Published online Dec 15, 2014. doi: 10.4239/wjd.v5.i6.882
Revised: October 2, 2014
Accepted: October 23, 2014
Published online: December 15, 2014
Processing time: 122 Days and 21.5 Hours
Hepatic glycogenosis (HG) in type 1 diabetes is a underrecognized complication. Mauriac firstly described the syndrome characterized by hepatomegaly with altered liver enzymes, growth impairment, delay puberty and Cushingoid features, during childhood. HG in adulthood is characterized by the liver disorder (with circulating aminotransferase increase) in the presence of poor glycemic control (elevation of glycated hemoglobin, HbA1c levels). The advances in the comprehension of the metabolic pathways driving to the hepatic glycogen deposition point out the role of glucose transporters and insulin mediated activations of glucokinase and glycogen synthase, with inhibition of glucose-6-phosphatase. The differential diagnosis of HG consists in the exclusion of causes of liver damage (infectious, metabolic, obstructive and autoimmune disease). The imaging study (ultrasonography and/or radiological examinations) gives information about the liver alterations (hepatomegaly), but the diagnosis needs to be confirmed by the liver biopsy. The main treatment of HG is the amelioration of glycemic control that is usually accompanied by the reversal of the liver disorder. In selected cases, more aggressive treatment options (transplantation) have been successfully reported.
Core tip: This review contain an extensive revision of the case reports described in literature; in particular glycemic control (elevation of glycated hemoglobin, HbA1c levels, presence of ketoacidosis and insulin dosage), imaging studies and bioptic findings are summarized and discussed. The pathophysiological mechanisms behind the accumulation of glycogen in hepatocytes in patient with poorly controlled type 1 diabetes mellitus are described in detail.
- Citation: Giordano S, Martocchia A, Toussan L, Stefanelli M, Pastore F, Devito A, Risicato MG, Ruco L, Falaschi P. Diagnosis of hepatic glycogenosis in poorly controlled type 1 diabetes mellitus. World J Diabetes 2014; 5(6): 882-888
- URL: https://www.wjgnet.com/1948-9358/full/v5/i6/882.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i6.882
Primary glycogenosis or glycogen storage disease is a well known hereditary disease affecting liver and muscles, characterized by the presence of hepatomegaly, hypoglycemia, muscle weakness and growth delay. On the contrary, secondary glycogenosis [hepatic glycogenosis (HG)] is less described in the literature, but it may be frequently observed and underrecognized in type 1 diabetes (T1D)[1]. Mauriac[2] firstly described the syndrome in 1930. The main features in prepuberal children are hepatomegaly with increased liver enzymes, growth impairment, delay puberty and Cushingoid features in poorly controlled T1D[3]. In young adults with T1D the syndrome is uncomplete, and, in fact, only hepatomegaly with increased liver enzymes are present. The latter alterations are often underrecognized or confused with fatty liver disease or non-alcoholic steatohepatitis (NASH), that is common in T2D[4]. In rare cases, glycogen storage hepatomegaly has been described also in T2D[5].
As pointed out by Wasserman[6], 4 grams of glucose circulates in the blood (a small fraction of the body mass) and 100 grams of glycogen are present in the liver. In glucose homeostasis, the liver plays a significant role for synthesis, storage and redistribution of carbohydrates, with opposite effects during hyperglycemic (glucose uptake and glycogen synthesis) and hypoglycemic conditions (glycogenolysis and gluconeogenesis)[7].
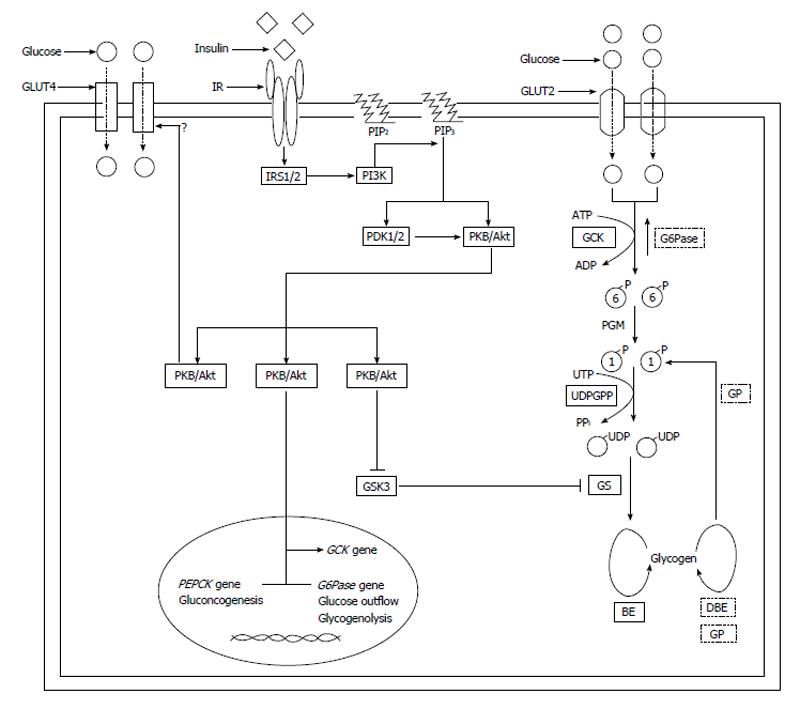
The glucose transport into cells is mediated by fourteen members of membrane glucose transporter (GLUT) molecules, divided into three families (Classes 1 to 3). The expression of the GLUTs varies between different cellular subtypes in liver (hepatocytes, endothelial cells, Kupffer cells and cholangiocytes)[8].
The liver is not considered as an insulin-sensitive tissues, such as skeletal and cardiac muscle, brown and white adipose tissue and endothelial cells. In fact, the transport of glucose into the hepatocytes is mainly mediated by the GLUT2 (insulin-independent, low-affinity, high-capacity with a Km of 10-20 mmol/L), but hepatocytes also express lower levels of GLUT1, GLUT3, GLUT4 (insulin-dependent), GLUT8, GLUT9, GLUT10[9-16] (Figure 1).
After the entrance, glucose is available for the intracellular metabolism. Glucokinase is a phosphorylating enzyme, acting with not stringent substrate specificity for glucose (it is able to phosphorylate hexoses like mannose or fructose in addition to glucose), to produce glucose-6-phosphate (G6P)[17]. There are four mammalian isoenzymes (hexokinases I-IV or A-D), displaying extensive sequence identities[18]. Glucokinase (GCK, or hexokinase IV or D) has a low affinity for glucose (S0.5 approximately equal to 6 mmol/L) and a rate of reaction with sigmoid dependence on intracellular glucose concentration (cooperativity), operating as an ultrasensitive physiological glucose sensor in hepatocytes with non-limiting glucose transport. If blood glucose is below 5 mmol/L (90 mg/dL) there is no significant effect of GCK on G6P production and subsequent steps, ensuring that hepatic glycogen synthesis is only engaged when blood glucose levels are high.
In the human liver, expression of GCK is strictly dependent on the presence of insulin, and the sterol regulatory element binding protein (SREBP1c), a master regulator of lipogenic enzymes, has been proposed to be a mediator of insulin induction of GCK[19].
Moreover, the GCK activity is modulated by the GCK regulatory protein (GCKRP) that binds and inhibits GCK, competitively with respect to glucose[20]. GCK is localized to the nucleus of the hepatocyte, where it is retained by GCKRP, but moves into the cytosol when glucose levels increase.
The hydrolysis of G6P to glucose (the inverse reaction of GCK) is mediated by the enzyme glucose-6-phosphatase (G6Pase), and its deficiency causes the impaired glycogenolysis of one type of the genetic accumulation of glycogen in hepatocytes, previously described by Von Gierke [glycogen storage disease type I (GSD1a)][21,22]. GSD1a has typical hypoglycemic events after a four to six hour fast (differentiating GDS1a from T1D), lactic acidosis, hypertriglyceridemia, and hyperuricemia[23].
The G6P is successively converted into G1P by phosphoglucomutase. Then, uridine diphosphate (UDP)-glucose pyrophosphorylase transforms G1P into UDP-glucose in the presence of uridine triphosphate, releasing inorganic pyrophosphate.
The G6P, after the phosphorylation by GCK, functions as an allosteric activator of the phosphorylated glycogen synthase (GS) for the glycogen synthesis[24]. Insulin significantly stimulates the glycogen synthesis in hepatocytes. Insulin binds the α-subunit of insulin receptor (IR) on the cellular surface of hepatocytes, inducing the dimerization of the α2β2 complex and the tyrosine kinase activity of the β-subunits. Then, the IR is autophosphorylated and the IR activation recruits and phosphorylates several substrates, including insulin receptor substrate 1-4. The downstream signaling proteins activates phosphotidylinositide-3-kinase (PI3K) to protein kinase B (PKB, also known as Akt signaling cascade), a pathway controlled via a multistep process[25]. In particular, the activation of PI3K converts phosphatidylinositol (3,4)-bisphosphate to phosphatidylinositol (3,4,5)-trisphosphate (PIP3). The 3-phosphoinositide-dependent protein kinase 1 and 2 (PDK1 and PKD2) phosphorylate and activate PKB/Akt, allowing to bindPIP3 at the plasma membrane. The activation of PKB/Akt phosphorylates and inhibits glycogen synthase kinase 3 (GSK3). GSK3 is a negative regulator of GS, through the phosphorylation at COOH-terminal residues. The result of insulin signal transduction is the GS dephosphorylation that activates the enzyme and the glycogen production. The GS is the rate-limiting enzyme for glycogen synthesis and it catalyzes the addition of α-1,4-linked glucose units from UDP-glucose to a nascent glycogen chain[26]. The UDP-glucose is the glycosyl donor in the reaction catalyzed by GS. There are two GS isoforms: the muscle GS (encoded by GYS1 gene), and the liver isoform (encoded by GYS2 gene)[27].
Glycogen is a branched polymer of glucose residues connected by α-1,4-glycosidic linkages formed by the enzyme GS and branchpoints formed viaα-1,6-glycosidic linkages, introduced by the branching enzyme, occurring every 8-12 glucose units.
New glycogen synthesis begin near the plasma membrane, at the periphery of the hepatocyte. Then, glycogen deposits grow from the periphery towards the interior of the cell. Through this way of glycogen deposition, hepatocytes may store large amounts of glycogen.
Glycogen degradation takes place in the reverse order. Glycogen phosphorylase (GP) is the key enzyme in glycogenolysis, yielding G1P[28]. When hepatocytes are depleted of glucose, the GP-mediated phosphorolysis of glycogen proceed from the interior to the exterior of the hepatocyte[29]. Phosphorylase kinase stimulates GP and protein phosphatase 1 inhibits phosphorylase kinase and GP.
Besides stimulating the glycogen synthesis, insulin severely inhibits hepatic glucose output, suppressing gluconeogenesis and glycogenolysis, by inhibiting expression and activity of the key enzymes phosphoenolpyruvate carboxykinase (PEPCK) and G6Pase[30].
The inhibition of gluconeogenesis and glycogenolysis are IR-mediated PI3K and Akt dependent effects. Akt translocates into the nucleus, where it phosphorylates FOXO1 (a member of the O-class of forkhead/winged helix transcription factors), inhibiting PEPCK and G6Pase gene transcription[31]. Moreover, Akt phosphorylates and inhibits CRTC2, cAMP response element binding protein-regulated transcription coactivator-2, also reducing hepatic gluoconeogenesis[32].
Adolescent diabetic patients with their metabolic activity, dietary intake, and disease state (high frequency of ketoacidosis and increase in exogenous insulin) represents a high-risk subjects, with diabetes control often deteriorating[33].
In T1D patients with poor glycemic control, two combined events are usually present, promoting hepatic glycogen deposition: hyperglycemia (as pointed out by increased blood glucose level and glycated hemoglobin, HbA1c) and consequent large amount of insulin (as demonstrated by elevated insulin dose as UI/kg of body weight/day). In hyperglycemia, glucose passively enters the hepatocytes by insulin-independent GLUT2, and it is rapidly phosphorylated, with inhibition of its release from hepatocytes[34]. The GCK convert the glucose into the G6P, with subsequent trapping in the hepatocyte. Then, an increased insulin administration promotes the polymerization of G6P in glycogen by GS, driving the large amount of glycogen synthesis in the presence of high cytoplasmic glucose concentrations[29]. Therefore, glycogen is trapped within the hepatocytes as a result of a combination of both hyperglycemia and insulin treatment. The consequent liver damage become evident with the blood release of aminotransferases.
Repeated ketoacidosis episodes in T1D increase the risk for hepatic glycogen overload, since diabetic ketoacidosis (a fatal complication of poor controlled diabetes) is usually treated with sustained levels of intravenous insulin (in the presence of high glucose blood concentrations).
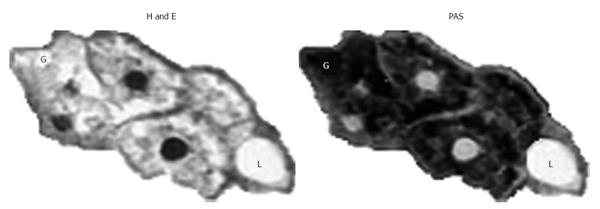
Nowadays Mauriac syndrome during childhood is uncommon especially with the advent of new insulin analogues and intensive insulin regimens. More frequently, patients affected are teenager or young adults and the diagnosis may be difficult[3]. During adulthood, the key symptoms are hepatomegaly, abdominal pain, and other symptoms such as nausea and vomiting. Laboratory findings are high levels of glucose, glycated hemoglobin (HbA1c, demonstrating a poor long-term glycemic control) and aminotransferases [aspartate and alanine, Aspartate-aminotransferase (AST) and Alanine-aminotransferase (ALT), respectively, suggesting liver damage][35]. The range of AST/ALT values is from 47/48 UI/L to 4000/1900 UI/L (Table 1). The investigations about hepatomegaly and elevated aminotransferases include investigations for infectious diseases, metabolic (such as Wilson disease), obstructive or oncologic causes and autoimmune liver tests to exclude all these possible causes and make the differential diagnosis[33]. The ultrasonographic examination of the liver is a simple and useful procedure to have information about the dimension and the characteristics of the liver tissue[34]. In few cases, T1D patients were submitted to an abdomen computed tomography scan. Unfortunately, HG cannot be clinically distinguished from non-alcoholic fatty liver disease or non-alcoholic steatohepatitis (NASH) by history, physical examination or ultrasound: the gold standard examination is the liver biopsy[36]. The preparation of the tissue is very important for the identification of the glycogen in tissue sections. The Carnoy’s solution is rapid acting, gives good nuclear preservation, retains glycogen and dissolves lipids[37]. The cytoplasmic swelling due to glycogen can be quickly demonstrated by the staining with Best’s carmine or periodic acid-Schiff (PAS) with and without diastase since the slides treated with diastase, that digest the glycogen, lack the PAS positive staining[34]. The main histological features of HG are marked glycogen accumulation leading to pale swollen hepatocyte, no or mild fatty change, no or minimal inflammation, no or minimal spotty lobular necrosis, and intact architecture with no significant fibrosis[35]. Best’s carmine is another common used stain for glycogen, that appears bright red in sections. On the contrary, in hematoxylin & eosin sections, pale hepatocytes loose their glycogen during tissue preparation and may give a hint to hepatic glycogenosis (Figure 2)[37] .
| Ref. | Sex | Age (yr) | BMI | AST (U/L) | ALT (U/L) | HbA1c (%) | Insulin (U/kg) | Glucose (mg/dL) | US exam | CT scan | Biopsy |
| [51] | M | 16 | 20 | 66 | 58 | 11.1 | 0.98 | 198 | X | X | |
| [52] | F | 17 | 138 | 164 | 12 | X | X | ||||
| [54] | M | 19 | 262 | 519 | 12.7a | X | X | ||||
| [55] | F | 19 | 27 | 98 | 49 | 7.9 | X | X | X | ||
| M | 37 | 769 | 844 | 16 | X | X | X | ||||
| [56] | F | 19 | 23 | 800 | 12.2a | X | X | ||||
| [57] | F | 3 | 300 | 350 | 9.5a | 1.5 | 522 | X | No | ||
| M | 16 | 100 | 200 | 1.3 | 810 | X | No | ||||
| [33] | M | 14 | 290 | 127 | 13.4 | 1.6 | X | X | X | ||
| F | 17 | 102 | 147 | 13.3a | 1.8 | X | |||||
| F | 16 | 567 | 316 | 12.2a | X | No | |||||
| [1] | F | 17 | 21.4 | 1620 | 629 | 13 | 0.9 | X | X | ||
| [58] | M | 16 | 21.1 | 578 | 526 | 11.0a | X | ||||
| [59] | F | 22 | 18.6 | 1028 | 365 | 13.8 | X | X | |||
| F | 26 | 23.6 | 914 | 307 | 12.9 | X | X | ||||
| F | 20 | 21 | 1310 | 346 | 13.6 | X | X | ||||
| [53] | F | 29 | 4000 | 1900 | 15.3a | X | X | ||||
| [60] | M | 13 | 1000 | 13 | 1.2 | X | X | X | |||
| [36] | F | 20 | 249 | 383 | 13.3a | X | X | ||||
| [61] | F | 13 | 113 | 8.8a | 890 | X | X | ||||
| [35] | F | 19 | 83 | 97 | a | 520 | X | ||||
| M | 12 | 47 | 49 | 13.5a | 635 | X | |||||
| F | 22 | 77 | 48 | 183 | X | ||||||
| M | 8 | H | H | X | |||||||
| F | 15 | N | N | X | |||||||
| M | 22 | 360 | 1100 | 16.0a | 404 | X | |||||
| M | 25 | 1128 | 1629 | 10.8 | X | ||||||
| M | 16 | H | H | a | X | ||||||
| M | 20 | 120 | N | 9.9 | 288 | X | |||||
| F | 18 | 57 | N | 10.8 | 137 | X | |||||
| M | 28 | 1544 | 1099 | H | X | ||||||
| M | 34 | 10 | 259 | X | |||||||
| M | 16 | 1354 | 1413 | 365 | X | ||||||
| F | 23 | 224 | 255 | X | |||||||
| [41] | F | 19 | 199 | 14.6a | b | X |
Navigator-gated and gradient-echo shimmed point-resolved spectroscopy with proton hydrogen1 (1H) magnetic resonance (MR) has been recently proposed to quantify liver glycogen concentrations in vivo, even if this measurement is more challenging than just lipid quantification[38]. In previous studies, an MR technique was used with (1-13C) glucose to measure changes in net hepatic glycogen concentration in normal and diabetic subjects[39,40].
To our best knowledge, in only one study the authors investigated the liver by the means of the MR imaging, with anatomical purposes[41].
Whereas it is well known that glycogen storage diseases, particularly type I, develop hepatic adenoma that potentially progress into hepatocellular carcinoma (HCC), to our best knowledge no data have been published about the association of diabetic glycogenosis and the progression of carcinogenesis to HCC[42-47].
The more the T1D patients (and their caregivers) obtain a good glycemic control, the more HG is expected to be minimal.
The Diabetes Control and Complication Trial (DCCT) is a well-known multicenter randomized trial that compared intensive with conventional therapy in insulin-dependent diabetes mellitus, demonstrating a prevention of diabetic complications[48]. The percentage of adolescent (13-18 years old) was 9%-19% of 1441 patients, with a 2.6-8.9 years of disease duration, a starting insulin dose of 0.62-0.72 U/kg of body weight/day and an insulin dose after 5 year of 0.46-1.10 U/kg of body weight/day[48,49].
As it has been described in the literature, the mean insulin dose in T1D patients with HG was significantly higher than in DCCT trial (1.33 U/kg), having been treated with supra-physiologic doses of insulin (Table 1).
Repeated ketoacidosis episodes in T1D significantly increase the risk for hepatic glycogen overload, since diabetic ketoacidosis (a fatal complication of poor controlled diabetes) is usually treated with sustained levels of intravenous insulin (in the presence of high glucose blood concentrations). As matter of fact, a high percentage of the HG cases described in the literature presented diabetic ketoacidosis, with a frequency of about 40% (14/35 cases), confirming the association of sustained insulin treatment and the development of HG.
With a significant difference from NASH, HG is completely reversible with a good metabolic control[50,51]. Adequate management of glucose and insulin levels can result in complete remission of clinical, laboratory and histological abnormalities[52]. Continuous subcutaneous insulin infusion should be considered as an option because the insulin requirements usually come down with improved glycemic control[41]. In severe and rare cases, pancreatic transplantation has been reported to be effective[53].
| 1. | Martocchia A, Risicato MG, Mattioli C, Antonelli M, Ruco L, Falaschi P. Association of diffuse liver glycogenosis and mild focal macrovesicular steatosis in a patient with poorly controlled type 1 diabetes. Intern Emerg Med. 2008;3:273-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Mauriac P. Grosventre, hepatomegalie, troble de la croissance chez les enfants diabetiques: traits depuis plusieursannesparl’insuline. Gaz Hebl Sci Med Bordeaux. 1930;26:402-410. |
| 3. | Mahesh S, Karp RJ, Castells S, Quintos JB. Mauriac syndrome in a 3-year-old boy. Endocr Pract. 2007;13:63-66. [PubMed] |
| 4. | Vo HDT, Klein GW, Loizides A, Zhou P, Liu Q, Pan DH. Glycogen hepatopathy in children with poorly controlled type 1 diabetes. Intern J Case Reports Images. 2011;2:1-4. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Tsujimoto T, Takano M, Nishiofuku M, Yoshiji H, Matsumura Y, Kuriyama S, Uemura M, Okamoto S, Fukui H. Rapid onset of glycogen storage hepatomegaly in a type-2 diabetic patient after a massive dose of long-acting insulin and large doses of glucose. Intern Med. 2006;45:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab. 2009;296:E11-E21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Vidal-Puig A, O’Rahilly S. Metabolism. Controlling the glucose factory. Nature. 2001;413:125-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Karim S, Adams DH, Lalor PF. Hepatic expression and cellular distribution of the glucose transporter family. World J Gastroenterol. 2012;18:6771-6781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr. 1999;19:379-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 394] [Article Influence: 14.6] [Reference Citation Analysis (13)] |
| 10. | Leturque A, Brot-Laroche E, Le Gall M. GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am J Physiol Endocrinol Metab. 2009;296:E985-E992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Bilir BM, Gong TW, Kwasiborski V, Shen CS, Fillmore CS, Berkowitz CM, Gumucio JJ. Novel control of the position-dependent expression of genes in hepatocytes. The GLUT-1 transporter. J Biol Chem. 1993;268:19776-19784. [PubMed] |
| 12. | Aschenbach JR, Steglich K, Gäbel G, Honscha KU. Expression of mRNA for glucose transport proteins in jejunum, liver, kidney and skeletal muscle of pigs. J Physiol Biochem. 2009;65:251-266. [PubMed] |
| 13. | Gould GW, Holman GD. The glucose transporter family: structure, function and tissue-specific expression. Biochem J. 1993;295:329-341. [PubMed] |
| 14. | McVie-Wylie AJ, Lamson DR, Chen YT. Molecular cloning of a novel member of the GLUT family of transporters, SLC2a10 (GLUT10), localized on chromosome 20q13.1: a candidate gene for NIDDM susceptibility. Genomics. 2001;72:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Doege H, Bocianski A, Joost HG, Schürmann A. Activity and genomic organization of human glucose transporter 9 (GLUT9), a novel member of the family of sugar-transport facilitators predominantly expressed in brain and leucocytes. Biochem J. 2000;350 Pt 3:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Dawson PA, Mychaleckyj JC, Fossey SC, Mihic SJ, Craddock AL, Bowden DW. Sequence and functional analysis of GLUT10: a glucose transporter in the Type 2 diabetes-linked region of chromosome 20q12-13.1. Mol Genet Metab. 2001;74:186-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Cárdenas ML, Cornish-Bowden A, Ureta T. Evolution and regulatory role of the hexokinases. Biochim Biophys Acta. 1998;1401:242-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 199] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci. 2009;66:27-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 268] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Iynedjian PB, Marie S, Gjinovci A, Genin B, Deng SP, Buhler L, Morel P, Mentha G. Glucokinase and cytosolic phosphoenolpyruvate carboxykinase (GTP) in the human liver. Regulation of gene expression in cultured hepatocytes. J Clin Invest. 1995;95:1966-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | van Schaftingen E, Veiga-da-Cunha M, Niculescu L. The regulatory protein of glucokinase. Biochem Soc Trans. 1997;25:136-140. [PubMed] |
| 21. | Von Gierke E. Hepato-nephromegalia glycogenika (Glykogenspeicherkrankheitder Leber und Nieren). Beitr Pathol Anat. 1929;82:497-513. |
| 22. | Mundy HR, Lee PJ. Glycogenosis type I and diabetes mellitus: a common mechanism for renal dysfunction? Med Hypotheses. 2002;59:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Santos BL, de Souza CF, Schuler-Faccini L, Refosco L, Epifanio M, Nalin T, Vieira SM, Schwartz IV. Glycogen storage disease type I: clinical and laboratory profile. J Pediatr (Rio J). 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | von Wilamowitz-Moellendorff A, Hunter RW, García-Rocha M, Kang L, López-Soldado I, Lantier L, Patel K, Peggie MW, Martínez-Pons C, Voss M. Glucose-6-phosphate-mediated activation of liver glycogen synthase plays a key role in hepatic glycogen synthesis. Diabetes. 2013;62:4070-4082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4:a011189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 828] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 26. | Roach PJ, Depaoli-Roach AA, Hurley TD, Tagliabracci VS. Glycogen and its metabolism: some new developments and old themes. Biochem J. 2012;441:763-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 520] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 27. | Irimia JM, Meyer CM, Peper CL, Zhai L, Bock CB, Previs SF, McGuinness OP, DePaoli-Roach A, Roach PJ. Impaired glucose tolerance and predisposition to the fasted state in liver glycogen synthase knock-out mice. J Biol Chem. 2010;285:12851-12861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Clark D, Haynes D. The glycogen storage disease (gsd/gsd) rat. Curr Top Cell Regul. 1988;29:217-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Ferrer JC, Favre C, Gomis RR, Fernández-Novell JM, García-Rocha M, de la Iglesia N, Cid E, Guinovart JJ. Control of glycogen deposition. FEBS Lett. 2003;546:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2003;285:E685-E692. [PubMed] |
| 31. | Okamoto MM, Anhe GF, Sabino-Silva R, dos Santos Ferreira Marques MF, Freitas HS, Tieko Mori RC, Melo KFS, Machado UF. Intensive insulin treatment induces insulin resistance in diabetic rats by impairing glucose metabolism-related mechanisms in muscle and liver. J Endocrinol. 2011;211:55-64. [PubMed] [DOI] [Full Text] |
| 32. | Wang Y, Inoue H, Ravnskjaer K, Viste K, Miller N, Liu Y, Hedrick S, Vera L, Montminy M. Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc Natl Acad Sci USA. 2010;107:3087-3092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Munns CF, McCrossin RB, Thomsett MJ, Batch J. Hepatic glycogenosis: reversible hepatomegaly in type 1 diabetes. J Paediatr Child Health. 2000;36:449-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Chatila R, West AB. Hepatomegaly and abnormal liver tests due to glycogenosis in adults with diabetes. Medicine (Baltimore). 1996;75:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 35. | Torbenson M, Chen YY, Brunt E, Cummings OW, Gottfried M, Jakate S, Liu YC, Yeh MM, Ferrell L. Glycogenic hepatopathy: an underrecognized hepatic complication of diabetes mellitus. Am J Surg Pathol. 2006;30:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 36. | Hudacko RM, Manoukian AV, Schneider SH, Fyfe B. Clinical resolution of glycogenic hepatopathy following improved glycemic control. J Diabetes Complications. 2008;22:329-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Eltoum I, Fredenburgh J, Myers RB, Grizzle WE. Introduction to theory and practice of fixation of tissues. J Histotechnology. 2001;24:173-190. [DOI] [Full Text] |
| 38. | Ouwerkerk R, Pettigrew RI, Gharib AM. Liver metabolite concentrations measured with 1H MR spectroscopy. Radiology. 2012;265:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Hwang JH, Perseghin G, Rothman DL, Cline GW, Magnusson I, Petersen KF, Shulman GI. Impaired net hepatic glycogen synthesis in insulin-dependent diabetic subjects during mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy study. J Clin Invest. 1995;95:783-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 148] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Petersen KF, Price TB, Bergeron R. Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: impact of type 1 diabetes. J Clin Endocrinol Metab. 2004;89:4656-4664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Imtiaz KE, Healy C, Sharif S, Drake I, Awan F, Riley J, Karlson F. Glycogenic hepatopathy in type 1 diabetes: an underrecognized condition. Diabetes Care. 2013;36:e6-e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Bannasch P, Mayer D, Hacker HJ. Hepatocellular glycogenosis and hepatocarcinogenesis. Biochim Biophys Acta. 1980;605:217-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Bannasch P, Klimek F, Mayer D. Early bioenergetic changes in hepatocarcinogenesis: preneoplastic phenotypes mimic responses to insulin and thyroid hormone. J Bioenerg Biomembr. 1997;29:303-313. [PubMed] |
| 44. | Su Q, Benner A, Hofmann WJ, Otto G, Pichlmayr R, Bannasch P. Human hepatic preneoplasia: phenotypes and proliferation kinetics of foci and nodules of altered hepatocytes and their relationship to liver cell dysplasia. Virchows Arch. 1997;431:391-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Scharf JG, Ramadori G, Dombrowski F. Analysis of the IGF axis in preneoplastic hepatic foci and hepatocellular neoplasms developing after low-number pancreatic islet transplantation into the livers of streptozotocin diabetic rats. Lab Invest. 2000;80:1399-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Bannasch P. Glycogenotic hepatocellular carcinoma with glycogen-ground-glass hepatocytes: a heuristically highly relevant phenotype. World J Gastroenterol. 2012;18:6701-6708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Ribback S, Calvisi DF, Cigliano A, Sailer V, Peters M, Rausch J, Heidecke CD, Birth M, Dombrowski F. Molecular and metabolic changes in human liver clear cell foci resemble the alterations occurring in rat hepatocarcinogenesis. J Hepatol. 2013;58:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16445] [Article Influence: 498.3] [Reference Citation Analysis (4)] |
| 49. | Delahanty LM, Nathan DM, Lachin JM, Hu FB, Cleary PA, Ziegler GK, Wylie-Rosett J, Wexler DJ. Association of diet with glycated hemoglobin during intensive treatment of type 1 diabetes in the Diabetes Control and Complications Trial. Am J Clin Nutr. 2009;89:518-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 50. | Abaci A, Bekem O, Unuvar T, Ozer E, Bober E, Arslan N, Ozturk Y, Buyukgebiz A. Hepatic glycogenosis: a rare cause of hepatomegaly in Type 1 diabetes mellitus. J Diabetes Complications. 2008;22:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Bua J, Marchetti F, Faleschini E, Ventura A, Bussani R. Hepatic glycogenosis in an adolescent with diabetes. J Pediatr. 2010;157:1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | van den Brand M, Elving LD, Drenth JP, van Krieken JH. Glycogenic hepatopathy: a rare cause of elevated serum transaminases in diabetes mellitus. Neth J Med. 2009;67:394-396. [PubMed] |
| 53. | Fridell JA, Saxena R, Chalasani NP, Goggins WC, Powelson JA, Cummings OW. Complete reversal of glycogen hepatopathy with pancreas transplantation: two cases. Transplantation. 2007;83:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Bohinc BN, Parker JC. What is the diagnosis? Hepatic glycogenosis. Endocr Pract. 2007;16:529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 55. | Sayuk GS, Elwing JE, Lisker-Melman M. Hepatic glycogenosis: an underrecognized source of abnormal liver function tests? Dig Dis Sci. 2007;52:936-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Cuthbertson DJ, Brennan G, Walsh S, Henry E. Hepatic glycogenosis: abnormal liver function tests in Type 1 diabetes. Diabet Med. 2007;24:322-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Carcione L, Lombardo F, Messina MF, Rosano M, De Luca F. Liver glycogenosis as early manifestation in type 1 diabetes mellitus. Diabetes Nutr Metab. 2003;16:182-184. [PubMed] |
| 58. | Merino Palacios C, Primo Vera J, Fernández Chinchilla J, Ferrando Marco J, Aragó Galindo M, García Ferrer L. Hypertransaminasemia in poorly-controlled type-1 diabetes mellitus. Rev Esp Enferm Dig. 2004;96:730-731; 731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 59. | Cha JH, Ra SH, Park YM, Ji YK, Lee JH, Park SY, Baik SK, Kwon SO, Cho MY, Kim MY. Three cases of glycogenic hepatopathy mimicking acute and relapsing hepatitis in type I diabetes mellitus. Clin Mol Hepatol. 2013;19:421-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Aljabri KS, Bokhari SA, Fageeh SM, Alharbi AM, Abaza MA. Glycogen hepatopathy in a 13-year-old male with type 1 diabetes. Ann Saudi Med. 2011;31:424-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Butts GT, Hairston FJ, Bishop PR, Nowicki MJ. Massive hepatomegaly in poorly controlled insulin-dependent diabetes mellitus. J Pediatr. 2014;164:214-214.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
P- Reviewer: Bannasch P, Pastromas S S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ