Published online Dec 15, 2014. doi: 10.4239/wjd.v5.i6.962
Revised: July 30, 2014
Accepted: September 11, 2014
Published online: December 15, 2014
Processing time: 179 Days and 23 Hours
AIM: To compare freeze-dried strawberry (FDS) beverage and strawberry-flavored drink effects on lipid profile and blood pressure in type 2 diabetic (T2D) patients.
METHODS: In a randomized, double-blind, controlled trial, 36 subjects with T2D (23 females; mean ± SE age: 51.57 ± 10 years) were randomly divided into two groups. Participants consumed two cups of either pure FDS beverage (each cup containing 25 g freeze-dried strawberry powder equivalent to one serving of fresh strawberries; intervention group) or an iso-caloric drink with strawberry flavoring (similar to the FDS drink in fiber content and color; placebo group) daily for 6 wk. Anthropometric measurements, 3 d, 24 h dietary recall, and fasting blood samples were collected at baseline and at weeks 6 intervention. After lying down and relaxing for approximately 10 min, each participant’s blood pressure was recorded in triplicate with 5 min intervals; recordings were made at baseline and the trial end-point. Each participant’s lipid profile was assessed before and after intervention.
RESULTS: Assessment at the weeks 6 intervention showed a significant reduction from baseline in total cholesterol levels and total cholesterol to high-density lipoprotein cholesterol (HDL-C) ratio in the intervention group (179.01 ± 31.86 to 165.9 ± 32.4 mg/L; P = 0.00 and 3.9 ± 0.88 to 3.6 ± 0.082 mg/L; P = 0.00 respectively), but the change was not significantly different between the two groups (P = 0.07, P = 0.29 respectively). Systolic blood pressure levels were significantly reduced from baseline in both the FDS and placebo drink groups (129.95 ± 14.9 to 114.3 ± 27.5 mmHg; P = 0.02 and 127.6 ± 15.6 to 122.9 ± 14.47 mmHg; P = 0.00 respectively), but the reduction was not significantly different between the two groups. Diastolic blood pressure was significantly reduced post-intervention in the FDS drink group compared to placebo group (78.7 ± 7.2 vs 84.4 ± 5.8; P = 0.01), the reduction was also significant within the FDS drink group (84.2 ± 8.03 to 78.7 ± 7.2; P = 0.00). Triglycerides, HDL-C concentrations and anthropometric indices showed no significant differences between or within groups.
CONCLUSION: Short-term FDS supplementation improved selected cardiovascular risk factors in subjects with T2D. Long-term effects on other metabolic biomarkers need to be investigated in future trials.
Core tip: Cardiovascular complications are the main cause of mortality in diabetes patients. Considering the role of flavonoids in modulating the latter complications, this study was designed to test the favorable impact of freeze-dried strawberry (FDS) drink, a flavonoid-rich beverage, on the metabolic profile of diabetes patients in a randomized, double-blind, placebo control trial. Lipid profile and blood pressure were improved in patients who consumed the FDS drink for 6 wk. Effects of the latter intervention on other atherosclerotic biomarkers have been discussed separately in Ann Nutr Metab 2013; 63: 256-264. This paper describes the further analysis of other metabolic biomarkers.
- Citation: Amani R, Moazen S, Shahbazian H, Ahmadi K, Jalali MT. Flavonoid-rich beverage effects on lipid profile and blood pressure in diabetic patients. World J Diabetes 2014; 5(6): 962-968
- URL: https://www.wjgnet.com/1948-9358/full/v5/i6/962.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i6.962
The increasing prevalence of type 2 diabetes (T2D) all over the world has highlighted the importance of cost-effective interventions in mitigating the common complications of this devastating disease[1]. Elevated serum triglycerides (TGs), reduced high-density lipoprotein cholesterol (HDL-C), increased blood pressure and enhanced fasting plasma glucose are among the most important complications experienced by patients with diabetes[2]. Diet is known to have a crucial impact on the main risk factors that are responsible for cardiovascular complications in T2D patients, exerting its effects by modulating plasma levels of lipids and lipoproteins, blood pressure, energy balance and oxidative modification or protection of plasma lipids and lipoproteins[3]. Higher consumption of fruits and vegetables are among the dietary recommendations for controlling common complications of T2D[4]. There is scarce evidence for the individual natural components, although flavonoids are thought to play a significant role in health effects of plant-based diets.
The proposed mechanisms underlying the protective role of flavonoids include regulating postprandial glucose, delaying the gastric emptying rate, and reducing active transport of glucose across intestinal brush border membrane. Inhibition of intestine sodium-glucose cotransporter-1 (Na-Glut-1) along with inhibition of α-amylase and α-glycosidase activity makes flavonoids potential candidate factors in the management of hyperglycemia[5,6]. Anthocyanins, a significant group of flavonoids in berries, have been shown to influence glucose absorption, insulin levels/secretion/action, and lipid metabolism, both in vitro and in vivo[7-9]. Due to high content of essential nutrients and flavonoids, especially anthocyanins, strawberries seem to have relevant biological impacts on human health. Few human investigations have been conducted on the cardiovascular effects of strawberries in T2D patients, despite these patients showing relative risk of cardiovascular disease (CVD) at rates 2- to 4-fold higher than those of non-diabetic subjects[10].
The main aim in this study was to assess the changes in lipid profile and blood pressure in subjects with T2D after consuming a freeze-dried strawberry (FDS) beverage or placebo drink for 6 wk. A secondary aim of this study was to provide more evidence on the beneficial effects of adding natural flavonoid-rich sources to the diets of diabetic patients and at achievable doses.
In order to attribute the effect of FDS beverage more precisely as compared to the flavonoids content of it, a placebo formula was specifically designed with similar fiber and calorie contents. A total of 40 subjects with T2D, aged between 35 and 60 years and with body mass index (BMI) of less than 35 kg/m2, were selected from Golestan Hospital in Ahavz, Iran for the present investigation. Participants were recruited via phone and advertisement. Patients with established T2D (i.e., for over 12 mo) and who had not received any lipid-lowering therapies were recruited to the study. Exclusion criteria consisted of being on medications for any chronic disease (cancer, CVD), smoking (current or stopped for less than 6 mo), lactose intolerance, alcohol consumption of more than 1 oz/d, ingestion of antioxidant supplements and vitamins, being under medical care (including taking medication) for any other disorders. Antidiabetic therapies included metformin, sulfonylurea and glitazone. The basic characteristics of participants are summarized in Table 1.
| Characteristic | Intervention | Control | P2 |
| n = 19 | n = 17 | ||
| Age in years | 51.9 ± 8.2 | 51.1 ± 13.8 | 0.710 |
| Sex, M:F | 6:13 | 5:12 | 0.433 |
| Weight at study baseline in kg | 75.79 ± 9.02 | 73.38 ± 11.98 | 0.550 |
| Weight at end-of-trial in kg | 75.84 ± 9.04 | 73.12 ± 11.89 | 0.750 |
| BMI at study baseline in kg/m2 | 27.36 ± 4.23 | 28.58 ± 4.7 | 0.330 |
| Duration of diabetes | 5.96 ± 5.1 | 9.00 ± 7.2 | 0.120 |
| Fasting blood glucose in mg/dL | 160.5 ± 51.3 | 201.7 ± 89.2 | 0.090 |
| HbA1C, % | 7.2 ± 1.6 | 7.5 ± 1.9 | 0.740 |
| Waist circumference in cm | 99.13 ± 9.06 | 100.56 ± 8.06 | 0.680 |
| Hypoglycemic agent use, n (%) | 17 (89.5) | 14 (82.3) | 0.4233 |
| Anti-hypertension agent use, n (%) | 5 (26.13) | 3 (17.46) | 0.2533 |
In order to detect a significance level of P < 0.05 and power of 80%, the sample size of 16 was calculated for each group. Considering a dropout rate of 20%, the sample size was increased to 20 for each group. Our intervention was conducted according to the Declaration of Helsinki and all procedures involving human subjects were approved by the Medical Research Ethics Committee at Ahvaz JondiShapour University of Medical Science.
This investigation was a double-blind, randomized, controlled clinical trial. A block randomization method was used to randomly assign the matched participants into one of two groups total. Patients were asked to refrain from ingesting flavonoid-rich foods (including other sources of berries, green tea, cocoa and soy products, which were identified for each participant by a screening food frequency questionnaire modified for flavonoids) for 2 wk prior to the study and throughout the intervention period. Subjects were instructed to consume daily either two cups of the FDS beverage (as intervention; containing 25 g pure freeze-dried strawberry powder) or a flavored beverage (as placebo; containing 12 g lactose, 4 g pectin and 4 g sugar-free instant strawberry drink powder) for 6 wk (Table 2). The interval between ingestion of the two cups was at least 6 h and all subjects were also instructed to avoid consuming the strawberry drink with any other snack, lunch or dinner. All participants were asked not to alter their lifestyle throughout the 6 wk trial. The FDS and placebo powders were identical in packaging as well as in taste and color upon dissolving into a glass of water. The researches distributed the FDS and placebo powder packs weekly to the participants. Compliance with the beverage consumption instructions was monitored via phone interviews twice a week.
| Nutrient composition of FDS powder | Per 50 ga |
| Carbohydrates in gram | 27.1 |
| Protein in gram | 4.05 |
| Energy in kcal | 108.4 |
| Moisture, % | 5 |
| Ash in gram | 3.17 |
| Vitamin C in milligram | 109.0 |
| Total phenolics in milligramb | 2006.0 |
| Total anthocyanins in milligramc | 154.0 |
| Phytosterols in milligram | 50 |
| Total dietary fiber in gram | 8 |
| Nutrient composition of placebo powder | Per 40 g |
| Carbohydrates in gram | 24 |
| Protein in gram | 0 |
| Energy in kcal | 98 |
| Total fiber in gram | 8 |
| Sugar-free instant drink powder with strawberry flavoring in gram | 8 |
Nutrient intake was estimated using a 24 h dietary recall exercise conducted for 3 d at pre- and post-study periods (Table 3). The 3 d averages of energy and macronutrient intakes were analyzed by Nutritionist Pro software (version 3.2, 2007; Axxya Systems, Stafford, TX, United States). All data entry was performed by a trained dietitian. Nutrient information was also obtained through food labels or recipes from participants.
| Run-in period | Throughout the study | |||||
| FDS supplement | Placebo | P2 | FDS supplement | Placebo | P2 | |
| n = 19 | n = 17 | n = 19 | n = 17 | |||
| Energy in kcal/d | 1760.36 ± 145.21 | 1697.04 ± 132.42 | 0.69 | 1784.03 ± 162.32 | 1624.42 ± 158.02 | 0.47 |
| Fat in g/d | 75.04 ± 5.17 | 69.88 ± 7.62 | 0.96 | 68.41 ± 4.68 | 73.21 ± 3.08 | 0.34 |
| SFA in g/d | 22.36 ± 1.65 | 21.62 ± 1.82 | 0.72 | 21.98 ± 1.60 | 21.23 ± 1.44 | 0.48 |
| PUFA in g/d | 19.39 ± 1.92 | 16.14 ± 1.51 | 0.023 | 19.79 ± 1.74 | 18.5 ± 1.81 | 0.46 |
| MUFA in g/d | 20.68 ± 1.70 | 21.32 ± 1.26 | 0.41 | 22.56 ± 1.51 | 21.98 ± 1.42 | 0.65 |
| Cholesterol in mg/d | 173.12 ± 14.23 | 158 ± 12.16 | 0.46 | 169.54 ± 12.50 | 160.02 ± 14.14 | 0.94 |
| Dietary fiber in g/d | 15.68 ± 1.20 | 14.73 ± 1.60 | 0.28 | 14.25 ± 1.83 | 14.21 ± 1.40 | 0.56 |
| Vitamin E in mg/d | 3.65 ± 1.72 | 4.51 ± 1.27 | 0.35 | 4.79 ± 1.50 | 4.15 ± 1.42 | 0.65 |
| Vitamin C in mg/d | 71.25 ± 25.02 | 68.42 ± 18.12 | 0.75 | 64.54 ± 16.32 | 69.47 ± 21.56 | 0.48 |
| Zinc in mg/d | 8.24 ± 1.32 | 9.80 ± 1.42 | 0.43 | 7.53 ± 1.25 | 8.67 ± 1.36 | 0.09 |
Body weight was measured using a scale (Seca, Hamburg, Germany), to 0.1 kg accuracy without shoes. Heights were measured using a stationary stadiometer (Seca), to 0.1 cm accuracy. Systolic and diastolic blood pressures (SBP and DBP respectively) were measured using the Spot Vital Signs device (Welch Allyn, Skaneateles Falls, NY). Participants were asked to lie down and relax for approximately 8 to 10 min, after which three blood pressure measurements were recorded with 5 min intervals.
Twelve hour overnight fasting blood samples were collected between 8:00 and 9:00 a.m. Serum and plasma samples were separated by centrifugation at 2000 rpm for 15 min using a 5810R centrifuge (Eppendorf, Hamburg, Germany). The serum samples were stored at -70 °C until further assay.
Serum concentrations of total cholesterol (TC), TGs, and HDL-C were measured using the standard enzymatic assay kits (Pars Azmoon Co., Tehran, Iran); specifically, TC and TGs were assessed using the cholesterol esterase/cholesterol oxidase method and glycerol phosphate oxidase method, respectively; the HDL-C concentration was measured after precipitation of B-containing lipoproteins.
FDS (intervention) powder was purchased from Chaucer Foods Co. (Paris, France). The flavored beverage (placebo) powder was supplied by Tabriz Chemistry Co. (Tabriz, Iran). All laboratory chemicals were purchased from Farzan Teb Co. (Tabriz, Iran).
Data were analyzed using SPSS for Windows (version 16.0; SPSS Inc., Chicago, IL, United States) and the results are expressed as mean ± SE. Normality of the distribution of variables was determined by the Kolmogorov-Smirnov test. The basic characteristics and nutrient intakes of participants in both groups were compared using independent sample t-test and χ2 test. The diabetes medication use in both groups was compared using Mann-Whitney U test. Analysis of covariance was used to identify any differences between the two groups post-intervention, adjusting for baseline measurements and covariates. Changes in anthropometric measurements, nutrient intakes and blood lipid parameters of the participants pre- and post-intervention were compared by paired sample t-tests. P values less than 0.05 were considered as statistically significant.
The study protocol was approved by the Medical Ethics Committee of Ahvaz JondiShapour University of Medical Sciences (Study No. ETH_393). The clinical trial registration number is IRCT201110117765N1.
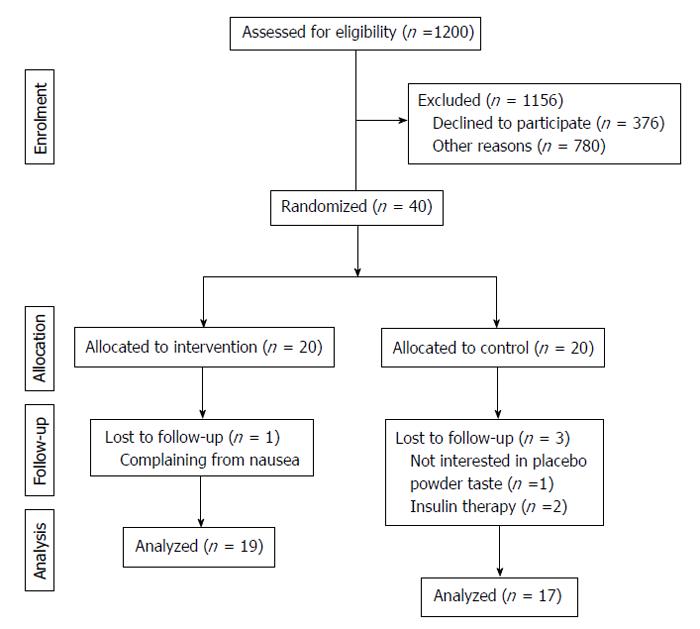
All participants completed the study, but 4 people were excluded from the statistical analysis. Among those 4 excluded patients, 3 from the placebo group experienced changes in medication or became uninterested in the taste of beverage and 1 did not consume the FDS drink due to unwillingness to continue (Figure 1). Except for the temporary gastrointestinal discomfort reported by some patients in both groups, all cases of which were alleviated during the first week, the participants demonstrated good compliance with the FDS and placebo beverage consumption.
Table 1 presents the baseline characteristics of the participants in the study groups. The two groups were statistically similar in most baseline characteristics. Weight and BMI remained unchanged during the study for both groups. No statistically significant difference was seen within and between groups in micro- and macro-nutrients dietary intake, except for polyunsaturated fatty acids intake at the beginning of intervention and at the end of the study, for which the difference in terms of dietary intake remained insignificant (Table 3).
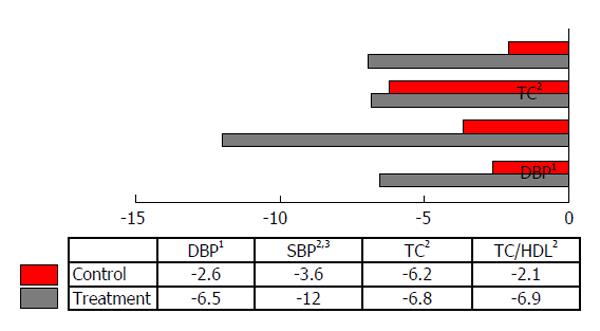
The lipid profiles were not significantly different between the FDS and placebo groups at baseline. Results of covariance analysis showed statistically significant differences within the FDS group for TC (P = 0.000) and TC:HDL-C ratio (P = 0.002) at the end of study, adjusted for monounsaturated fatty acid intake (Table 4). FDS beverage consumption caused a 13.8% decrease in TC and a 7.1% decrease in TC:HDL-C ratio compared to baseline (Figure 2). No significant differences in the lipid profiles were observed between the two groups at baseline and 6 wk post-intervention (Table 4).
| Groups | |||
| Intervention | Control | Pa | |
| n = 19 | n = 17 | ||
| TC in mg/L | |||
| Baseline | 179.01 ± 31.86 | 196.35 ± 50.5 | 0.19 |
| 6 wk | 165.9 ± 32.4 | 183.29 ± 49.9 | 0.07 |
| Change 0-6 wk | -13.1 ± 16.45 | -13.05 ± 42 | 0.80 |
| %CI for change | -7.57 to 20.32 | -8.5 to 34.67 | |
| P for change within group | 0.0001 | 0.216 | |
| LDL-C in mg/dL | |||
| Baseline | 95.84 ± 26.45 | 116.51 ± 48.8 | 0.13 |
| 6 wk | 92.96 ± 28.03 | 108.19 ± 40.2 | 0.19 |
| Change 0-6 wk | -2.87 ± 0.47 | -8.3 ± 0.13 | 0.60 |
| %CI for change | -6.8 to 12.53 | -15.52 to -32.17 | |
| P for change within group | 0.54 | 0.46 | |
| HDL-C in mg/dL | |||
| Baseline | 47.38 ± 13.67 | 46.54 ± 12.32 | 0.84 |
| 6 wk | 48.36 ± 12.62 | 47.7 ± 12.26 | 0.88 |
| Change 0-6 wk | 0.97 ± 2.4 | 1.2 ± 3.1 | 0.78 |
| %CI for change | -2.1 to 0.18 | -2.8 to 0.38 | |
| P for change within group | 0.098 | 0.12 | |
| TGs in mg/dL | |||
| Baseline | 184.6 ± 87.6 | 195.2 ± 84.2 | 0.81 |
| 6 wk | 166.37 ± 99.59 | 183.2 ± 84.4 | 0.65 |
| Change 0-6 wk | -18.28 ± 58.7 | -11.88 ± 90.56 | 0.80 |
| %CI for change | -10.5 to 46.6 | -34.6 to 58.4 | |
| P for change within group | 0.19 | 0.59 | |
| TC/HDL-C | |||
| Baseline | 3.9 ± 0.88 | 4.4 ± 1.5 | 0.19 |
| 6 wk | 3.6 ± 0.82 | 4.3 ± 1.2 | 0.29 |
| Change 0-6 wk | -0.28 ± 0.35 | -0.35 ± 0.08 | 0.40 |
| %CI for change | 0.11 to 0.45 | -0.06 to 1.01 | |
| P for change within group | 0.0021 | 0.08 | |
| LDL-C/HDL-C | |||
| Baseline | 2.1 ± 0.68 | 2.6 ± 1.2 | 0.16 |
| 6 wk | 1.9 ± 0.62 | 2.3 ± 0.94 | 0.24 |
| Change 0-6 wk | -0.12 ± 0.36 | -0.27 ± 0.06 | 0.57 |
| %CI for change | 0.11 to 0.45 | -0.06 to 1.01 | |
| P for change within group | 0.183 | 0.33 | |
| SBP in mmHg | |||
| Baseline | 129.95 ± 14.9 | 127.6 ± 15.6 | 0.74 |
| 6 wk | 114.3 ± 27.5 | 122.9 ± 14.47 | 0.25 |
| Change 0-6 wk | -15.94 ± 27.98 | -4.7 ± 6.2 | 0.57 |
| %CI for change | 2.45 to 29.43 | 1.49 to 7.91 | |
| P for change within group | 0.0231 | 0.0071 | |
| DBP in mmHg | |||
| Baseline | 84.2 ± 8.03 | 86.76 ± 6.3 | 0.168 |
| 6 wk | 78.7 ± 7.2 | 84.4 ± 5.8 | 0.0141 |
| Change 0-6 wk | -5.5 ± 7 | -2.3 ± 6.7 | 0.16 |
| %CI for change | 0.11 to 0.45 | -0.06 to 1.01 | |
| P for change within group | 0.0031 | 0.134 | |
SBP was significantly decreased in both the FDS and placebo groups, compared to baseline. DBP was also significantly reduced in the FDS group compared to the placebo group (Table 4).
The potential role of berries, a natural source of flavonoids, in improving lipid profile has been indicated by an emerging body of evidence. Strawberry puree supplementation in combination with other berries has been shown to increase HDL-C and decrease SBP (vs a control group) in subjects with cardiovascular risk factors[11]. Yet, scant human interventions have been carried out in order to prove this protective role of berries in subjects with diabetes. In order to confirm the recommendation of adding two servings of fruits with low glycemic index for proper control of diabetic complications[12], we tested a 50 g freeze-dried strawberry powder (equivalent to approximately 500 g or two servings of fresh strawberries) to investigate the beneficial effects of strawberries in a standard freeze-dried form on lipid profile and blood pressure levels in subjects with T2D. The effects of FDS beverage consumption on glaciated hemoglobin and atherosclerosis biomarkers in this study have been indicated in a separate paper[13].
In previous studies[11,14,15], plain water was mainly used as the placebo beverage; however, for better elucidation of the role of polyphenols content of berries, we used a fiber- and energy-matched placebo powder. To our best knowledge, this is the first double-blind, placebo controlled trial carried out with iso-caloric/fiber placebo beverage, investigating favorable effects of FDS beverage in T2D patients. Results from previous in vitro studies indicate that anthocyanin might affect expression of genes involved in cell cycling, signal transduction, and lipid and carbohydrate metabolism in adipose tissue cells[8,9,16].
Clinical trials involving cranberry and mixed berries extract supplementations have led to improved dyslipidemia in T2D patients and patients with hyperlipidemia[17,18]. The 6 wk FDS supplementation also improved glycated hemoglobin (HbA1c) in our intervention study[13]. However, in the present study, no significant changes were observed in low-density lipoprotein-cholesterol (LDL-C) and HDL-C after the 6 wk supplementation with FDS or placebo beverage. These findings might be due to near-normal baseline levels of LDL-C and HDL-C in our intervention and control groups. Decreases in plasma TC and the TC:HDL-C ratio were significantly greater in the FDS-supplemented group compared to the baseline (Figure 2). Our findings are similar to the previous studies reporting the effects of freeze-dried strawberries in lowering TC and LDL-C in subjects with metabolic syndrome[11,14,15].
The change in lipid profile was not significant between the intervention and control groups in this study, which might be due to the similar fiber content of the placebo drink and the FDS beverage. However, this study was specifically designed to assess the effects of the flavonoids content of the FDS beverage. Further investigations with a fiber-free placebo (as a third group) are needed to study the favorable effects of the whole content of berry products in diabetic patients.
The FDS supplementation in this study significantly decreased SBP and DBP (Table 4). These findings are in agreement with the results from a study, in which the anti-hypertensive effects of freeze-dried blueberries were assessed in obese subjects with metabolic syndrome or of mixed berry supplementation in those subjects with CVD risk factors[17,19,20]. Although, some studies have shown no significant changes in blood pressure after FDS supplementation in subjects with metabolic syndrome, which might be due to smaller sample size and/or shorter duration of intervention[14,15].
The impact of berries or anthocyanin in mitigating hypertension has been explained as enhancing endothelial nitric oxide synthase levels in endothelial cells, decreasing vasoconstriction via nitric oxide-mediated pathway, and reducing renal oxidative stress[16,17,21,22]. SBP was also significantly decreased in the control group at 6 wk post-intervention (Table 4). The latter might be attributable to the effects of the soluble fiber content of the placebo drink, indicating the possible role of fiber in FDS beverage, which could partially contribute to the reduction in SBP.
It should be mentioned that the lack of a dose-response treatment in a cross-over intervention and of the use of more sensitive biomarkers are among our study’s limitations. Gastrointestinal discomforts were anticipated, considering the excessive fiber intake accompanying the placebo drink[6]. Those who completed the entire 6 wk study period experienced this temporary gastrointestinal discomfort during the first week, which was alleviated thereafter (but which equated to a 15% drop-out rate). However, the FDS beverage was well tolerated by participants (with only a total 5% drop-out) rate. It is likely that the administration of the FDS or placebo beverage in two equal doses throughout the day and the instruction of participants to avoid consuming the drinks along with a main meal or other snacks contributed to the good tolerance. Precise adjustment for total fiber intake, longer duration of intervention, and administration of freeze-dried berry products in three or four doses throughout the day could improve tolerability while exerting more beneficial effects in future investigations.
In conclusion, our study suggests a cardio-protective role of dietary achievable doses of strawberries in subjects with T2D. These findings justify further research to provide more evidence to support the inclusion of strawberries as a part of healthy dietary practices for diabetic patients.
Amir Mansour Vatankhah, MSc, from the Research Center of Tabriz University of Medical Science is appreciated for his kind help in laboratory tests.
Increasing prevalence of type 2 diabetes (T2D) has lead to a great focus on reasonable interventions for mitigating its disease-related complications. Diet has a crucial impact on the main risk factors of cardiovascular complications in T2D patients. Flavonoids, as a natural component of a plant-based diet, might play a significant role in improving the complications of T2D. Still there is a need for more precise controlled trials on cardiovascular effects of these sources, such as berries, in diabetic patients.
Strawberries, as a rich source of flavonoids, may have biological impacts on human health through their inhibition of the main mechanism in hyperglycemia and improving blood pressure. This study was aimed to provide more evidence to support the beneficial effects of adding natural flavonoid-rich food sources at dietary achievable doses in diabetic patients. The authors investigated the changes in lipid profile and blood pressure after consumption of a freeze-dried strawberry (FDS) beverage or placebo drink by diabetic patients.
Beneficial effects of flavonoids on cardiovascular complications have emerged as a subject of considerable research interest. This study, therefore, was carried out to investigate effects of FDS beverage on lipid profile and blood pressure in comparison to a placebo drink that was specifically designed to resemble the FDS beverage in taste, color, and fiber and energy content, after a 6-wk course of supplementation in patients with diabetes. This is the first time that a randomized controlled trial has been carried out on the effect of FDS on T2D complications.
Considering the favorable effects observed upon adding two servings of fruits with low glycemic index to the dietary plan of diabetic patients, this study might suggest a suitable method of supplementing the daily dietary plan of such patients with flavonoid-rich fruits and beverages.
FDS is a term used to describe organic strawberries that have been dried using the freeze-drying technique, which is considered the most effective method for protecting the micronutrients and phytochemical content of fruits and vegetables under drying conditions. Freeze-drying enables us to take advantage of using flavonoid-rich fruits and vegetables while sustaining the highest possible quality during every season.
This study is the first randomized control trial that has been carried out to study the effects of FDS on T2D mellitus complications. Lipid profile and blood pressure were improved in patients who consumed the FDS beverage for 6 wk. The study is interesting because it demonstrates the efficacy of dietetic changes related to atherosclerosis in patients affected with T2D mellitus.
| 1. | Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9344] [Cited by in RCA: 9031] [Article Influence: 410.5] [Reference Citation Analysis (1)] |
| 2. | Dembinska-Kiec A, Mykkänen O, Kiec-Wilk B, Mykkänen H. Antioxidant phytochemicals against type 2 diabetes. Br J Nutr. 2008;99 E Suppl 1:ES109-ES117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581-4589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1451] [Cited by in RCA: 1201] [Article Influence: 46.2] [Reference Citation Analysis (1)] |
| 4. | van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care. 2002;25:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 390] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 5. | Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord. 2004;28 Suppl 4:S12-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 300] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Kobayashi K, Saito Y, Nakazawa I, Yoshizaki F. Screening of crude drugs for influence on amylase activity and postprandial blood glucose in mouse plasma. Biol Pharm Bull. 2000;23:1250-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Tsuda T, Ueno Y, Kojo H, Yoshikawa T, Osawa T. Gene expression profile of isolated rat adipocytes treated with anthocyanins. Biochim Biophys Acta. 2005;1733:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Jayaprakasam B, Vareed SK, Olson LK, Nair MG. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem. 2005;53:28-31. [PubMed] [DOI] [Full Text] |
| 9. | Martineau LC, Couture A, Spoor D, Benhaddou-Andaloussi A, Harris C, Meddah B, Leduc C, Burt A, Vuong T, Mai Le P. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine. 2006;13:612-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Ray A, Huisman MV, Tamsma JT, van Asten J, Bingen BO, Broeders EA, Hoogeveen ES, van Hout F, Kwee VA, Laman B. The role of inflammation on atherosclerosis, intermediate and clinical cardiovascular endpoints in type 2 diabetes mellitus. Eur J Intern Med. 2009;20:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Basu A, Rhone M, Lyons TJ. Berries: emerging impact on cardiovascular health. Nutr Rev. 2010;68:168-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 12. | Jenkins DJ, Srichaikul K, Kendall CW, Sievenpiper JL, Abdulnour S, Mirrahimi A, Meneses C, Nishi S, He X, Lee S. The relation of low glycaemic index fruit consumption to glycaemic control and risk factors for coronary heart disease in type 2 diabetes. Diabetologia. 2011;54:271-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Moazen S, Amani R, Homayouni Rad A, Shahbazian H, Ahmadi K, Taha Jalali M. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: a randomized double-blind controlled trial. Ann Nutr Metab. 2013;63:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Basu A, Wilkinson M, Penugonda K, Simmons B, Betts NM, Lyons TJ. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: baseline and post intervention effects. Nutr J. 2009;8:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Jenkins DJ, Nguyen TH, Kendall CW, Faulkner DA, Bashyam B, Kim IJ, Ireland C, Patel D, Vidgen E, Josse AR. The effect of strawberries in a cholesterol-lowering dietary portfolio. Metabolism. 2008;57:1636-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Lazzè MC, Pizzala R, Perucca P, Cazzalini O, Savio M, Forti L, Vannini V, Bianchi L. Anthocyanidins decrease endothelin-1 production and increase endothelial nitric oxide synthase in human endothelial cells. Mol Nutr Food Res. 2006;50:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Lee IT, Chan YC, Lin CW, Lee WJ, Sheu WH. Effect of cranberry extracts on lipid profiles in subjects with Type 2 diabetes. Diabet Med. 2008;25:1473-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, Mattila P, Jula A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr. 2008;87:323-331. [PubMed] |
| 19. | Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, Cao L, Ling W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009;90:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, Aston CE, Lyons TJ. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140:1582-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 326] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 21. | Kalea AZ, Clark K, Schuschke DA, Klimis-Zacas DJ. Vascular reactivity is affected by dietary consumption of wild blueberries in the Sprague-Dawley rat. J Med Food. 2009;12:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Xu JW, Ikeda K, Yamori Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension. 2004;44:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
P- Reviewer: Gómez-Sáez J, Salceda R, Sasaoka T S- Editor: Ji FF L- Editor: AmEditor E- Editor: Liu SQ