Published online Dec 15, 2014. doi: 10.4239/wjd.v5.i6.894
Revised: September 4, 2014
Accepted: October 1, 2014
Published online: December 15, 2014
Processing time: 170 Days and 2 Hours
Diabetes mellitus (DM) is a systemic and complex disease with micro and macrovascular complications that result from impaired metabolic pathways and genetic susceptibilities. DM has been accepted as an epidemic worldwide during the last two decades. A substantial gap in our knowledge exists regarding the pathophysiology of this metabolic disorder despite the improved diagnostic tools and therapeutic approaches. Sirtuins are a group of NAD+ dependent enzymes that are involved in cellular homeostasis due to their deacetylating activity. In the present review, we aimed to discuss the role of associated sirtuins in the pathogenesis and treatment of diabetes mellitus.
Core tip: Diabetes mellitus has been accepted as an epidemic worldwide during the last two decades. Despite the diagnostic tools and therapeutic approaches, the pathophysiology of this metabolic disorder and cellular defensive mechanisms are unknown. The maintenance of cellular homeostasis requires a well-organized network between glucose, amino acid and lipid metabolism. Sirtuins are a group of NAD+ dependent proteins that are involved in cellular homeostasis due to their deacetylating activity. Of these, sirtuin 1, -3 and -4 have been the most extensively investigated. In the present review, we aimed to discuss the role of associated sirtuins in glucose and lipid metabolism and in the pathogenesis and treatment of diabetes mellitus.
- Citation: Turkmen K, Karagoz A, Kucuk A. Sirtuins as novel players in the pathogenesis of diabetes mellitus. World J Diabetes 2014; 5(6): 894-900
- URL: https://www.wjgnet.com/1948-9358/full/v5/i6/894.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i6.894
Diabetes mellitus is one of the leading causes of cardiovascular morbidity and mortality despite the emergence of new diagnostic tools and therapeutic applications in clinical practice[1]. According to American Diabetes Association data, there are 17.5 million diagnosed and 6.6 million undiagnosed diabetics in the United States[2]. Hence, diabetes and its complications represent a significant economic burden. Hyperglycemia, insulin resistance, advanced glycation end products, polyol, hexosamine and protein kinase C pathways collectively contribute to the classical pathogenesis of diabetes complications. However, to date, we know that only serum glucose control is not sufficient to overcome the major cardiovascular (CV) events[3,4]. In this regard, novel risk factors including adipokines such as adiponectin, apelin, obestatin, leptin and resistin, chronic inflammation, and the renin-angiotensin-aldosterone system were found to be involved in the pathogenesis of diabetes and its chronic complications[5]. It would be wise to search the main mechanisms of these undesirable pathophysiologic events responsible for increased CV morbidity and mortality in diabetic patients. In addition, treatment of these various entities separately is illogical. Therefore, the main pathogenetic mechanisms should be determined and new therapeutic agents should be identified to treat diabetes.
Mammalian sirtuins are a group of proteins that include seven NAD+ dependent enzymes with homology to the silent information regulator 2 (Sir2) family of Saccharomyces cerevisae[6]. Activation or deactivation of the enzymes occur as a consequence of this deacetylation. Since both carbohydrate and lipid metabolism are affected in diabetes, it would be wise to consider that sirtuins may be the responsible key proteins that fight against the detrimental effects of these disorders. With this background, in this review, we sought to highlight the role of sirtuins as novel players in the pathogenesis of diabetes mellitus.
The main function of sirtuins is to deacetylate the important proteins for cellular homeostasis that regulate a wide variety of processes regarding protein, carbohydrate and lipid metabolism, mitochondrial homeostasis and programmed cell death mechanisms such as apoptosis and autophagy[7]. Sirtuins remove the acetyl groups from lysine residues of transcription factors, histones, specific enzymes including manganese superoxide dismutase and peroxisome proliferator activated receptor-γ coactivator-1α (PGC-1α) and other miscellaneous proteins that have important roles in cellular homeostasis[8]. As a consequence of the deacetylation, nicotinamide and 2’-0-acetyl-adenosine di phosphate (ADP) ribose are generated[9].
Experimental data showed the beneficial effects of decreasing food intake by 30% without malnutrition, also named calorie restriction (CR), on aging that could be mediated by sirtuin overexpression and this effect leads to increasing lifespan[10]. Increased intracellular NAD+ concentrations and CR are the main effectors that can stimulate sirtuin activation. In energy rich conditions, NAD+ is reduced to nicotine-amide adenine di nucleotide (NADH) and the proportion of NAD+ to NADH is reduced during glycolysis, cyclic acid cycling, lipid β-oxidation and protein catabolism[11]. Two main sources of NAD+ are the salvage pathway of nicotinamide catalyzed by the enzyme, nicotinamide phosphoribosyltransferase, and de novo synthesis from tryptophan metabolism[12].
Recent experimental studies showed that sirtuins can be found and activated in kidney, liver, spleen, lung, heart, muscle, brain, testis, ovary, thymus, pancreas, white and brown adipose tissue[13]. The localization of Sirtuin (SIRT) proteins differ and matter in the cell, hence, the different localizations develop various physiologic and possibly pathologic metabolic effects under certain stress conditions. SIRT1 resides both in the nucleus and cytoplasm and SIRT2 is primarily found in the cytoplasm, however, it can be transferred into the nucleus in a cell cycle-dependent manner. SIRT3, -4 and-5 exist in the mitochondrion. The last two members of the SIRT protein family, SIRT6 and-7 are found in the nucleus and the nucleolus of the cell, respectively[14]. Table 1 summarizes the characteristic features of sirtuins.
| Sirtuin group | Enzyme localization | Enzyme activity |
| SIRT1 | Cytoplasm and nucleus | Deacetylase |
| SIRT2 | Cytoplasm and nucleus | Deacetylase |
| SIRT3 | Cytoplasm, mitochondrion and nucleus | Deacetylase |
| SIRT4 | Mitochondrion | ADP-Ribosyl transferase |
| SIRT5 | Mitochondrion | Deacetylase |
| SIRT6 | Nucleus | Deacetylase and ADP-Ribosyl transferase |
| SIRT7 | Nucleus | Deacetylase |
SIRT1 is the most studied member of the sirtuins, probably because of its generalized effects on the cell cycle, mitochondria metabolism, energy homeostasis, inflammation, oxidative stress and apoptosis[15]. SIRT1 can directly deacetylate nuclear histone proteins that results in repression of gene transcription[16]. On the other hand, the metabolic effects of SIRT1 depend on the deacetylation of non-histone proteins such as insulin receptor substrate 2, PGC-1α, peroxisome-proliferator-activated receptor (PPAR)-α, PPAR-γ, mitochondrial uncoupling protein 2 (UCP-2), liver X receptor, farnesoid X receptor and sterol-regulatory-element binding protein[17-21]. Due to its deacetylation activity, SIRT1 regulates insulin secretion, adipogenesis and myogenesis.
In contrast to other sirtuins, SIRT4 has an additional ADP-ribosyltransferase activity that is also involved in telomere maintenance, genomic stability and longevity[22,23].
SIRT5 is a mitochondrial sirtuin. The main activity of SIRT5 is translocating SIRT3 to the nucleus[24].
SIRT6 has auto-ADP-ribosyltransferase activity[25] and its main function includes genomic stability of cells in terms of DNA repair and modulating telomere maintenance[26].
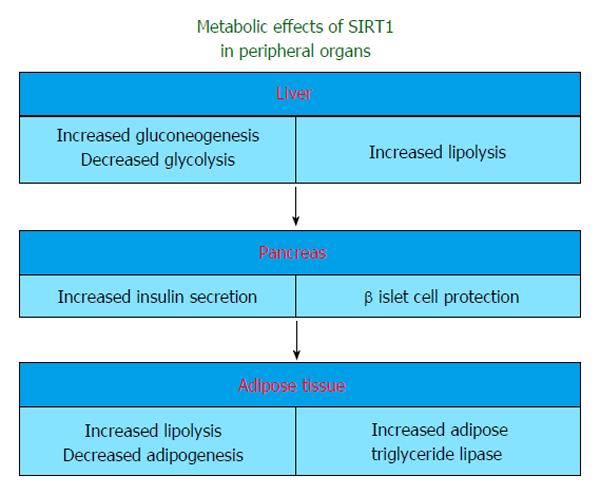
Sirtuins, especially SIRT1, influence many steps of glucose metabolism in liver, pancreas, muscle and adipose tissue (Figure 1). The main regulator of these reactions is the deactylated form of PGC-1α in SIRT1 activated states[27].
Forkhead box group O (FOXO), a group of transcriptional factors, can sense nutrient deprivation and promote cellular homeostasis[28]. FOXO1 regulates glucose metabolism[29] and feeding behaviors[30]. During the fasting state, the balance between insulin and glucagon (decreased insulin vs increased glucagon) stimulates gluconeogenesis via cAMP response element-binding protein regulated transcription coactivator 2 and FOXO1[31,32].
The link between FOXO proteins, Signal transducer and activator of transcription 3 (STAT3) and SIRT1 regarding hepatic glucose metabolism has been identified. FOXO1,-3a,-4 were found to be closely associated with increased expression of gluconeogenesis genes and decreased expression of glucokinase[33,34]. SIRT1 also regulates gluconeogenesis via deacetylation and thereby deactivates STAT3 which can inhibit the transcription of gluconeogenic genes in normal conditions[35].
The role of sirtuins in the pancreas has been demonstrated. Experimental data of SIRT1 overexpression suggested that serum insulin and cholesterol were diminished along with a reduction in adipose tissue volume and decreased obesity-induced insulin resistance[36,37]. Recently, beside experimental data, Song et al[38] also observed that adipose tissue SIRT1 may play a key role in the regulation of whole body metabolic homeostasis, and downregulation of SIRT1 in visceral adipose tissue may contribute to the metabolic abnormalities that are associated with visceral obesity in diabetic and obese women. SIRT1 deficient mice also exhibit low levels of serum glucose and insulin[39]. Despite the repetitive results of the studies regarding the CR induced SIRT1 expression, Moynihan et al[21] demonstrated that increased dosage of mammalian Sir2 in pancreatic beta cells enhanced glucose-stimulated insulin secretion in mice. Bordone et al[39] also pointed out that insulin secretion was reduced in SIRT1 knock-out mice and in pancreatic β islet cell lines in which SIRT1 had been knocked down by RNA interference. This effect partially depends on the SIRT1-mediated inhibition of UCP-2 in pancreatic islet β-cells[21]. UCP-2 is a mitochondrial inner membrane protein that regulates mitochondrial ATP synthesis. SIRT1 knock-out mice exhibit increased UCP-2 in β-cells along with low levels of serum insulin[39]. Increased pancreatic secretion of insulin and ATP were also demonstrated in UCP-2 knock-out mice[40]. In light of these studies, SIRT1 might be a positive regulator rather than a supressor of insulin in the postprandial fed state.
Insulin sensitivity is considered to be an important part of glucose metabolism. Protein tyrosine phosphatase 1B (PTP1B) is involved in glucose metabolism and diet-induced obesity[41]. PTP1B which is a tyrosine phosphatase for the insulin receptor, can be repressed via deacetylation. In accordance, resveratrol, an activator of SIRT1 may also inhibit PTP1B. Thus, SIRT1 might improve insulin sensitivity in insulin-resistant conditions by reducing PTP1B activity[42].
SIRT2 is a cytosolic deacetylase which was originally identified as a tubulin deacetylase. It was subsequently demonstrated that SIRT2 can also transiently shuttle into the nucleus in a cell cycle-dependent manner[43]. It is possible that besides their tubulin deacetylating function, nuclear proteins may be another target of SIRT2. In addition, researchers showed that SIRT2 was prominently expressed in adipocytes[44]. Krishnan et al[45] also found that SIRT2 was predominantly localized to the nucleus in adipocytes. PGC-1α has been strongly associated with energy expenditure[46]. The acetylation of PGC-1α has been reported to be critical in regulating its activity. In this regard, SIRT2 was found to deacetylate PGC-1α. The identification of PGC-1α as a SIRT2 substrate suggests that SIRT2 regulates adipocyte mitochondrial activity. Additionally, SIRT2 can deacetylate FOXO1 and FOXO3. Hence, SIRT2 was found to be closely associated with DNA repair, cell cycle, metabolism, apoptosis, and aging[47]. It has also been demonstrated that SIRT2 may increase the expression of the antioxidant mitochondrial superoxide dismutase due to its ability to deacetylate FOXO3 and consequently increase FOXO3 DNA-binding activity[48].
SIRT3 has beneficial effects on glucose metabolism by increasing insulin sensitivity and decreasing serum glucose. Hirschey et al[49] showed that high-fat diet feeding induces hepatic mitochondrial protein hyperacetylation in mice and downregulation of the major mitochondrial protein deacetylase SIRT3. They concluded that increased obesity, insulin resistance, hyperlipidemia, and steatohepatitis were prominent in mice lacking SIRT3 compared to wild-type mice. The same group also identified a single nucleotide polymorphism which encoded a point mutation in the SIRT3 protein. In this regard, impaired mitochondrial protein acetylation and polymorphism of SIRT3 have been shown to be closely associated with the metabolic syndrome[49].
Another important sirtuin involved in glucose metabolism is SIRT4. One of the target enzymes of SIRT4 is glutamate dehydrogenase (GDH) which converts glutamate to α-ketoglutarate in the mitochondrion[50]. SIRT4 inhibits amino-acid induced insulin secretion by repressing GDH[51]. During the fasting state, SIRT4 is inhibited in liver. This induces gluconeogenesis from amino acids and fats and the inhibition of SIRT4 allows insulin secretion from β-cells. However, SIRT4 is activated and the reactions mentioned above are reversed in the fed state[50].
In the early stages of type 2 diabetes mellitus, insulin resistance is the dominant feature and as a result hyperinsulinemia occurs. Impaired glucose uptake and utilization follow this stage and hyperglycemia and hyperinsulinemia contribute to pancreatic β islet cell destruction in the following stages of diabetes[52]. SIRT1 induces gluconeogenesis and inhibits glycolysis in liver during fasting by deacetylating FOXO1 and PGC1α. One of the most important questions is what are the changes in gluconeogenesis and glycolysis in diabetes mellitus? Rodgers et al[53] showed that hepatic PGC-1α is upregulated and gluconeogenesis is increased which can further aggravate hyperglycemia in diabetic mice. Yechoor et al[54] demonstrated that SIRT3 mRNA is down-regulated in muscle insulin receptor knock-out mice. Hallows et al[55] showed that SIRT3 induces ketogenesis by activating acetylCo-A synthetase in mammalian cells. Hence, one might expect that SIRT3 may play an important role in the increased ketogenesis observed during diabetes mellitus.
SIRT1, -3 and -4 play an important role in the pathogenesis of hepatosteatosis which is commonly seen in diabetic patients[56]. When taken together, inhibition of SIRT1 and 3 and/or activation of SIRT4 might be attributed to this heightened risk of hepatosteatosis in the progression of diabetes mellitus.
Dong et al[57] reported that there was an association between the SIRT5 and SIRT6 gene variants with atherosclerosis. Several important relationships were found between gender and risk factors including smoking (for the associations with SIRT5 and UCP-4), hypertension (for the associations with SIRT3, SIRT5, and UCP-5), and diabetes (for the associations with SIRT5 and UCP-5). These results suggest that genetic variants in sirtuins may have an influence on the development of vascular aging phenotypes, independent of common risk factors.
A plant polyphenol, resveratrol, was found to be the first drug to activate SIRT1[58]. Recent research demonstrated that the positive effects of resveratrol on glucose metabolism and insulin sensitivity were closely associated with AMPK subunit α activation of this agent rather than the stimulatory effect on SIRT1. Um et al[59] showed that resveratrol did not improve glucose tolerance and insulin sensitivity in AMPK α knock-out mice. On the other hand, Timmers et al[60] recently demonstrated the beneficial effects of resveratrol in obese patients in terms of lowering systolic blood pressure, serum lipid and glucose levels and inflammation parameters.
There are conflicting results about the effects of novel synthetic SIRT1 activators on glucose and lipid metabolism. SIRT1 activators might induce insulin secretion and sensitivity, reduce adipogenesis, but also induce gluconeogenesis in the liver which may worsen hyperglycemia in diabetes mellitus. Recently, Yamazaki et al[61] showed that treatment of mice with nonalcoholic fatty liver disease with a synthetic SIRT1 activator, SRT1720, might decrease the serum lipid levels, oxidative stress and inflammation. In addition, Feige et al[62] suggested that activation of SIRT1 via SRT1720 protected the organism from diet-induced obesity and insulin resistance by increasing oxidation of fatty acids in liver, adipose tissue and skeletal muscle.
Nicotinamide mononucleotide (NMN), a NAD+ intermediate, is another molecule that has been demonstrated to have beneficial effects and improved glucose and lipid levels in aging-induced diabetes[63]. The role of NMN regarding diabetic nephropathy has also been studied. Recent studies showed that SIRT1 in proximal tubule cells protects against albuminuria in diabetes by maintaining NMN concentrations around glomeruli and controlling podocyte function[64,65]. In addition, SIRT1 was found to be closely associated with the survival of cells in an affected kidney by modulating their responses to various stress stimuli, SIRT1 also takes part in arterial blood pressure control, protects against cellular apoptosis in renal tubules by inducing catalase and triggers autophagy. Hence, activation of SIRT1 may become a novel target in the treatment of diabetic nephropathy[66].
Niacin (vitamin B3), is also an important intermediate for the biosynthesis of NAD+ that can used for the activation of SIRT1[67].
Metformin, a commonly used anti-diabetic drug, decreases insulin resistance and hyperglycemia by inhibiting gluconeogenesis and hepatic glucose output, and activation of free fatty acid oxidation in skeletal muscle[68]. Some of these beneficial effects of metformin were attributed to SIRT1 activation via the AMPK pathway[69].
Calorie restriction results in a desirable metabolic profile and improvement in mitochondrial function in humans by activating several genes including SIRT1[70]. In this regard, CR with increased physical activity should be encouraged especially in obese diabetic patients.
In contrast to the above-mentioned data regarding the beneficial effects of SIRT1 activation, Marampon et al[71] recently demonstrated that an angiotensin converting enzyme inhibitor, zofenoprilat, triggered SIRT1 downregulation via p38 activation. They concluded that zofenoprilat negatively controlled angiotensin I receptor protein expression through SIRT1 and this would be associated with improved cardiovascular morbidity and mortality especially in hypertensive and diabetic patients. Hence, further research is needed to clarify the exact role of the SIRT1-related pathways in the pathogenesis of diabetes and hypertension.
In summary, SIRT1 may represent a new therapeutic target for the prevention of insulin resistance, obesity, diabetes mellitus and its chronic complications[72]. However, to date, among the treatment options mentioned above, using metformin along with CR may the optimal choices in obese type 2 diabetic patients.
Calorie restriction, oxidative stress, and various endogenous proteins might decrease nicotinamide and increase the NAD/NADH ratio that trigger sirtuins. In the fasting state, sirtuins inhibit insulin release in the pancreas and prevent β-cell degeneration, promote gluconeogenesis and insulin signaling, inhibit glycolysis and adipose tissue differentiation, and prevent ketogenesis, especially in diabetes mellitus. Activation of sirtuins may result in various beneficial metabolic effects which makes these proteins target new drugs, especially for the future treatment of metabolic disorders including diabetes and obesity. However, there are many missing pieces in the puzzle. Hence, further experimental and clinical studies are needed to highlight the exact roles of sirtuins in diabetes mellitus.
| 1. | Skyler JS, Oddo C. Diabetes trends in the USA. Diabetes Metab Res Rev. 2002;18 Suppl 3:S21-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | American Diabetes Association. Economic costs of diabetes in the US In 2007. Diabetes Care. 2008;31:596-615. [PubMed] |
| 3. | Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5314] [Cited by in RCA: 5383] [Article Influence: 299.1] [Reference Citation Analysis (1)] |
| 4. | Gerstein HC, Riddle MC, Kendall DM, Cohen RM, Goland R, Feinglos MN, Kirk JK, Hamilton BP, Ismail-Beigi F, Feeney P. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:34i-43i. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63:250-259. [PubMed] |
| 6. | Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 339] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Guarente L. Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011;364:2235-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 436] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 8. | Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschöp MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92:1479-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 573] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 9. | Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1209] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 10. | Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 335] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 11. | Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 626] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 12. | McCreanor GM, Bender DA. The metabolism of high intakes of tryptophan, nicotinamide and nicotinic acid in the rat. Br J Nutr. 1986;56:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Sakamoto J, Miura T, Shimamoto K, Horio Y. Predominant expression of Sir2alpha, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett. 2004;556:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 153] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623-4635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 1102] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 15. | Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D. Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin Sci (Lond). 2013;124:153-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature. 2007;450:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 345] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 17. | Walker AK, Yang F, Jiang K, Ji JY, Watts JL, Purushotham A, Boss O, Hirsch ML, Ribich S, Smith JJ. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 18. | Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 912] [Cited by in RCA: 897] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 19. | Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 511] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 20. | Zhang J. The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem. 2007;282:34356-34364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Méneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 502] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 22. | Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 646] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 23. | Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332:1443-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 684] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 24. | Nakamura Y, Ogura M, Tanaka D, Inagaki N. Localization of mouse mitochondrial SIRT proteins: shift of SIRT3 to nucleus by co-expression with SIRT5. Biochem Biophys Res Commun. 2008;366:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 856] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 26. | Lombard DB, Schwer B, Alt FW, Mostoslavsky R. SIRT6 in DNA repair, metabolism and ageing. J Intern Med. 2008;263:128-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2318] [Cited by in RCA: 2538] [Article Influence: 120.9] [Reference Citation Analysis (0)] |
| 28. | Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1101] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 29. | Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 451] [Cited by in RCA: 432] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 30. | Sasaki T, Kim HJ, Kobayashi M, Kitamura YI, Yokota-Hashimoto H, Shiuchi T, Minokoshi Y, Kitamura T. Induction of hypothalamic Sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology. 2010;151:2556-2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1116] [Cited by in RCA: 1196] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 32. | Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 800] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 33. | Yeagley D, Guo S, Unterman T, Quinn PG. Gene- and activation-specific mechanisms for insulin inhibition of basal and glucocorticoid-induced insulin-like growth factor binding protein-1 and phosphoenolpyruvate carboxykinase transcription. Roles of forkhead and insulin response sequences. J Biol Chem. 2001;276:33705-33710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Ayala JE, Streeper RS, Desgrosellier JS, Durham SK, Suwanichkul A, Svitek CA, Goldman JK, Barr FG, Powell DR, O’Brien RM. Conservation of an insulin response unit between mouse and human glucose-6-phosphatase catalytic subunit gene promoters: transcription factor FKHR binds the insulin response sequence. Diabetes. 1999;48:1885-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Inoue H, Ogawa W, Ozaki M, Haga S, Matsumoto M, Furukawa K, Hashimoto N, Kido Y, Mori T, Sakaue H. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med. 2004;10:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 308] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 36. | Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 532] [Cited by in RCA: 520] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 37. | Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 545] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 38. | Song YS, Lee SK, Jang YJ, Park HS, Kim JH, Lee YJ, Heo YS. Association between low SIRT1 expression in visceral and subcutaneous adipose tissues and metabolic abnormalities in women with obesity and type 2 diabetes. Diabetes Res Clin Pract. 2013;101:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 544] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 40. | Imai S, Johnson FB, Marciniak RA, McVey M, Park PU, Guarente L. Sir2: an NAD-dependent histone deacetylase that connects chromatin silencing, metabolism, and aging. Cold Spring Harb Symp Quant Biol. 2000;65:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1686] [Cited by in RCA: 1718] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 42. | Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 591] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 43. | North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1234] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 44. | Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007;6:105-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 400] [Cited by in RCA: 386] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 45. | Krishnan J, Danzer C, Simka T, Ukropec J, Walter KM, Kumpf S, Mirtschink P, Ukropcova B, Gasperikova D, Pedrazzini T. Dietary obesity-associated Hif1α activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev. 2012;26:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 46. | Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 911] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 47. | Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 977] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 48. | Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 499] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 49. | Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stančáková A, Goetzman E, Lam MM, Schwer B. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 662] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 50. | Bordone L, Guarente L. Sirtuins and beta-cell function. Diabetes Obes Metab. 2007;9 Suppl 2:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282:33583-33592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 306] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 52. | Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 675] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 53. | Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci USA. 2007;104:12861-12866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 438] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 54. | Yechoor VK, Patti ME, Ueki K, Laustsen PG, Saccone R, Rauniyar R, Kahn CR. Distinct pathways of insulin-regulated versus diabetes-regulated gene expression: an in vivo analysis in MIRKO mice. Proc Natl Acad Sci USA. 2004;101:16525-16530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 55. | Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci USA. 2006;103:10230-10235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 675] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 56. | Nasrin N, Wu X, Fortier E, Feng Y, Bare’ OC, Chen S, Ren X, Wu Z, Streeper RS, Bordone L. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 2010;285:31995-32002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 57. | Dong C, Della-Morte D, Wang L, Cabral D, Beecham A, McClendon MS, Luca CC, Blanton SH, Sacco RL, Rundek T. Association of the sirtuin and mitochondrial uncoupling protein genes with carotid plaque. PLoS One. 2011;6:e27157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2852] [Cited by in RCA: 2815] [Article Influence: 122.4] [Reference Citation Analysis (0)] |
| 59. | Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 522] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 60. | Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 1001] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 61. | Yamazaki Y, Usui I, Kanatani Y, Matsuya Y, Tsuneyama K, Fujisaka S, Bukhari A, Suzuki H, Senda S, Imanishi S. Treatment with SRT1720, a SIRT1 activator, ameliorates fatty liver with reduced expression of lipogenic enzymes in MSG mice. Am J Physiol Endocrinol Metab. 2009;297:E1179-E1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 62. | Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 592] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 63. | Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 1071] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 64. | Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, Hosoya K, Komatsu M, Kaneko Y, Kanda T. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19:1496-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 393] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 65. | Bible E. Diabetic nephropathy: Sirt1 attenuates diabetic albuminuria. Nat Rev Nephrol. 2013;9:696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Polak-Jonkisz D, Laszki-Szcząchor K, Rehan L, Pilecki W, Filipowski H, Sobieszczańska M. Nephroprotective action of sirtuin 1 (SIRT1). J Physiol Biochem. 2013;69:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Sauve AA. NAD+ and vitamin B3: from metabolism to therapies. J Pharmacol Exp Ther. 2008;324:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 68. | Caton PW, Nayuni NK, Kieswich J, Khan NQ, Yaqoob MM, Corder R. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol. 2010;205:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 69. | Zheng Z, Chen H, Li J, Li T, Zheng B, Zheng Y, Jin H, He Y, Gu Q, Xu X. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes. 2012;61:217-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 70. | Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 634] [Cited by in RCA: 585] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 71. | Marampon F, Gravina GL, Scarsella L, Festuccia C, Lovat F, Ciccarelli C, Zani BM, Polidoro L, Grassi D, Desideri G. Angiotensin-converting-enzyme inhibition counteracts angiotensin II-mediated endothelial cell dysfunction by modulating the p38/SirT1 axis. J Hypertens. 2013;31:1972-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Kitada M, Kume S, Kanasaki K, Takeda-Watanabe A, Koya D. Sirtuins as possible drug targets in type 2 diabetes. Curr Drug Targets. 2013;14:622-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
P- Reviewer: Ido Y, Lazzeri C, Russo MA S- Editor: Ji FF L- Editor: Webster JR E- Editor: Liu SQ