrix. Both cell layers are grown from infant fore skin and looks and feels like human skin[164,165] | It’s used for full-thickness neuropathic DFU of greater than 3 wk duration, resistant to standard therapy (also without tendon, muscle, capsule, or bone exposure) and is contraindicated in infected ulcers[167] | Veves et al[168] Falanga et al[169] Edmonds[170] Steinberg et al[171] |
| Dermagraft (Organogenesis Inc, Canton, Mass) | An allogeneic living-dermis equivalent and includes neonatal fibroblasts from human fore skin cultured on a polyglactin scaffold[164,165] | It’s used for DFU of greater than 6 wk duration, full thickness in depth but without tendon, muscle, joint, or bone exposure and is contraindicated in infected ulcers[164,167] | Marston et al[172] Gentzkow et al[173] |
| Oasis (Cook Biotech, West Lafayette, IN) | An acellular biomaterial derived from porcine small intestine submucosa, contains numerous crucial dermal components including collagen, glycosaminoglycans (hyaluronic acid), proteoglycans, fibronectin, and bioactive growth factors such as fibroblast growth factor-2, transforming growth factor β1, and VEGF[164,165] | It’s used for full-thickness DFU[174] | Niezgoda et al[174] |
BES product cells are seeded into the scaffolds and cultured in vitro. In vitro incubation establishes the cells and allows the cell-secreted ECM and growth factors to accumulate in the scaffold. The cells within live cell scaffolds are believed to accelerate DFU healing by actively secreting growth factors during the repair process[164,165]. In addition, it seems that BES can provide the cellular substrate and molecular components necessary to accelerate wound healing and angiogenesis. They act as biologic dressings and as delivery systems for growth factors and ECM components through the activity of live human fibroblasts contained in the dermal elements[162,163,170].
Despite the advantages of BES, they cannot be used in isolation to treat DFU. Peripheral ischemia, which is one of the pathological characteristics of DFU, is a critical contributing factor that affects BES transplantation. Hence, surgical revascularization and decompression as well as wound bed preparation are considered as essential prerequisites for BES applications. In addition, this method needs control of the infection[77,175]. Therefore, the above-mentioned points may result in high long-term costs and cause major concern for use of this treatment[176].
DFU has demonstrated the benefits from growth factors (GFs) such as platelet derived growth factor (PDGF), fibroblast growth factor, vascular endothelial growth factor, insulin-like growth factors (IGF1, IGF2), epidermal growth factor, and transforming growth factor b[177]. Among the aforementioned GFs, only recombinant human PDGF (rhPDGF) (Becaplermin or Regranex), which is a hydrogel that contains 0.01% of PDGF-BB (rhPDGF-BB), has demonstrated increased healing rates when compared with controls in a number of clinical trials[178-181] and has shown sufficient DFU repair efficacy to earn Food and Drug Administration (FDA) approval[182]. In one randomized placebo controlled trial involving patients with full thickness DFU, Becaplermin demonstrated a 43% increase in complete closure vs placebo gel (50% vs 35%)[183]. In another randomized placebo-controlled trial, Sibbald et al[184] demonstrated that patients with infection-free chronic foot ulcers treated with the best clinical care and once-daily applications of 100 μg/g Becaplermin gel had a significantly greater chance of 100% ulcer closure by 20 wk than those receiving the best clinical care plus placebo (vehicle gel) alone.
GFs have been shown to stimulate chemotaxis and mitogenesis of neutrophils, fibroblasts, monocytes, and other components that form the cellular basis of wound healing[178,185]. Despite FDA approval and other reviewed studies, the clinical use of Becaplermin remains limited because of its high cost[186] and uncertain patient-specific clinical benefits[187,188]. Some studies have indicated that endogenous PDGF stimulates tumor infiltrating fibroblasts found in human melanoma cells and is overexpressed at all stages of human astrocytoma growth[164]. So, it would be biologically possible that topical administration of recombinant PDGF could promote cancer.
Foot ulcers in patients with diabetes is common, and frequently leads to lower limb amputation unless a prompt, rational, multidisciplinary approach to therapy is taken. The main components of management that can ensure successful and rapid healing of DFU include education, blood sugar control, wound debridement, advanced dressing, offloading, surgery, and advanced therapies, which are used clinically. These approaches should be used whenever feasible to reduce high morbidity and risk of serious complications resulting from foot ulcers.
We thank Diabetes Research Center of Ahvaz Jundishapur University of Medical Sciences, Iran, for their help in editing.
| 1. | Shahbazian H, Yazdanpanah L, Latifi SM. Risk assessment of patients with diabetes for foot ulcers according to risk classification consensus of International Working Group on Diabetic Foot (IWGDF). Pak J Med Sci. 2013;29:730-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (5)] |
| 2. | Ramachandran A, Snehalatha C, Shetty AS, Nanditha A. Trends in prevalence of diabetes in Asian countries. World J Diabetes. 2012;3:110-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 288] [Cited by in RCA: 314] [Article Influence: 22.4] [Reference Citation Analysis (4)] |
| 3. | Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4438] [Cited by in RCA: 4429] [Article Influence: 276.8] [Reference Citation Analysis (4)] |
| 4. | Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2650] [Cited by in RCA: 2700] [Article Influence: 180.0] [Reference Citation Analysis (3)] |
| 5. | Aalaa M, Malazy OT, Sanjari M, Peimani M, Mohajeri-Tehrani M. Nurses’ role in diabetic foot prevention and care; a review. J Diabetes Metab Disord. 2012;11:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, Armstrong DG, Woo K, Boeni T, Ayello EA, Kirsner RS. Diabetic foot ulcers: Part II. Management. J Am Acad Dermatol. 2014;70:21.e1-2124; quiz 21.e1-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 7. | Cavanagh PR, Lipsky BA, Bradbury AW, Botek G. Treatment for diabetic foot ulcers. Lancet. 2005;366:1725-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 319] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 8. | Leone S, Pascale R, Vitale M, Esposito S. [Epidemiology of diabetic foot]. Infez Med. 2012;20 Suppl 1:8-13. [PubMed] |
| 9. | Richard JL, Schuldiner S. [Epidemiology of diabetic foot problems]. Rev Med Interne. 2008;29 Suppl 2:S222-S230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Nather A, Bee CS, Huak CY, Chew JL, Lin CB, Neo S, Sim EY. Epidemiology of diabetic foot problems and predictive factors for limb loss. J Diabetes Complications. 2008;22:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Bakri FG, Allan AH, Khader YS, Younes NA, Ajlouni KM. Prevalence of Diabetic Foot Ulcer and its Associated Risk Factors among Diabetic Patients in Jordan. J Med J. 2012;46:118-125. |
| 12. | Iraj B, Khorvash F, Ebneshahidi A, Askari G. Prevention of diabetic foot ulcer. Int J Prev Med. 2013;4:373-376. [PubMed] |
| 13. | Fard AS, Esmaelzadeh M, Larijani B. Assessment and treatment of diabetic foot ulcer. Int J Clin Pract. 2007;61:1931-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 14. | Snyder RJ, Hanft JR. Diabetic foot ulcers--effects on QOL, costs, and mortality and the role of standard wound care and advanced-care therapies. Ostomy Wound Manage. 2009;55:28-38. [PubMed] |
| 15. | Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev. 2001;17:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Ragnarson Tennvall G, Apelqvist J. Health-economic consequences of diabetic foot lesions. Clin Infect Dis. 2004;39 Suppl 2:S132-S139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, Landsman AS, Lavery LA, Moore JC, Schuberth JM. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45:S1-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 482] [Article Influence: 24.1] [Reference Citation Analysis (1)] |
| 19. | Bortoletto MS, de Andrade SM, Matsuo T, Haddad Mdo C, González AD, Silva AM. Risk factors for foot ulcers--a cross sectional survey from a primary care setting in Brazil. Prim Care Diabetes. 2014;8:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Waaijman R, de Haart M, Arts ML, Wever D, Verlouw AJ, Nollet F, Bus SA. Risk factors for plantar foot ulcer recurrence in neuropathic diabetic patients. Diabetes Care. 2014;37:1697-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 21. | Monteiro-Soares M, Boyko EJ, Ribeiro J, Ribeiro I, Dinis-Ribeiro M. Predictive factors for diabetic foot ulceration: a systematic review. Diabetes Metab Res Rev. 2012;28:574-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 22. | McEwen LN, Ylitalo KR, Herman WH, Wrobel JS. Prevalence and risk factors for diabetes-related foot complications in Translating Research Into Action for Diabetes (TRIAD). J Diabetes Complications. 2013;27:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 658] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 24. | Formosa C, Gatt A, Chockalingam N. Diabetic foot comp-lications in Malta: prevalence of risk factors. Foot (Edinb). 2012;22:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Malgrange D. [Physiopathology of the diabetic foot]. Rev Med Interne. 2008;29 Suppl 2:S231-S237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Sawacha Z, Gabriella G, Cristoferi G, Guiotto A, Avogaro A, Cobelli C. Diabetic gait and posture abnormalities: a biomechanical investigation through three dimensional gait analysis. Clin Biomech (Bristol, Avon). 2009;24:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Ledoux WR, Shofer JB, Cowley MS, Ahroni JH, Cohen V, Boyko EJ. Diabetic foot ulcer incidence in relation to plantar pressure magnitude and measurement location. J Diabetes Complications. 2013;27:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Amemiya A, Noguchi H, Oe M, Ohashi Y, Ueki K, Kadowaki T, Mori T, Sanada H. Elevated plantar pressure in diabetic patients and its relationship with their gait features. Gait Posture. 2014;40:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Fernando ME, Crowther RG, Pappas E, Lazzarini PA, Cunningham M, Sangla KS, Buttner P, Golledge J. Plantar pressure in diabetic peripheral neuropathy patients with active foot ulceration, previous ulceration and no history of ulceration: a meta-analysis of observational studies. PLoS One. 2014;9:e99050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Bacarin TA, Sacco IC, Hennig EM. Plantar pressure distribution patterns during gait in diabetic neuropathy patients with a history of foot ulcers. Clinics (Sao Paulo). 2009;64:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 31. | Schaper NC, Apelqvist J, Bakker K. The international consensus and practical guidelines on the management and prevention of the diabetic foot. Curr Diab Rep. 2003;3:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | DiPreta JA. Outpatient assessment and management of the diabetic foot. Med Clin North Am. 2014;98:353-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 33. | Markowitz JS, Gutterman EM, Magee G, Margolis DJ. Risk of amputation in patients with diabetic foot ulcers: a claims-based study. Wound Repair Regen. 2006;14:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Patout CA, Birke JA, Horswell R, Williams D, Cerise FP. Effectiveness of a comprehensive diabetes lower-extremity amputation prevention program in a predominantly low-income African-American population. Diabetes Care. 2000;23:1339-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Driver VR, Madsen J, Goodman RA. Reducing amputation rates in patients with diabetes at a military medical center: the limb preservation service model. Diabetes Care. 2005;28:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Frykberg RG. Diabetic foot ulcers: pathogenesis and management. Am Fam Physician. 2002;66:1655-1662. [PubMed] |
| 37. | Sumpio BE, Aruny J, Blume PA. The multidisciplinary approach to limb salvage. Acta Chir Belg. 2004;104:647-653. [PubMed] |
| 38. | Wraight PR, Lawrence SM, Campbell DA, Colman PG. Creation of a multidisciplinary, evidence based, clinical guideline for the assessment, investigation and management of acute diabetes related foot complications. Diabet Med. 2005;22:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Malekian Ragheb S, Naderi Beni M. Management of a diabetic foot ulcer by specialist nurses in Iran. Wounds International. 2013;4:20-23. |
| 40. | Aydin K, Isildak M, Karakaya J, Gürlek A. Change in amputation predictors in diabetic foot disease: effect of multidisciplinary approach. Endocrine. 2010;38:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Lepäntalo M, Apelqvist J, Setacci C, Ricco JB, de Donato G, Becker F, Robert-Ebadi H, Cao P, Eckstein HH, De Rango P. Chapter V: Diabetic foot. Eur J Vasc Endovasc Surg. 2011;42 Suppl 2:S60-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (2)] |
| 42. | Seaman S. The role of the nurse specialist in the care of patients with diabetic foot ulcers. Foot Ankle Int. 2005;26:19-26. [PubMed] |
| 43. | Mensing C, Boucher J, Cypress M, Weinger K, Mulcahy K, Barta P, Hosey G, Kopher W, Lasichak A, Lamb B. National standards for diabetes self-management education. Diabetes Care. 2005;28 Suppl 1:S72-S79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Malone JM, Snyder M, Anderson G, Bernhard VM, Holloway GA, Bunt TJ. Prevention of amputation by diabetic education. Am J Surg. 1989;158:520-523; discussion 523-524. [PubMed] |
| 45. | Annersten Gershater M, E Pilhammar E, Apelqvist J, Alm-Roijer C. Patient education for the prevention of diabetic foot ulcers: Interim analysis of a randomised controlled trial due to morbidity and mortality of participants. EDN. 2011;8:102-107. [DOI] [Full Text] |
| 46. | Dorresteijn JA, Kriegsman DM, Assendelft WJ, Valk GD. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev. 2012;10:CD001488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | American Diabetes Association. Standards of medical care in diabetes--2006. Diabetes Care. 2006;29 Suppl 1:S4-42. [PubMed] |
| 48. | Faglia E, Favales F, Morabito A. New ulceration, new major amputation, and survival rates in diabetic subjects hospitalized for foot ulceration from 1990 to 1993: a 6.5-year follow-up. Diabetes Care. 2001;24:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 49. | Bowering CK. Diabetic foot ulcers. Pathophysiology, assessment, and therapy. Can Fam Physician. 2001;47:1007-1016. [PubMed] |
| 50. | McMurry JF. Wound healing with diabetes mellitus. Better glucose control for better wound healing in diabetes. Surg Clin North Am. 1984;64:769-778. [PubMed] |
| 51. | Pomposelli JJ, Baxter JK, Babineau TJ, Pomfret EA, Driscoll DF, Forse RA, Bistrian BR. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 433] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 52. | Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5771] [Cited by in RCA: 5338] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 53. | Tallis A, Motley TA, Wunderlich RP, Dickerson JE, Waycaster C, Slade HB. Clinical and economic assessment of diabetic foot ulcer debridement with collagenase: results of a randomized controlled study. Clin Ther. 2013;35:1805-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Lebrun E, Tomic-Canic M, Kirsner RS. The role of surgical debridement in healing of diabetic foot ulcers. Wound Repair Regen. 2010;18:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 55. | Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2010;CD003556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Brem H, Sheehan P, Boulton AJ. Protocol for treatment of diabetic foot ulcers. Am J Surg. 2004;187:1S-10S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 57. | Enoch S, Harding K. Wound bed preparation: the science behind the removal of barrier to healing. Wounds. 2003;15:213-229. |
| 58. | Jain AC. A New Classification (Grading System) of Debridement in Diabetic Lower Limbs-an Improvization and Standardization in Practice of Diabetic Lower Limb Salvage around the World. Medicine Science. 2014;3:991-1001. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg. 1996;183:61-64. [PubMed] |
| 60. | Piaggesi A, Schipani E, Campi F, Romanelli M, Baccetti F, Arvia C, Navalesi R. Conservative surgical approach versus non-surgical management for diabetic neuropathic foot ulcers: a randomized trial. Diabet Med. 1998;15:412-417. [PubMed] |
| 61. | Saap LJ, Falanga V. Debridement performance index and its correlation with complete closure of diabetic foot ulcers. Wound Repair Regen. 2002;10:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Cardinal M, Eisenbud DE, Armstrong DG, Zelen C, Driver V, Attinger C, Phillips T, Harding K. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair Regen. 2009;17:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 63. | Attinger CE, Bulan E, Blume PA. Surgical débridement. The key to successful wound healing and reconstruction. Clin Podiatr Med Surg. 2000;17:599-630. [PubMed] |
| 64. | Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1743] [Article Influence: 83.0] [Reference Citation Analysis (1)] |
| 65. | Warriner RA, Wilcox JR, Carter MJ, Stewart DG. More frequent visits to wound care clinics result in faster times to close diabetic foot and venous leg ulcers. Adv Skin Wound Care. 2012;25:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312 744 wounds. JAMA Dermatol. 2013;149:1050-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 67. | Sherman RA. Maggot therapy takes us back to the future of wound care: new and improved maggot therapy for the 21st century. J Diabetes Sci Technol. 2009;3:336-344. [PubMed] |
| 68. | Armstrong DG, Mossel J, Short B, Nixon BP, Knowles EA, Boulton AJ. Maggot debridement therapy: a primer. J Am Podiatr Med Assoc. 2002;92:398-401. [PubMed] |
| 69. | Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol. 2001;2:219-227. [PubMed] |
| 70. | Sherman RA. Maggot therapy for foot and leg wounds. Int J Low Extrem Wounds. 2002;1:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Sherman RA. Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabetes Care. 2003;26:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 72. | van Veen LJ. Maggot debridement therapy: a case study. J Wound Ostomy Continence Nurs. 2008;35:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Armstrong DG, Salas P, Short B, Martin BR, Kimbriel HR, Nixon BP, Boulton AJ. Maggot therapy in “lower-extremity hospice” wound care: fewer amputations and more antibiotic-free days. J Am Podiatr Med Assoc. 2005;95:254-257. [PubMed] |
| 74. | Paul AG, Ahmad NW, Lee HL, Ariff AM, Saranum M, Naicker AS, Osman Z. Maggot debridement therapy with Lucilia cuprina: a comparison with conventional debridement in diabetic foot ulcers. Int Wound J. 2009;6:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 75. | Scott RG, Loehne HB. 5 questions--and answers--about pulsed lavage. Adv Skin Wound Care. 2000;13:133-134. [PubMed] |
| 76. | Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11 Suppl 1:S1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 769] [Article Influence: 33.4] [Reference Citation Analysis (1)] |
| 77. | Ramundo J, Gray M. Enzymatic wound debridement. J Wound Ostomy Continence Nurs. 2008;35:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 78. | Langer V, Bhandari PS, Rajagopalan S, Mukherjee MK. Enzymatic debridement of large burn wounds with papain-urea: Is it safe? Med J Armed Forces India. 2013;69:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Jarczyk G, Jackowski M, Szpila K, Boszek G, Kapelaty S. Use of Lucilia sericata blowfly maggots in the treatment of diabetic feet threatened with amputation. Acta Angiologica. 2008;14:42-55. |
| 80. | Bowling FL, Salgami EV, Boulton AJ. Larval therapy: a novel treatment in eliminating methicillin-resistant Staphylococcus aureus from diabetic foot ulcers. Diabetes Care. 2007;30:370-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off-loading the diabetic foot wound: a randomized clinical trial. Diabetes Care. 2001;24:1019-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 356] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 82. | Armstrong DG, Lavery LA, Nixon BP, Boulton AJ. It’s not what you put on, but what you take off: techniques for debriding and off-loading the diabetic foot wound. Clin Infect Dis. 2004;39 Suppl 2:S92-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. J Vasc Surg. 2010;52:37S-43S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 84. | Boulton AJ. Pressure and the diabetic foot: clinical science and offloading techniques. Am J Surg. 2004;187:17S-24S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Rathur HM, Boulton AJ. Pathogenesis of foot ulcers and the need for offloading. Horm Metab Res. 2005;37 Suppl 1:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Rathur HM, Boulton AJ. The diabetic foot. Clin Dermatol. 2007;25:109-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 87. | Mueller MJ, Diamond JE, Sinacore DR, Delitto A, Blair VP, Drury DA, Rose SJ. Total contact casting in treatment of diabetic plantar ulcers. Controlled clinical trial. Diabetes Care. 1989;12:384-388. [PubMed] |
| 88. | Piaggesi A, Viacava P, Rizzo L, Naccarato G, Baccetti F, Romanelli M, Zampa V, Del Prato S. Semiquantitative analysis of the histopathological features of the neuropathic foot ulcer: effects of pressure relief. Diabetes Care. 2003;26:3123-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D. Delivery of care to diabetic patients with foot ulcers in daily practice: results of the Eurodiale Study, a prospective cohort study. Diabet Med. 2008;25:700-707. [PubMed] [DOI] [Full Text] |
| 90. | Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care. 2008;31:2118-2119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 91. | Fife CE, Carter MJ, Walker D, Thomson B, Eckert KA. Diabetic foot ulcer off-loading: The gap between evidence and practice. Data from the US Wound Registry. Adv Skin Wound Care. 2014;27:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 92. | Katz IA, Harlan A, Miranda-Palma B, Prieto-Sanchez L, Armstrong DG, Bowker JH, Mizel MS, Boulton AJ. A randomized trial of two irremovable off-loading devices in the management of plantar neuropathic diabetic foot ulcers. Diabetes Care. 2005;28:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 93. | Armstrong DG, Lavery LA, Wu S, Boulton AJ. Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds: a randomized controlled trial. Diabetes Care. 2005;28:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 94. | Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1841] [Cited by in RCA: 1838] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 95. | Moura LI, Dias AM, Carvalho E, de Sousa HC. Recent advances on the development of wound dressings for diabetic foot ulcer treatment--a review. Acta Biomater. 2013;9:7093-7114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 516] [Article Influence: 39.7] [Reference Citation Analysis (3)] |
| 96. | Hilton JR, Williams DT, Beuker B, Miller DR, Harding KG. Wound dressings in diabetic foot disease. Clin Infect Dis. 2004;39 Suppl 2:S100-S103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 97. | Hansson C. Interactive wound dressings. A practical guide to their use in older patients. Drugs Aging. 1997;11:271-284. [PubMed] |
| 99. | Mason J, O’Keeffe C, Hutchinson A, McIntosh A, Young R, Booth A. A systematic review of foot ulcer in patients with Type 2 diabetes mellitus. II: treatment. Diabet Med. 1999;16:889-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 100. | Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg. 2002;137:822-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 246] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 101. | Jude EB, Apelqvist J, Spraul M, Martini J. Prospective randomized controlled study of Hydrofiber dressing containing ionic silver or calcium alginate dressings in non-ischaemic diabetic foot ulcers. Diabet Med. 2007;24:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 102. | Dumville JC, Deshpande S, O’Meara S, Speak K. Hydrocolloid dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013;8:CD009099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3573] [Cited by in RCA: 2844] [Article Influence: 948.0] [Reference Citation Analysis (0)] |
| 103. | Dumville JC, Deshpande S, O’Meara S, Speak K. Foam dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2011;CD009111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 104. | Dumville JC, O’Meara S, Deshpande S, Speak K. Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2012;2:CD009110. [PubMed] |
| 105. | Bergin SM, Wraight P. Silver based wound dressings and topical agents for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2006;CD005082. [PubMed] |
| 106. | Lo SF, Chang CJ, Hu WY, Hayter M, Chang YT. The effectiveness of silver-releasing dressings in the management of non-healing chronic wounds: a meta-analysis. J Clin Nurs. 2009;18:716-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 107. | Thomas DR, Goode PS, LaMaster K, Tennyson T, Parnell LK. A comparison of an opaque foam dressing versus a transparent film dressing in the management of skin tears in institutionalized subjects. Ostomy Wound Manage. 1999;45:22-24, 27-28. [PubMed] |
| 108. | Fletcher J. Using film dressings. Nurs Times. 2003;99:57. [PubMed] |
| 109. | Carter MJ, Tingley-Kelley K, Warriner RA. Silver treatments and silver-impregnated dressings for the healing of leg wounds and ulcers: a systematic review and meta-analysis. J Am Acad Dermatol. 2010;63:668-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 110. | Capobianco CM, Stapleton JJ, Zgonis T. Soft tissue reconstruction pyramid in the diabetic foot. Foot Ankle Spec. 2010;3:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 111. | Blume PA, Paragas LK, Sumpio BE, Attinger CE. Single-stage surgical treatment of noninfected diabetic foot ulcers. Plast Reconstr Surg. 2002;109:601-609. [PubMed] |
| 112. | Armstrong DG, Lavery LA, Stern S, Harkless LB. Is prophylactic diabetic foot surgery dangerous? J Foot Ankle Surg. 1996;35:585-589. [PubMed] |
| 113. | Hinchliffe RJ, Valk GD, Apelqvist J, Armstrong DG, Bakker K, Game FL, Hartemann-Heurtier A, Löndahl M, Price PE, van Houtum WH. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2008;24 Suppl 1:S119-S144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 114. | Armstrong DG, Frykberg RG. Classifying diabetic foot surgery: toward a rational definition. Diabet Med. 2003;20:329-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of Achilles tendon lengthening on neuropathic plantar ulcers. A randomized clinical trial. J Bone Joint Surg Am. 2003;85-A:1436-1445. [PubMed] |
| 116. | Lin SS, Lee TH, Wapner KL. Plantar forefoot ulceration with equinus deformity of the ankle in diabetic patients: the effect of tendo-Achilles lengthening and total contact casting. Orthopedics. 1996;19:465-475. [PubMed] |
| 117. | Lepäntalo M, Biancari F, Tukiainen E. Never amputate without consultation of a vascular surgeon. Diabetes Metab Res Rev. 2000;16 Suppl 1:S27-S32. [PubMed] |
| 118. | Sumpio BE, Lee T, Blume PA. Vascular evaluation and arterial reconstruction of the diabetic foot. Clin Podiatr Med Surg. 2003;20:689-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 119. | Faglia E, Mantero M, Caminiti M, Caravaggi C, De Giglio R, Pritelli C, Clerici G, Fratino P, De Cata P, Dalla Paola L. Extensive use of peripheral angioplasty, particularly infrapopliteal, in the treatment of ischaemic diabetic foot ulcers: clinical results of a multicentric study of 221 consecutive diabetic subjects. J Intern Med. 2002;252:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 120. | van Baal JG. Surgical treatment of the infected diabetic foot. Clin Infect Dis. 2004;39 Suppl 2:S123-S128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 121. | Attinger CE, Brown BJ. Amputation and ambulation in diabetic patients: function is the goal. Diabetes Metab Res Rev. 2012;28 Suppl 1:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 122. | Frykberg RG, Armstrong DG, Giurini J, Edwards A, Kravette M, Kravitz S, Ross C, Stavosky J, Stuck R, Vanore J. Diabetic foot disorders: a clinical practice guideline. American College of Foot and Ankle Surgeons. J Foot Ankle Surg. 2000;39:S1-60. [PubMed] |
| 123. | Abou-Zamzam AM, Gomez NR, Molkara A, Banta JE, Teruya TH, Killeen JD, Bianchi C. A prospective analysis of critical limb ischemia: factors leading to major primary amputation versus revascularization. Ann Vasc Surg. 2007;21:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 124. | Oliveira N, Rosa P, Borges L, Dias E, Oliveira F, Cássio I. Treatment of diabetic foot complications with hyperbaric oxygen therapy: a retrospective experience. Foot Ankle Surg. 2014;20:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 125. | Strauss MB. Hyperbaric oxygen as an intervention for managing wound hypoxia: its role and usefulness in diabetic foot wounds. Foot Ankle Int. 2005;26:15-18. [PubMed] |
| 126. | Cianci P. Advances in the treatment of the diabetic foot: Is there a role for adjunctive hyperbaric oxygen therapy? Wound Repair Regen. 2004;12:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 127. | Landau Z. Topical hyperbaric oxygen and low energy laser for the treatment of diabetic foot ulcers. Arch Orthop Trauma Surg. 1998;117:156-158. [PubMed] |
| 128. | Barnes RC. Point: hyperbaric oxygen is beneficial for diabetic foot wounds. Clin Infect Dis. 2006;43:188-192. [PubMed] |
| 129. | Thackham JA, McElwain DL, Long RJ. The use of hyperbaric oxygen therapy to treat chronic wounds: A review. Wound Repair Regen. 2008;16:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 130. | Abidia A, Laden G, Kuhan G, Johnson BF, Wilkinson AR, Renwick PM, Masson EA, McCollum PT. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial. Eur J Vasc Endovasc Surg. 2003;25:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 250] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 131. | Ma L, Li P, Shi Z, Hou T, Chen X, Du J. A prospective, randomized, controlled study of hyperbaric oxygen therapy: effects on healing and oxidative stress of ulcer tissue in patients with a diabetic foot ulcer. Ostomy Wound Manage. 2013;59:18-24. [PubMed] |
| 132. | Kessler L, Bilbault P, Ortéga F, Grasso C, Passemard R, Stephan D, Pinget M, Schneider F. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: a prospective randomized study. Diabetes Care. 2003;26:2378-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 133. | Löndahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care. 2010;33:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 134. | Löndahl M. Hyperbaric oxygen therapy as treatment of diabetic foot ulcers. Diabetes Metab Res Rev. 2012;28 Suppl 1:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 135. | Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2012;4:CD004123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 136. | O’Reilly D, Pasricha A, Campbell K, Burke N, Assasi N, Bowen JM, Tarride JE, Goeree R. Hyperbaric oxygen therapy for diabetic ulcers: systematic review and meta-analysis. Int J Technol Assess Health Care. 2013;29:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 137. | Gill AL, Bell CN. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM. 2004;97:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 307] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 138. | Al-Waili NS, Butler GJ. Effects of hyperbaric oxygen on inflammatory response to wound and trauma: possible mechanism of action. ScientificWorldJournal. 2006;6:425-441. [PubMed] |
| 139. | Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127 Suppl 1:131S-141S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 140. | Niinikoski JH. Clinical hyperbaric oxygen therapy, wound perfusion, and transcutaneous oximetry. World J Surg. 2004;28:307-311. [PubMed] |
| 141. | Peters EJ, Lavery LA, Armstrong DG, Fleischli JG. Electric stimulation as an adjunct to heal diabetic foot ulcers: a randomized clinical trial. Arch Phys Med Rehabil. 2001;82:721-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 142. | Petrofsky JS, Lawson D, Berk L, Suh H. Enhanced healing of diabetic foot ulcers using local heat and electrical stimulation for 30 min three times per week. J Diabetes. 2010;2:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 143. | Lundeberg TC, Eriksson SV, Malm M. Electrical nerve stimulation improves healing of diabetic ulcers. Ann Plast Surg. 1992;29:328-331. [PubMed] |
| 144. | Baker LL, Chambers R, DeMuth SK, Villar F. Effects of electrical stimulation on wound healing in patients with diabetic ulcers. Diabetes Care. 1997;20:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 145. | Thakral G, Lafontaine J, Najafi B, Talal TK, Kim P, Lavery LA. Electrical stimulation to accelerate wound healing. Diabet Foot Ankle. 2013;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 146. | Barnes R, Shahin Y, Gohil R, Chetter I. Electrical stimulation vs. standard care for chronic ulcer healing: a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Invest. 2014;44:429-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 147. | Vikatmaa P, Juutilainen V, Kuukasjärvi P, Malmivaara A. Negative pressure wound therapy: a systematic review on effectiveness and safety. Eur J Vasc Endovasc Surg. 2008;36:438-448. [PubMed] |
| 148. | Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366:1704-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 597] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 149. | DeFranzo AJ, Argenta LC, Marks MW, Molnar JA, David LR, Webb LX, Ward WG, Teasdall RG. The use of vacuum-assisted closure therapy for the treatment of lower-extremity wounds with exposed bone. Plast Reconstr Surg. 2001;108:1184-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 150. | Espensen EH, Nixon BP, Lavery LA, Armstrong DG. Use of subatmospheric (VAC) therapy to improve bioengineered tissue grafting in diabetic foot wounds. J Am Podiatr Med Assoc. 2002;92:395-397. [PubMed] |
| 151. | Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS. Mechanisms and clinical applications of the vacuum-assisted closure (VAC) Device: a review. Am J Clin Dermatol. 2005;6:185-194. [PubMed] |
| 152. | Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative-pressure wound dressings for diabetic foot wounds. Ann Vasc Surg. 2003;17:645-649. [PubMed] |
| 153. | Eto¨z A, O¨zgenel Y, O¨ zcan M. The use of negative pressure wound therapy on diabetic foot ulcers: a preliminary controlled trial. Wounds. 2004;16:264-269. |
| 154. | McCallon SK, Knight CA, Valiulus JP, Cunningham MW, McCulloch JM, Farinas LP. Vacuum-assisted closure versus saline-moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy Wound Manage. 2000;46:28-32, 34. [PubMed] |
| 155. | Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care. 2008;31:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 377] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 156. | Akbari A, Moodi H, Ghiasi F, Sagheb HM, Rashidi H. Effects of vacuum-compression therapy on healing of diabetic foot ulcers: randomized controlled trial. J Rehabil Res Dev. 2007;44:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 157. | Sadat U, Chang G, Noorani A, Walsh SR, Hayes PD, Varty K. Efficacy of TNP on lower limb wounds: a meta-analysis. J Wound Care. 2008;17:45-48. [PubMed] |
| 158. | Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H. A systematic review of topical negative pressure therapy for acute and chronic wounds. Br J Surg. 2008;95:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 159. | Mouës CM, van den Bemd GJ, Meerding WJ, Hovius SE. An economic evaluation of the use of TNP on full-thickness wounds. J Wound Care. 2005;14:224-227. [PubMed] |
| 160. | Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg. 2006;118:390-397; discussion 398-400. [PubMed] |
| 161. | Kim PJ, Heilala M, Steinberg JS, Weinraub GM. Bioen-gineered alternative tissues and hyperbaric oxygen in lower extremity wound healing. Clin Podiatr Med Surg. 2007;24:529-46, x. [PubMed] |
| 162. | Teng YJ, Li YP, Wang JW, Yang KH, Zhang YC, Wang YJ, Tian JH, Ma B, Wang JM, Yan X. Bioengineered skin in diabetic foot ulcers. Diabetes Obes Metab. 2010;12:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 163. | Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2:305-313. [PubMed] |
| 164. | Richmond NA, Vivas AC, Kirsner RS. Topical and biologic therapies for diabetic foot ulcers. Med Clin North Am. 2013;97:883-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 165. | Futrega K, King M, Lott WB, Doran MR. Treating the whole not the hole: necessary coupling of technologies for diabetic foot ulcer treatment. Trends Mol Med. 2014;20:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 166. | Kirsner RS, Warriner R, Michela M, Stasik L, Freeman K. Advanced biological therapies for diabetic foot ulcers. Arch Dermatol. 2010;146:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 167. | O’Loughlin A, McIntosh C, Dinneen SF, O’Brien T. Review paper: basic concepts to novel therapies: a review of the diabetic foot. Int J Low Extrem Wounds. 2010;9:90-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 168. | Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 501] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 169. | Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999;7:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 250] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 170. | Edmonds M. Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Low Extrem Wounds. 2009;8:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 171. | Steinberg JS, Edmonds M, Hurley DP, King WN. Confir-matory data from EU study supports Apligraf for the treatment of neuropathic diabetic foot ulcers. J Am Podiatr Med Assoc. 2010;100:73-77. [PubMed] |
| 172. | Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 476] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 173. | Gentzkow GD, Iwasaki SD, Hershon KS, Mengel M, Prendergast JJ, Ricotta JJ, Steed DP, Lipkin S. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350-354. [PubMed] |
| 174. | Niezgoda JA, Van Gils CC, Frykberg RG, Hodde JP. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18:258-266. [PubMed] |
| 175. | Lorenzi G, Crippa M, Rossi G, Ferrari S, Terzi A, Motolese A. Open and endovascular revascularization combined with regenerative dermal skin graft in the treatment of ischemic ulcers. Ital J Vasc Endovasc Surg. 2005;12:61-64. |
| 176. | Dinh TL, Veves A. The efficacy of Apligraf in the treatment of diabetic foot ulcers. Plast Reconstr Surg. 2006;117:152S-157S; discussion 158S-159S. [PubMed] |
| 177. | Papanas N, Maltezos E. Becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Clin Interv Aging. 2008;3:233-240. [PubMed] |
| 178. | Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003;90:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 179. | Wieman TJ. Clinical efficacy of becaplermin (rhPDGF-BB) gel. Becaplermin Gel Studies Group. Am J Surg. 1998;176:74S-79S. [PubMed] |
| 180. | Knighton DR, Ciresi KF, Fiegel VD, Austin LL, Butler EL. Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF). Ann Surg. 1986;204:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 321] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 181. | Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg. 2006;117:143S-149S; discussion 150S-151S. [PubMed] |
| 182. | Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2237] [Cited by in RCA: 2646] [Article Influence: 147.0] [Reference Citation Analysis (2)] |
| 183. | Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21:822-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 471] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 184. | Sibbald RG, Torrance G, Hux M, Attard C, Milkovich N. Cost-effectiveness of becaplermin for nonhealing neuropathic diabetic foot ulcers. Ostomy Wound Manage. 2003;49:76-84. [PubMed] |
| 185. | Hogge J, Krasner D, Nguyen H, Harkless LB, Armstrong DG. The potential benefits of advanced therapeutic modalities in the treatment of diabetic foot wounds. J Am Podiatr Med Assoc. 2000;90:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 186. | Lantis JC, Boone D, Gendics C, Todd G. Analysis of patient cost for recombinant human platelet-derived growth factor therapy as the first-line treatment of the insured patient with a diabetic foot ulcer. Adv Skin Wound Care. 2009;22:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 187. | Fang RC, Galiano RD. A review of becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Biologics. 2008;2:1-12. [PubMed] |
| 188. | Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen. 1999;7:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 320] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
P- Reviewer: Carter MJ S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/