SALIVA AS A DIAGNOSTIC TOOL: CURRENT KNOWLEDGE
Saliva, an exocrine secretion of the salivary glands, containing water (99%), electrolytes, proteins, and enzymes, provides sensory perception of food, and aids chewing, swallowing, and digestion of food[1]. Saliva protects tissues against desiccation, penetration, ulceration, potential carcinogens, and assists in wound healing[2]. Whole saliva comprises of a mixture of fluids, secreted from the salivary glands (submandibular, sublingual, and parotid, and the minor gland), gingival fold, oral mucosa transudate, and, mucous from the nasal cavity and pharynx, that vary in rheological properties and the composition of their secretions[3-6]. The parotid gland secretions are largely composed of water and electrolytes, while the submandibular and sublingual glands produce both serous and mucous secretions, with mucin being the most abundant protein in saliva[7]. Saliva also contains cystatins, proline-rich peptides, and other molecules that are found in blood[4,8]. Saliva is hypotonic to plasma and is actively involved in exchange of sodium (Na+), chloride (Cl-), potassium (K+) and bicarbonate (HCO3-) ions with plasma[7]. Proteins and other substances from blood have been shown to enter saliva intracellularly through passive diffusion or active transport, and paracellularly through ultrafiltration at tight junctions between cells[9]. Saliva can be collected by passive drool technique or by using oral swabs. In healthy individuals, depending on age and gender, the unstimulated salivary flow rate is between 0.1-2 mL/min[10]. Additional factors influencing unstimulated salivary flow and composition include individual hydration, body posture, lighting, smoking, circadian and circannual rhythms, and medications[1].
The use of saliva as an alternative diagnostic tool to blood offers certain advantages. Salivary composition has been observed to be influenced by systemic changes allowing identification of biomarkers for disease conditions. Since saliva collection is non-invasive and relatively stress-free, saliva can serve as a potential alternative diagnostic fluid in infants, toddlers, youth and adults. However, despite its diagnostic potential, saliva has not yet been established as an analytical tool due to insufficient information regarding salivary biochemical composition and its correlation with plasma levels. Salivary Na, K, total protein, IgA and amylase activity has been shown to increase linearly with age. For example, salivary amylase activity has been shown to be variable and significantly different between infants and toddlers[11]. However, in healthy adults (mean age 22 years), no significant differences were observed in salivary concentrations of glucose, inorganic phosphate, total protein, Mg2+, Cl- and Ca2+ between men and women participants[12]. Interestingly, recent studies demonstrate the diagnostic utility of saliva with implications for cardiovascular disease, systemic and local inflammation, hepatic damage and insulin resistance[8,13,14].
Currently, saliva testing is used in areas of toxicology, endocrinology, infectious diseases, and forensics, with established diagnostic tests available for alcohol detection, HIV infections, hormonal analyses, and drug testing[15,16]. Several studies have demonstrated the use of saliva for detection of antibodies against HIV-1 and HIV-2 under non-laboratory settings[17,18]. The United States Food and Drug Administration (FDA) has recently approved OraQuick, the first over-the-counter, in-home self-testing HIV kit, which uses an oral sample for rapid detection of antibodies against HIV[19]. The assessment of hormones in saliva has been widely studied for routine clinical use[20-22]. The FDA has recently approved the use of enzyme immunoassay technique for in vitro diagnostic assay of salivary cortisol for adrenal cortical function and screening for Cushing’s and Addison’s disease[23]. In this review, we explore the potential of using saliva as a non-invasive diagnostic tool for the measurement of biomarkers of insulin-resistance and inflammation.
GLUCOSE IN SALIVA
Salivary glucose has been shown to significantly correlate (r = 0.5216, P < 0.05) with serum glucose in healthy subjects (n = 15). In individuals with newly diagnosed type 2 diabetes (n = 106), salivary glucose demonstrated strong correlation with serum glucose (r = 0.7686, P < 0.01) and serum HbA1c (r = 0.5662, P < 0.01). Type 2 diabetic patients had significantly higher (P < 0.01) mean salivary glucose values (4.22 ± 3.59 mg/mL) compared to healthy controls (1.23 ± 0.52 mg/mL)[24]. Pendyala et al[25] have also evaluated serum and salivary glucose in diabetic (men = 26, women = 14) and non-diabetic (men = 28, women = 12) individuals[25]. These authors observed significant correlation between fasting salivary and plasma glucose in both diabetic (r = 0.40) and non-diabetic (r = 0.58) groups. Further, they reported a significant difference in fasting salivary glucose (P < 0.001) between diabetic (10.93 ± 1.93 mg/mL) and non-diabetic controls (6.08 ± 1.16 mg/mL). Further, a recent systematic review reported a meaningful increase in salivary glucose concentration in type 2 diabetes that was associated with HbA1c values, suggesting that salivary glucose levels may be a potential biomarker for type 2 diabetes mellitus[26]. Ongoing research is focused on the development of nanotechnology-based biochip sensors for salivary glucose measurements. Such a novel biochemical sensor that provides a compact, high-throughput device for real-time glucose measurements may have implications in point-of-care clinical settings[27].
INSULIN IN SALIVA
Salivary insulin, assayed in normal and type 1 diabetic subjects by Pasic and Pickup demonstrated significant correlation between mean serum insulin and salivary insulin (r = 0.81, P < 0.01 in non-diabetics and r = 0.91, P < 0.001 in type 1 diabetics)[28]. However, because several individual profiles showed marked discrepancies between the timing and magnitude of insulin changes, these authors did not recommend salivary insulin concentrations as a reliable index of insulinemia. More recently, studies by Fabre et al[29] demonstrated that salivary insulin concentrations were approximately 10 times lower than serum insulin concentrations[29]. These authors showed a significant correlation (r = 0.92, P < 0.001) between salivary and serum insulin concentrations in 130 boys and 147 girls, aged 6-14 years, suggesting that salivary insulin measurements may be a feasible approach, but suggest the need for additional studies to validate these findings. However, there were no reports that assessed surrogate measures of insulin resistance, including the Homeostasis Assessment Model-estimated insulin resistance (HOMA-IR) or the Quantitative Insulin Sensitivity Check Index[30,31].
CORTISOL IN SALIVA
One of the most widely studied salivary biomarker of stress is the glucocorticoid hormone, cortisol[32,33]. Elevated cortisol production can lead to hypertension, central obesity, insulin resistance and glucose intolerance[34]. In a study of overweight Latino youth (n = 211, boys = 119, girls = 92, age between 8 and 13 years) at risk for type 2 diabetes, cortisol was shown to negatively influence insulin sensitivity, and was inversely correlated with fasting glucose (r = 0.23, P < 0.01), β-cell function (r = -0.24, P < 0.05), and acute insulin response to glucose (r = -0.27, P < 0.05)[35]. HPA-axis dysfunction has been associated with various psychological and pathophysiological conditions, and hyperactivity of hypothalamic-pituitary-adrenal (HPA) axis has been observed in individuals with type 2 diabetes[36,37].
Saliva contains free, biologically active cortisol as opposed to total cortisol present in serum or plasma. Further, the concentration of cortisol in saliva is independent of the salivary flow rate and is strongly correlated with circulating cortisol concentrations[33,36]. Cortisol follows a diurnal pattern and any disruption in the rhythm would also be indicative of an HPA dysfunction. The average salivary cortisol concentrations in healthy subjects were reported to be higher in the morning (0.20-1.41 μg/mL) compared to afternoon values (0.04-0.41 μg/mL)[33]. Björntorp et al[36] have reported the use of salivary cortisol measurements to monitor the activity of HPA axis. In their study, circulatory perturbations in cortisol expression, which are indicative of increased risk of endocrine abnormalities, insulin resistance, central obesity, dyslipidemia, hypertension and type 2 diabetes, were reflected in the salivary cortisol levels[36].
Data from the Multi-Ethnic Study of Atherosclerosis has demonstrated associations between salivary cortisol and markers of inflammation including interleukin (IL)-6, IL-10 and tumor necrosis factor (TNF)-α in plasma[38]. In this study, IL-6 was found to be most consistently related to cortisol profiles, and higher IL-6 levels were inversely associated with lower cortisol awakening response. In obese individuals (men, n = 91; women, n = 103) between the ages 19 to 35 years, significant associations were observed between cortisol levels and body fat distribution[39].
Salivary cortisol concentrations are known to increase within 5 min of increases in plasma cortisol, and are generally well correlated with plasma values[40]. There are several salivary cortisol kits available commercially, which commonly use immunoassay techniques or the more recent liquid chromatography-tandem mass spectrophotometry technique. In clinical settings, salivary cortisol is frequently used in the diagnosis of Cushing’s syndrome with reported sensitivities and specificities of 90%[41,42]. Saiyudthong et al[43] have conducted a study to compare salivary cortisol levels in healthy individuals (n = 83, aged 18-25 years), measured by enzyme-linked immunosorbent assay (ELISA) and electrochemiluminescence (ECL). Salivary cortisol showed a positive correlation with serum values (r = 0.84, P < 0.001) measured using ECL. Further, there was no significant difference between salivary cortisol measured by ELISA and ECL, suggesting ECL as an alternative detection technique for salivary cortisol measurement[43].
ADIPOKINES IN SALIVA
Adipose tissue produces several pro-inflammatory and anti-inflammatory factors, including the adipokines leptin, adiponectin, resistin, and visfatin, as well as cytokines such as TNF-α, IL-6, and chemokines such as monocyte chemoattractant protein-1 (MCP-1). These have been shown to participate in the pathogenesis of insulin resistance, adipogenesis and inflammation[44-48].
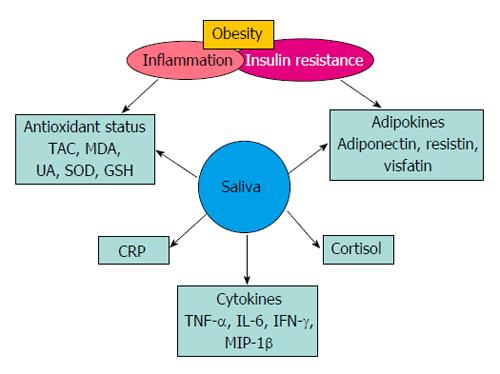
Recent studies have shown that resistin, visfatin, and adiponectin concentrations can be measured using saliva (Figure 1)[45,46,49]. Mamali et al[45] have examined associations between serum and salivary concentrations of adiponectin, resistin and visfatin in healthy individuals (men, n = 17; women, n = 33) with a mean age of 34 ± 14 years, body mass index (BMI) 22.4 ± 3.6 and body fat percentage 22.4 ± 8.4. In this study, mean salivary (10.92 ng/mL) and serum (12.27 μg/mL) adiponectin levels were shown to be marginally correlated (r = 0.347, P = 0.019). There was a significant positive correlation (r = 0.441, P < 0.01) between salivary (1.69 ng/mL) and serum (7.78 ng/mL) resistin values, and no statistical correlation between salivary (9.51 ng/mL) and serum (21.41 ng/mL) visfatin values[45]. Further, the study reported that the differences were not significant between men and women. Similarly, Toda et al[50] have demonstrated significant correlation (P < 0.05) between plasma and salivary adiponectin values in healthy female participants (n = 30, age > 43 years)[50]. In this study, the authors have compared plasma adiponectin (11.7 μg/mL) concentrations with salivary adiponection in saliva samples collected directly in a test tube (0.89 ng/mL), and with cotton wads using the Salivette system (0.82 ng/mL). There was a significant correlation (P < 0.05) between plasma and test-tube saliva samples, and not with the Salivette samples. Salivary detection of proteins such as adiponectin depends largely on salivary processing methods, and the recovery of proteins from saliva. Thanakun et al[51] have demonstrated filtration as an alternative saliva processing technique, to the commonly used centrifugation method. In this study, adiponectin levels, following filtration, were comparable to those after centrifugation[51]. In another study, these authors have demonstrated significant association (r = 0.211, P = 0.018) between salivary and plasma adiponection, using ELISA technique, in both healthy individuals (n = 46) and patients with metabolic syndrome (n = 82). The authors, however, did not observe significant difference in salivary adiponectin between the 2 study groups[52].
Figure 1 Salivary biomarkers of inflammation and insulin resistance.
TAC: Total antioxidant capacity; MDA: Malondialdehyde; UA: Uric acid; SOD: Superoxide dismutase; GSH: Glutathione reductase; CRP: C-reactive protein; TNF-α: Tumor necrosis factor-alpha; IL-6: Interleukin-6; IFN-γ: Interferon gamma; MIP-1β: Macrophage inflammatory protein-1 beta.
In a second study, Yin et al[46] have reported significantly higher salivary resistin concentrations (P > 0.05) in individuals with newly diagnosed type 2 diabetes (men, n = 18; women, n = 20) compared to non-diabetic subjects. Salivary resistin was significantly correlated with serum resistin concentrations at different time points of oral glucose tolerance test, and was not affected by an oral glucose load. Further, there was a positive correlation of serum and salivary resistin concentrations with BMI and HOMA-IR in both control and diabetic groups[46]. The studies together indicate that while assay validation and the method of saliva sample collection can play a key role in biomarker quantification and standardization, saliva has the potential to be further explored as a diagnostic tool for adipokine analyses. More research needs to be directed towards developing saliva processing techniques, which can substantially increase the recovery of proteins. Higher protein yields can positively contribute towards improving outcomes of studies determining correlations between saliva and serum concentrations of adipokines.
INFLAMMATORY BIOMARKERS IN SALIVA
Inflammation can be caused by a variety of conditions including oxidative stress, overweight/obesity, improper oral hygiene and nutritional deficiencies[1,13,53]. Chronic low-grade inflammation has been associated with systemic diseases, insulin resistance and development of type 2 diabetes[54,55]. Focusing on the need to establish rapid, non-invasive and easy-to-use strategies for disease diagnosis, there has been growing interest in evaluating the potential of saliva for inflammatory marker profiling.
Studies have indicated that the most commonly explored biomarkers of inflammation include antioxidant status and C-reactive protein (CRP) concentrations[53,56-58]. Spectrophotometric assays quantifying levels of thiobarbituric acid reacting substances (TBARS) are used to evaluate salivary antioxidant status, while CRP concentrations are measured using ELISA kits or high-sensitivity immunoturbidimetric assays[53,56-59]. However, these tests lack the sensitivity for detection of CRP in saliva. To address the issue of sensitivity, researchers have developed a “lab-on-the-chip” technique for salivary CRP measurements. This novel technique utilizes a microchip assay system that offers the advantages of increased sensitivity (10 pg/mL of CRP) with lower noise-to-signal ratio. The lab-on-the-chip system captures optical signals generated by chemical and immunological reactions performed on microspheres (280 microns in diameter) implanted in silicon microchip wells[60]. Saliva collection techniques reported in clinical studies include the use of unstimulated passive drool or the filter paper method[59,61]. However, it has been observed that correlations between salivary biomarkers were not strong enough to support one collection method over another[61].
Williamson et al[61] have reported the presence of 27 cytokine biomarkers including IL-1β, IL-1 receptor agonist, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-17, eotaxin, basic fibroblast growth hormone, growth-colony stimulating factor, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, interferon-inducible protein 10, MCP-1, macrophage inflammatory proteins (MIPs)-1α, MIP-1β, platelet-derived growth factors BB, TNF-α, and vascular endothelial growth factor in the saliva of healthy adults. These cytokines were measured using a commercially available cytokine multiplex assay kit that combines the use of fluorescent flow cytometry and ELISA technology. These authors observed that out of the 27 cytokines tested, only 3 cytokines including IL-6, IFN-γ and MIP-1β, found in saliva samples collected by passive drool, showed significant correlation (P < 0.05) with plasma levels[61].
Recently, a novel clinical approach termed, salivary transcriptome diagnostics, has been evaluated to provide a robust, high-throughput and reproducible tool for salivary biomarker detection. Using microarray analysis and quantitative polymerase chain reaction, this method has demonstrated high sensitivity (91%) and specificity (91%) for inflammatory biomarkers including IL-8 and IL-1β[62]. Another emerging technique called the oral fluid nanosensor test (OFNASET), offers a rapid and simultaneous detection of multiple salivary proteins, including IL-8 and IL-1β, for point-of-care disease screening and detection. OFNASET involves the use of advanced electrochemical-based molecular analysis platforms including self-assembled monolayers, bionanotechnology, cyclic enzymatic amplification, microfluids, hybridization-based detection, and molecular purification[63].
A recent study in healthy adolescent girls (11-17 years), observed that cytokines including GM-CSF, IL-1β, IL-2, IL-6, IL-8, IL-12p70, TNF-α, adiponectin, and cotinine were detectable in saliva. However, the cytokine concentrations, except IL-8 and IL-1β, were lower than serum values and variable at baseline. Further, there were no serum-saliva associations in the levels of cytokines tested[64]. It has been suggested that lack of correlation between salivary and plasma cytokine biomarkers may be due to the impact of oral environment, and the influence of local immunity. It has also been indicated that the variability in cytokine levels may be due to distinct diurnal patterns, reflecting the time of saliva collection[65].
Salivary concentrations of TNF-α and IL-6 have been shown to be elevated in individuals with type 2 diabetes and periodontal disease (n = 20, mean age = 57 ± 4 years), compared to healthy subjects (n = 21) with periodontal disease[66]. In this study, salivary TNF-α and IL-6 were assayed with ELISA-sandwich technique using commercially available immunoassay kits. In type 2 diabetic patients with periodontal disease, both salivary and serum TNF-α and IL-6 concentrations were significantly higher compared to healthy individuals with periodontal disease. Further, there was a significant correlation (r = 0.500, P = 0.057) between salivary and serum IL-6 concentrations, and between salivary IL-6 and parameters including age, BMI, blood glucose and HbA1c. Salivary TNF-α also showed a significant positive correlation (r = 0.674, P = 0.0006) with serum concentrations in diabetics with periodontal disease. However, salivary TNF-α was not correlated with age, BMI, blood glucose and HbA1c.
In overweight and obese children (mean age 14.5 years), BMI adjusted for age and gender was shown to be significantly associated with reduced flow rate of stimulated whole saliva (1.2 mL/min), compared to the salivary flow rate (2.0 mL/min) in normal-weight children. This suggested that childhood obesity may cause stimulated whole saliva flow rate to fall below the median value of 1.5 mL/min, which can negatively impact oral health in children[67]. Further, overweight and obese children, between 7 and 10 years of age, have demonstrated a significant decrease in salivary concentrations of phosphate (P < 0.001) and peroxidase activity (P < 0.001), and an increase in free sialic acid (P = 0.004) and protein (P = 0.003) levels compared to normal weight control group suggesting the influence of BMI on stimulated whole saliva composition[68].
CRP IN SALIVA
CRP is a sensitive marker of systemic inflammation and an independent risk factor for cardiovascular diseases in both adults and children[69,70]. In a study of 170 black South African children (age 10 ± 2 years; boys, n = 70; girls, n = 100) salivary CRP concentrations, determined using a commercially available CRP ELISA kit, showed that obese children (n = 53, boys = 24, girls = 29, mean BMI = 26.2 ± 5 kg/m2) had significantly higher (P < 0.05) salivary CRP concentration (7.31 ± 0.93 pg/mL) compared to normal-weight control group (6.77 ± 0.92 pg/mL)[57]. Further, obese children were also shown to have significantly higher (P < 0.05) salivary CRP secretion rate (7.25 ± 0.99 pg/min) compared to normal weight children (6.68 ± 0.98 pg/min).
In healthy individuals (men, n = 13; women, n = 12) between 20 to 35 years age, salivary CRP concentrations have been shown to be in the range of 35-217 pg/mL for saliva collected using the passive drool method. Use of acid-stimulation for saliva collection have shown lower salivary CRP concentrations (38-171 pg/mL) compared to saliva collected using mechanical stimulation (32-213 pg/mL)[71]. In this study, a commercially available ELISA kit (AlphaLISA, PerkinElmer, MA, United States) was used for quantification of salivary CRP. Another study has reported salivary CRP concentrations in the range of 118 to 24156 pg/mL in healthy participants (n = 61) between 20 and 54 years of age[72]. In this study, saliva samples were collected using the unstimulated passive drool method and salivary CRP was measured with a commercial ELISA kit (Salimetrics LLC, Carlsbad, CA). The observed differences in salivary CRP range among healthy individuals may be explained by differences in pre-processing techniques, and the use of different assay kits. Further, these authors have shown that salivary and serum CRP concentrations were correlated (r = 0.72). Further, it was shown that salivary CRP concentrations could predict serum CRP concentrations with 89% accuracy at higher mean serum values.
However, Qvarnstrom et al[58] have reported that salivary CRP was not significantly associated with metabolic syndrome in patients with or without coronary artery disease[58]. In this study, out of 250 participants with coronary artery disease, 81 had metabolic syndrome, and salivary lysozyme was shown to be significantly associated with metabolic syndrome (P = 0.02), independent of CRP concentrations. While comparing saliva and plasma CRP concentrations, Dillon et al[59] have reported that CRP concentrations in saliva of healthy adults (n = 69) ranged between 0.05 to 64.3 μg/L, which were significantly lower compared to plasma CRP concentrations (0.14 to 31.1 mg/L). Further, regression analysis showed no correlation between CRP concentrations in saliva and plasma (R2 = 0.001)[59]. In this study, unstimulated whole saliva samples were obtained by the passive drool method and salivary CRP concentrations were measured using a commercial kit (Salimetrics). Interestingly, salivary CRP concentrations have been shown to be positively correlated with serum concentrations in patients (n = 56) with acute myocardial infarction[73]. In this study, CRP showed the highest median concentration, for diseased over control subjects, in both serum (4.29) and saliva (72.25) followed by matrix metalloproteinase-9, IL-1β, soluble intercellular adhesion molecule 1, myeloperoxidase, adiponectin and MCP-1. Receiver-operating characteristic curve analysis showed that CRP had a significantly higher area under the curve for saliva (area under the curve = 0.78, P < 0.05). The current developments in identifying and standardizing potential inflammatory biomarkers in saliva suggest that substantial research is required to standardize and validate the use of clinically relevant biomarkers in disease diagnosis[74].
ANTIOXIDANT STATUS IN SALIVA
Oxidative stress is another major cause of obesity-induced inflammation resulting from increased production of free radicals and/or low antioxidant status. Oral inflammation is associated with elevated systemic inflammation, and has been linked with increased risk of insulin resistance and diabetes[24]. In a study by Al-Rawi[56], the oxidative status of type 2 diabetic patients was evaluated by measuring salivary and serum levels of malondialdehyde (MDA), uric acid (UA), superoxide dismutase and reduced glutathione (GSH). Salivary concentrations of MDA were lower (between 0.29-0.98 μmol/L) compared to serum MDA values (0.85-4.31 μmol/L) in all the study groups. However, salivary MDA was significantly higher in participants with type 2 diabetes compared to control subjects. Further, UA and GSH concentrations were significantly elevated (P < 0.001) in saliva of diabetic patients, while salivary GSH showed no significant change compared to the control group[56].
Type 2 diabetes has also been associated with decreased total antioxidant capacity (TAC) evaluated by spectrophotometric measurement of TBARS[25]. In this study, salivary TAC content (1.24 ± 0.18) was significantly lower in diabetes group (n = 30, 13 men and 17 women) compared to healthy controls (n = 30, 4.6 ± 0.31). Further, there was a significant decrease (P < 0.01) in the salivary flow rates in subjects with diabetes (0.38 ± 0.16) compared to the healthy individuals (0.65 ± 0.10). A recent study has demonstrated increased concentrations of pro-inflammatory cytokines in unstimulated whole saliva samples collected from pregnant women with diabetes (n = 63). The findings of this study suggested that changes in saliva properties were more pronounced in long-term cases of diabetes and partly correlated with HbA1c[75].
SALIVA RESEARCH: EMERGING STUDIES
Currently, there has been an increasing focus on proteomic analysis of saliva. Research is directed to identify and catalog human salivary proteins. Recently, a NIDCR-supported research consortium has compiled an extensive list of whole saliva proteins, using mass spectrophotometric techniques. This research group has identified 597 salivary proteins that are also found in the plasma[76].
Studies are being conducted to develop sensitive and reliable saliva-based diagnostic assays with the potential to be used in a clinical setting. Researchers have evaluated the use of a Luciferase Immunoprecipitation System for detection of autoantibodies in salivary and lacrimal gland secretions of patients with Sjogren’s Syndrome (SjS)[27]. This assay has been reported to detect autoantibodies in 67% of SjS patients with 100% specificity suggesting its potential use as an alternative to serum.
Interestingly, approximately 50 microRNAs have been identified in whole saliva, which currently are being studied for their potential to serve as biomarkers of oral cancer[77]. Scientists have also developed a surface immobilized optical protein sensor to detect IL-8 with implications for use in cancer detection. To overcome the challenge of detecting low concentrations of biomarkers in saliva, the authors propose use of confocal optical sensors[78].
CONCLUSION
While low-grade inflammation, a hallmark of obesity, may be a pivotal mechanism linking obesity to its numerous systemic complications, these require invasive procedures, such as blood drawing. Recently, interest in the use of saliva as a diagnostic fluid has increased exponentially because of its non-invasive nature and potential to be used in population-based screening programs, confirmatory diagnosis, risk stratification, prognosis determination, and therapy response. Salivary cortisol is becoming widely used as a screening test for the diagnosis of hypercortisolism and as a biomarker of psychological stress. Current literature for diagnostic potential of salivary biomarkers suggests that salivary CRP, TNF-α, IL-6, and IFN-γ are elevated in overweight/obesity and inflammatory conditions in children, and adults. These salivary biomarkers demonstrate moderate-to-strong correlation with serum biomarkers, in healthy as well as obese and diabetic individuals. Salivary markers of antioxidant status, including malondialdehyde and uric acid, show promise but will need to be explored further. While some studies show that salivary resistin and adiponectin concentrations are significantly correlated with serum values, and are known to be been elevated in obesity and diabetes, additional studies are needed to characterize such biomolecules in saliva and their relevance to inflammatory, metabolic, and cardiovascular conditions.
In conclusion, while saliva has the potential to become a premier diagnostic sample, substantial future research is required to standardize saliva collection techniques, validate salivary biomarkers of inflammation and insulin-resistance, across various life-stages and conditions, and establish reference ranges, before it can be used as a diagnostic fluid for cardiometabolic risk assessment.