Published online Feb 15, 2015. doi: 10.4239/wjd.v6.i1.184
Peer-review started: August 29, 2014
First decision: September 30, 2014
Revised: October 22, 2014
Accepted: December 16, 2014
Article in press: December 17, 2014
Published online: February 15, 2015
Processing time: 155 Days and 20.3 Hours
Diabetes mellitus is a powerful risk factor of coronary artery disease (CAD), leading to death and disability. In recent years, given the accumulating evidence that prediabetes is also related to increasing risk of CAD including cardiovascular events, a new guideline has been proposed for the treatment of blood cholesterol for primary prevention of cardiovascular events. This guideline recommends aggressive lipid-lowering statin therapy for primary prevention in diabetes and other patients. The ultimate goal of patient management is to inhibit progression of systemic atherosclerosis and prevent fatal cardiovascular events such as acute coronary syndrome (ACS). Because disruption of atherosclerotic coronary plaques is a trigger of ACS, the high-risk atheroma is called a vulnerable plaque. Several types of novel diagnostic imaging technologies have been developed for identifying the characteristics of coronary atherosclerosis before the onset of ACS, especially vulnerable plaques. According to coronary angioscopic evaluation, atherosclerosis severity and plaque vulnerability were more advanced in prediabetic than in nondiabetic patients and comparable to that in diabetic patients. In addition, pharmacological intervention by statin therapy changed plaque color and complexity, and the dynamic changes in plaque features are considered plaque stabilization. In this article, we review the findings of atherosclerosis in prediabetes, detected by intravascular imaging modalities, and the therapeutic implications.
Core tip: Coronary artery disease is the principal cause of death and disability in not only diabetes but also prediabetes patients. Aggressive statin therapy is an established method of primary prevention of cardiovascular disease events in diabetic patients. According to the findings of coronary imaging modalities detecting atherosclerotic lesions, statin therapy in prediabetes may be beneficial for reducing atherosclerotic cardiovascular risk.
- Citation: Kurihara O, Takano M, Seino Y, Shimizu W, Mizuno K. Coronary atherosclerosis is already ongoing in pre-diabetic status: Insight from intravascular imaging modalities. World J Diabetes 2015; 6(1): 184-191
- URL: https://www.wjgnet.com/1948-9358/full/v6/i1/184.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i1.184
Diabetes is categorized as a metabolic disease char-acterized by hyperglycemia arising from abnormal insulin secretion from the pancreas and/or lack (or absence) of insulin action. Diabetes causes damage, dysfunction, or failure of various organs involving heart and blood vessels[1]. It is well known that diabetes promotes atherosclerotic disease of systemic and coronary arteries and increases the mortality rate from cardiovascular disease[2,3]. However, myocardial ischemia owing to coronary artery disease (CAD) is occasionally absent from the typical symptoms in patients with diabetes[4]. As a result, severe multivessel disease of the coronary arteries can manifest as silent myocardial ischemia before treatment is begun. A delayed recognition of CAD can worsen the prognosis in many diabetic patients[5]. Moreover, a recent study showed that impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) are also causes of adverse cardiovascular events[6,7].
A goal of CAD management is to prevent cardio-vascular diseases such as acute coronary syndrome (ACS). The principal pathogenesis of ACS is disruption of atheromatous coronary plaques and subsequent flow-limiting thrombus formation[8,9]. Plaque disruption, the trigger of this serious event, is pathologically classified as a rupture, and shallower intimal injury is termed erosion. Additionally, previous pathological studies showed that the majority of disrupted plaques have a large lipid core under a thin fibrous cap, hence the term thin-cap fibroatheroma (TCFA). Not only plaque rupture but also superficial calcified nodules are possible origins of ACS[10-12]. A vulnerable plaque is defined as a future high-risk plaque provoking ACS, and TCFA is representative of a vulnerable plaque. In recent years, novel intraco-ronary imaging modalities have been developed for detecting such vulnerable plaques.
Coronary angiography remains the gold standard for diagnosis of CAD and the following catheter intervention therapy. However, an angiogram represents a 2-dimensional silhouette of the coronary artery, and the angiogram does not supply certain information about the vessel wall components or the atherosclerotic plaque. Therefore, a coronary angiogram is not capable of detecting vulnerable plaques, including TCFA. Thus, supplemental CAD diagnostic modalities, including various intravascular imaging devices such as intravascular ultrasound (IVUS), coronary angioscopy (CAS), and optical coherence tomography (OCT), have been developed to discriminate each component of the plaque and to identify the presence of vulnerable plaques.
IVUS is an intravascular imaging modality that supplies cross-sectional images of the coronary artery including the lumen and vessel wall. High-frequency (20-40 MHz) IVUS visualizes 3 layers of the vessel wall: the intima, media, and adventitia. IVUS allows in vivo qualitative measurements of the lumen and plaque area (and volume). Conventional grayscale IVUS images have major limitation of precise tissue characterization except calcification. Although a large plaque burden and microcalcifications, factors of plaque vulnerability, are detected by grayscale IVUS, this imaging system is not able to identify TCFA[13]. Because of these limitations, 3 modalities using radiofrequency analysis, virtual histology IVUS (VH-IVUS; Volcano Therapeutics, Rancho Cordova, CA, United States)[14], iMAP-IVUS (Boston Scientific, Santa Clara, CA, United States)[15], and integrated backscatter IVUS (IB-IVUS; YD Co., Nara, Japan)[16] are now available in clinical settings.
VH-IVUS takes into account detailed qualitative and quantitative assessment of the vessel wall components. The axial resolution of VH-IVUS is just about 150-250 μm. Ex vivo studies have shown that power spectrum-related parameters from raw backscattered ultrasound signals permit discrimination of plaque components[17]. These parameters are used in a classification scheme to yield a tissue color map for each plaque characteristic as follows: dark green indicates fibrous, yellow-green indicates fibrofatty, red indicates necrotic core, and white indicates dense calcium. VH-derived TCFA was defined as at least 3 consecutive frames with a plaque burden of at least 40% and without overlying fibrous tissue[18]. A recent prospective study using VH-IVUS, the PROSPET trial, has shown that the VH-derived TCFA with a minimal luminal area ≤ 4 mm2 and a plaque burden ≥ 70% was the highest-risk plaque type leading to adverse cardiovascular events[19].
CAS using optic fibers is a technology that permits direct visualization of the lumen surface of the coronary artery and provides detailed information about plaque morphology and the presence of a thrombus with high resolution (50 μm). CAS clearly identifies irregularities of the lumen surface, such as ulceration, fissures, and tears. Disrupted plaques are involved in these plaques with irregularities (or complexity). In addition, CAS is an extremely sensitive detector of a thrombus. Angiographic stenosis of the lesion progresses despite healing of the silent plaque disruption in the nonculprit lesions[20].
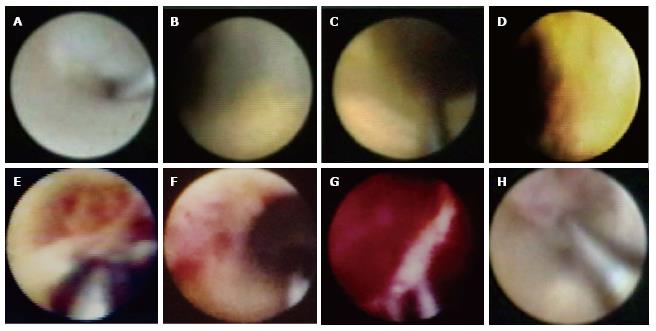
Based on angioscopic analysis, an atherosclerotic plaque is defined as a nonmobile, protruding structure that can be clearly delimited from the adjacent vessel wall. Although a normal vessel wall appears glistening white, plaques can be yellow or white according to the surface color. The color of the plaque is classified semiquantitatively: (1) grade 0 is white; (2) grade 1 is light yellow; (3) grade 2 is medium yellow; and (4) grade 3 is intense yellow. The majority of yellow plaques contain lipid-rich tissue or a necrotic core according to comparative validation using OCT and IVUS. The grade of yellow plaques is affected by the thickness of the fibrous cap covering lipidic tissue. A high-intensity yellow plaque is identical to a TCFA. On the contrary, white plaques contain fibrous tissue or a thick fibrous cap covering the lipidic plaque[21-24]. Yellow plaques are detected not only in the culprit lesion but also in the nonculprit lesions of ACS[25-29]. Representative CAS images of plaques and thrombi are shown in Figure 1.
Prospective studies demonstrated that the incidence of ACS is higher in patients with intense yellow or multiple yellow plaques than in patients without yellow plaques[30,31]. These findings indicate that intense yellow or multiple yellow plaques detected by CAS might be vulnerable and could cause future coronary events.
OCT imaging employs a near-infrared range light source, at approximately 1300 nm. OCT has a 10-fold higher image resolution (10-15 μm) than IVUS, and its image quality is superior to that of other imaging devices. In addition, OCT provides accurate tissue characterization of the plaque. Normal vessel walls appear as a 3-layer structure on OCT images as well as IVUS. The vascular media is seen as a dark band delineated by the internal elastic lamina and external elastic lamina. Fibrous plaques consist of homogeneous and low-attenuation areas. Lipid-rich plaques exhibit a high-attenuation mass with a diffuse border. A calcified plaque is presented as high-attenuation mass with a clear border[32-36].
OCT is the only intravascular imaging technology with high spatial resolution that can measure the fibrous cap thickness[37,38].
In diabetic patients, coronary angiograms characteristically reveal diffuse long lesions in multiple small vessels[39,40]. In an IVUS study, plaques in diabetic patients were characterized by an increased amount of dense calcium, a necrotic core, and a high frequency of VH-TCFA[41]. In addition, IVUS studies showed that the levels of hemoglobin A1c (HbA1c) was associated with atheroma volume and the severity of coronary atherosclerosis[42,43]. CAS showed that diabetes was an independent predictor of plaque disruption in the nonculprit vessel[44]. In an OCT study, patients with diabetes had large lipid plaque volumes and a high prevalence of calcified plaque and thrombus[45].
Free fatty acids and insulin resistance, which are elevated by hyperglycemia, stimulate molecular mechanisms and alter the function and structure of blood vessels, including increased oxidative stress and activation of protein kinase C and the receptor for advanced glycation end products. Consequently, hyperglycemia decreases the availability of nitric oxide, increases the production of endothelin, and activates transcription factors such as nuclear factor-κB and activator protein-1. These factors bring about systemic vasoconstriction and inflammation and promote systemic atherosclerosis[46-48]. A similar glucose metabolism disorder occurs in the prediabetic state[49,50].
The American Diabetes Association defines prediabetes as IFG, IGT, and HbA1c values ranging 5.7%-6.4%[1]. The patients with IFG and IGT should be informed of their increased risk for diabetes as well as CAD. The HbA1c value is more commonly used to diagnose diabetes, and an HbA1c level of 5.7%-6.4% also indicates a relatively high risk for future diabetes and CAD[1].
The low concordance in the relationships between IFG, IGT, and HbA1c, as well as the diagnoses of prediabetes using these parameters, accentuates the various dysfunctions of glucose metabolism. A dys-function in hepatic insulin resistance manifests as IFG, whereas muscle insulin resistance represents IGT[51]. Although data for IFG and IGT are provided by the daily glucose snapshot, HbA1c levels after chronic exposure (over 60-90 d) to basal and postprandial hyperglycemia reflects a combination of IFG and IGT[52].
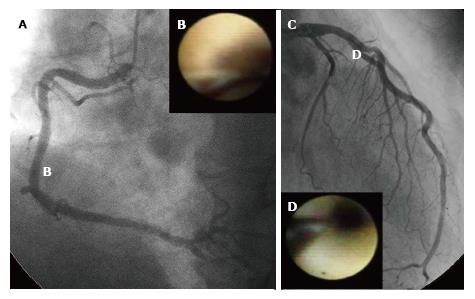
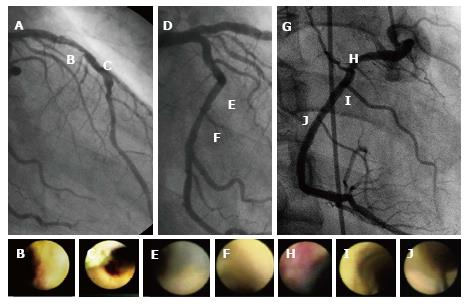
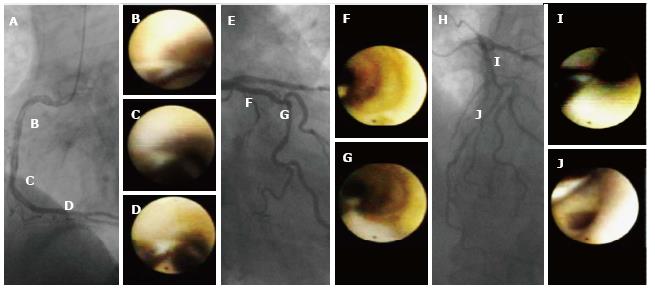
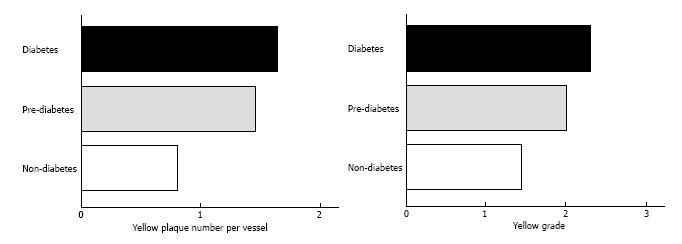
An angiographic study revealed that atherosclerosis of coronary arteries developed not only in patients with diabetes but also in those with IGT[53]. An IVUS study showed that even patients with a prediabetic status detected by IGT and IFG exhibited abundant lipid-rich plaques in their coronary arteries[54]. Recently, we used CAS to identify yellow vulnerable plaques in the coronary arteries of patients with prediabetes and diabetes compared with controls. Representative images of patients without diabetes and with prediabetes and diabetes are shown in Figures 2-4. Our findings indicate that both the degree of coronary atherosclerosis and the plaque vulnerability were more advanced in patients with prediabetes than in those without diabetes, and were comparable to patients with diabetes. We showed that the number of yellow plaques (0.80 ± 0.64 vs 1.45 ± 0.81 vs 1.63 ± 0.99; P = 0.011) and yellow grade (1.44 ± 1.03 vs 2.00 ± 0.86 vs 2.30 ± 0.70; P = 0.047) in patients with prediabetes were greater than those in patients without diabetes, but similar to those in patients with diabetes (Figure 5)[55].
Recently, the American College of Cardiology and the American Heart Association proposed a new guideline for the treatment of hyperlipidemia to reduce the risk of cardiovascular events and recommended aggressive statin therapy for both primary and secondary prevention of atherosclerotic cardiovascular disease (ACVD) events in diabetes patients[56]. Moreover, for patients with diabetes without preexisting CAD, the American Diabetes Association currently recommends starting pharmacological therapy at a low-density lipoprotein cholesterol (LDL-C) level of ≥ 130 mg/dL with a goal of < 100 mg/dL[57]. In an angioscopic investigation, lowering LDL-C by statin induces reduction of color intensity in yellow plaques, and the phenomenon is regarded as its effect on plaque stabilization[58].
Four major groups were identified that would benefit from statin therapy to reduce ACVD risk: (1) patients with clinical ACVD (secondary prevention); (2) patients with primary elevations of LDL-C ≥ 190 mg/dL (primary prevention); (3) patients 40-75 years of age who have diabetes and LDL-C 70-189 mg/dL (primary prevention); and (4) patients up to 75 years of age without diabetes and with an estimated 10-year ACVD risk ≥ 7.5% and LDL-C 70-189 mg/dL (primary prevention). Selected patients with < 5% 10-year ACVD risk who are < 40 or > 75 years of age may also benefit from statin therapy[56]. The 10-year ACVD risk was estimated from age, sex, race, blood cholesterol level, history of hypertension and diabetes, smoking habits, etc. In the group with 10-year ACVD risk < 7.5%, a benefit of statin therapy was not completely established. Regarding prediabetes, we should pay attention to this group and consider the benefit of statin therapy.
Because coronary atherosclerosis is already present in prediabetes and our angioscopic examination revealed that the level of coronary atherosclerosis with prediabetes is almost equal to that in patients with diabetes, even for patients with prediabetes, earlier pharmacological therapy should be recommended.
We should consider the risk of CAD in the prediabetic state with mild glucose metabolism disorder, and further clinical investigations are required to establish an exact risk stratification and prevent future cardiovascular events in patients with prediabetes.
| 1. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1:S62-S69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3654] [Cited by in RCA: 4362] [Article Influence: 272.6] [Reference Citation Analysis (0)] |
| 2. | Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2769] [Cited by in RCA: 2561] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 3. | Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4846] [Cited by in RCA: 4507] [Article Influence: 161.0] [Reference Citation Analysis (1)] |
| 4. | Wingard DL, Barrett-Connor EL, Scheidt-Nave C, McPhillips JB. Prevalence of cardiovascular and renal complications in older adults with normal or impaired glucose tolerance or NIDDM. A population-based study. Diabetes Care. 1993;16:1022-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1310] [Cited by in RCA: 1308] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 6. | Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 761] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 7. | Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, Cameron AJ, Dwyer T, Taylor HR, Tonkin AM. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 524] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 8. | Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992;326:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2165] [Cited by in RCA: 2003] [Article Influence: 58.9] [Reference Citation Analysis (1)] |
| 9. | Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med. 1992;326:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1030] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 10. | Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1874] [Cited by in RCA: 1830] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 11. | Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108:1772-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 801] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 12. | Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 1017] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 13. | Schmermund A, Erbel R. Unstable coronary plaque and its relation to coronary calcium. Circulation. 2001;104:1682-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106:2200-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 791] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 15. | Sathyanarayana S, Carlier S, Li W, Thomas L. Charact-erisation of atherosclerotic plaque by spectral similarity of radiofrequency intravascular ultrasound signals. EuroIntervention. 2009;5:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Kawasaki M, Takatsu H, Noda T, Ito Y, Kunishima A, Arai M, Nishigaki K, Takemura G, Morita N, Minatoguchi S. Noninvasive quantitative tissue characterization and two-dimensional color-coded map of human atherosclerotic lesions using ultrasound integrated backscatter: comparison between histology and integrated backscatter images. J Am Coll Cardiol. 2001;38:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Moore MP, Spencer T, Salter DM, Kearney PP, Shaw TR, Starkey IR, Fitzgerald PJ, Erbel R, Lange A, McDicken NW. Characterisation of coronary atherosclerotic morphology by spectral analysis of radiofrequency signal: in vitro intravascular ultrasound study with histological and radiological validation. Heart. 1998;79:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Rodriguez-Granillo GA, García-García HM, Mc Fadden EP, Valgimigli M, Aoki J, de Feyter P, Serruys PW. In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol. 2005;46:2038-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 297] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 19. | Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2252] [Cited by in RCA: 2526] [Article Influence: 168.4] [Reference Citation Analysis (1)] |
| 20. | Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 569] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 21. | Kawasaki M, Takatsu H, Noda T, Sano K, Ito Y, Hayakawa K, Tsuchiya K, Arai M, Nishigaki K, Takemura G. In vivo quantitative tissue characterization of human coronary arterial plaques by use of integrated backscatter intravascular ultrasound and comparison with angioscopic findings. Circulation. 2002;105:2487-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Takano M, Jang IK, Inami S, Yamamoto M, Murakami D, Okamatsu K, Seimiya K, Ohba T, Mizuno K. In vivo comparison of optical coherence tomography and angioscopy for the evaluation of coronary plaque characteristics. Am J Cardiol. 2008;101:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H. Implication of plaque color classification for assessing plaque vulnerability: a coronary angioscopy and optical coherence tomography investigation. JACC Cardiovasc Interv. 2008;1:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Yamamoto M, Takano M, Okamatsu K, Murakami D, Inami S, Xie Y, Seimiya K, Ohba T, Seino Y, Mizuno K. Relationship between thin cap fibroatheroma identified by virtual histology and angioscopic yellow plaque in quantitative analysis with colorimetry. Circ J. 2009;73:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Sakai S, Mizuno K, Yokoyama S, Tanabe J, Shinada T, Seimiya K, Takano M, Ohba T, Tomimura M, Uemura R. Morphologic changes in infarct-related plaque after coronary stent placement: a serial angioscopy study. J Am Coll Cardiol. 2003;42:1558-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Okamatsu K, Takano M, Sakai S, Ishibashi F, Uemura R, Takano T, Mizuno K. Elevated troponin T levels and lesion characteristics in non-ST-elevation acute coronary syndromes. Circulation. 2004;109:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Mizuno K, Satomura K, Miyamoto A, Arakawa K, Shibuya T, Arai T, Kurita A, Nakamura H, Ambrose JA. Angioscopic evaluation of coronary-artery thrombi in acute coronary syndromes. N Engl J Med. 1992;326:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 395] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 28. | Mizuno K, Miyamoto A, Satomura K, Kurita A, Arai T, Sakurada M, Yanagida S, Nakamura H. Angioscopic coronary macromorphology in patients with acute coronary disorders. Lancet. 1991;337:809-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 160] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Takano M, Inami S, Ishibashi F, Okamatsu K, Seimiya K, Ohba T, Sakai S, Mizuno K. Angioscopic follow-up study of coronary ruptured plaques in nonculprit lesions. J Am Coll Cardiol. 2005;45:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Uchida Y, Nakamura F, Tomaru T, Morita T, Oshima T, Sasaki T, Morizuki S, Hirose J. Prediction of acute coronary syndromes by percutaneous coronary angioscopy in patients with stable angina. Am Heart J. 1995;130:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 121] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Ohtani T, Ueda Y, Mizote I, Oyabu J, Okada K, Hirayama A, Kodama K. Number of yellow plaques detected in a coronary artery is associated with future risk of acute coronary syndrome: detection of vulnerable patients by angioscopy. J Am Coll Cardiol. 2006;47:2194-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH, Halpern EF. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 857] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 33. | Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, Choi KB, Shishkov M, Schlendorf K, Pomerantsev E. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 657] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 34. | Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 643] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 35. | Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, Shishkov M, Houser S, Aretz HT, Halpern EF. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111:1551-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 676] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 36. | Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Assessment of coronary arterial plaque by optical coherence tomography. Am J Cardiol. 2006;97:1172-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 37. | Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2834] [Cited by in RCA: 2929] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 38. | Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1194] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 39. | Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000;102:1014-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 484] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 40. | Kip KE, Faxon DP, Detre KM, Yeh W, Kelsey SF, Currier JW. Coronary angioplasty in diabetic patients. The National Heart, Lung, and Blood Institute Percutaneous Transluminal Coronary Angioplasty Registry. Circulation. 1996;94:1818-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 291] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 41. | Nasu K, Tsuchikane E, Katoh O, Fujita H, Surmely JF, Ehara M, Kinoshita Y, Tanaka N, Matsubara T, Asakura Y. Plaque characterisation by Virtual Histology intravascular ultrasound analysis in patients with type 2 diabetes. Heart. 2008;94:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Nicholls SJ, Tuzcu EM, Kalidindi S, Wolski K, Moon KW, Sipahi I, Schoenhagen P, Nissen SE. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol. 2008;52:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 268] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 43. | Berry C, Noble S, Grégoire JC, Ibrahim R, Levesquie S, Lavoie MA, L’Allier PL, Tardif JC. Glycaemic status influences the nature and severity of coronary artery disease. Diabetologia. 2010;53:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Wang Z, Inami S, Kirinoki S, Yamamoto H, Takagi G, Aoki S, Kato K, Takano H, Asai K, Yasutake M. Angioscopic study of silent plaque disruption in nonischemic related coronary artery in patients with stable ischemic heart disease. Int Heart J. 2010;51:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Kato K, Yonetsu T, Kim SJ, Xing L, Lee H, McNulty I, Yeh RW, Sakhuja R, Zhang S, Uemura S. Comparison of nonculprit coronary plaque characteristics between patients with and without diabetes: a 3-vessel optical coherence tomography study. JACC Cardiovasc Interv. 2012;5:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 46. | Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 934] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 47. | Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA. 1992;89:11059-11063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 508] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 48. | Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1773] [Cited by in RCA: 1836] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 49. | Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 418] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 50. | Ferrannini E, Gastaldelli A, Iozzo P. Pathophysiology of prediabetes. Med Clin North Am. 2011;95:327-339, vii-viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 51. | Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 987] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 52. | Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, Sacks DB. Tests of glycemia in diabetes. Diabetes Care. 2004;27:1761-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 431] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 53. | Kataoka Y, Yasuda S, Morii I, Otsuka Y, Kawamura A, Miyazaki S. Quantitative coronary angiographic studies of patients with angina pectoris and impaired glucose tolerance. Diabetes Care. 2005;28:2217-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Amano T, Matsubara T, Uetani T, Nanki M, Marui N, Kato M, Yoshida T, Arai K, Yokoi K, Ando H. Abnormal glucose regulation is associated with lipid-rich coronary plaque: relationship to insulin resistance. JACC Cardiovasc Imaging. 2008;1:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 55. | Kurihara O, Takano M, Yamamoto M, Shirakabe A, Kimata N, Inami T, Kobayashi N, Munakata R, Murakami D, Inami S. Impact of prediabetic status on coronary atherosclerosis: a multivessel angioscopic study. Diabetes Care. 2013;36:729-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2564] [Cited by in RCA: 2920] [Article Influence: 224.6] [Reference Citation Analysis (0)] |
| 57. | Haffner SM. Dyslipidemia management in adults with diabetes. Diabetes Care. 2004;27 Suppl 1:S68-S71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 180] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 58. | Takano M, Mizuno K, Yokoyama S, Seimiya K, Ishibashi F, Okamatsu K, Uemura R. Changes in coronary plaque color and morphology by lipid-lowering therapy with atorvastatin: serial evaluation by coronary angioscopy. J Am Coll Cardiol. 2003;42:680-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
P- Reviewer: Charoenphandhu N, Gorrell MD S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/