Published online Jun 15, 2014. doi: 10.4239/wjd.v5.i3.385
Revised: February 18, 2014
Accepted: April 16, 2014
Published online: June 15, 2014
Processing time: 202 Days and 12 Hours
Protein kinase C-β (PKCβ), a member of the lipid-activated serine/threonine PKC family, has been implicated in a wide range of important cellular processes. Very recently, the novel role of PKCβ in the regulation of triglyceride homeostasis via regulating mitochondrial function has been explored. In this review, I aim to provide an overview of PKCβ regarding regulation by lipids and recently gained knowledge on its role in energy homeostasis. Alterations in adipose PKCβ expression have been shown to be crucial for diet-induced obesity and related metabolic abnormalities. High-fat diet is shown to induce PKCβ expression in white adipose tissue in an isoform- and tissue-specific manner. Genetically manipulated mice devoid of PKCβ are lean with increased oxygen consumption and are resistant to high-fat diet-induced obesity and hepatic steatosis with improved insulin sensitivity. Available data support the model in which PKCβ functions as a “diet-sensitive” metabolic sensor whose induction in adipose tissue by high-fat diet is among the initiating event disrupting mitochondrial homeostasis via intersecting with p66Shc signaling to amplify adipose dysfunction and have systemic consequences. Alterations in PKCβ expression and/or function may have important implications in health and disease and warrants a detailed investigation into the downstream target genes and the underlying mechanisms involved. Development of drugs that target the PKCβ pathway and identification of miRs specifically controlling PKCβ expression may lead to novel therapeutic options for treating age-related metabolic disease including fatty liver, obesity and type 2 diabetes.
Core tip: Nutrition has important long-term consequences for health. It is one of the lifestyle factors that contribute to the development and progression of obesity (increased fat accumulation), diabetes, and cardiovascular diseases. In fact, obesity rates are increasing dramatically worldwide and obesity amplifies the risk of developing various age-related chronic diseases, such as type 2 diabetes and cardiovascular disease. The prevention or management of chronic diseases is a global priority since they constitute a serious strain on health care systems and account for more than half of the deaths worldwide. Although correct lifestyle remains the mainstream solution to this problem, pharmacological strategies are also being actively seeked. Current antiobesity strategies have not controlled increasing epidemic of obesity and obesity-related disorders. We hope that a better knowledge of the molecular players and biochemical mechanism linking dietary fat to fat accumulation and development of glucose intolerance are critically needed. This review examines a way of metabolizing dietary fat into heat instead of storing them as fat, and the possibility that the “browning” of white fat is regulated by a diet-inducible kinase Protein kinase C-β (PKCβ) may help us explore new translational approaches to combat obesity, improve insulin sensitivity and potentially increase longevity. Finally, attenuation of inflammation in fat by PKCβ inhibition could have profound clinical consequences because of the large size of the fat organ and its central metabolic role.
- Citation: Mehta KD. Emerging role of protein kinase C in energy homeostasis: A brief overview. World J Diabetes 2014; 5(3): 385-392
- URL: https://www.wjgnet.com/1948-9358/full/v5/i3/385.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i3.385
Protein kinase C (PKC) family is the largest serine/threonine-specific kinase family known to comprise approximately 2% of the human kinome[1]. PKCs are broadly conserved in eukaryotes, ranging in complexity from a single isoform in budding yeast (Saccharomyces cerevisiae) to 5 isoforms in Drosophila melanogaste and 12 in mammals[2,3]. Three distinct subfamilies can be identified according to their dependency on three combinations of activators: conventional (α, βI, βII, γ) require phosphatidylserine, diacylglycerol, and Ca2+; novel (δ, ε, η, θ) need phosphatidylserine (PS) and DAG but not Ca2+; atypical PKCs (λ/l, ζ) are insensitive to both DAG and Ca2+. PKC isoforms differ in primary structure, tissue distribution, subcellular localization, in vitro mode of action, response to extracellular signals, and substrate specificity. The role of individual PKC isoform is thought to be determined through sub isoform-specific activation processes or isoform-specific substrates in the region downstream of the PKC pathway[4]. Specific role of each isoform is beginning to be understood using isoform-specific transgenic and knockout mouse models. PKCs have been extensively discussed in the literature, and the aim of this review is to focus on the functions of PKCβ in the context of obesity and related metabolic syndromes.
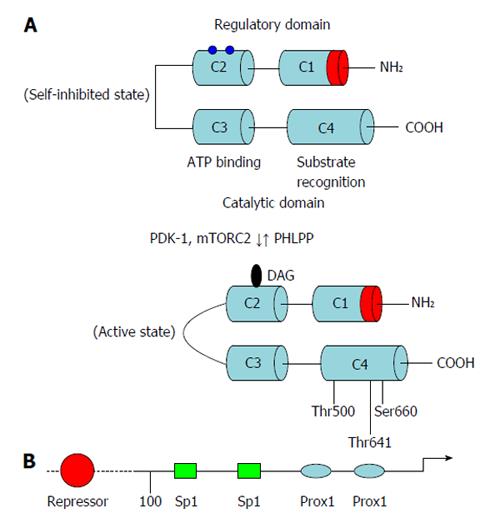
PKCβ is unique among all PKC isoforms in that a single gene locus encodes two proteins, PKCβI and PKCβII, which are generated by alternative splicing of C-terminal exons and are shown to be physiologically relevant[5]. The difference between these two isoforms resides in the C-terminal V5 domains, which still exhibit a moderate homology (45%) at their amino acid sequences[6,7]. PKCβ is highly expressed in the brain and adipose tissue, and widely expressed at a lower level in multiple tissues including liver, kidney, and skeletal muscle. Analysis of the primary structure of PKCβ reveals the presence of four domains conserved across PKC isoforms (C1-C4) and five variable domains that are divergent (V1-V5). Two functional domains have been described: an amino terminal regulatory domain and a carboxyl terminal catalytic domain. The regulatory domain (V1-V3) contains the so-called pseudosubstrate site which is thought to interact with the catalytic domain to retain PKCβ in an inactive conformation. The regulatory domain also contains sites for the interaction of PKC with PS, DAG/phorbol ester, and Ca2+. The Ca2+ dependency is mediated by the C2 region, while phorbol-ester binding requires the presence of two cysteine-rich zinc finger regions within the C1 domain. The catalytic domain contains two conserved regions, C3 and C4, which are essential for the kinase activity and the binding of adenosine-5’-triphosphate (ATP)/substrate (Figure 1).
In addition to the above specific inputs, other regulatory processes influence the function of PKCβ, including phosphorylation and interaction with specific binding partners. PKCβ is processed by three distinct phosphorylation events before it is competent to respond to the coactivators and is phosphorylated at three conserved serine/threonine residues in the C-terminal domain[8]. Phosphorylation at the activation loop (Thr500) is generally proposed to be first and to be followed by two ordered phosphorylations at the C-terminal tail, the turn motif (Thr641 in PKCβII) and then the hydrophobic motif (Ser660 in PKCβII). The phosphorylation of the turn motif depends on the mTORC2 complex; this phosphorylation triggers autophosphorylation of the hydrophobic motif[9,10]. The fully-phosphorylated “mature” PKCβ is in a closed conformation in which the pseudosubstrate occupies the substrate-binding cavity, thus autoinhibiting the kinase. Signals that cause hydrolysis of phosphatidylinositol-4,5-bisphosphate result in translocation of PKCβ to the membrane by a low-affinity interaction where it binds DAG via the C1 domain. Engaging both the C1 and C2 domains on the membrane results in a high-affinity membrane interaction that results in release of the pseudosubstrate, allowing downstream signaling. The membrane-bound conformation is highly phosphatase-sensitive, so that prolonged membrane binding results in dephosphorylation of PKCβ by pleckstrin homology domain Leucine-rich repeat protein phosphatase and PP2A, and subsequent degradation[11]. Binding of Hsp70 to the dephosphorylated turn motif on the C-terminus stabilizes PKCβ, allowing it to become rephosphorylated and reenter the pool of signaling-competent PKC. PKCβ that is not rescued by hsp70 is ubiquitinated by E3 ligases such as the recently discovered RINCK and degraded[12].
PKCβ is also responsive to oxidative stress[13-15]. Why is PKCβ sensitive to oxidative stress? In the PKCβ structure, two pairs of zinc fingers are found within the regulatory domain. They are sites of DAG and phorbol ester binding. Each zinc finger is formed by a structure that is composed of six cysteine residues and two zinc atoms. The high level of cysteine residues renders the regulatory domain susceptible to redox regulation[16,17]. The oxidant destroys the zinc finger conformation, and the autoinhibition is relieved, resulting in a PKCβ form that is catalytically active in the absence of Ca2+ or phospholipids[18].
Besides the lipid activation at the post-transcriptional level, PKCβ expression also fluctuates in response to high-fat diet intake. It is shown that feeding high-fat diet (HFD) for 12 wk induces adipose PKCβ expression in an isoform and tissue-specific manner[19]. The molecular mechanism(s) underlying transcription induction have yet to be elucidated but previous studies have cloned and sequenced PKCβ promoter[20-22]. A putative 5’-promoter region for PKCβ is identified and suggested that there is heterogeneity in the active promoter region dependent upon the cellular context. Analysis of the 5’-promoter of PRKCB revealed that a region between -110 bp and -48 bp contains two Sp1 binding sites which are important for basal expression of PKCβ gene. In addition two PROX1 sites are also present 3’ to Sp1 sites and are involved in inhibiting Sp1-mediated basal transcription of PKCβ promoter[23]. In fact, an inverse relationship between PROX1 and PKCβ levels exist in colon cancer cell lines. It was also found that treatment with a demethylating agent, 5-aza-2’-deoxycytidine, restored PKCβ mRNA expression in PROX1-expressing cells, suggesting that the 5’-promoter of PKCβ is methylated in these cells[23]. Actually, a CpG island in this region, in particular a CpG site within the distal Sp1 site is identified in this study, leading to downregulation of PKCβ transcription. Hypermethylation of PROX1 sites inhibits direct Sp1 binding to this region in PROX1 overexpressing cells. Finally, previous studies have also identified a repressor region located upstream of -110 bp in the PKCβ promoter and the identity of the nuclear factor(s) binding to this region has not been characterized.
A significant conceptual advance in our understanding of the importance of PKCβ signaling in obesity has come from realization that mice deficient in PKCβ express higher levels of genes that regulate fatty acid oxidation and proteins involved in energy dissipation, highlighting its role as a corepressor and in controlling the balance between energy consumption and energy expenditure[24]. On the contrary, genes involved in FA synthesis and gluconeogenesis seem to be downregulated in the absence of PKCβ[25,26]. As a consequence, PKCβ mice are lean, with a significant reduction of body fat and body weight compared to WT mice and are resistant to HFD-induced obesity and hepatic steatosis so that these mice maintain their insulin sensitivity[19]. Moreover, PKCβ levels are shown to be elevated in adipose tissue of leptin-deficient (ob/ob) mice and deletion of PKCβ in ob/ob mice attenuates obesity syndrome of these mice[26]. An important mechanistic insight is the revelation that in PKCβ-deficient mice white adipose tissue (WAT) express genes characteristic of BAT including peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1α), fatty acid transporter carnitine palmitoyltransferase, and uncoupling protein-1 (UCP-1). Targeted disruption in mice of several genes directly involved in energy metabolism and fat accumulation also leads to lean phenotype with a marked increase in UCP-1 expression in adipocytes, particularly in white fat depots[27-29]. Thus total energy consumption is increased significantly in PKCβ-null mice, presumably as a consequence of energy dissipation in WAT resulting from the expression of UCP-1 and increased mitochondrial activity. The ability of white and brown adipocytes in each depot to reversibly switch into one another has been reported, but the extent to which this occurs and the precise mechanisms involved are not fully understood. The search for regulators that could mediate conversion of white adipocytes (energy storing) into brown adipocytes (energy consuming) has led to the identification of PGC-1α, FOXC2 and positive regulatory domain-containing 16 as transcriptional regulators that have been found to promote a brown fat genetic program, while retinoblastoma protein and RIP140 have been described to favor a white adipose phenotype[27-30]. Another important aspect of these studies relates to possible connection between PKCβ and β-adrenergic receptor levels in WAT. Results presented argue strongly in favor of an inverse relationship between PKCβ and β3-adrenergic receptor expression[26]. The proposed relationship is consistent with earlier reports showing that sustained PKC activation suppressed β-ARs expression at the transcriptional level[31-33]. The net consequence of PKCβ-mediated adipose dysfunction could have profound clinical consequences because of the large size of the fat organ and its central metabolic role. Interestingly, in agreement with the above animal studies, adipose PKCβ activation is subsequently linked to obese side effects of antipsychotic drugs in humans[34]. Moreover, in agreement with its role in energy homeostasis, PKCβ is shown to be required for adipocyte differentiation[35], PKCβ inhibition promotes insulin signaling in adipocytes[36,37], and PKCβ promoter polymorphism is associated with insulin resistance in humans[38].
The role of PKCβ in obesity is further supported by its potential involvement in angiogenesis. To ensure a sufficient supply of nutrients and oxygen and to transport fatty acids and adipokines, an extended microvasculature is mandatory for adipose tissue. Adipogenesis and angiogenesis are two closely related processes during adipose tissue enlargement, as shown in animal studies and in vitro models[39,40]. As adipocyte hypertrophy endures, local adipose tissue hypoxia may occur due to hypoperfusion since the diameter of fat cells overgrows the diffusion limit of oxygen. As a result, hypoxia-inducible transcription factors are expressed triggering the expression of angiogenic factors [vasuclar endothelial growth factor (VEGF), hepatocyte growth factor, plasminogen activator inhibitor-1]. In view of role of PKCβ/HuR in regulating VEGF expression at the post-transcriptional level, simultaneous induction of PKCβ is expected to promote VEGF expression[41,42].
Finally, specific overexpression of a constitutively active PKCβII mutant in mouse skeletal muscle demonstrated that this splice variant of PKCβ not only induces insulin resistance, but also affects the levels of several genes involved in lipid metabolism[43]. Thus impairment in the expression of PGC-1α, acyl CoA oxidase and hormone-sensitive lipase, but enhanced expression of the lipogenic transcription factor sterol response element-binding protein 1c in skeletal muscle, were associated with decreased lipid oxidation and increased intra-myocellular lipid deposition. In addition to these direct effects in muscle, these animals showed defects in insulin action in the liver and brain, as well as hepatic lipid accumulation similar to that seen in fat-fed animals.
Several studies have emphasized the association between enhanced mitochondria-derived H2O2 and insulin resistance, particularly in the context of excessive nutrient intake that results in metabolic imbalance[44-47]. Oxidative stress has also been described clinically, as well as in WAT of many additional mouse models of obesity, such as the KKAy and db/db mice. Systemic markers of oxidative stress increase with adiposity, consistent with the role of reactive oxygen species (ROS) in the development of obesity-induced insulin resistance. Available data suggest that an increase in ROS significantly affects WAT biology and leads to deregulated expression of inflammatory cytokines such as tumor necrosis factor-α, interleukin-6, and macrophage chemoattractant protein-1, and insulin resistance, which could contribute to obesity-associated diabetes and cardiovascular diseases[47]. Moreover, oxidative stress induced by ROS stimulates fat tissue development both in vitro and in vivo. H2O2-induced oxidative stress is shown to facilitate the differentiation of preadipocytes into adipocytes by accelerating mitotic clonal expansion[48]. Antioxidants such as flavonoids and N-acetyl-cysteine inhibit both adipogenic transcription factors C/EBP-β and PPAR-γ expression, as well as adipogenic differentiation in 3T3-L1 preadipocytes[49,50]. N-acetyl cysteine (NAC) was also shown to reduce ROS levels and fat accumulation in a concentration-dependent manner[50]. Moreover, animals on a HFD with the antioxidant NAC exhibited lower visceral fat and body weight[51]. Finally, ROS scavenging is associated with fat reduction in obese Zucker rats[52].
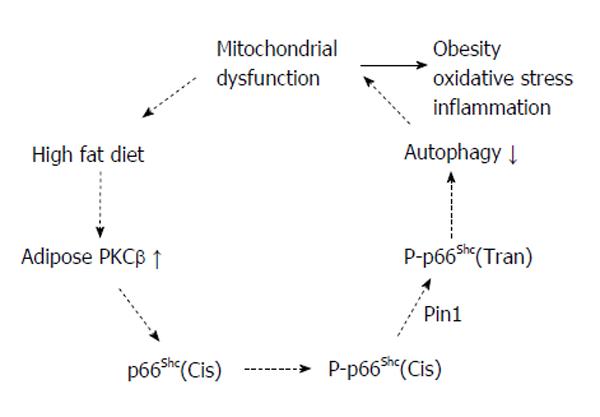
Recent studies have highlighted a novel, unexpected signaling pathway bridging the oxidative challenge of a cell to the activation of PKCβ/p66Shc-controlled mitochondrial lifespan[53,54]. PKCβ activated by oxidative stress is shown to be required for phosphorylation of the Ser36 of p66Shc and the effect of PKCβ overexpression on mitochondrial Ca2+ signaling was not observed in p66Shc-/- cells. Importantly, the mitochondrial consequences of hydrogen peroxide are blocked by hispidine, a specific PKCβ inhibitor. The pathway emerging from these studies is the following: during oxidative stress PKCβ is activated and induces p66Shc phosphorylation, thus allowing p66Shc to be recognized by Pin1, isomerised and imported into mitochondria after dephosphorylation by type 2 protein serine/threonine phosphatase. The p66Shc protein translocated into the appropriate cell domain, can exert the oxidoreductase activity, generating H2O2 and inducing the opening of MPTP. This event in turn perturbs mitochondria structure and function. Identification of a novel signaling mechanism, which is operative in the pathophysiological condition of oxidative stress, may open new possibilities for pharmacologically addressing the process of organ deterioration during aging. The above studies are among the first to dissect the downstream target genes and regulatory properties of the PKCβ protein, and therefore make an important contribution to our understanding of the molecular basis to the lean phenotype exhibited by PKCβ-/- mice. Based on a very recent demonstration that PKCβ/p66Shc mitochondrial axis inhibits autophagy[55] and the evolving role of autophagy in energy homeostasis[56-61], it is possible that a combination of adipose PKCβ activation, mitochondrial dysfunction and insufficient autophagy may contribute to the development of diet-induced obesity. In addition to mitochondrial effects, PKCβ is an upstream regulator of NOX but this signaling axis actively produces superoxide across the membranes of neutrophils and phagosomes[62-65]. Accumulating data so far implicates mitochondria as the main source for regulation of autophagy by ROS production in adipocytes[66], whereas NOX contributes to activation of selective, bacterial autophagy[67] (Figure 2).
Although biological function of PKCβ in energy homeostasis appears to be mostly linked with events occurring at the mitochondria, however, increasing evidence has implied a role for this kinase in nuclear functions, suggesting this may be a pathway to communicate signals generated at the plasma membrane to the nucleus. For example, Goss et al[68] first showed that PKCβ translocates to the nucleus at G2/M, concomitant with the phosphorylation of lamin B1. Subsequently, a considerable number of nuclear proteins have been identified which are in vivo and/or in vitro substrates for PKCβ. These proteins include: histone H3, DNA topoisomerase I and IIa, DNA polymerase α and β, cyclic AMP-response element-binding protein, retinoblastoma protein, and vitamin D receptor[69-73]. It has even been shown that PKCβI co-localizes with androgen receptor and lysine-specific demethylase 1 on target gene promoters and phosphorylation of histone H3 at threonine 6 by PKCβI is the key event that prevents lysine-specific demethylase 1 from demethylating histone H3 lysine 4[69]. Finally, activated PKCβ indirectly can affect other signaling cascades, including PI3-kinase/Akt pathway, extracellular signal-regulated kinase, and p38 pathway which can impact nuclear events[74-79]. It is thus clear that characterization of PKCβ downstream signaling in the nucleus and its relevance to energy homeostasis is another facets that requires in-depth investigation.
The above findings are applicable to the pathogenesis of obesity and type 2 diabetes since mitochondrial loss in WAT correlates with the development of obesity and type 2 diabetes[80,81]. Indeed, mitochondrial DNA copy number, mitochondrial mass, and mitochondrial activity are all decreased in the white adipose tissue of mouse models of obesity, such as ob/ob and db/db mice[82,83]. Similarly in patients with insulin resistance, type 2 diabetes, and severe obesity, the abundance of mitochondria and the expression of key genes pertinent to mitochondrial function are significantly reduced in white adipose tissue, in concert with decreased adipocyte oxygen consumption rates and ATP production[84,85]. The mitochondrial dysfunction, which could impair substrate oxidation in adipose tissue, is thought to participate in metabolic impairment capacity, thereby accentuating the development of obesity and associated pathologies, such as type 2 diabete. As a result, WAT mitochondria are emerging as highly attractive organelles for therapeutic interventions with the potential to impact upon systemic metabolism. Interestingly, the insulin-sensitizing effects of thiazolidinediones are closely matched by robust increases in adipose tissue mitochondrial biogenesis[86].
We have reviewed recent advances pertaining to the potential role of PKCβ in regulating energy homeostasis and contribution to the development of metabolic syndrome. Evidence gathered recently point to an essential role for PKCβ in diet-induced obesity. As a signaling pathway, PKCβ is highly sensitive to changes in environment and fluctuations in lipid supply activate adipose PKCβ, which in turn appears to promote fat accumulation via modulating mitochondrial function. A positive loop between oxidative stress and PKCβ/p66Shc is promising and may be the major mechanism underlying contribution of PKCβ activation in generating oxidative stress observed in the obese state. The main gap in our understanding today lies in the specific, molecular and chemical mechanisms of PKCβ-mediated energy homeostasis. What are the mitochondrial and nuclear targets of PKCβ physiologically relevant to energy homeostasis? How is the dietary lipid signals transmitted to the PKCβ promoter? Is PKCβ regulatory signaling network dysregulated in metabolic disease states? Can PKCβ inhibition be adopted to prevent human obesity? These important questions should be the target of future studies. The manipulation of PKCβ levels, activity, or signaling might represent a therapeutic approach to combat obesity and associated metabolic disorders.
| 1. | Parker PJ. Protein kinase C: a structurally related family of enzymes Protein kinase C: current concepts and future perspectives. Epand RM, Lester DS, editors. 1992;3-24. |
| 2. | Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484-496. [PubMed] |
| 3. | Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495-28498. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1122] [Cited by in RCA: 1176] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 4. | Mochly-Rosen D, Khaner H, Lopez J. Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci USA. 1991;88:3997-4000. [PubMed] |
| 5. | Ono Y, Kikkawa U, Ogita K, Fujii T, Kurokawa T, Asaoka Y, Sekiguchi K, Ase K, Igarashi K, Nishizuka Y. Expression and properties of two types of protein kinase C: alternative splicing from a single gene. Science. 1987;236:1116-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 260] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Parker PJ, Parkinson SJ. AGC protein kinase phosphorylation and protein kinase C. Biochem Soc Trans. 2001;29:860-863. [PubMed] |
| 7. | Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361-371. [PubMed] |
| 8. | Newton AC. Lipid activation of protein kinases. J Lipid Res. 2009;50 Suppl:S266-S271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1972] [Cited by in RCA: 2122] [Article Influence: 96.5] [Reference Citation Analysis (6)] |
| 10. | Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 507] [Article Influence: 28.2] [Reference Citation Analysis (14)] |
| 11. | Gould CM, Kannan N, Taylor SS, Newton AC. The chaperones Hsp90 and Cdc37 mediate the maturation and stabilization of protein kinase C through a conserved PXXP motif in the C-terminal tail. J Biol Chem. 2009;284:4921-4935. [PubMed] |
| 12. | Chen D, Gould C, Garza R, Gao T, Hampton RY, Newton AC. Amplitude control of protein kinase C by RINCK, a novel E3 ubiquitin ligase. J Biol Chem. 2007;282:33776-33787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Rimessi A, Rizutto R, Pinton P. Differential recruitment of PKC isoforms in HeLa cells during redox stress. Cell Stress and Chaperons. 2007;12:291-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Scivittaro V, Ganz MB, Weiss MF. AGEs induce oxidative stress and activate protein kinase C-beta(II) in neonatal mesangial cells. Am J Physiol Renal Physiol. 2000;278:F676-F683. [PubMed] |
| 15. | Almeida M, Han L, Ambrogini E, Bartell SM, Manolagas SC. Oxidative stress stimulates apoptosis and activates NF-kappaB in osteoblastic cells via a PKCbeta/p66shc signaling cascade: counter regulation by estrogens or androgens. Mol Endocrinol. 2010;24:2030-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Giorgi C, Agnoletto C, Baldini C, Bononi A, Bonora M, Marchi S, Missiroli S, Patergnani S, Poletti F, Rimessi A. Redox control of protein kinase C: cell- and disease-specific aspects. Antioxid Redox Signal. 2010;13:1051-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 17. | Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 530] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 18. | Lin YL, Shivji MK, Chen C, Kolodner R, Wood RD, Dutta A. The evolutionary conserved zinc finger motif in the largest subunit of human protein kinase A is required for DNA replication and mismatch repair but not for nucleotide excision repair. J Biol Chem. 1998;3:1453-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Huang W, Bansode R, Mehta M, Mehta KD. Loss of protein kinase Cbeta function protects mice against diet-induced obesity and development of hepatic steatosis and insulin resistance. Hepatology. 2009;49:1525-1536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Obeid LM, Blobe GC, Karolak LA, Hannun YA. Cloning and characterization of the major promoter of the human protein kinase C beta gene. Regulation by phorbol esters. J Biol Chem. 1992;267:20804-20810. [PubMed] |
| 21. | Niino YS, Ohno S, Suzuki K. Positive and negative regulation of the transcription of the human protein kinase C beta gene. J Biol Chem. 1992;267:6158-6163. [PubMed] |
| 22. | Mahajna J, King P, Parker P, Haley J. Autoregulation of cloned human protein kinase C beta and gamma gene promoters in U937 cells. DNA Cell Biol. 1995;14:213-222. [PubMed] |
| 23. | Hagiwara K, Ito H, Murate T, Miyata Y, Ohashi H, Nagai H. PROX1 overexpression inhibits protein kinase C beta II transcription through promoter DNA methylation. Genes Chromosomes Cancer. 2012;51:1024-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Bansode RR, Huang W, Roy SK, Mehta M, Mehta KD. Protein kinase C deficiency increases fatty acid oxidation and reduces fat storage. J Biol Chem. 2008;283:231-236. [PubMed] |
| 25. | Yamamoto T, Watanabe K, Inoue N, Nakagawa Y, Ishigaki N, Matsuzaka T, Takeuchi Y, Kobayashi K, Yatoh S, Takahashi A. Protein kinase Cbeta mediates hepatic induction of sterol-regulatory element binding protein-1c by insulin. J Lipid Res. 2010;51:1859-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Huang W, Bansode RR, Bal NC, Mehta M, Mehta KD. Protein kinase Cβ deficiency attenuates obesity syndrome of ob/ob mice by promoting white adipose tissue remodeling. J Lipid Res. 2012;53:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11:257-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 327] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 28. | Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes Dev. 2009;23:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 29. | Hansen JB, Kristiansen K. Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem J. 2006;398:153-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 669] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 31. | Li Z, Vaidya VA, Alvaro JD, Iredale PA, Hsu R, Hoffman G, Fitzgerald L, Curran PK, Machida CA, Fishman PH. Protein kinase C-mediated down-regulation of beta1-adrenergic receptor gene expression in rat C6 glioma cells. Mol Pharmacol. 1998;54:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Fève B, Elhadri K, Quignard-Boulangé A, Pairault J. Transcriptional down-regulation by insulin of the beta 3-adrenergic receptor expression in 3T3-F442A adipocytes: a mechanism for repressing the cAMP signaling pathway. Proc Natl Acad Sci USA. 1994;91:5677-5681. [PubMed] |
| 33. | Spencer MJ, Croall DE, Tidball JG. Calpains are activated in necrotic fibers from mdx dystrophic mice. J Biol Chem. 1995;270:10909-10914. [PubMed] |
| 34. | Pavan C, Vindigni V, Michelotto L, Rimessi A, Abatangelo G, Cortivo R, Pinton P, Zavan B. Weight gain related to treatment with atypical antipsychotics is due to activation of PKC-β. Pharmacogenomics J. 2010;10:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Zhou Y, Wang D, Li F, Shi J, Song J. Different roles of protein kinase C-betaI and -delta in the regulation of adipocyte differentiation. Int J Biochem Cell Biol. 2006;38:2151-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Kleiman E, Carter G, Ghansah T, Patel NA, Cooper DR. Developmentally spliced PKCbetaII provides a possible link between mTORC2 and Akt kinase to regulate 3T3-L1 adipocyte insulin-stimulated glucose transport. Biochem Biophys Res Commun. 2009;388:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Liberman Z, Plotkin B, Tennenbaum T, Eldar-Finkelman H. Coordinated phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 and protein kinase C betaII in the diabetic fat tissue. Am J Physiol Endocrinol Metab. 2008;294:E1169-E1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Osterhoff MA, Heuer S, Pfeiffer M, Tasic J, Kaiser S, Isken F, Spranger J, Weickert MO, Möhlig M, Pfeiffer AF. Identification of a functional protein kinase Cbeta promoter polymorphism in humans related to insulin resistance. Mol Genet Metab. 2008;93:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1427] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 40. | Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 532] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 41. | Amadio M, Bucolo C, Leggio GM, Drago F, Govoni S, Pascale A. The PKCbeta/HuR/VEGF pathway in diabetic retinopathy. Biochem Pharmacol. 2010;80:1230-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3598] [Cited by in RCA: 3459] [Article Influence: 203.5] [Reference Citation Analysis (0)] |
| 43. | Hennige AM, Heni M, Machann J, Staiger H, Sartorius T, Hoene M, Lehmann R, Weigert C, Peter A, Bornemann A. Enforced expression of protein kinase C in skeletal muscle causes physical inactivity, fatty liver and insulin resistance in the brain. J Cell Mol Med. 2010;14:903-913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3370] [Cited by in RCA: 3928] [Article Influence: 187.0] [Reference Citation Analysis (0)] |
| 45. | Curtis JM, Grimsrud PA, Wright WS, Xu X, Foncea RE, Graham DW, Brestoff JR, Wiczer BM, Ilkayeva O, Cianflone K. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59:1132-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 46. | Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1933] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 47. | Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, Kang L, Rabinovitch PS, Szeto HH. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1040] [Cited by in RCA: 1004] [Article Influence: 59.1] [Reference Citation Analysis (6)] |
| 48. | Lee H, Lee YJ, Choi H, Ko EH, Kim JW. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem. 2009;284:10601-10609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 345] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 49. | Park HS, Kim SH, Kim YS, Ryu SY, Hwang JT, Yang HJ, Kim GH, Kwon DY, Kim MS. Luteolin inhibits adipogenic differentiation by regulating PPARgamma activation. Biofactors. 2009;35:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Calzadilla P, Sapochnik D, Cosentino S, Diz V, Dicelio L, Calvo JC, Guerra LN. N-acetyl-cysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int J Mol Sci. 2011;12:6936-6951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 51. | Kim JR, Ryu HH, Chung HJ, Lee JH, Kim SW, Kwun WH, Baek SH, Kim JH. Association of anti-obesity activity of N-acetylcysteine with metallothionein-II down-regulation. Exp Mol Med. 2006;38:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Carpéné C, Iffiú-Soltesz Z, Bour S, Prévot D, Valet P. Reduction of fat deposition by combined inhibition of monoamine oxidases and semicarbazide-sensitive amine oxidases in obese Zucker rats. Pharmacol Res. 2007;56:522-530. [PubMed] |
| 53. | Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 399] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 54. | Pinton P, Rizzuto R. p66Shc, oxidative stress and aging: importing a lifespan determinant into mitochondria. Cell Cycle. 2008;7:304-308. [PubMed] |
| 55. | Patergnani S, Marchi S, Rimessi A, Bonora M, Giorgi C, Mehta KD, Pinton P. PRKCB/protein kinase C, beta and the mitochondrial axis as key regulators of autophagy. Autophagy. 2013;9:1367-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim do H, Hur KY, Kim HK. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19:83-92. [PubMed] |
| 57. | Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1085] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 58. | Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009;119:3329-3339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 59. | Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci USA. 2009;106:19860-19865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 536] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 60. | Nuñez CE, Rodrigues VS, Gomes FS, Moura RF, Victorio SC, Bombassaro B, Chaim EA, Pareja JC, Geloneze B, Velloso LA. Defective regulation of adipose tissue autophagy in obesity. Int J Obes (Lond). 2013;37:1473-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 61. | Jansen HJ, van Essen P, Koenen T, Joosten LA, Netea MG, Tack CJ, Stienstra R. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology. 2012;153:5866-5874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 62. | He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 63. | Dekker LV, Leitges M, Altschuler G, Mistry N, McDermott A, Roes J, Segal AW. Protein kinase C-beta contributes to NADPH oxidase activation in neutrophils. Biochem J. 2000;1:285-289. [PubMed] |
| 64. | Gray RD, Lucas CD, Mackellar A, Li F, Hiersemenzel K, Haslett C, Davidson DJ, Rossi AG. Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J Inflamm (Lond). 2013;10:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 65. | Kitada M, Koya D, Sugimoto T, Isono M, Araki S, Kashiwagi A, Haneda M. Translocation of glomerular p47phox and p67phox by protein kinase C-beta activation is required for oxidative stress in diabetic nephropathy. Diabetes. 2003;52:2603-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 166] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 66. | Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 1030] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 67. | Huang J, Canadien V, Lam GY, Steinberg BE, Dianauer MC, Magalhaes MAO, Giogauer M, Grinstein S, Brumell JH. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci USA. 2009;106:6226-6231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 471] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 68. | Goss VL, Hocevar BA, Thompson LJ, Stratton CA, Burns DJ, Fields AP. Identification of nuclear beta II protein kinase C as a mitotic lamin kinase. J Biol Chem. 1994;269:19074-19080. [PubMed] |
| 69. | Metzger E, Imhof A, Patel D, Kahl P, Hoffmeyer K, Friedrichs N, Müller JM, Greschik H, Kirfel J, Ji S. Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature. 2010;464:792-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 70. | Teng H, Ballim RD, Mowla S, Prince S. Phosphorylation of histone H3 by protein kinase C signaling plays a critical role in the regulation of the developmentally important TBX2 gene. J Biol Chem. 2009;284:26368-26376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Clarke DL, Sutcliffe A, Deacon K, Bradbury D, Corbett L, Knox AJ. PKCbetaII augments NF-kappaB-dependent transcription at the CCL11 promoter via p300/CBP-associated factor recruitment and histone H4 acetylation. J Immunol. 2008;181:3503-3514. [PubMed] |
| 72. | Hsieh JC, Jurutka PW, Nakajima S, Galligan MA, Haussler CA, Shimizu Y, Shimizu N, Whitfield GK, Haussler MR. Phosphorylation of the human vitamin D receptor by protein kinase C. Biochemical and functional evaluation of the serine 51 recognition site. J Biol Chem. 1993;268:15118-15126. [PubMed] |
| 73. | Suzuma K, Takahara N, Suzuma I, Isshiki K, Ueki K, Leitges M, Aiello LP, King GL. Characterization of protein kinase C beta isoform’s action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc Natl Acad Sci USA. 2002;99:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Sauma S, Yan Z, Ohno S, Friedman E. Protein kinase C beta 1 and protein kinase C beta 2 activate p57 mitogen-activated protein kinase and block differentiation in colon carcinoma cells. Cell Growth Differ. 1996;7:587-594. [PubMed] |
| 75. | Formisano P, Oriente F, Fiory F, Caruso M, Miele C, Maitan MA, Andreozzi F, Vigliotta G, Condorelli G, Beguinot F. Insulin-activated protein kinase Cbeta bypasses Ras and stimulates mitogen-activated protein kinase activity and cell proliferation in muscle cells. Mol Cell Biol. 2000;20:6323-6333. [PubMed] |
| 76. | Patel NA, Yamamoto M, Illingworth P, Mancu D, Mebert K, Chappell DS, Watson JE, Cooper DR. Phosphoinositide 3-kinase mediates protein kinase C beta II mRNA destabilization in rat A10 smooth muscle cell cultures exposed to high glucose. Arch Biochem Biophys. 2002;403:111-120. [PubMed] |
| 77. | Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55:691-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 78. | Wu D, Peng F, Zhang B, Ingram AJ, Kelly DJ, Gilbert RE, Gao B, Krepinsky JC. PKC-beta1 mediates glucose-induced Akt activation and TGF-beta1 upregulation in mesangial cells. J Am Soc Nephrol. 2009;20:554-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Guo K, Liu Y, Zhou H, Dai Z, Zhang J, Sun R, Chen J, Sun Q, Lu W, Kang X. Involvement of protein kinase C beta-extracellular signal-regulating kinase 1/2/p38 mitogen-activated protein kinase-heat shock protein 27 activation in hepatocellular carcinoma cell motility and invasion. Cancer Sci. 2008;99:486-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31:364-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 426] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 81. | Kusminski CM, Scherer PE. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol Metab. 2012;23:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 82. | Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006;49:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 295] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 83. | Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes. 2007;56:1751-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 280] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 84. | Dahlman I, Forsgren M, Sjögren A, Nordström EA, Kaaman M, Näslund E, Attersand A, Arner P. Downregulation of electron transport chain genes in visceral adipose tissue in type 2 diabetes independent of obesity and possibly involving tumor necrosis factor-alpha. Diabetes. 2006;55:1792-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 85. | Bogacka I, Ukropcova B, McNeil M, Gimble JM, Smith SR. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. J Clin Endocrinol Metab. 2005;90:6650-6656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 86. | Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 470] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
P- Reviewer: Zdravkovic M S- Editor: Gou SX L- Editor: A E- Editor: Liu SQ