Published online Feb 15, 2015. doi: 10.4239/wjd.v6.i1.208
Peer-review started: September 9, 2014
First decision: November 3, 2014
Revised: November 24, 2014
Accepted: December 16, 2014
Article in press: December 17, 2014
Published online: February 15, 2015
Processing time: 143 Days and 23.8 Hours
AIM: To provide an update on glycaemic control in European patients with type 2 diabetes mellitus (T2DM). We present the Greek population data of the study.
METHODS: An observational multicenter, cross-sectional study evaluating glycaemic control and a range of other clinical and biological measures as well as quality of life (QoL) and treatment satisfaction in 375 patients with T2DM enrolled by 25 primary care sites from Greece.
RESULTS: The mean age of the patients was 63.5 years and the male/female ratio 48.9%/51.1%. 79.7% of the patients exerted none or light physical activity, 82.4% were overweight or obese and 32.9% did not meet HbA1c target of less than 7.0% (53 mmol/mol). Patients reported high satisfaction to continue with treatment, high satisfaction with administered treatment and increased willingness to recommend treatment to others (mean Diabetes Treatment Satisfaction Questionnaire score 29.1 ± 5.6). However, 80% of the patients reported that their QoL would be better without diabetes. Finally, the most challenging parameter reported was the lack of freedom to eat and drink.
CONCLUSION: This analysis of the Greek Panorama study results showed that a considerable percentage of T2DM patients in Greece do not achieve glycaemic target levels, despite the favourably reported patient satisfaction from administered therapy. Additionally, the majority of primary care T2DM patients in Greece depict the negative effect of the disease in their QoL.
Core tip: Diabetes is a common, chronic disease with serious complications. Despite the multiple antidiabetic treatment options and the clear treatment guidelines, a significant proportion of type 2 diabetes patients do not achieve the glycaemic goals. Few studies have examined the quality of life in these patients. PANORAMA was a Pan-European multinational study that provided an update of the glycaemic control and quality of life in patients with diabetes. The Greek results of this study showed that a significant proportion of Greek patients were not under glycaemic control despite the high satisfaction that they had from their treatment. A negative impact of the disease in quality of life was also noted.
- Citation: Avramopoulos I, Moulis A, Nikas N. Glycaemic control, treatment satisfaction and quality of life in type 2 diabetes patients in Greece: The PANORAMA study Greek results. World J Diabetes 2015; 6(1): 208-216
- URL: https://www.wjgnet.com/1948-9358/full/v6/i1/208.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i1.208
Type 2 diabetes mellitus (T2DM) is a chronic and complex metabolic disease characterized by hyperglycaemia, as a result of insulin resistance, impaired insulin secretion and excessive or abnormal glucagon release. It is well established that its prevalence increases globally especially in the developed countries, and this increased prevalence is associated with deleterious changes in lifestyle, unhealthy eating patterns and reduced physical activity[1]. Epidemiological studies in the Greek population have shown that diabetes prevalence is also on the rise, increasing from 5.7% in 2001 to 10.4% in 2006[2]. At the same time cardiovascular (CV) risk factors, tightly related to T2DM, such as obesity, hypertension and hypercholesterolemia demonstrated even a greater increase[3]. Many effective pharmacological treatments for diabetes are now available that can be initiated after the behavioural modifications of exercise and diet. However, despite the progress in treatment strategies, many patients still face difficulties in achieving or maintaining HbA1c target levels. Moreover, diabetes is often accompanied by complications, stemming from various reasons including non-adherence to treatment and delayed adjustment of treatment regimen leading to progressive loss of b-cell function[4,5]. These complications have a negative impact on patients’ satisfaction with treatment as well as patients’ quality of life (QoL)[6-8]. Moreover, living with diabetes, reduces health related QoL which is often manifested as loss of functional ability, restrictions and barriers to everyday activities, limitations to work capacity and poor general health, while on the other hand may propagate psychological disorders such as anxiety and depression[7,9-12]. Although the advantages and disadvantages of treatment intensification on glycaemic control and various clinical measures have been the focus of several recent investigations[13-16], the data available on patients’ QoL and treatment satisfaction, especially in primary care, are still sparse. The Pan-European study PANORAMA has attempted to satisfy the need for a more up-to-date national and European data on glycaemic control from a pool of T2DM patients treated with diet, oral anti-diabetes drugs (OAD) and/or injectables. Given the alarming reports of increased prevalence of T2DM in Greece, the present study aimed at investigating the level of glycaemic control in T2DM patients and describing diabetes treatment satisfaction, QoL and fear of hypoglycaemic episodes in the Greek population of the PANORAMA study.
The objectives, design and methodology of the study have recently been published by Bradley et al[17]. PANORAMA was an observational multicentre, multinational (Belgium, France, Germany, Greece, Italy, The Netherlands, Spain, Turkey and United Kingdom), cross-sectional study (NCT00916513), evaluating glycaemic control and a range of other clinical and biological measures as well as health-related QoL and treatment satisfaction in patients with T2DM. In Italy and Turkey, physicians were recruited both from hospitals and primary care practices due to country-specific healthcare systems, whereas the physicians from the other participating countries were recruited from the primary care setting only. The group of participating investigators in Greece (n = 25) included both diabetologists/endocrinologists (n = 10) and internists (n = 15).
Eligible patients enrolled in the study were aged ≥ 40 years, diagnosed with T2DM at least one year prior to study initiation and had at least 1-year of available medical records at the participating site. Patients were given dietary and exercise advice, and could have been treated with OADs with or without insulin as well as with GLP-1 receptor agonists, with treatment unchanged within the previous 3 mo. The study excluded patients with type 1 diabetes and/or history of diabetic ketoacidosis, secondary diabetes and pregnant women. Also, excluded from the study were patients treated with systemic corticosteroids other than replacement therapy, patients already participating in a clinical trial and patients unable to complete the questionnaires. The PANORAMA study accommodated two methods of enrolling patients, a randomized and a sequential method[17]. In Greece, patient enrolment followed the sequential method, where each participating physician sequentially enrolled patients that attended the participating centre for a routine visit.
Once patients had signed the informed consent, data from their medical records were collected during a single study visit (index visit). These data included patient’s socio-demographic and anthropometric characteristics (age, gender, weight, height, educational level, socioeconomic status, alcohol consumption, smoking status, physical activity), biological measures [blood glucose, HbA1c levels, lipids: LDL-C, HDL-C, triglycerides (TG), total cholesterol (TC)] and disease-related variables (duration of diabetes, current and past diabetes treatment regimens, hypoglycaemic episodes, macrovascular and microvascular complications). The HbA1c levels of each patient were recorded by the physician at a single index visit using Bayer’s A1CNow® device (certified test by the United States National Glycohemoglobin Standardization Program). This HbA1c measurement determined whether treatment goals had been achieved.
Patients’ reported outcomes (PROs), using validated translations of standard and widely used assessment tools, were recorded via the DTSQ (Diabetes Treatment Satisfaction Questionnaire), ADDQoL (Audit of Diabetes Dependent QoL), worry subscale of HSF-II (Hypoglycaemia Fear Survey-II) and EQ5D (EuroQoL health utility questionnaire). Composite scores were calculated according to defined algorithms for each instrument.
DTSQ, is a self-administered instrument that has demonstrated validity and reliability in diabetes populations and is recommended by the World Health Organization (WHO) and the International Diabetes Federation (IDF). The DTSQ assesses treatment satisfaction over the few weeks before its completion. The treatment satisfaction score is the sum of six of the items of the DTSQ for each respondent. Each of the treatment satisfaction scale item is scored from 6 to 0 with a higher score indicating greater satisfaction. The treatment satisfaction score can range between 36 (very satisfied) and 0 (very dissatisfied). The two additional items measuring perceived frequency of hypo - and hyperglycaemia are scored from 0 (none of the time) to 6 (most of the time)[18,19].
ADDQoL is an individualized measure of the impact of diabetes on QoL. It is a self-administered questionnaire with 21 items. The first 19 items concern specific life domains such as social and work life and are scored on a 5-point impact scale, accompanied by a related importance rating scale for each domain used to assess the importance of each aspect of life for the individual’s QoL. Weight impact scores can range from +3 (maximum positive impact of diabetes) to -9 (maximum negative impact of diabetes). The 2 remaining overview items are scored separately and include a single diabetes-specific QoL item measuring the impact of diabetes on QoL that is scored from +1 (maximum positive impact of diabetes) to -3 (maximum negative impact of diabetes) and a single item, present QoL, that is scored from +3 (excellent) to -3 (extremely bad) to measure overall QoL[20,21].
The worry subscale of HSF-II consists of 18 items, rated by patients using a 5-point Likert scale ranging from 0 (never) to 4 (almost always). The 18 items are preceded by the statement “‘Because my blood sugar could drop, I worried about …”. Scores on the “worry” subscale range from 0 to 72, with 0 representing “least worry”[22,23].
Statistical analysis was performed using the SAS system (SAS for Windows v8.2) according to Statistical Analysis Plan prepared prior to database lock. Data were summarised by standard summary statistics. The continuous variables of age, duration of T2DM and HbA1c at index visit were expressed as mean ± SD. Additionally, the categorical variables of demographics, disease characteristics, treatment regimens, physicians’ perceptions for not reaching HbA1c goals and corrective actions taken, blood and lipid profiles and microvascular and macrovascular complications were expressed as frequencies.
The Pan-European data presenting the current level of glycaemic control and its associated factors in T2DM patients, as well as the data for the Spanish subgroup were published by Depablos-Velasco et al[24,25]. The PANORAMA study in Greece enrolled 375 patients. Their mean age was 63.5 ± 10.0 years with males and females proportionally represented (48.9% men vs 51.1 % women). Obesity was observed in 42.8% of the patients, while 21.9% were current smokers and 79.7% reported no or very light (less than once a week) physical activity. Demographic and other basic patients’ characteristics are presented in Table 1.
| n (%) | |
| Age (yr, mean ± SD) | 63.5 (± 10.0) |
| Gender (males) | 183 (48.9) |
| Physical activity | |
| None | 84 (22.5) |
| Light (less than 1 time/wk) | 214 (57.2) |
| Intense (1 to 2 times/wk) | 48 (12.8) |
| Intense (3 or more times/wk) | 28 (7.5) |
| Body massindex | |
| Normal (18.5-25 kg/m2) | 66 (17.6) |
| Overweight (25-30 kg/m2) | 148 (39.6) |
| Obese (≥ 30 kg/m2) | 160 (42.8) |
| Smoking status | |
| Never smoker | 205 (54.8) |
| Former smoker | 87 (23.3) |
| Current smoker | 82 (21.9) |
| Alcohol consumption (units per week) | |
| Males | 2.1 (3.3) |
| Females | 0.6 (1.6) |
Mean duration of T2DM in the Greek PANORAMA study population was 9.7 ± 8.8 years with 63.5% of patients presenting the disease for more than 5 years (Table 2). In total, 26.9% of patients suffered microvascular complications, with the most frequent being diabetic nephropathy and chronic diabetic polyneuropathy. In parallel, 24.0% of patients presented macrovascular disease. Coronary heart disease was the most prevalent complication (Table 3).
| mean ± SD | |
| Average duration of type 2 diabetes in years (n = 375) | 9.7 (± 8.8) |
| Duration of type 2 diabetes | n (%) |
| < 5 yr | 137 (36.5) |
| ≥ 5 yr | 238 (63.5) |
| Years on insulin treatment, (n = 82, yr, mean ± SD) | 4.8 (± 7.2) |
| n (%) | |
| Microvascular complications | |
| Any complication | 101 (26.9) |
| Chronic diabetic polyneuropathy-Asymptomatic | 33 (8.8) |
| Chronic diabetic polyneuropathy-Symptomatic | 29 (7.7) |
| Autonomic neuropathy | 6 (1.6) |
| Diabetic retinopathy | 27 (7.2) |
| Diabetic nephropathy | 49 (13.1) |
| Diabetic nephropathy-Microalbuminuria | 32 (8.5) |
| Diabetic nephropathy-Proteinuria | 12 (3.2) |
| Diabetic nephropathy-Renal insufficiency | 9 (2.4) |
| Diabetic nephropathy-Dialysis | 0 (0) |
| Macrovascular complications | |
| Any complication | 91 (24.0) |
| Coronary heart disease | 70 (18.7) |
| Cerebrovascular disease | 10 (2.7) |
| Peripheral artery disease | 21 (6.6) |
Exercise and dietary advice only, was the treatment of 5.3% of the study population. Hence, the majority of the patients (94.7%) were under pharmacological treatment consisting of OADs only (65.1%), 2.7% received GLP-1 agonists, and 24.3% insulin with or without OADs. Regarding OADs, metformin was used by 73.3% patients, while fixed-dose combinations were administered to 12.3% of the patients. The most frequently administered oral hypoglycaemic agents are presented in Table 4.
| Treatment regimen | n (%) |
| No diet, no orals, no injectables (no available data) | 10 (2.7) |
| Only diet and/or exercise | 20 (5.3) |
| Only OADs | 244 (65.1) |
| On oral plus insulin | 63 (16.8) |
| Only on insulin | 28 (7.5) |
| On GLP-1 analogues ± insulin1 | 10 (2.7) |
| Oral hypoglycaemic agents | 316 (84.3) |
| Sylphonylureas | 121 (32.3) |
| Meglitinides/Glinides | 12 (3.2) |
| Biguanides | 275 (73.3) |
| Thiazolidinediones | 41 (10.9) |
| DPP-4 inhibitors | 94 (25.1) |
| Alpha glucosidase inhibitors | 13 (3.5) |
| Fixed-dose combinations | 46 (12.3) |
| Thiazolidinediones + metformin | 3 (6.5) |
| DPP4 inhibitors + metformin | 43 (93.5) |
The patients’ mean HbA1c, recorded in the index visit of the PANORAMA study, was 6.7% ± 1.0% (50 mmol/mol), while the 32.9% of the patients failed to meet HbA1c target levels presenting with HbA1c ≥ 7.0% (53 mmol/mol) (Table 5). When physicians were asked about the reasons for not reaching HbA1c target, the most frequent answer was poor patient adherence to dietary and exercise recommendations (39.5%), while other common reasons were failure of current drug regimen, resistance or reluctance of the patient to intensify the medication regimen, poor patient adherence to self-monitoring of blood glucose levels, and reluctance of physician to intensify the regimen due to fear of hypoglycaemia. In order to achieve HbA1c target, reported actions taken by the physician included retraining of patients in diet/lifestyle recommendations that need to be adopted (educational approach) (42.7%) and intensification of dose of the current anti-hyperglycaemic medication (27.5%). The addition of another OAD agent was chosen as corrective action in 11.2% of the cases. Initiation of insulin treatment, with or without changing OAD medication, was recorded in a small percentage of cases (Tables 6 and 7).
| Glycaemic control | n (%) |
| HbA1c value at index visit (mean ± SD) | 6.7 (± 1.0) (50 mmol/mol) |
| HbA1c value at index visit | |
| < 6.5% (47 mmol/mol) | 179 (47.9) |
| ≥ 6.5% (47 mmol/mol) | 195 (52.1) |
| < 7.0% (53 mmol/mol) | 251 (67.1) |
| ≥ 7.0% (53 mmol/mol) | 123 (32.9) |
| Reasons | n (%) |
| Therapeutic failure of current drug regimen | 52 (13.9) |
| Poor patient adherence to diet and exercise | 148 (39.5) |
| Poor patient adherence to self-monitoring of blood glucose levels | 44 (11.7) |
| Poor patient adherence to recommendations | 26 (6.9) |
| Resistance/reluctance of the patient to intensify his/her medication regimen | 46 (12.3) |
| Reluctance of physician to intensify medication regimen | 3 (0.8) |
| Reluctance of physician to intensify medication regimen-Fear of hypoglycaemia | 44 (11.7) |
| Reluctance of physician to intensify medication regimen-Fear of unwanted side effects | 14 (3.7) |
| Reluctance of physician to intensify medication regimen-Fear of interaction with other medications | 6 (1.6) |
| Reluctance of physician to intensify medication regimen-Cost of treatment | 9 (2.4) |
| Reluctance of physician to intensify medication regimen-Fear of additional weight gain | 13 (3.5) |
| Actions taken | n (%) |
| No specific actions | 45 (12.0) |
| Educational approach | 160 (42.7) |
| Increase dose of current medication | 103 (27.5) |
| Addition of new oral antihyperglycaemic medication | |
| Sylphonylureas | 8 (2.1) |
| Meglitinides/Glinides | 4 (1.1) |
| Biguanides | 5 (1.3) |
| Thiazolidinediones | 3 (0.8) |
| DPP-4 inhibitors | 13 (3.5) |
| Combination treatment | 9 (2.4) |
| Start insulin therapy without changing oral diabetes medication | 9 (2.4) |
| Start insulin therapy changing oral diabetes medication | 14 (3.7) |
| Other action | 9 (2.4) |
More than half of the population did not attain LDL-cholesterol (LDL-C) target < 100 mg/dL (2.586 mmol/L) with 55.8% of the patients appearing with LDL-C ≥ 100 mg/dL (2.586 mmol/L). Similarly, 40.4% of the population appeared with triglyceride (TG) levels ≥ 150 mg/dL (1.6935 mmol/L), while 24.3% of the population was off-target at ≤ 40mg/dL (1.0344 mmol/L) for HDL-cholesterol (HDL-C). Additionally, the majority of patients did not also achieve blood pressure targets since 69.8% of the study’s patients reported systolic/diastolic blood pressure ≥ 130/80 mmHg (target ≤ 130/80 mmHg) (Table 8).
| n (%) | |
| Hypertension | |
| SBP/DBP < 130/80 mmHg | 113 (30.2) |
| Triglycerides | |
| < 150 mg/dL (1.6935 mmol/L) | 221 (59.6) |
| LDL-C | |
| < 100 mg/dL (2.586 mmol/L) | 160 (44.2) |
| HDL-C | |
| > 40 mg/dL (1.0344 mmol/L) | 278 (75.7) |
DTSQ questionnaire: In the PANORAMA study, the Greek population’s mean DTSQ score reported by the patients was 29.1 ± 5.6. Patients reported high satisfaction grades in all domains of the questionnaire; satisfaction with treatment, convenience, flexibility and understanding of diabetes, willingness to recommend treatment to someone else and satisfaction to continue with current treatment. Unacceptably high or unacceptably low glucose levels were rarely reported. DTSQ scores were presented in Figure 1.
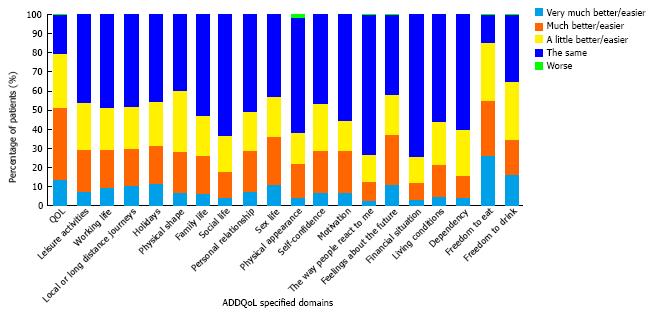
ADDQoL questionnaire: The mean ADDQoL questionnaire score reported by patients was -2.0 ± 1.9. Overall, 79.5% of patients reported that their QoL would be better if they did not have diabetes. Following analysis of the individual questionnaire components, the most affected parameters of ADDQoL were freedom to eat and drink (Figure 2).
HFS questionnaire: The mean HFS questionnaire total score for the Greek PANORAMA population was 14.2 ± 14.7. Taking into consideration that the score for the greatest fear equals to 72, the reported HFS score in the study represents an overall mild fear of hypoglycaemia. In particular, 15.3% of the patients were frequently afraid of having a hypoglycaemic episode while alone or during sleep where no one would be present to help (Figure 3).
The European PANORAMA study investigated the level of glycaemic control in Europe in addition to patients’ treatment satisfaction and QoL[17]. Here, the Greek PANORAMA study results from primary care T2DM patients in Greece are discussed next. The study was performed in 2009-2010 and enrolled 375 subjects from 25 participating study centres.
Previously, other large, multinational European studies have attempted to assess the level of glycaemic control across Europe. The RECAP-DM study for example, that included Finland, France, Germany, Norway, Poland, Spain and United Kingdom, provided data on glycaemic control on T2DM patients who intensified their treatment by adding either a sulphonylurea or a thiazolidinedione to their standard metformin treatment. Approximately, 26% of European out-patients had adequate glycaemic control, i.e., HbA1c < 6.5% (47 mmol/mol), after a mean of 2.6 years of combined oral antihyperglycaemic therapy. It was observed that glycaemic control modestly declined over time, even though more patients were being treated with insulin[26]. Similarly, another earlier European, epidemiological survey on T2DM that provided data on glycaemic control was the CODE-2 study, of which many participating countries were also included in the PANORAMA study. In the CODE-2 study 69% of the patients did not attain the HbA1c target of less than 7% (53 mmol/mol), as opposed to the 37.4% of the patients from the PANORAMA study and 32.9% of the Greek population from the PANORAMA study[24,27]. The much better glycaemic control observed in PANORAMA and its Greek population in comparison to other studies can be attributed to the fact that patients were enrolled in the study only if medical records for at least the past 1 year existed in the study site. This could suggest that the study population was more closely followed.
Regarding CV risk factor control in T2DM patients from the Greek PANORAMA study, data showed that a large percentage of patients failed to meet the recommended target levels for LDL-C, triglycerides and especially blood pressure. This is in line with previous studies conducted in Greece, showing that a considerable percentage of patients do not meet treatment goals for better CV risk control[28,29]. CV risk factors continue to be the most critical determinants of mortality and morbidity in T2DM patients, and account for more than half of the observed mortality and morbidity in this population.
The issue of CV risk factor control emerges as a great challenge in the management and treatment of T2DM patients, especially when joint standards of medical care for patients with diabetes are considered. For example, the present data indicate inadequate control for LDL-C with 55.8% of patients not achieving LDL-C target < 100 mg/dL (2.586 mmol/L). This observation highlights the difficulty in regulating LDL-C and the status of current available therapies and drugs.
The difficulty in total cardiovascular risk reduction observed in our results was also clearly shown in the total PANORAMA population recently published by de Pablos-Velasco et al[24], that reported that the joint triple target for HbA1c, blood lipids (total cholesterol) and blood pressure was achieved only in the 7.5% of the patients. This observation denotes an unmet medical need and that despite new improvements in pharmacotherapy, still a great deal of work is warranted for better T2DM disease management.
The majority of the Greek PANORAMA study population perceived positively their diabetes treatment. The high scores of the DTSQ questionnaire in the Greek PANORAMA study (29.1 ± 5.6 out of 36), are attributed to the high level of satisfaction reported in the sections concerning satisfaction with treatment, satisfaction to continue with treatment and willingness to recommend treatment to someone else.
DTSQ outcomes have been shown to correlate significantly with the duration of diabetes and the perceived glucose control by the patients, showing that the longer the diabetes duration and the less controlled glucose levels the more patients appear unsatisfied with their treatment[19]. On the other hand, satisfaction also appears to be sensitive to treatment changes[13,30] and differences between treatment groups[31]. Furthermore, in a study about diabetes patients’ perception of their disease, a clear correlation was demonstrated between patients’ responses to the questionnaire, demographic characteristics, the health status and the type of their anti-diabetic treatment[32].
The overall ADDQoL questionnaire mean score in the present study suggests that diabetes exerts a negative impact on patients’ perception of QoL. The QoL parameter identified to be most commonly, negatively affected by diabetes in the study population was freedom to eat as wish, a parameter valued as important or very important from 80.1% of the patients.
Use of the ADDQoL in people with type 1 or type 2 diabetes has shown, on average, almost universally, negative impact of diabetes on all domains[13]. Significantly improved T2DM management has also been shown in non-insulin treated patients without complications in comparison to those insulin-treated with complications[33]. The ADDQoL has also proven useful in detecting the negative impact of diabetes on QoL despite the high levels of treatment satisfaction, measured by the DTSQ[34].
Lastly, the results of the use of the HSF questionnaire as a measure of the impact of hypoglycaemia in the patients’ QoL, suggested a presence of a mild fear of hypoglycaemic episodes among the study population. History of hypoglycaemic episodes seems to also play an important role in shaping patients’ perceptions on hypoglycaemic events[35].
It was clear from the present data that patients were more often worried about having a hypoglycaemic episode while alone, at a time where no one would be available to help, or during sleep which by itself yields a negative impact on QoL.
The PANORAMA study has some inherent limitations such as the mixing of sampling techniques and the cross-sectional design of the study that cannot determine the causal nature of the associations[24]. In the present study, the A1CNow® (Bayer) was used to reduce the high variability of blood glucose measurements between centres. In addition, patient recruitment in Greece followed a sequential, rather than randomized manner, which was adopted in other European participating countries. This may raise concerns towards specific variables that could be affected by the lack of randomization at selection, such as duration of diabetes and diabetes-related problems or macro/microvascular complications, since the patients selected solely by their attendance to a participating centre may be more prone to clinic/hospital visits or diabetes related comorbidities and complications than others.
In conclusion, the Greek Panorama study data analysis demonstrated that a considerable part of the T2DM patient population does not achieve glycaemic target levels despite the coincident patient satisfaction by their administered antidiabetic treatment. Despite this high level of satisfaction, a mild fear of hypoglycaemia was detected and a considerable percentage of primary care patients, approximately 1 in 3, did not meet glycaemic goals. In parallel, the majority of the study’s population reported that their QoL would be better without diabetes. Finally, since CV risk factors are proven to be inadequately controlled among T2DM patients in Greece, further intensification efforts regarding treatment and management are required to enable better management towards diminishing CV risk and to improve treatment of type 2 diabetes patients.
The authors would like to thank George Kraniou from Pharmassist Ltd., for providing assistance in the manuscript preparation, which was funded by AstraZeneca/Bristol-Myers Squibb.
Diabetes mellitus is a common disease with a rising prevalence worldwide. Its complications are divided to macrovascular disease (atherosclerosis) and microvascular disease which includes diabetic neuropathy, retinopathy and nephropathy. Glycaemic control is of paramount importance in patients with diabetes and it can be estimated by glycated haemoglobin (HbA1c) blood levels. According to treatment guidelines, for most patients with diabetes, a level of HbA1c lower than 7% is recommended. There are many antidiabetic medications of different categories that can be administered for blood glucose control as monotherapy or in combination in order to achieve the treatment targets.
Despite the multiple available antidiabetic treatment options, a large proportion of patients with diabetes do not achieve the glycaemic goals. Furthermore, only a few studies have examined the impact of diabetes on the quality of life as well as the patients’ perception of their treatment.
PANORAMA study was a multinational Pan-European study that provided an update on glycaemic control in European patients with type 2 diabetes mellitus. The authors present the results from the Greek population of the study which showed that a large proportion of patients with diabetes do not achieve the glycaemic targets. The other cardiovascular risk factors as LDL cholesterol, triglycerides and blood pressure were also out of control in the majority of Greek patients that were enrolled in our study. Although the majority of the Greek PANORAMA study population perceived positively their diabetes treatment, most of them reported that their life has a negative impact from diabetes, especially because they do not have the freedom to eat and drink.
The primary care physician must know that most of the Greek patients with diabetes are not under control. More effort is needed to achieve glycaemic targets and cardiovascular risk factors control in these patients. Apart from treatment targets though, it must also be remembered that quality of life in these patients is reduced because of diabetes mellitus. More emphasis must be given on this issue by the physician.
The control level of blood glucose seems to be better compared to Steno study.
| 1. | Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4438] [Cited by in RCA: 4430] [Article Influence: 276.9] [Reference Citation Analysis (4)] |
| 2. | Panagiotakos DB, Pitsavos C, Skoumas Y, Lentzas Y, Stefanadis C. Five-year incidence of type 2 diabetes mellitus among cardiovascular disease-free Greek adults: findings from the ATTICA study. Vasc Health Risk Manag. 2008;4:691-698. [PubMed] |
| 3. | Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas I, Stefanadis C. Prevalence and five-year incidence (2001-2006) of cardiovascular disease risk factors in a Greek sample: the ATTICA study. Hellenic J Cardiol. 2009;50:388-395. [PubMed] |
| 4. | Davies M. The reality of glycaemic control in insulin treated diabetes: defining the clinical challenges. Int J Obes Relat Metab Disord. 2004;28 Suppl 2:S14-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 5. | Fritsche A, Häring H. At last, a weight neutral insulin? Int J Obes Relat Metab Disord. 2004;28 Suppl 2:S41-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Voorham J, Haaijer-Ruskamp FM, Wolffenbuttel BH, de Zeeuw D, Stolk RP, Denig P. Differential effects of comorbidity on antihypertensive and glucose-regulating treatment in diabetes mellitus--a cohort study. PLoS One. 2012;7:e38707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15:205-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27:2800-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 317] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 9. | UK Prospective Diabetes Study Group. Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). U.K. Prospective Diabetes Study Group. Diabetes Care. 1999;22:1125-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 359] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care. 1996;19:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 341] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Egede LE. Diabetes, major depression, and functional disability among U.S. adults. Diabetes Care. 2004;27:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Kovacs M, Iyengar S, Goldston D, Stewart J, Obrosky DS, Marsh J. Psychological functioning of children with insulin-dependent diabetes mellitus: a longitudinal study. J Pediatr Psychol. 1990;15:619-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ. 2002;325:746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 680] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 14. | Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6292] [Cited by in RCA: 5684] [Article Influence: 315.8] [Reference Citation Analysis (0)] |
| 15. | Anderson RT, Narayan KM, Feeney P, Goff D, Ali MK, Simmons DL, Sperl-Hillen JA, Bigger T, Cuddihy R, O’Conner PJ. Effect of intensive glycemic lowering on health-related quality of life in type 2 diabetes: ACCORD trial. Diabetes Care. 2011;34:807-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Biderman A, Noff E, Harris SB, Friedman N, Levy A. Treatment satisfaction of diabetic patients: what are the contributing factors? Fam Pract. 2009;26:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Bradley C, de Pablos-Velasco P, Parhofer KG, Eschwège E, Gönder-Frederick L, Simon D. PANORAMA: a European study to evaluate quality of life and treatment satisfaction in patients with type-2 diabetes mellitus--study design. Prim Care Diabetes. 2011;5:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Bradley C, Lewis KS. Measures of psychological well-being and treatment satisfaction developed from the responses of people with tablet-treated diabetes. Diabet Med. 1990;7:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 270] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Bradley C. The Diabetes Treatment Satisfaction Questionnaire: DTSQ. Handbook of Psychology and Diabetes: a guide to psychological measurement in diabetes research and practice. Chur: Harwood Academic Publishers 1994; . |
| 20. | Bradley C, Speight J. Patient perceptions of diabetes and diabetes therapy: assessing quality of life. Diabetes Metab Res Rev. 2002;18 Suppl 3:S64-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 246] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res. 1999;8:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 420] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Irvine A, Cox D, Gonder-Frederick L. The Fear of Hypoglycaemia Scale. Handbook of Psychology and Diabetes; a guide to measurement in diabetes research and practice, ed. Bradley C. Chur, Switzerland: Harwood Academic Publishers 1994; 133-155. |
| 24. | de Pablos-Velasco P, Parhofer KG, Bradley C, Eschwège E, Gönder-Frederick L, Maheux P, Wood I, Simon D. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol (Oxf). 2014;80:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 25. | Depablos-Velasco P, Salguero-Chaves E, Mata-Poyo J, Derivas-Otero B, García-Sánchez R, Viguera-Ester P. Quality of life and satisfaction with treatment in subjects with type 2 diabetes: results in Spain of the PANORAMA study. Endocrinol Nutr. 2014;61:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Alvarez Guisasola F, Mavros P, Nocea G, Alemao E, Alexander CM, Yin D. Glycaemic control among patients with type 2 diabetes mellitus in seven European countries: findings from the Real-Life Effectiveness and Care Patterns of Diabetes Management (RECAP-DM) study. Diabetes Obes Metab. 2008;10 Suppl 1:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Liebl A, Mata M, Eschwège E. Evaluation of risk factors for development of complications in Type II diabetes in Europe. Diabetologia. 2002;45:S23-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Guallar E, Banegas JR, Blasco-Colmenares E, Jiménez FJ, Dallongeville J, Halcox JP, Borghi C, Massó-González EL, Tafalla M, Perk J. Excess risk attributable to traditional cardiovascular risk factors in clinical practice settings across Europe - The EURIKA Study. BMC Public Health. 2011;11:704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Hermans MP, Brotons C, Elisaf M, Michel G, Muls E, Nobels F. Optimal type 2 diabetes mellitus management: the randomised controlled OPTIMISE benchmarking study: baseline results from six European countries. Eur J Prev Cardiol. 2013;20:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Witthaus E, Stewart J, Bradley C. Treatment satisfaction and psychological well-being with insulin glargine compared with NPH in patients with Type 1 diabetes. Diabet Med. 2001;18:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Lewis KS, Bradley C, Knight G, Boulton AJ, Ward JD. A measure of treatment satisfaction designed specifically for people with insulin-dependent diabetes. Diabet Med. 1988;5:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Marra G. The DIAB.& amp; TE.S Project: how patients perceive diabetes and diabetes therapy. Acta Biomed. 2004;75:164-170. [PubMed] |
| 33. | Sundaram M, Kavookjian J, Patrick JH, Miller LA, Madhavan SS, Scott VG. Quality of life, health status and clinical outcomes in Type 2 diabetes patients. Qual Life Res. 2007;16:165-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Speight J, Bradley C. ADDQoL indicates negative impact of diabetes on quality of life despite high levels of satisfaction with treatment. Diabetologia. 2000;43 Supplement 1:A225-A225. |
| 35. | Marrero DG, Guare JC, Vandagriff JL, Fineberg NS. Fear of hypoglycemia in the parents of children and adolescents with diabetes: maladaptive or healthy response? Diabetes Educ. 1997;23:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
P- Reviewer: Panchu P, Tamemoto H S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/