Published online Feb 15, 2014. doi: 10.4239/wjd.v5.i1.17
Revised: December 2, 2013
Accepted: December 12, 2013
Published online: February 15, 2014
Processing time: 127 Days and 10.2 Hours
Cardiac autonomic neuropathy (CAN) is an often overlooked and common complication of diabetes mellitus. CAN is associated with increased cardiovascular morbidity and mortality. The pathogenesis of CAN is complex and involves a cascade of pathways activated by hyperglycaemia resulting in neuronal ischaemia and cellular death. In addition, autoimmune and genetic factors are involved in the development of CAN. CAN might be subclinical for several years until the patient develops resting tachycardia, exercise intolerance, postural hypotension, cardiac dysfunction and diabetic cardiomyopathy. During its sub-clinical phase, heart rate variability that is influenced by the balance between parasympathetic and sympathetic tones can help in detecting CAN before the disease is symptomatic. Newer imaging techniques (such as scintigraphy) have allowed earlier detection of CAN in the pre-clinical phase and allowed better assessment of the sympathetic nervous system. One of the main difficulties in CAN research is the lack of a universally accepted definition of CAN; however, the Toronto Consensus Panel on Diabetic Neuropathy has recently issued guidance for the diagnosis and staging of CAN, and also proposed screening for CAN in patients with diabetes mellitus. A major challenge, however, is the lack of specific treatment to slow the progression or prevent the development of CAN. Lifestyle changes, improved metabolic control might prevent or slow the progression of CAN. Reversal will require combination of these treatments with new targeted therapeutic approaches. The aim of this article is to review the latest evidence regarding the epidemiology, pathogenesis, manifestations, diagnosis and treatment for CAN.
Core tip: Cardiac autonomic neuropathy (CAN) is a complication of diabetes mellitus that is often under-diagnosed but can lead to severe morbidity and mortality, due to the associated cardiovascular burden. New evidence has emerged surrounding its complex pathways, but its full pathogenesis is yet to be understood. CAN manifests in a spectrum of subclinical and clinical presentations, ranging from resting tachycardia to cardiomyopathy. Heart rate variability and scintigraphy have enabled the diagnosis at a subclinical stage, thus providing the opportunity for better prevention and treatment. However, no definite therapeutic approaches have been adopted to date, emphasizing the need for newer targeted treatments.
- Citation: Dimitropoulos G, Tahrani AA, Stevens MJ. Cardiac autonomic neuropathy in patients with diabetes mellitus. World J Diabetes 2014; 5(1): 17-39
- URL: https://www.wjgnet.com/1948-9358/full/v5/i1/17.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i1.17
Diabetes mellitus (DM) is a global epidemic affecting at least 8.3% of the global population and 371 million people worldwide with a significant proportion (50%) remaining undiagnosed. It is estimated that almost one in six people are currently at risk of developing diabetes-related complications[1]. Cardiovascular disease (CVD) is the leading cause of mortality and morbidity in patients with diabetes and subsequently the primary goal of diabetes treatment is to reduce the burden of CVD as well as the vascular complications associated with diabetes[2,3]. Much of the CVD prevention strategies in patients with DM are based on lowering blood pressure and LDL-cholesterol levels and improving glycaemic control[4-7]. Despite that, CVD remains very common and a major cause of mortality and morbidity in patients with DM. Hence, better understanding of pathogenesis of CVD is crucial to develop new therapeutic targets.
Cardiac autonomic neuropathy (CAN) is a very common and often overlooked diabetes-related complication that has a major impact on CVD, mortality and morbidity in patients with DM[8,9]. Improving our understanding of the pathogenesis of CAN and its role in CVD, offers the potential of new treatment targets that might reduce the burden of CVD in patients with diabetes. This review aims to provide an overview of the epidemiology, pathogenesis, cardiovascular consequence, diagnosis, and treatments of CAN, with particular emphasis on the latest developments in the field.
We conducted a review of the original papers and review articles indexed in PubMed, Medline and Google Scholar between 1975 and 2013. We have used several terms individually or in combination including: diabetes, autonomic neuropathy, CAN, cardiovascular, cardiac, autonomic, neuropathy, dysfunction. Only articles in English and in adult population were reviewed.
Based on the CAN Subcommittee of the Toronto Consensus Panel on Diabetic Neuropathy[10], CAN is defined as the impairment of cardiovascular autonomic control in patients with established DM following the exclusion of other causes. CAN, especially at the early stages, can be sub-clinical and thus as the disease progresses, it becomes clinically evident.
The prevalence of CAN varies between 1%-90% in patients with type 1 DM (T1DM) and 20%-73% in patients with T2DM (Table 1). This huge variation in CAN prevalence is due to the inconsistency in the criteria used to diagnose CAN and significant differences in the study populations, particularly in relation to CAN risk factors (such as age, gender and DM duration amongst others).
| Ref. | Year | Country | N of subjects | Type of DM | Population characteristics | Diagnostic test | Criteria applied | Prevalence(%) | Comments |
| O’Brien et al[111] | 1991 | United Kingdom | 506 | IDDM | Mean age 45 yr, mean DM duration 15 yr, female 42% | HRV in response to (1) rest (2) single deep breath (3) Valsalva manoeuvre or (4) standing | At least two positive of the tests mentioned in the previous column | 17 | Prevalence of CAN was associated with the presence of other DM complications |
| Ziegler et al[223] | 1992 | Germany | 130 | Newly diagnosed IDDM | CV of HRV, low- and mid- frequency bands of spectral analysis, MCR, Valsalva manoeuvre or lying-to standing | At least three positive of the tests mentioned in the previous column | 7.7 | ||
| Austria | 647 | Total IDDM | 25.3 | ||||||
| Switzerland | 524 | Non-IDDM | 34.3 | ||||||
| Kennedy et al[11] | 1995 | United States | 290 | IDDM | Listed pancreas transplantation recipients | HRV Valsalva manoeuvre | 90 88 | ||
| DCCT research group[19] | 1998 | United States | 1441 | IDDM (1) primary prevention cohort (absence of end–organ damage such as retinopathy and microalbuminuria) (2) secondary intervention cohort (mild/ moderate retinopathy +/- microalbuminuria) | Mean age 27 yr, female 47% duration of DM 1-5 yr (mean 2.6) primary prevention cohort 1-15 yr (mean 8.8) secondary intervention cohort | HRV | R-R variation < 15 | 1.6-6.2 | These figures represent baseline characteristics |
| Valsalva manoeuvre | Valsalva ratio < 1.5 | 5.5-6.3 | |||||||
| Postural BP | Diastolic BP drop > 10 mmHg | 0 | |||||||
| Kempler et al[28] (EURODIAB IDDM) | 2002 | 16 European countries | 3250 | T1DM | Mean age 32 yr, mean DM duration 14 yr, female 49% | (1) R-R response to standing (2) Postural BP | R-R ratio < 1.04 or drop > 20 mmHg in systolic BP | 36 | Correlation with age, DM duration and HbA1c |
| Gaede et al[5,224] (the Steno type 2 study) | 2003 | Denmark | 160 | T2DM | Mean age 55 yr, female 27%, HbA1C 8.8% at baseline | (1) R-R response to breathing (2)Postural BP | R-R variation < 6 or drop > 25 mmHg in systolic BP | 27.5 | This figure represents baseline findings |
| Valensi et al[27] | 2003 | France | 245 | T1DM | Mean age 39.6 yr, mean DM duration 8.6 yr, female 43% | R-R response to | Criteria for abnormal tests were based on Armstrong et al[225] | Rate of moderate/severe CAN was higher in T1DM (18.2% and 4.8%) than in T2DM (12.3% and 2.3%) (P = 0.031) | |
| 151 | T2DM | (1) deep breathing | 21.2 | ||||||
| (2) Valsalva and | 20.7 | ||||||||
| (3) standing | 33.5 | ||||||||
| At least two positive tests (classed as moderate CAN) | 20 | ||||||||
| Low et al[23] | 2004 | United States | 83 | T1DM | Mean age 59 yr, white 99%, female 48% | (1) Sudomotor axon-reflex test (2) Valsalva manoeuvre (3) BP and HR response to standing (4) R-R response to deep breathing | CASS ≥ 1 in two domains or ≥ 2 in one domain (sudomotor, cardiovagal, adrenergic) | 54 | This study focuses on DAN but encompasses several cardiac autonomic tests |
| 148 | T2DM | 73 | |||||||
| Pop-Busui et al[18] (DCCT/EDIC study) | 2009 | United States | 620 | IDDM-former intensive Tx group IDDM-former conventional Tx group | Mean age 47 yr in both groups, mean DM duration 26 yr, female 49% and 46% respectively | R-R response to (1) deep breathing (2) Valsalva manoeuvre (3) postural BP | R-R < 15 or R-R 15-19.9 and Valsalva ratio < 1.5 or drop > 15 mmHg in diastolic BP | 29 | 13/14 yr post closeout of DCCT |
| 591 | 35 |
CAN has been detected at time of diagnosis of diabetes in patients with either T1DM or T2DM irrespective of age, suggesting that CAN presentation is not limited by age or type of diabetes and can occur before DM is evident clinically[11-15]. However, the duration of diabetes is an independent factor for developing CAN irrespective to diabetes type[10,16]. CAN is detected in about 7% of both T1DM and T2DM at the time of initial diagnosis[17], and it is estimated that the risk for developing CAN increases annually by approximately 6% and 2% in patients with T1DM and T2DM respectively[17-19].
Poor glycaemic control is a major risk factor for CAN progression[14,19-21]. In the Diabetes Control and Complications Trial (DCCT), intensive glycaemic control resulted in a 50% decrease in CAN incidence over the 6.5 years follow-up period[19]. This protective effect persisted 14 years after the end of the study despite the disappearance of HbA1c differences that were achieved between the groups during the randomised phase of trial[18]. Similarly, CAN has been shown to be associated with conventional CVD risk factors, such as hypertension, smoking, hyperlipidaemia and obesity[22-24]. In the Steno-2 trial of patients with T2DM and microalbuminuria, intensive pharmacological intervention targeting hypertension, hyperlipidaemia and microalbuminuria combined with behavioural treatment (exercise, diet and smoking cessation) reduced the risk of autonomic neuropathy over the course of a 7.8 years follow-up (HR = 0.37, 95%CI: 0.18-0.79)[5]. After a mean of 5.5 years following the end of the study, the same protective effect against the development of autonomic neuropathy persisted (RR = 0.53, 95%CI: 0.34-0.81, P = 0.004). There was also reduction in the risk for developing CVD (RR = 0.43, 95%CI: 0.19-0.94, P = 0.04) and overall mortality (RR = 0.54, 95%CI: 0.32-0.89, P = 0.02) in this study[25].
Moreover, in a large cohort of more than 1000 patients with T2DM the incidence of CAN over a 7.5 years follow-up correlated with age (P < 0.001) and microvascular disease (P = 0.035)[26]. Diabetic nephropathy (including microalbuminura), diabetic retinopathy and diabetic polyneuropathy have been widely identified as clinical predictors of CAN[23,24,27], which is not surprising as diabetic microvascular complications share common mechanisms and risk factors. The impact of gender on CAN is controversial. In a multi-centre, cross sectional study of 3250 patients with DM, CAN prevalence was no different between men and women (35% male vs 37% female)[28]. However, in the action to control cardiovascular risk in diabetes trial including more than 8000 patients with T2DM CAN was more prevalent in women (2.6% in men vs 4.7% in women for moderate severity CAN and 1.4% in men vs 2.2% in women for severe CAN, P < 0.01 for all three definitions of CAN in the study)[29].
Ethnicity has also been postulated to be a risk factor for CAN as South Asians seem to have lower rates of peripheral neuropathy than White Europeans with DM[30]. More specifically, the prevalence of small fibre neuropathy was significantly lower in Indian Asians than in Europeans (32% vs 43% respectively, P = 0.03) and mean nerve conduction velocity Z scores (measuring large fibre neuropathy) were superior in Asians compared to Europeans (mean ± SD 0.07 ± -0.62 vs -0.11 ± 0.60, P = 0.007). However, using heart rate variability (HRV) spectral analysis as well as frequency and time domain analysis showed no difference in CAN prevalence between South Asians and white Europeans (Tahrani et al, unpublished data).
The exact pathogenesis of CAN is complex and remains unclear. Most of the proposed mechanisms of neuronal injury are based on models of somatic rather than autonomic neuropathy. Although many of these mechanisms might be shared between autonomic and somatic neuropathies, differences do exist as shown by the Steno-2 trial (described above) in which the multi-factorial intervention (including intensive metabolic control and lifestyle changes) slowed down the progression of autonomic but not somatic neuropathy.
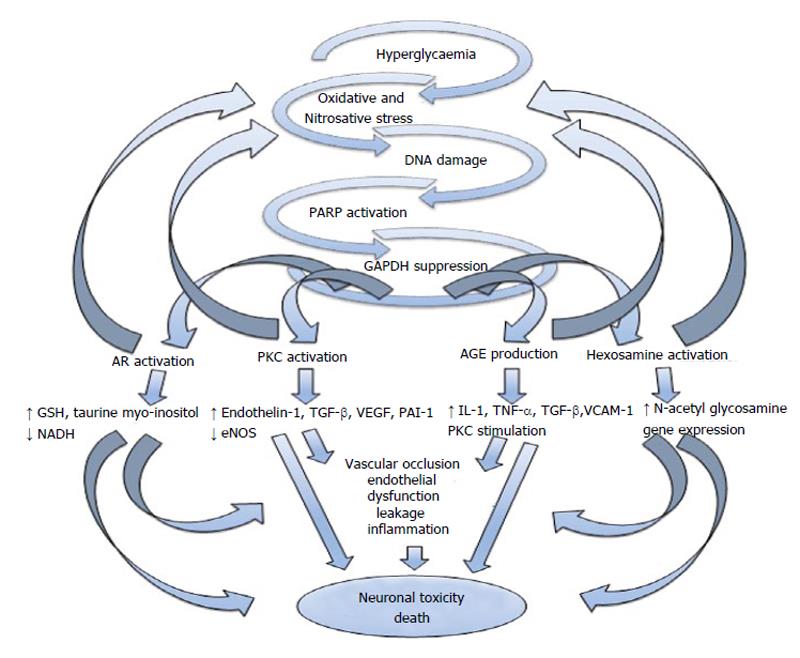
The pathogenesis of CAN is likely to be multi-factorial[31] and to involve several mechanisms and pathways that lead to neuronal ischaemia or direct neuronal death/dysfunction (Figure 1). Hyperglycaemia and the adverse metabolic environment in patients with DM result in increased oxidative and nitrosative stress[17], which can cause direct neuronal damage/dysfunction as well as endothelial dysfunction resulting in neuronal ischaemia. Neuronal axons are rich in mitochondria which makes them particularly susceptible to the direct and indirect effects on oxidative and nitrosative stress[32].
Increased oxidative stress results in poly ADP-ribose polymerase activation which when coupled with other activated downstream pathways including the polyol pathway, advanced glycation endproducts production, protein kinase C and the hexosamine pathway are thought to contribute to glucose toxicity[33-36]. These different pathways can in return exacerbate oxidative stress and can induce changes in gene expression, transcription factors, diverse cellular products disrupting several cellular functions and the communication between the cell and the surrounding matrix all of which leads to neuronal dysfunction and death[37-39]. These pathways also result in impaired microvascular-- regulation and endothelial dysfunction by different mechanisms, including increase in plasminogen activator inhibitor-1 and endothelin-1 production and impairment of endothelial nitric oxide (NO) synthase and NO actions[40,41]. This can lead to reduction of neurovascular perfusion, dysfunction and cellular apoptosis[42].
The role of autoimmunity has also been explored particularly in patients with T1DM. The presence of complement-fixing antibodies against sympathetic and parasympathetic tissues in patients with insulin-dependent diabetes and their correlation with CAN was described in the early 90s[43,44]. In a study of 78 patients with DM, the prevalence of phospholipid autoantibodies (PLA) in the patient’s serum was significantly higher in those tested positive for autonomic neuropathy (88% of the patients with autonomic neuropathy vs 32% of those without, P < 0.001) and there was a strong correlation between the PLA titre and total neuropathy score (r2 = 0.58, P = 0.0002)[45]. Granberg et al[46] demonstrated in a group of patients with T1DM that patients positive for complement-fixing antibodies to the sympathetic ganglion, vagus nerve and adrenal medulla had a significant higher risk to develop cardiac autonomic dysfunction (measured by the E/I ratio during deep inspiration and HRV to postural change) over a 6-year follow-up (RR = 7.5, 95%CI: 1.72-32.80). There are, however, conflicting reports whether these auto-antibodies contribute to the pathogenesis of autonomic neuropathy or represent rather incidental findings and can be attributed to autoimmunity against concurrent conditions, such as thyroid disease[47]. A recent study of mixed T1DM and T2DM patients concluded that neither peripheral nor CAN was associated with the presence or the levels of Neuropeptide Y Autoantibodies[48].
Several studies have shown a protective effect of residual β-cell function (i.e., C-peptide levels) on the development and incidence of microvascular complications (including CAN) in patients with T1DM[49,50]. The exact mechanisms for these associations are not clear but it is thought that the C-peptide activates Na/K channels, lowers inflammation and improves NO bioavailability and endothelial function[51,52]. Small RCTs have shown beneficial effect of C-peptide treatment on CAN parameters[53].
More recently data suggesting genetic predisposition to CAN have emerged. In a study of 154 patients with T2DM, TCF7L2 gene was found to be strongly associated with the presence of CAN, as assessed by deep breathing, lying to standing, Valsalva manoeuvre and postural hypotension tests (OR = 8.28, P = 0.022 for the rs7903146 allele)[54]. Another study on healthy Japanese individuals showed that the T393C polymorphism of the gene encoding the Gs-protein-α-subunit (GNAS1) is significantly associated with cardiovascular autonomic dysfunction, detected with power spectral analysis (P < 0.05 for TT + TC vs CC polymorphism)[55]. Twins studies, however, failed to show an association between CAN and genetic factors[56].
Obstructive sleep apnoea (OSA) is emerging as another possible factor in the development of CAN. OSA is very common in patients with diabetes and has been associated with increased sympathetic tone in patients without diabetes[57,58]. The interrelationship between OSA and CAN in patients with DM requires further investigation and is likely to be bidirectional. While the intermittent hypoxia that occurs in OSA could lead to increased oxidative stress, nitrosative stress, and impaired microvascular complications which could lead to CAN[59], CAN on the other hand could lead to changes in upper airways tone and changes in respiratory drive which could predispose the patient to OSA. One recent study presented in the Diabetic Neuropathy Study Group of the European Association for the Study of Diabetes 2012 meeting showed that the prevalence of CAN was similar in patients with T2DM with and without OSA, but CAN severity was worse in the OSA group (Tahrani et al[59], unpublished data). Furthermore, the presence of CAN was associated with more severe apnoea/hypopnea episodes (Tahrani et al[59], unpublished data).
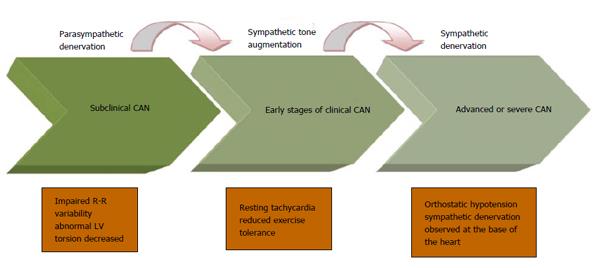
DM affects the autonomic (as well as the peripheral) nervous system in an ascending length-dependent manner. The vagus nerve, which anatomically is the longest autonomic nerve and physiologically mediates 75% of the overall parasympathetic activity, tends to be involved early in the course of CAN development. The early stages of CAN therefore involve reduction in parasympathetic activity, which results in sympathetic predominance. This increase in sympathetic tone continues until the latest stage of CAN when sympathetic denervation ensues, which spreads gradually from the apex to the base of the heart[60,61].
CAN is divided into a sub-clinical and a clinical stage. During the initial sub-clinical stage, CAN is detected through abnormalities in frequency and time domains of the spectral analysis of HRV and the Baroreflex Sensitivity (BRS) tests, as well as an increased torsion of the left ventricle (LV) on cardiac imaging before the development of abnormalities in standard cardiac autonomic reflex testing (CART) (please see below for details)[62-67]. Studies have shown that these abnormalities can even be present at the time of diagnosis of DM[63]. CAN progresses and parasympathetic denervation is followed by compensatory sympathetic overdrive, resulting in abnormal CARTs followed by symptomatic CAN in which the clinical manifestations become apparent (please see below). At the stage of sympathetic denervation, autonomic dysfunction correlates clinically with postural hypotension[63] (Figure 2). The time scale for the progression of subclinical CAN to the development of abnormal CART is unclear; similarly the natural history of the development of early cardiac abnormalities (such as torsion or deficits in myocardial perfusion or cardiac energetic) and its relationship to subclinical CAN is also unclear. But we estimate that many patients with sub-clinical CAN will develop abnormal CART and early features of cardiac involvement within 5 years of developing abnormal frequency and time domain parameters.
Resting tachycardia is a common manifestation of CAN that occurs at a relatively early stage of the disease. A HR of 90-130 beats per minute (bpm) can be observed and is associated with a reduction in parasympathetic tone followed by increased sympathetic activity as CAN progresses[68]. A fixed HR which does not change during sleep, exercise or stress is a sign of complete cardiac denervation[69]. Moreover, poor HR response to adenosine is associated with higher risk for adverse cardiac events[70], including all-cause and CVD mortality[71]. Hence, resting HR can be used as a diagnostic and prognostic tool in patients with DM after excluding other causes of tachycardia[10].
Impaired blood pressure, HR and cardiac stroke volume in response to exercise in the absence of structural or coronary cardiac disease are all features of CAN[69]. As disease progresses, the parasympathetic-sympathetic imbalance can lead to further impairment of the above parameters[68] which limits the diagnostic utility of exercise tolerance testing in these patients due to increased false-negative outcomes caused by blunted HR response[72]. In addition, patients with CAN should be tested using stress cardiac imaging (usually echocardiography) prior to starting an exercise program, especially those with high-risk profile[69].
Orthostatic hypotension is a manifestation of advanced CAN. Orthostatic hypotension is defined as the reduction in systolic blood pressure by > 20 mmHg or in diastolic blood pressure by > 10 mmHg 2 min following postural change from supine to standing[17,19,69]. Orthostatic hypotension occurs as a result of the impairment of the sympathetic response to postural change secondary to poor norepinephrine response and abnormalities in the baro-receptor sensitivity, resulting in inadequate HR response and peripheral vasoconstriction[23,69]. Orthostatic hypotension can be aggravated by many medications that are commonly used in patients with DM such as diuretics, vasodilators, tricyclic antidepressants and insulin[63]. Similar to resting tachycardia, assessing the presence of orthostatic hypotension is of prognostic value as a marker of advanced CAN[10]. In the middle-aged general population, orthostatic hypotension has been shown to be an independent prognostic factor for CVD and all-cause mortality[73].
CAN is associated with a prolonged subjective angina threshold (which is defined as the time between the observation of 1 mm ST depression on the electrocardiogram and the development of symptoms of angina pectoris); thus rendering patients with CAN susceptible for experiencing silent myocardial ischaemia and potentially infarction, despite being asymptomatic[74]. A meta-analysis of 12 cross-sectional studies showed that CAN is associated with silent ischaemia in patients with DM (the Mantel-Haenszel estimate for prevalence rate risk was 1.96, 95%CI: 1.53-2.51)[17]. A study of 120 patients with DM and no previous CVD found evidence that CAN (detected using the Valsalva manoeuvre, the deep breath test and lying-to-standing HRV) was a better predictor of major cardiac events [i.e., myocardial infarction or myocardial infarction (MI)] than the presence of silent ischaemia (OR = 4.16, 95%CI: 1.01-17.19) but when CAN was combined with silent ischaemia the risk was even higher (5 out of 10 had a major event)[75]. A study from Spain that included 217 patients with T1DM and T2DM, found that the presence of autonomic neuropathy is independently associated with increased risk for developing silent ischaemia (as demonstrated by positive exercise test) (OR = 6.5, 95%CI: 1.3-7.9) especially when combined with other cardiovascular risk factors such as microalbuminuria[76]. In the Detection of Ischaemia in Asymptomatic Diabetic subjects study which included 1123 patients with T2DM, CAN (defined as abnormal Valsalva manoeuvre) was also a predictor of silent ischaemia (defined using stress cardiac perfusion imaging) (OR = 5.6, 95%CI: 2.6-12.4, P = 0.0001)[77].
It is evident that patients with DM and CAN are at high risk of sustaining a major cardiovascular event during exercise, due to the limited perception of ischaemic pain which could delay the appropriate and timely response to ischaemia. A recent statement from the Toronto Consensus Panel on Diabetic Neuropathy has emphasised the importance of integration of cardiac autonomic function testing into the current risk stratification pathways for patients with DM and established CVD risk factors[10].
The mechanisms underpinning relationship between CAN and silent ischaemia are not clear. Several mechanisms have been proposed including altered pain threshold, impaired afferent myocardial autonomic pathways and ischaemic processes not detected by routine electrocardiography. There has also been debate over whether the relationship between them is indeed a causative one, or both CAN and silent ischaemia are a product of coronary artery disease observed in diabetes[78,79].
Diabetic cardiomyopathy is a clinical entity that is characterised by changes in the biochemical signalling in the presence of a sympathetic-vagal imbalance resulting ultimately in left ventricular hypertrophy and remodelling, and therefore cardiac dysfunction in patients with DM in the absence of coronary artery disease[63]. Diabetic cardiomyopathy results in variable degrees of systolic and predominantly diastolic dysfunction in the absence of structural or valvular cardiac disease, coronary vessel disease, or hypertension[80,81]. Changes in the diastolic and/or systolic function can be identified on various diagnostic imaging modalities in otherwise asymptomatic patients and can precede the occurrence of macrovascular diabetic complications[82]. Frequently, the only detectable abnormality in the early stages of CAN is an isolated diastolic dysfunction with a normal LV ejection fraction[83] associated with high CVD morbidity[84,85].
Conventional echocardiography studies, with or without Doppler technique, showed that CAN is associated with significant reduction in the peak diastolic filling and an increase in the atrial component of diastole[69]. The introduction of new diagnostic modalities, such as the cardiac magnetic resonance imaging has allowed even more sensitive means of diagnosing and classifying diabetic cardiomyopathy even in the early stages by examining myocardial twist, torsion and strain[86]. Torsion is a measure of the apical rotation along the long axis of the heart and is followed by a rapid untwisting, occurring during the isovolumic relaxation phase[87]. Both torsion and maximal torsion rate have been found to be increased in patients with T2DM and preserved systolic function[86]. In patients with T1DM, increased torsion appears to be independent of energetic deficits but related to microvascular perfusion deficits and correlates with changes in sympathetic denervation[88,89]. Myocardial Perfusion Reserve (another diagnostic tool used for the detection of microvascular abnormalities) has been shown to detect the early stages of CAN in asymptomatic patients and to assess CAN severity[90].
There are several proposed mechanisms for the development of diabetic cardiomyopathy. The parasympathetic denervation observed in the early stages of the disease leads to a dominant sympathetic tone[91], which promotes a cascade of intrinsic metabolic changes, including the release of high myocardial catecholamine levels and catecholamine toxicity[92,93]. This catecholamine rise has been shown to induce mitochondrial uncoupling[94,95], switching energy generation on a cardiac level from myocardial glucose to free fatty acids, which is considered an inefficient energy source[96] and therefore increases the oxygen demand[94,95]. These alterations on the cardiac biochemical and cellular level, lead ultimately to programmed cell death and fibrosis[97,98], elevated oxygen consumption relevant to the cardiac work[99,100] and finally hypertrophy and remodelling of the LV[101]. Crucial mediators in the above process are the mitochondrial reactive oxygen species[102,103], insulin resistance[104] and calcium dependent apoptosis[102,105,106].
On a macroscopic level, diastolic dysfunction in CAN is associated with delayed relaxation, impaired filling and increased stiffness of the LV[107]. The previously described sympathetic predominance is a stimulator of the rennin-angiotensin-aldosterone axis, resulting in increased HR, cardiac output and peripheral vasoconstriction[108]. Studies have shown that this alteration on the cardiac profile can lead to reduction of coronary blood perfusion and diastolic dysfunction in patients with evidence of early microangiopathy[60]. Sympathetic overdrive may also lead LV wall stress and LV hypertrophy. Pop-Busui et al have recently shown in a large cohort of the DCCT/EDIC study, that CAN is associated with a mass increase as well as a concentric remodelling of the LV, independent of other risk factors[109].
CAN is associated with an increased mortality risk (Table 2). This was described in longitudinal studies in the early 1990s showing a 50% increase in 5 year-mortality risk in patients with DM and autonomic neuropathy compared to those without[110-113]. In a meta-analysis of 15 studies on the basis that they included patients with DM who had baseline assessment of HRV using one or more tests described by Maser et al[114] showed that the pooled estimated relative mortality risk was 2.14, (95%CI: 1.83-2.51, P < 0.0001), for those who had CAN. CAN was also found to have the strongest association with mortality amongst other risk factors in the EURODIAB IDDM Complications Study[115].
| Ref. | Country | N ofsubjects | Type of DM | FUp (yr) | Diagnostic test for CAN | Criteria applied | Mortality figures(expressed in HR, RR and incidence) | Comments |
| Veglio et al[226] | Italy | 316 | T1DM | 5 | (1) Resting heart rate (2) HRV during deep breathing (3) BP response to standing | ≥ 2 abnormal tests | Relative risk: 3.55 (1.4-8.9) and 2.21 (0.62-7.84, P = 0.22) after multivariate analysis for all-cause mortality | The mortality rates were 13% and 4% in the presence and absence of CAN respectively |
| Gerritsen et al[164] the Hoorn Study | Nether-lands | 446 | Non-DM | 9 | Seven parameters assessing HRV and BP response to: (1) 3-min breathing and (2) six deep breaths | Cut–off set as the lowest 25th percentile of non-diabetic group | Only E/I had a statistically significant association with mortality- Relative Risk: 2.25 (1.13–4.45) for all cause and 2.04 (0.74–5.65) for CVD mortality | An additional four parameters showed a tendency (P < 0.10) for association with acc- cause mortality: mean NN, LF power, HF power, and BRS |
| Chen et al[227] | Taiwan | 159 | T2DM | 7.7 | HRV response to: (1) single deep breath (2) six consecutive breaths (3) standing, (4) Valsalva manoeuvre | ≥ 3 abnormal tests | All cause mortality: 29% vs 12% with and without CAN respectively CVD mortality: 9% vs 2% in pts with and without CAN | The 8-yr survival rate for pts with abnormal CAN tests was 63.6% in males and 76.4% in females, compared with 80.9% and 93.3% for patients with normal CAN tests |
| 612 | T2DM | |||||||
| Wheeler et al[228] | United States | 843 | T1DM and T2DM | HRV response to deep breathing and postural BP | Drop in BP ≥ 30 mmHg and HRV divided into 5 quintiles HRV < 10 bpm at baseline abnormal E/I | Hazard Ratio: 1.49 (1.01-2.19) for all-cause mortality and 1.08 (0.69-1.70) for CVD mortality in the lowest quintile of HRV. Relative Risk for orthostatic hypotension: 0.65 (0.69-1.70) Relative risk: 4.9 (2.1-11.5, P < 0.0001) after adjustment for traditional CVD risk factors Hazard Ratio: 0.92 (0.87–0.98, P = 0.005) for HRV (1 beat/min increase) | Of the 142 patients for whom cause of death was available, 75 deaths (49.7%) were due to CVD. The lowest quintile of HRV was associated with a 50% increase in mortality after adjusting for other risk factors During follow-up, 33 Patients died from cardiovascular causes, During follow-up 54 of 104 patients died: 41 patients (80.4%) with diabetic nephropathy and 13 patients (24.5%) with normoalbuminuria. Thirty patients (55%) died from cardio-vascular causes | |
| Astrup et al[229] | Denmark | 388 | T1DM (197 with macro-, 191 normo- albuminuria) | 10.1 | HRV to deep breathing | |||
| Astrup et al[230] | Denmark | 104 | T2DM (51 with nephropathy, 52 with normal albuminuria) | 9.2 | HRV to deep breathing | |||
| Soedamah- Muthu et al[115] the EURODIAB PCS | 16 European countries | 2787 | T1DM | 7 | HRV response to standing and postural BP | R-R ratio of < 1.04 and drop in systolic BP ≥ 20 mmHg | Hazard Ratio: 3.61 (1.49–8.76) for CVD mortality and 2.83 (1.82–4.38) for all-cause mortality. | Autonomic neuropathy and microalbuminuria were the most important independent predictors of mortality |
| Lykke et al[231] | Denmark | 391 | T1DM | 10 | HRV and QTc | All cause mortality Hazard Ratio: 2.5 (0.9–6.8, P = 0.071) in pts with abnormal HRV and 2.3 (1.3-4.0, P = 0.005) in those with abnormal QT combined hazard ratio 6.7 (1.8-25, P = 0.005) | Out of 34 patients with both tests abnormal, 15 died in the 10 yr period (14 from cardiovascular causes) | |
| Ziegler et al[232] MONICA/ KORA Augsburg Cohort study | Germany | 1560 | Non-DM | 9 | HRV, QTc interval and QTD | Group (1) Lowest quartile for SDNN, CV and max-min R-R intervals Group (2) QTc > 440, Group (3) QTD > 60 ms | All-cause mortality Relative Risk: 0.93 (0.65-1.34)/2.02 (1.29-3.17)/0.98 (0.60-1.60) in patients without DM and 1.74 (0.95-3.18)/3.00 (1.34-6.71)/0.42 (0.06-3.16) in patients with DM for group 1/2/3 respectively | Prolonged QTc interval was an independent predictor of mortality both in patients with and without DM, Low HRV trended towards an increased risk of mortality by 73% in patients with DM but not the population without DM |
| 160 | DM | |||||||
| Beijers et al[233] the Hoorn Study | Nether-lands | 376 | Non-DM | 13.6 | HRV and BP response to: (1) 3-min breathing, (2) six deep breaths (3) standing | Calculated z-score for each parameter and averaged into a total CAD score | Relative risk: 2.54 (1.60–4.04) for CVD mortality and 2.11 (1.58–2.81) for all cause mortality, | CAN was associated with all-cause and CVD mortality independent to other CVD risk factors and microalbuminuria |
| 114 | T2DM | |||||||
| Pop-Busui et al[29] | United States and Canada | 8135 | T2DM | 3.5 | HRV and QTI computed from 10-s resting electrocardiograms | CAN1: lowest quartile of SDNN and highest QTI quartile, CAN2: CAN1 and resting heart rate, CAN3: CAN1 and peripheral neuropathy | Hazard ratios: 1.55 (1.09-2.21)/2.14 (1.37-2.37)/2.07 (1.14-3.76) for all-cause and 1.94 (1.20-3.12)/2.62 (1.40-4.91)/2.95 (1.33-6.53) for CVD mortality in CAN1/CAN2/CAN3 respectively | CAN was independently associated with overall and CVD mortality after adjusting for baseline CVD, DM duration, traditional CVD risk factors and medications |
Even in patients with high CVD risk profile such as the population of the ACCORD trial, CAN was an independent predictor of all-cause mortality (HR = 2.14, 95%CI: 1.37-3.37) as well as CVD mortality (HR = 2.62, 95%CI: 1.4-4.91) after a mean follow-up of 3.5 years[29]. Interestingly the relationship between CAN and mortality was similar regardless of treatment allocation to the intensive or standard glycaemic control groups[29].
CAN was also found to be associated with a higher mortality risk in patients who had myocardial infarction[116], suggesting that screening for CAN in patients with DM who suffered a myocardial infarction can be used for risk stratification[117].
CAN is also associated with increased risk of sudden cardiac death[112,113,118]. This can be explained by the increased rate of fatal cardiac arrhythmias due to the imbalance between the sympathetic and parasympathetic autonomic function[119], as well as cardiac sympathetic denervation[67]. QT prolongation which has been associated with autonomic neuropathy in several studies[120-122], can also provide an alternative mechanism, rendering patients with CAN more susceptible to suffer life-threatening cardiac arrhythmias, including Torsades de Pointes[69]. The exact relationship between CAN and sudden cardiac death remains, however, under question. As shown by the Rochester Diabetic Neuropathy Study, sudden death cases are also related to severe coronary artery disease or LV dysfunction rather than CAN itself[123]. Nonetheless, as we discussed above, CAN seems to contribute to cardiovascular mortality even in those with established coronary artery disease.
Several mechanisms have been implicated in explaining the relationship between CAN and mortality in patients with DM. Autonomic neuropathy can lead to impaired response to hypoxic state[124], reduced hypoglycaemia awareness and prolonged hypoglycaemic episodes[111]. The observed mortality can also be attributed to a direct effect of autonomic neuropathy and its microvascular complications[125] as well as to an indirect association with end-organ complications, such as nephropathy, left ventricular hypertrophy and diastolic dysfunction[100]. In addition, the lack of the physiological nocturnal parasympathetic dominance in patients with CAN can lead to nocturnal hypertension, causing LV hypertrophy[126,127] and increasing the CVD burden[93,128].
Patients with CAN exhibit 2- to 3-times fold increase in perioperative morbidity (perioperative complications, impaired wound healing, impaired drug metabolism) and mortality[129,130]. Patients with CAN are more likely to require vasopressor support in the theatre setting[130]. They are also prone to experience a blood pressure and HR reduction during the induction of anaesthesia, as well as severe intraoperative hypothermia[131]. The above findings can be explained by an impairment or absence of the normal vasoconstrictive response to vasodilating anaesthesia in patients with CAN[130].
Unlike the strong links between CAN and CVD, there is only limited data regarding the impact of CAN on cerebrovascular disease. In a study conducted by Töyry et al[132] that included 133 patients with T2DM, CAN was found to be an independent risk factor for developing stroke after 10 years of follow-up (OR = 6.7, 95%CI: 1.5-29.9 for HRV response to deep breathing and OR = 1.1, 95%CI: 1.01-1.2 for lying-standing BP). In a sub-analysis of the Appropriate Blood Pressure Control in Diabetes population, including 950 patients with T2DM over a 5-year period, CAN was significantly associated with the occurrence of stroke, independent to other risk factors[133]. The later was also confirmed by a recent study including 1458 patients with T2DM who were followed up for 7 years[134].
Several authors have hypothesized that CAN is involved in the pathogenesis of diabetic nephropathy, although causation has not been proven[135]. Sympathetic overactivity has been shown to cause glomerular and tubular dysfunction in diabetic animal models via indirect (hypertension and angiotensin II) and direct (vascular smooth muscles proliferation, vasoconstriction, podocytes injury) insults[136]. CAN is associated with increased CVD morbidity and mortality[63,135] and with haemodynamic changes such as lack of nocturnal BP dipping (causing increased intra-glomerular pressure resulting in albuminuria)[137] and diurnal postural falls in BP (resulting in lower intra-glomerular pressure)[138] and endothelial dysfunction in humans. In addition, CAN is associated with deficits in erythropoietin production and, as a result, erythropoietin-deficiency anaemia[137]. Subsequently, CAN patients are deprived from the direct nephroprotective action of erythropoietin and thus, anaemia becomes a strong predictor of nephropathy and progression of chronic kidney disease[68]. In streptozotocin-diabetic rats, sympathetic overactivation has been shown to be involved in the pathogenesis of diabetic nephropathy[139] and renal denervation was shown to prevent glomerular hyperfiltration[140]. Hence it is plausible that CAN is involved in the development and progression of diabetic nephropathy. Several studies examined the association between CAN and either albuminuria and/or glomerular filtration rate[141-145], but all these studies had a cross-sectional design, hence causation cannot be proven, particularly that the pathogenesis of CAN is similar to other microvascular complications including diabetic nephropathy. Longitudinal studies are scarce and limited to a small number of patients with T1DM[138,146]. Hence, data regarding the longitudinal impact of CAN on diabetic nephropathy in patients with T2DM is lacking.
CAN has been proposed as a contributing factor in the development of lower limb vascular and neurological complications. Autonomic neuropathy can cause alterations in microvascular blood flow (MBF), which predispose to changes in skin structure and quality and impairment of sweat glands’ innervation resulting in dry skin and increased risk of oedema and foot deformity which increases pressure on certain areas causing ulceration[147]. It is also believed that CAN, through the sympathetic denervation of the lower limb vasculature, can induce lower extremity hyperaemia, increase inflammation and erosion into the joints/bones and therefore contribute in Charcot’s neuroarthropathy. As a result, the patient with Charcot will typically present with prominent peripheral pulses due to vasodilatation and autonomic neuropathy. Power Spectral Analysis and HRV has been employed in trials for the detection of autonomic neuropathy in patients with Charcot’s disease[148]. Similarly to Charcot’s arthropathy, patients with recurrent vascular neuropathic ulcers appear to share analogous cardiac autonomic dysfunction, as shown by the use of HRV, Valsalva ratio and orthostatic hypotension[149].
Ewing et al[150] proposed in early 1970s five simple non-invasive tests to measure cardiac autonomic function based on the HR and blood pressure response to certain physiological manoeuvres. These tests include: (1) the HR response to deep breathing, which assesses beat to beat HR variation (R-R variation) during paced deep breathing [expiration-to-inspiration ratio (E:I)]; (2) the HR response to standing, which is expressed as the 30:15 ratio which is the ratio of the longest R-R interval (between the 20th and 40th beat) to the shortest R-R interval (between beats 5-25) elicited by a change from horizontal to vertical position; (3) the Valsalva manoeuvre which evaluates the HR response during and after a provoked increase in the intra-thoracic and intra-abdominal pressures (the patient normally exhales for a period of 15 seconds against a fixed resistance); (4) the blood pressure response to standing, which assesses the baro-reflex mediated blood pressure change following postural change; and finally; and (5) the blood pressure response to sustained handgrip, as defined by the diastolic blood pressure increase caused by the sustained muscle contraction with the use of a handgrip dynamometer[17]. The first two tests reflect defects in the parasympathetic activity (i.e., the ability of the vagal nerve to slow the HR during the procedures which increases the R-R interval and hence increases the ratios), while the last two also describe changes in the sympathetic function (i.e., the ability to provide appropriate BP and HR response to the activity involved)[151,152]. The autonomic changes that occur during the Valsalva manoeuvre are complex and involve both the sympathetic and parasympathetic systems[153], although the Valsalva ratio mostly represents parasympathetic activity. For more details about the autonomic changes during Valsalva please see[17].
While the above described CARTs have been widely used since their introduction, there is no evidence on the superiority of one test over another when it comes to assessing CAN[10]. However, the HR response to deep breathing is the most commonly utilised, because of its high reproducibility and specificity[154] and its ease of use[10,155]. All the tests are considered to be valid markers of autonomic dysfunction, given that end organ failure is excluded and parameters such as concomitant illness, use of over the counter medications and lifestyle factors (exercise, smoking, exercise) are taken into consideration[156].
A reduction in HRV has been associated with the early stages of clinical CAN. In healthy individuals, the beat-to-beat variability with aspiration is predominantly affected by the direct sympathetic and parasympathetic activity[62,157], as well as various other stimuli, including certain neurohumoral factors (catecholamines, thyroid hormones), temperature changes and mechanical and ionic changes in the sinus node[158]. The efferent sympathetic and vagal stimulation is characterised by synchronised discharges, modulated by central and peripheral oscillators, with the former referring to vasomotor and respiratory centres and the later to respiratory movements and arterial pressure. These synchronous neural discharges can manifest as short and long -term oscillations in the HR[63].
The R-R intervals recorded under paced breathing are transformed to generate the time and frequency domains. Conceptually, if the faster respiratory sinus arrhythmia signal and the slower mean HR changes could each be separated from the patient’s cardiogram and analyzed independently, the result would yield a measure of Vagal outflow from the respiratory sinus arrhythmia and a measure of sympathetic activity from the changes in mean HR. Effectively this is what is accomplished in the frequency- or spectral-domain. Spectral analysis of respiratory sinus arrhythmia provides the indication of where in the frequency domain the Vagus is influencing the heart. The frequency domains are generated using continuous wavelet transform method (Fourier transform) and separated to three basic components: very-low-frequency, low-frequency (LF) and high-frequency (HF)[61,159]. HF represents vagal activity, whereas LF is attributed to the combined effect of sympathetic and parasympathetic influence[62,160]. Modern software (such as ANSAR technology) adjusts for the respiratory rate, hence simplifying the process. Parameters generated include: respiratory frequency (Rfa, 0.15 to 0.4 Hz, represents parasympathetic function), and LF (Lfa, 0.04 to 0.15 Hz, represents sympathetic function). The HRV and BP are recorded with the patient in sitting position during resting, deep breathing, Valsalva manoeuvre and standing position.
The electrocardiogram (ECG) recordings were initially longer in duration, usually over a period of 24 h but recent data has demonstrated that recording of shorter duration can provide equally reliable information[16,158,161]. Time domain analysis is a useful tool in the assessment of parasympathetic activity by measuring the normal R-R intervals, whereas the frequency domain is based on the spectral analysis of R-R interval and other cardiovascular and respiratory signals based on short-term ECG recordings (2-5 min)[69,158].
The key element in the accurate use and interpretation of HRV models is the standardisation of the conditions under which the test is carried, including age, blood pressure, HR, tobacco smoking or caffeine use and, above all, respiration control[69].
The BRS measures the cardiac vagal and sympathetic baro-reflex function. The idea behind its function is that an increase in the BP normally induces a reflective increase in the vagal cardiac efferents and a reduction to the efferent sympathetic activity, resulting in bradycardia and hypotension, due to the reduction in cardiac output as well as the peripheral vasodilation[158]. A reduction in BP induces opposite responses. Thus, to correctly measure the baro-reflex function, both the vagal efferent activity (evidenced by changes in HR in response to changes in BP), and the sympathetic efferent activity (affecting the arterial vessels) should be taken into account.
In practice, the term “baro-reflex sensitivity” normally applies to the cardiac-vagal arm, and to methods measuring changes in HR in response to changes in (systolic) BP. The test can be performed either with the use of pharmacological methods (intravenous bolus injection of epinephrine)[162] or non-pharmacological techniques (physical manoeuvres such as postural change). Although the former is considered the gold standard to date for evaluating BRS, both of them correlate well clinically with each other[163]. Both techniques require a continuous measure of BP and a continuous and synchronised measure of HR (R-R interval)[158].
BRS can be used for detecting sub-clinical CAN[63], since BRS can be abnormal in diabetes, before the demonstration of any clinical signs of CAN or other conventional autonomic function tests detect any abnormalities[64,65]. Several studies on patients with diabetes have concluded that BRS is a strong independent risk factor for mortality[164], especially in cohorts suffering from heart failure or following a myocardial infarction[162,165].
The use of Single-photon emission computed tomography (SPECT) and/or positron emission tomography (PET) and sympathetic neurotransmitter analogues, such as the 123I-metaiodobenylguanide (123I-MIBG) (SPECT), the 11C-metahydroxyephedrine (11C-HED) (PET) and 11C-epinephrine has enabled the quantitative scintigraphic evaluation of cardiac sympathetic innervation[63].
123I-MIBG undergoes rapid uptake in the myocardium but as it is semi-quantitative is not a precise indicator of neuronal uptake[158]. Metabolically stable 11C-HED demonstrates a highly specific uptake by the sympathetic nerves mediated by norepinephrine transporters[166]. It is important, however, to take myocardial perfusion (which affects the delivery of the tracer of interest) into consideration before interpreting the results of these imaging techniques. Retention defects of both 123I-MIBG and 11C-HED have been reported in patients with T1DM and T2DM and have been variably correlated with abnormal but also normal CARTs[60,67,167]. The consistent pattern of sympathetic denervation in patients with T1DM supports the notion that 11C-HED can be used to monitor the population of sympathetic nerves and evaluate the regional autonomic deficits of sympathetic innervations[66,166,167]. In patients with CAN and T1DM, the wash rates of 11C-epinephrine have been shown to correlate well with those of 11C-HED[158]. The development of microvascular complications has been associated with the augmentation in sympathetic tone and adrenergic hyper-responsiveness, by the use of 11C-HED[63]. As CAN reaches an advanced stage, a heterogenous pattern of 11C-HED retention is observed, with a reduced 11C-HED retention in the distal LV and a persistent or increased 11C-HED retention seen proximally, indicating a proximal to distal pattern of sympathetic denervation of the LV[63].
Increases in the sympathetic nervous tone and elevated epinephrine levels can affect the retention of sympathetic neurotransmitter analogues, making the interpretation of the above scintigraphic models rather challenging. Furthermore, the lack of standardisation, the high cost and the demand on highly skilled operators, restricts the role of scintigraphy as a valuable research tool and not a part of daily clinical routine[68].
When it comes to radiation exposure, 123I-MIBG lacks a β-particle emission and has a half-life of 13.2 h, whereas its energy of the primary imaging photon is calculated at 159 keV (kiloelectron volt)[168]. When compared to 131I, 123I-labelled agent is to be considered the radiopharmaceutical of choice as it has a more favourable dosimetry and better radiation profile. Whole-body radiation is markedly lower using 11C-HED PET [effective dose equivalent in adults, 1.2 milliSieverts (mSv)] compared with 123I-MIBG scintigraphy (effective dose equivalent in adults, 6.0 mSv)[169]. The radiation dose to the whole body from 20 milliCuri (mCi) 11C-HED is 0.186 rad, less than that from 0.5 mCi 131I-MIBG IBG (0.45 rad) or 10 mCi 123I-MIBG (0.53 rad)[170].
Muscle sympathetic nerve activity (MSNA) is based on the ability to record efferent sympathetic nerve signals in the skeletal muscles either at rest or in response to physiological perturbations with the use of microelectrodes into a fascicle or a distal sympathetic nerve of the skin or muscle (microneurography)-usually the peroneal nerve[171].
MSNA is the most direct measure of peripheral sympathetic activity and therefore a useful research tool. However, its invasiveness, cost and time-consuming nature is not recommended for routine autonomic assessment[158].
Occasionally, various tests have been proposed for the assessment, diagnosis and monitoring of CAN. A recent study on 167 patients with type I diabetes conducted by the University of Liege, found the use of pulsatile stress, which measures the arterial stiffness, correlates well with baro-reflex sensitivity, suggesting therefore that arterial stiffness can be used as a marker of CAN[172]. The association between arterial stiffness (expressed as carotid-femoral wave velocity (PWV)) had already been explored by another study. After multivariable linear regression, the association between CAN (E/I index in particular) and PWV not only remained significant but E/I index was the strongest predictor of PWV in the model (β coefficient: -0.326, 95%CI: (-3.110)-(-0.750), P = 0.002)[173]. Catecholamine kinetics, most specifically epinephrine and norepinephrine plasma clearance have been labelled as the biochemical equivalent of MSNA but they have failed to date to produce reliable diagnostic data[158].
Another aspect of autonomic function is the assessment of cutaneous MBF. The skin offer an accessible organ to asses MBF and endothelial function, which is often involved in the development of micro and macrovascular diabetes, correlates with systematic endothelial function measures and myocardial microcirculation[174]. Several methods are available to assess skin MBF[175]. Laser Doppler (LD) allows the determination of blood flow under basal conditions or following physical (e.g., heating) or pharmacological (e.g., acetylcholine and/or sodium nitroprusside) stimulation; allowing the differentiation between endothelial-dependant and independent responses[174]. Furthermore, LD allows the measurement of nerve axon reflex-related vasodilation following acetylcholine iontophoresis which is the result of C-fibre stimulation[176]. LD techniques include LD flowmetry, LD perfusion imaging and laser speckle contrast imaging[158,174].
Another assessment of the peripheral autonomic system is intra epidermal nerve fibre density (IENFD) using immuno staining[177]. IENFD is highly sensitive and specific to diagnose small fibre neuropathy (88%-98% and 88.8%-95% respectively)[178]. IENFD correlates also inversely with thermal thresholds[178]. In addition, IENFD innervates the sweat glands. Reduction in sweat production in the feet contributes to the development of dry skin/callus and hence predispose to the development of foot ulceration. This function can be assessed by several methods such as Neuropad[147] and Sudoscan[179].
HR responses to deep breathing, standing and Valsalva manoeuvre, as well as blood pressure response to standing (CART) are considered as the gold standard in clinical testing for autonomic neuropathy[10]. Their applicability in bedside clinical practice is based on their sensitivity, specificity, reproducibility, ease and safety of use and standardisation.
According to the CAN Subcommittee of the Toronto Consensus Panel statement following the 8th international symposium on diabetic neuropathy in 2010[10], the criteria for diagnosis and staging of CAN are as follows: (1) A single abnormal CART result suffices for the diagnosis of possible or early CAN; (2) The presence of two or three abnormal test among the seven autonomic cardiovascular indices (5 CARTS, time-domain and frequency-domain HRV tests) are required for the diagnosis of definite or confirmed CAN; and (3) The presence of orthostatic hypotension in addition to the above criteria signifies the presence of severe of advanced CAN.
The majority of diabetes patients with CAN have subclinical or asymptomatic disease, rendering the diagnosis and appreciation of CAN in clinical practice rather difficult[63]. Once CAN reaches the stage that becomes clinically evident, the disease might have reached an advanced level and management becomes more difficult. Screening for early CAN is therefore considered good clinical practice several reasons as summarised in Figure 3[10].
The Toronto Diabetic Neuropathy Expert Group in a recent statement have recommended that screening should be considered for patients at time of diagnosis of T2DM and within 5 years of diagnosis of T1DM, particularly in patients with other macro- and/or microvascular complications[180]. Patients with a history of poor glycaemic control are especially at risk for developing CAN, as demonstrated in several studies, suggesting that this clinical group may benefit from screening[17]. Due to its impact on exercise tolerance, testing for CAN should be a part of the screening in patients that are about to begin a new exercise programme that involves more intense physical activity than brisk walking[69,181]. Evidence also suggests that screening for CAN could be incorporated into the perioperative assessment of patients with poor glycaemic control and coronary artery disease, due to the association between CAN and haemodynamic instability peri- and intra-operatively[182]. Finally, testing for CAN could potentially be of benefit in patients with DM that have suffered MI, as this would serve in the risk stratification of this subgroup and assist into adapting a more aggressive therapeutic approach for those at risk of sudden cardiac death or life threatening arrhythmias.
CAN treatment can either be symptomatic or aimed at slowing or reversing CAN progression. However, effective therapies to slow or reverse CAN progression are rather limited as the complete underlying pathogenesis remains unclear. However, based on our current understanding of CAN pathogenesis and risk factors, several potential treatments have been examined.
Lifestyle changes have been shown to have a beneficial impact on the prevention of CAN progression in the Steno-2 trial[5] and the Diabetes Prevention Program (DPP)[183]. In the Steno-2 study, patients with T2DM and microalbuminuria were randomised to a multi-factorial cardiovascular risk factor intervention that included behavioural therapy (diet, physical exercise and smoking cessation) and pharmacological intervention (to control BP, lipids and hyperglycaemia) or conventional treatment in accordance to the national guidelines. After an average of 7.8 years of follow-up, the risk for developing CAN was significantly lower on the intervention arm (49% in the intensive group vs 65% in the conventional group, HR = 0.37, 95%CI: 0.18-0.79, P = 0.002). In the DPP, lifestyle modification demonstrated superior results in the improvement of autonomic dysfunction (assessed with HRV and QT indexes) as compared to the use of metformin or placebo.
Weight loss and dietary intervention accompanied[69] or not[184] by supervised training was associated with improvement on CAN indices. Aerobic training has also been shown to improve CAN, with some indication that mild physical exercise is recommended in less severe CAN cases. A recent review summarising the evidence for the impact of life style interventions on CAN has concluded that moderate endurance and aerobic exercise in both T1DM and T2DM, improve HRV and cardiac autonomic function significantly, in favour of parasympathetic dominance, independent of BMI, glycaemic or BP control and duration of diabetes[185].
Hyperglycaemia is a major risk factor for CAN development and progression. Intensive glycaemic control has been shown to slow the progression and prevent/delay the development of CAN[18,66,186,187]. In the DCCT trial, intensive glycaemic control in a group of patients with T1DM reduced the CAN incidence by 50% over 6.5 years follow-up compared with conventional therapy (7% vs 14% respectively)[19]. These beneficial effects persisted 13-14 years after close-out of the trial[18]. Although both former treatment arms exhibited deterioration in CAN during follow-up after the end of the DCCT, the former intensive treatment group continued to demonstrate a statistically significant slower decline in CAN.
PET cardiac imaging with the use of 11C-HED showed similar beneficial effects in a 3-year prospective trial. Good glycaemic control (defined as mean HbA1c < 8%) was associated with reduction of sympathetic denervation as opposed to the group of poor diabetes control (HbA1c ≥ 8%)[167]. In the SEARCH CVD study, 354 young patients with T1DM were assessed for the presence of sub-clinical autonomic dysfunction, as demonstrated by the use of HRV parameters and the presence parasympathetic loss with sympathetic override. Poor glycaemic control, as defined by HbA1C > 7.5%, was independently associated with the presence of subclinical CAN as compared to a frequency-matched control group without DM[188].
The effects of glycaemic control in T2DM are not similarly encouraging. Data from recent studies have failed to demonstrate differences in the incidence of CAN based on the application of intensive therapy in T2DM patients[189,190]. The sensitivity of tests utilised for the diagnosis of CAN in those trials, however have been questioned, suggesting that more research is needed to investigate the relationship between metabolic control and CAN in patients with T2DM.
There is limited but increasing data on the use of pharmacotherapy targeting specific pathogenic pathways. The use of the specific antioxidant α-lipoic acid improved CAN in patients with T2DM in a 4-mo controlled randomised trial[191]. In animal models, the pharmacological agents FP15 and FeTMPS, which act by catalysing the decomposition of peroxynitrite, have shown promising results in improving neuronal function[192-194]. The use of glucagon-like peptide 1 analogues or the dipeptidyl peptidase 4 inhibitors have demonstrated cardioprotective[195] and neuroprotective properties[196], raising the possibility of their use for treatment not only for peripheral neuropathy, but autonomic neuropathy as well. In small scale studies, aldose reductase inhibitors have been shown to improve LV function in patients with DAN without any alteration on CAN indices[197]. There is also evidence suggesting the vitamin E and C-peptide can both improve HRV indices[10]. In a randomised controlled trial, vitamin E when compared to placebo managed to increase the R-R interval (P < 0.05) and the HF component of HRV (HF; P < 0.05) in 50 patients with T2DM over a period of 4 mo[198]. Small RCTs have shown beneficial effect of C-peptide treatment on CAN parameters[53]. In a recent randomised placebo-controlled trial of 44 patients with T1DM, treatment with a triple antioxidant regime (allopurinol, α-lipoic acid and nicotinamide) over the course of 2 years failed to prevent progression of CAN and had no benefit on myocardial perfusion as demonstrated with scintigraphic imaging modalities[199]. Further research is required to confirm these findings and explore other potential pathogenetic therapies.
There is substantial data to support the use of certain pharmacological agents in the improvement of the left ventricular dysfunction associated with autonomic neuropathy in diabetes. In patients with heart failure, the use of bisoprolol[200] or the addition of spironolactone to enalapril, furosemide and digoxin[201], demonstrated a beneficial effect on autonomic function, as shown by HRV testing and sympatho-vagal balance respectively. The use of angiotensin-converting enzyme (ACE) inhibitors could potentially improve the parasympathetic/sympathetic balance[202] and improve prognosis in cardiac failure[203]. The addition of angiotensin receptor blockers to ACE inhibitors may be superior to monotherapy[204-206], due to the enhanced blockade on the renin-angiotensin-aldosterone axis[207]. In a small study by Didangelos et al[208], including 62 patients with type I and type II DM, the use of ACE inhibitors or ARBs, as well their combination, managed to improve both diabetic autonomic neuropathy and LV diastolic dysfunction.
Treatment of orthostatic hypotension is required in symptomatic patients with autonomic neuropathy. There are several strategies available, including lifestyle and behavioural measures as well as pharmacological options. The former include advice provided to the patients to avoid sudden changes in body posture, eat smaller and more frequent meals, avoid drugs-precipitants of postural hypotension (diuretics, tricyclic antidepressants, α-adrenoreceptor antagonists), perform physical counter-manoeuvres (leg crossing, stooping and squatting), increase fluid and salt intake, avoid physical activity that leads to straining and finally use garments over legs and abdomen[69,209].
If the above measures fail to improve symptoms, pharmacological intervention may be considered. A risk-benefit consideration should take place for each individual before starting a medication, especially weighing up the risk of developing marked supine hypertension against the benefit of preserving the erect blood pressure. Should a pharmacological agent be considered appropriate by the clinician, there are several options available[210-212].
Midodrine, a peripheral selective α1-adrenergic agonist, is considered a first line agent that acts through peripheral vasoconstriction of arterioles and veins. It remains to date the only drug approved by the food and drug administration (FDA) for the treatment of orthostatic hypotension[213,214]. However, post-market trials to prove drug’s efficacy are still ongoing and the final results on midodrine’s benefits are scheduled to be published in 2014, 18 years after the drug was given FDA approval[215].
9-α-fluorohydrocortisone, a synthetic mineralocorticoid, is another first line option that acts through sodium retention and plasma expansion[216]. In a double-blinded crossover study, 9-α-fluorohydrocortisone treated successfully the orthostatic hypotension of patients with diabetes and autonomic neuropathy[216]. 9-α-fluorohydrocortisone doses between 100 and 400 micrograms decreased significantly the orthostatic hypotension in 14 symptomatic patients with DM over a mean period of 12 mo (P < 0.001)[217]. Extra care should be taken when prescribed in patients with cardiac failure, as it can lead to fluid overload. There is usually a period of 10-14 d before its effects can become clinically evident[212].
Somatostatin and somatostatin analogues (octreotide) inhibit the release of vasoactive peptides from the GI tract and thus increase splanchnic vasoconstriction, leading to increase in mean blood pressure[218]. The use of long acting octreotide in patients with autonomic neuropathy increased the mean systolic BP from 83.8 ± 7.1 mmHg to 104.1 ± 3.1 mmHg (P < 0.025) within eight weeks, improving orthostatic dizziness and fatigue[219]. In a study of 18 patients with idiopathic orthostatic hypotension, octreotide reduced postural, postprandial and exertion-induced hypotension, as demonstrated by 24-h ambulatory blood pressure profiles and cusum analyses[220].
Other available pharmacological strategies include the use of erythropoietin which can increase the erect BP through the increase of red cell mass and circulating volume, the improvement of anaemia and its regulatory effect on vascular tone[221] and desmopressin acetate whose efficacy is mainly observed in morning time hypotension[212]. Finally, caffeine and acarbose can potentially be used in the management of post-prandial hypotension[212]. In a case report of 58 years old patient with DM and severe postprandial hypotension refractory to the use of midodrine and octreotide, acarbose (an alpha-glucosidase inhibitor) reduced the postural drop from 50 mmHg to 18 mmHg, improving the patients symptoms dramatically[222].
Unfortunately, despite the different options available, postural hypotension remains a difficult condition to treat and many patients require multiple therapies and develop severe intractable disabling symptoms. Beta blockers might help controlling the tachycardia in some patients[69].
CAN is very common and is an underdiagnosed complication of DM. CAN is associated with significant increase in morbidity and mortality and plays an important role in the development of diabetic cardiomyopathy and silent ischaemia. Clinicians interpreting exercise tolerance testing should be aware of the reduced accuracy of this test in patients with CAN. In addition, CAN might play a role in the pathogenesis of diabetes-related microvascular complications and the development of lower limb complications. However, before CAN is symptomatic and evident clinically, patients might have sub-clinical CAN for several years. The time scale for the progression from sub-clinical to clinically evident CAN is unknown. In addition, the time scale for the progression from early abnormalities (such as increased LV torsion) to clinically detectable cardiac disease is also unknown. Recent guidelines have recommended screening for CAN in patients with diabetes and issued guidance regarding the criteria used to diagnose CAN. CAN is assessed using several methods including CARTs, HRV, and imaging amongst others. The use of HRV and spectral analysis has simplified CAN testing which nonetheless remains time consuming. Despite our improved understanding of the pathogenesis of CAN, disease modifying treatment is lacking. Improving glycaemic control, life style changes and CVD risk factors management are the mainstay of treatment, which generally slow the progression of CAN rather than reversing it.
Further research exploring the natural history of CAN and the natural history of the impact of CAN on CVD is needed. Better understanding of CAN pathogenesis is also required in order to develop disease modifying treatments. OSA is increasingly recognised as an important contributor to the development of microvascular complications in DM, hence it is important to clarify the relationship between CAN and OSA as this might identify new treatment targets.
| 1. | Federation ID. IDF Diabetes Atlas 2012 Update. 2012; Available from: http: //www.idf.org/diabetesatlas/5e/Update2012. |
| 2. | Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2337] [Cited by in RCA: 2181] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 3. | Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1310] [Cited by in RCA: 1308] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 4. | Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2728] [Cited by in RCA: 2633] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 5. | Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3042] [Cited by in RCA: 2892] [Article Influence: 125.7] [Reference Citation Analysis (0)] |
| 6. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 12808] [Article Influence: 457.4] [Reference Citation Analysis (0)] |
| 7. | Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4930] [Cited by in RCA: 4304] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 8. | Maser RE, Pfeifer MA, Dorman JS, Kuller LH, Becker DJ, Orchard TJ. Diabetic autonomic neuropathy and cardiovascular risk. Pittsburgh Epidemiology of Diabetes Complications Study III. Arch Intern Med. 1990;150:1218-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Maser RE, Lenhard JM, DeCherney SG. Cardiovascular Autonomic Neuropathy: The Clinical Significance of Its Determination. Endocrinologist. 2000;10:27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P; on behalf of the Toronto Consensus Panel on Diabetic Neuropathy*. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;Jun 22; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 634] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 11. | Kennedy WR, Navarro X, Sutherland DE. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology. 1995;45:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Pfeifer MA, Weinberg CR, Cook DL, Reenan A, Halter JB, Ensinck JW, Porte D. Autonomic neural dysfunction in recently diagnosed diabetic subjects. Diabetes Care. 1984;7:447-453. [PubMed] |
| 13. | Verrotti A, Chiarelli F, Blasetti A, Morgese G. Autonomic neuropathy in diabetic children. J Paediatr Child Health. 1995;31:545-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Ziegler D. Diabetic cardiovascular autonomic neuropathy: prognosis, diagnosis and treatment. Diabetes Metab Rev. 1994;10:339-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 172] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Javorka K, Javorková J, Petrásková M, Tonhajzerová I, Buchanec J, Chromá O. Heart rate variability and cardiovascular tests in young patients with diabetes mellitus type 1. J Pediatr Endocrinol Metab. 1999;12:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care. 2010;33:434-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 17. | Vinik AI, Freeman R, Erbas T. Diabetic autonomic neuropathy. Semin Neurol. 2003;23:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, Sommer C, Cleary PA, Lachin JM, Herman WH. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation. 2009;119:2886-2893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 19. | The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia. 1998;41:416-423. [PubMed] |
| 20. | Karavanaki K, Baum JD. Prevalence of microvascular and neurologic abnormalities in a population of diabetic children. J Pediatr Endocrinol Metab. 1999;12:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Young RJ, Ewing DJ, Clarke BF. Nerve function and metabolic control in teenage diabetics. Diabetes. 1983;32:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956-962. [PubMed] |
| 23. | Low PA, Benrud-Larson LM, Sletten DM, Opfer-Gehrking TL, Weigand SD, O’Brien PC, Suarez GA, Dyck PJ. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care. 2004;27:2942-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Witte DR, Tesfaye S, Chaturvedi N, Eaton SE, Kempler P, Fuller JH. Risk factors for cardiac autonomic neuropathy in type 1 diabetes mellitus. Diabetologia. 2005;48:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 25. | Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2459] [Cited by in RCA: 2384] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 26. | Ko SH, Park SA, Cho JH, Song KH, Yoon KH, Cha BY, Son HY, Yoo KD, Moon KW, Park YM. Progression of cardiovascular autonomic dysfunction in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care. 2008;31:1832-1836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Valensi P, Pariès J, Attali JR. Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications--the French multicenter study. Metabolism. 2003;52:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 160] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Kempler P, Tesfaye S, Chaturvedi N, Stevens LK, Webb DJ, Eaton S, Kerényi Z, Tamás G, Ward JD, Fuller JH. Autonomic neuropathy is associated with increased cardiovascular risk factors: the EURODIAB IDDM Complications Study. Diabet Med. 2002;19:900-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:1578-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 384] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 30. | Abbott CA, Chaturvedi N, Malik RA, Salgami E, Yates AP, Pemberton PW, Boulton AJ. Explanations for the lower rates of diabetic neuropathy in Indian Asians versus Europeans. Diabetes Care. 2010;33:1325-1330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120:1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 514] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 32. | Leinninger GM, Edwards JL, Lipshaw MJ, Feldman EL. Mechanisms of disease: mitochondria as new therapeutic targets in diabetic neuropathy. Nat Clin Pract Neurol. 2006;2:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Soriano FG, Virág L, Szabó C. Diabetic endothelial dysfunction: role of reactive oxygen and nitrogen species production and poly(ADP-ribose) polymerase activation. J Mol Med (Berl). 2001;79:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Obrosova IG, Julius UA. Role for poly(ADP-ribose) polymerase activation in diabetic nephropathy, neuropathy and retinopathy. Curr Vasc Pharmacol. 2005;3:267-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Yamagishi S, Uehara K, Otsuki S, Yagihashi S. Differential influence of increased polyol pathway on protein kinase C expressions between endoneurial and epineurial tissues in diabetic mice. J Neurochem. 2003;87:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3956] [Cited by in RCA: 3769] [Article Influence: 235.6] [Reference Citation Analysis (11)] |
| 37. | Williams B, Gallacher B, Patel H, Orme C. Glucose-induced protein kinase C activation regulates vascular permeability factor mRNA expression and peptide production by human vascular smooth muscle cells in vitro. Diabetes. 1997;46:1497-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Russell JW, Sullivan KA, Windebank AJ, Herrmann DN, Feldman EL. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis. 1999;6:347-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 314] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 39. | Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R-28R. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 609] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 40. | Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J Clin Invest. 1998;101:160-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 295] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 41. | Wada R, Yagihashi S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann N Y Acad Sci. 2005;1043:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 42. | Sayeski PP, Kudlow JE. Glucose metabolism to glucosamine is necessary for glucose stimulation of transforming growth factor-alpha gene transcription. J Biol Chem. 1996;271:15237-15243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Rabinowe SL, Brown FM, Watts M, Smith AM. Complement-fixing antibodies to sympathetic and parasympathetic tissues in IDDM. Autonomic brake index and heart-rate variation. Diabetes Care. 1990;13:1084-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Ejskjaer N, Arif S, Dodds W, Zanone MM, Vergani D, Watkins PJ, Peakman M. Prevalence of autoantibodies to autonomic nervous tissue structures in Type 1 diabetes mellitus. Diabet Med. 1999;16:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Vinik AI, Leichter SB, Pittenger GL, Stansberry KB, Holland MT, Powers AC, Suwanwalaikorn S. Phospholipid and glutamic acid decarboxylase autoantibodies in diabetic neuropathy. Diabetes Care. 1995;18:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Granberg V, Ejskjaer N, Peakman M, Sundkvist G. Autoantibodies to autonomic nerves associated with cardiac and peripheral autonomic neuropathy. Diabetes Care. 2005;28:1959-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Stroud CR, Heller SR, Ward JD, Hardisty CA, Weetman AP. Analysis of antibodies against components of the autonomic nervous system in diabetes mellitus. QJM. 1997;90:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Skärstrand H, Dahlin LB, Lernmark A, Vaziri-Sani F. Neuropeptide Y autoantibodies in patients with long-term type 1 and type 2 diabetes and neuropathy. J Diabetes Complications. 2013;27:609-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Panero F, Novelli G, Zucco C, Fornengo P, Perotto M, Segre O, Grassi G, Cavallo-Perin P, Bruno G. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care. 2009;32:301-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 552] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 51. | Cotter MA, Ekberg K, Wahren J, Cameron NE. Effects of proinsulin C-peptide in experimental diabetic neuropathy: vascular actions and modulation by nitric oxide synthase inhibition. Diabetes. 2003;52:1812-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Luppi P, Cifarelli V, Tse H, Piganelli J, Trucco M. Human C-peptide antagonises high glucose-induced endothelial dysfunction through the nuclear factor-kappaB pathway. Diabetologia. 2008;51:1534-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Johansson BL, Borg K, Fernqvist-Forbes E, Kernell A, Odergren T, Wahren J. Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with Type 1 diabetes mellitus. Diabet Med. 2000;17:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 181] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Ciccacci C, Di Fusco D, Cacciotti L, Morganti R, D’Amato C, Novelli G, Sangiuolo F, Spallone V, Borgiani P. TCF7L2 gene polymorphisms and type 2 diabetes: association with diabetic retinopathy and cardiovascular autonomic neuropathy. Acta Diabetol. 2013;50:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Yasuda K, Matsunaga T, Moritani T, Nishikino M, Gu N, Yoshinaga M, Nagasumi K, Yamamura T, Aoki N, Tsuda K. T393C polymorphism of GNAS1 associated with the autonomic nervous system in young, healthy Japanese subjects. Clin Exp Pharmacol Physiol. 2004;31:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Osztovits J, Horváth T, Littvay L, Steinbach R, Jermendy A, Tárnoki A, Tárnoki D, Métneki J, Kollai M, Jermendy G. Effects of genetic vs. environmental factors on cardiovascular autonomic function: a twin study. Diabet Med. 2011;28:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 277] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 58. | Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 947] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 59. | Tahrani AA, Ali A, Raymond NT, Begum S, Dubb K, Mughal S, Jose B, Piya MK, Barnett AH, Stevens MJ. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 60. | Pop-Busui R, Kirkwood I, Schmid H, Marinescu V, Schroeder J, Larkin D, Yamada E, Raffel DM, Stevens MJ. Sympathetic dysfunction in type 1 diabetes: association with impaired myocardial blood flow reserve and diastolic dysfunction. J Am Coll Cardiol. 2004;44:2368-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 61. | Taskiran M, Rasmussen V, Rasmussen B, Fritz-Hansen T, Larsson HB, Jensen GB, Hilsted J. Left ventricular dysfunction in normotensive Type 1 diabetic patients: the impact of autonomic neuropathy. Diabet Med. 2004;21:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation. 1994;90:1826-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 742] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 63. | Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol. 2012;8:405-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 64. | Frattola A, Parati G, Gamba P, Paleari F, Mauri G, Di Rienzo M, Castiglioni P, Mancia G. Time and frequency domain estimates of spontaneous baroreflex sensitivity provide early detection of autonomic dysfunction in diabetes mellitus. Diabetologia. 1997;40:1470-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 164] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 65. | Weston PJ, James MA, Panerai RB, McNally PG, Potter JF, Thurston H. Evidence of defective cardiovascular regulation in insulin-dependent diabetic patients without clinical autonomic dysfunction. Diabetes Res Clin Pract. 1998;42:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Stevens MJ, Dayanikli F, Raffel DM, Allman KC, Sandford T, Feldman EL, Wieland DM, Corbett J, Schwaiger M. Scintigraphic assessment of regionalized defects in myocardial sympathetic innervation and blood flow regulation in diabetic patients with autonomic neuropathy. J Am Coll Cardiol. 1998;31:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Stevens MJ, Raffel DM, Allman KC, Dayanikli F, Ficaro E, Sandford T, Wieland DM, Pfeifer MA, Schwaiger M. Cardiac sympathetic dysinnervation in diabetes: implications for enhanced cardiovascular risk. Circulation. 1998;98:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 139] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Pop-Busui R. What do we know and we do not know about cardiovascular autonomic neuropathy in diabetes. J Cardiovasc Transl Res. 2012;5:463-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (2)] |
| 69. | Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 832] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 70. | Hage FG, Wackers FJ, Bansal S, Chyun DA, Young LH, Inzucchi SE, Iskandrian AE. The heart rate response to adenosine: a simple predictor of adverse cardiac outcomes in asymptomatic patients with type 2 diabetes. Int J Cardiol. 2013;167:2952-2957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Nauman J, Janszky I, Vatten LJ, Wisløff U. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA. 2011;306:2579-2587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 72. | Albers AR, Krichavsky MZ, Balady GJ. Stress testing in patients with diabetes mellitus: diagnostic and prognostic value. Circulation. 2006;113:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J. 2010;31:85-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 74. | Ambepityia G, Kopelman PG, Ingram D, Swash M, Mills PG, Timmis AD. Exertional myocardial ischemia in diabetes: a quantitative analysis of anginal perceptual threshold and the influence of autonomic function. J Am Coll Cardiol. 1990;15:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 85] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 75. | Valensi P, Sachs RN, Harfouche B, Lormeau B, Paries J, Cosson E, Paycha F, Leutenegger M, Attali JR. Predictive value of cardiac autonomic neuropathy in diabetic patients with or without silent myocardial ischemia. Diabetes Care. 2001;24:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 76. | Chico A, Tomás A, Novials A. Silent myocardial ischemia is associated with autonomic neuropathy and other cardiovascular risk factors in type 1 and type 2 diabetic subjects, especially in those with microalbuminuria. Endocrine. 2005;27:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Wackers FJ, Young LH, Inzucchi SE, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Wittlin SD, Heller GV, Filipchuk N. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 548] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 78. | Airaksinen KE, Ikäheimo MJ, Linnaluoto MK, Niemelä M, Takkunen JT. Impaired vagal heart rate control in coronary artery disease. Br Heart J. 1987;58:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Airaksinen KE, Koistinen MJ. Association between silent coronary artery disease, diabetes, and autonomic neuropathy. Fact of fallacy. Diabetes Care. 1992;15:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (Lond). 2004;107:539-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 81. | Sakamoto K, Yamasaki Y, Nanto S, Shimonagata T, Morozumi T, Ohara T, Takano Y, Nakayama H, Kamado K, Nagata S. Mechanism of impaired left ventricular wall motion in the diabetic heart without coronary artery disease. Diabetes Care. 1998;21:2123-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Didangelos TP, Arsos GA, Karamitsos DT, Athyros VG, Karatzas ND. Left ventricular systolic and diastolic function in normotensive type 1 diabetic patients with or without autonomic neuropathy: a radionuclide ventriculography study. Diabetes Care. 2003;26:1955-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Vanninen E, Mustonen J, Vainio P, Länsimies E, Uusitupa M. Left ventricular function and dimensions in newly diagnosed non-insulin-dependent diabetes mellitus. Am J Cardiol. 1992;70:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Ansari M, Alexander M, Tutar A, Massie BM. Incident cases of heart failure in a community cohort: importance and outcomes of patients with preserved systolic function. Am Heart J. 2003;146:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Lenzen MJ, Scholte op Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, Cleland J, Komajda M. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J. 2004;25:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 260] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 86. | Chung J, Abraszewski P, Yu X, Liu W, Krainik AJ, Ashford M, Caruthers SD, McGill JB, Wickline SA. Paradoxical increase in ventricular torsion and systolic torsion rate in type I diabetic patients under tight glycemic control. J Am Coll Cardiol. 2006;47:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 87. | Dong SJ, Hees PS, Siu CO, Weiss JL, Shapiro EP. MRI assessment of LV relaxation by untwisting rate: a new isovolumic phase measure of tau. Am J Physiol Heart Circ Physiol. 2001;281:H2002-H2009. [PubMed] |
| 88. | Piya MK, Shivu GN, Tahrani A, Dubb K, Abozguia K, Phan TT, Narendran P, Pop-Busui R, Frenneaux M, Stevens MJ. Abnormal left ventricular torsion and cardiac autonomic dysfunction in subjects with type 1 diabetes mellitus. Metabolism. 2011;60:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Shivu GN, Abozguia K, Phan TT, Ahmed I, Weaver R, Narendran P, Stevens M, Frenneaux M. Increased left ventricular torsion in uncomplicated type 1 diabetic patients: the role of coronary microvascular function. Diabetes Care. 2009;32:1710-1712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Shivu GN, Phan TT, Abozguia K, Ahmed I, Wagenmakers A, Henning A, Narendran P, Stevens M, Frenneaux M. Relationship between coronary microvascular dysfunction and cardiac energetics impairment in type 1 diabetes mellitus. Circulation. 2010;121:1209-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Axelrod S, Lishner M, Oz O, Bernheim J, Ravid M. Spectral analysis of fluctuations in heart rate: an objective evaluation of autonomic nervous control in chronic renal failure. Nephron. 1987;45:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Felten SY, Peterson RG, Shea PA, Besch HR, Felten DL. Effects of streptozotocin diabetes on the noradrenergic innervation of the rat heart: a longitudinal histofluorescence and neurochemical study. Brain Res Bull. 1982;8:593-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 93. | Poulsen PL, Hansen KW, Mogensen CE. Increase in nocturnal blood pressure and progression to microalbuminuria in diabetes. N Engl J Med. 2003;348:260-24; author reply 260-24;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Hirabara SM, Silveira LR, Alberici LC, Leandro CV, Lambertucci RH, Polimeno GC, Cury Boaventura MF, Procopio J, Vercesi AE, Curi R. Acute effect of fatty acids on metabolism and mitochondrial coupling in skeletal muscle. Biochim Biophys Acta. 2006;1757:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Schrauwen P, Hoeks J, Hesselink MK. Putative function and physiological relevance of the mitochondrial uncoupling protein-3: involvement in fatty acid metabolism. Prog Lipid Res. 2006;45:17-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Collins-Nakai RL, Noseworthy D, Lopaschuk GD. Epinephrine increases ATP production in hearts by preferentially increasing glucose metabolism. Am J Physiol. 1994;267:H1862-H1871. [PubMed] |
| 97. | Francis GS. Diabetic cardiomyopathy: fact or fiction. Heart. 2001;85:247-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 607] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 99. | Katz AM. Potential deleterious effects of inotropic agents in the therapy of chronic heart failure. Circulation. 1986;73:III184-III190. [PubMed] |
| 100. | Eichhorn EJ, Bristow MR. Medical therapy can improve the biological properties of the chronically failing heart. A new era in the treatment of heart failure. Circulation. 1996;94:2285-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 314] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 101. | Yaplito-Lee J, Weintraub R, Jamsen K, Chow CW, Thorburn DR, Boneh A. Cardiac manifestations in oxidative phosphorylation disorders of childhood. J Pediatr. 2007;150:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Givertz MM, Sawyer DB, Colucci WS. Antioxidants and myocardial contractility: illuminating the “Dark Side” of beta-adrenergic receptor activation. Circulation. 2001;103:782-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Scacco S, Vergari R, Scarpulla RC, Technikova-Dobrova Z, Sardanelli A, Lambo R, Lorusso V, Papa S. cAMP-dependent phosphorylation of the nuclear encoded 18-kDa (IP) subunit of respiratory complex I and activation of the complex in serum-starved mouse fibroblast cultures. J Biol Chem. 2000;275:17578-17582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2006;291:H1489-H1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 345] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 105. | Iwai-Kanai E, Hasegawa K, Araki M, Kakita T, Morimoto T, Sasayama S. alpha- and beta-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation. 1999;100:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 137] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 106. | Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 466] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 107. | Mandinov L, Eberli FR, Seiler C, Hess OM. Diastolic heart failure. Cardiovasc Res. 2000;45:813-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 228] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 108. | Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clin Exp Hypertens. 2001;23:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 109. | Pop-Busui R, Cleary PA, Braffett BH, Martin CL, Herman WH, Low PA, Lima JA, Bluemke DA. Association between cardiovascular autonomic neuropathy and left ventricular dysfunction: DCCT/EDIC study (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications). J Am Coll Cardiol. 2013;61:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 110. | Navarro X, Kennedy WR, Sutherland DE. Autonomic neuropathy and survival in diabetes mellitus: effects of pancreas transplantation. Diabetologia. 1991;34 Suppl 1:S108-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 111. | O’Brien IA, McFadden JP, Corrall RJ. The influence of autonomic neuropathy on mortality in insulin-dependent diabetes. Q J Med. 1991;79:495-502. [PubMed] |
| 112. | Rathmann W, Ziegler D, Jahnke M, Haastert B, Gries FA. Mortality in diabetic patients with cardiovascular autonomic neuropathy. Diabet Med. 1993;10:820-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 182] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 113. | Sampson MJ, Wilson S, Karagiannis P, Edmonds M, Watkins PJ. Progression of diabetic autonomic neuropathy over a decade in insulin-dependent diabetics. Q J Med. 1990;75:635-646. [PubMed] |
| 114. | Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26:1895-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 476] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 115. | Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care. 2008;31:1360-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 116. | Miettinen H, Lehto S, Salomaa V, Mähönen M, Niemelä M, Haffner SM, Pyörälä K, Tuomilehto J. Impact of diabetes on mortality after the first myocardial infarction. The FINMONICA Myocardial Infarction Register Study Group. Diabetes Care. 1998;21:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 429] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 117. | Katz A, Liberty IF, Porath A, Ovsyshcher I, Prystowsky EN. A simple bedside test of 1-minute heart rate variability during deep breathing as a prognostic index after myocardial infarction. Am Heart J. 1999;138:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med. 1980;49:95-108. [PubMed] |
| 119. | Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2735] [Cited by in RCA: 2610] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 120. | Pappachan JM, Sebastian J, Bino BC, Jayaprakash K, Vijayakumar K, Sujathan P, Adinegara LA. Cardiac autonomic neuropathy in diabetes mellitus: prevalence, risk factors and utility of corrected QT interval in the ECG for its diagnosis. Postgrad Med J. 2008;84:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 121. | Sivieri R, Veglio M, Chinaglia A, Scaglione P, Cavallo-Perin P. Prevalence of QT prolongation in a type 1 diabetic population and its association with autonomic neuropathy. The Neuropathy Study Group of the Italian Society for the Study of Diabetes. Diabet Med. 1993;10:920-924. [PubMed] |
| 122. | Veglio M, Borra M, Stevens LK, Fuller JH, Perin PC. The relation between QTc interval prolongation and diabetic complications. The EURODIAB IDDM Complication Study Group. Diabetologia. 1999;42:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 123. | Suarez GA, Clark VM, Norell JE, Kottke TE, Callahan MJ, O’Brien PC, Low PA, Dyck PJ. Sudden cardiac death in diabetes mellitus: risk factors in the Rochester diabetic neuropathy study. J Neurol Neurosurg Psychiatry. 2005;76:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 124. | Page MM, Watkins PJ. Cardiorespiratory arrest and diabetic autonomic neuropathy. Lancet. 1978;1:14-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 189] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 125. | Clarke BF, Ewing DJ, Campbell IW. Diabetic autonomic neuropathy. Diabetologia. 1979;17:195-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 231] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 126. | Furlan R, Guzzetti S, Crivellaro W, Dassi S, Tinelli M, Baselli G, Cerutti S, Lombardi F, Pagani M, Malliani A. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81:537-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 443] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 127. | Spallone V, Bernardi L, Ricordi L, Soldà P, Maiello MR, Calciati A, Gambardella S, Fratino P, Menzinger G. Relationship between the circadian rhythms of blood pressure and sympathovagal balance in diabetic autonomic neuropathy. Diabetes. 1993;42:1745-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 128. | Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation. 1992;85:I77-I91. [PubMed] |
| 129. | Latson TW, Ashmore TH, Reinhart DJ, Klein KW, Giesecke AH. Autonomic reflex dysfunction in patients presenting for elective surgery is associated with hypotension after anesthesia induction. Anesthesiology. 1994;80:326-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Burgos LG, Ebert TJ, Asiddao C, Turner LA, Pattison CZ, Wang-Cheng R, Kampine JP. Increased intraoperative cardiovascular morbidity in diabetics with autonomic neuropathy. Anesthesiology. 1989;70:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 131. | Kitamura A, Hoshino T, Kon T, Ogawa R. Patients with diabetic neuropathy are at risk of a greater intraoperative reduction in core temperature. Anesthesiology. 2000;92:1311-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 132. | Töyry JP, Niskanen LK, Länsimies EA, Partanen KP, Uusitupa MI. Autonomic neuropathy predicts the development of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke. 1996;27:1316-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 133. | Cohen JA, Estacio RO, Lundgren RA, Esler AL, Schrier RW. Diabetic autonomic neuropathy is associated with an increased incidence of strokes. Auton Neurosci. 2003;108:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 134. | Ko SH, Song KH, Park SA, Kim SR, Cha BY, Son HY, Moon KW, Yoo KD, Park YM, Cho JH. Cardiovascular autonomic dysfunction predicts acute ischaemic stroke in patients with Type 2 diabetes mellitus: a 7-year follow-up study. Diabet Med. 2008;25:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 135. | Maser RE, Lenhard MJ. Cardiovascular autonomic neuropathy due to diabetes mellitus: clinical manifestations, consequences, and treatment. J Clin Endocrinol Metab. 2005;90:5896-5903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 136. | Joles JA, Koomans HA. Causes and consequences of increased sympathetic activity in renal disease. Hypertension. 2004;43:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 137. | Spallone V, Gambardella S, Maiello MR, Barini A, Frontoni S, Menzinger G. Relationship between autonomic neuropathy, 24-h blood pressure profile, and nephropathy in normotensive IDDM patients. Diabetes Care. 1994;17:578-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 138. | Sundkvist G, Lilja B. Autonomic neuropathy predicts deterioration in glomerular filtration rate in patients with IDDM. Diabetes Care. 1993;16:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 139. | Salman IM, Ameer OZ, Sattar MA, Abdullah NA, Yam MF, Abdullah GZ, Abdulkarim MF, Khan MA, Johns EJ. Renal sympathetic nervous system hyperactivity in early streptozotocin-induced diabetic kidney disease. Neurourol Urodyn. 2011;30:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 140. | Luippold G, Beilharz M, Mühlbauer B. Chronic renal denervation prevents glomerular hyperfiltration in diabetic rats. Nephrol Dial Transplant. 2004;19:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 141. | Moran A, Palmas W, Field L, Bhattarai J, Schwartz JE, Weinstock RS, Shea S. Cardiovascular autonomic neuropathy is associated with microalbuminuria in older patients with type 2 diabetes. Diabetes Care. 2004;27:972-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 142. | Sterner NG, Nilsson H, Rosén U, Lilja B, Sundkvist G. Relationships among glomerular filtration rate, albuminuria, and autonomic nerve function in insulin-dependent and non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1997;11:188-193. [PubMed] |
| 143. | Smulders YM, Jager A, Gerritsen J, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Cardiovascular autonomic function is associated with (micro-)albuminuria in elderly Caucasian sujects with impaired glucose tolerance or type 2 diabetes: the Hoorn Study. Diabetes Care. 2000;23:1369-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 144. | Lafferty AR, Werther GA, Clarke CF. Ambulatory blood pressure, microalbuminuria, and autonomic neuropathy in adolescents with type 1 diabetes. Diabetes Care. 2000;23:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 145. | Poulsen PL, Ebbehøj E, Hansen KW, Mogensen CE. 24-h blood pressure and autonomic function is related to albumin excretion within the normoalbuminuric range in IDDM patients. Diabetologia. 1997;40:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 146. | Forsén A, Kangro M, Sterner G, Norrgren K, Thorsson O, Wollmer P, Sundkvist G. A 14-year prospective study of autonomic nerve function in Type 1 diabetic patients: association with nephropathy. Diabet Med. 2004;21:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 147. | Kempler P, Amarenco G, Freeman R, Frontoni S, Horowitz M, Stevens M, Low P, Pop-Busui R, Tahrani A, Tesfaye S, Várkonyi T, Ziegler D, Valensi P; on behalf of the Toronto Consensus Panel on Diabetic Neuropathy*. Gastrointestinal autonomic neuropathy, erectile-, bladder- and sudomotor dysfunction in patients with diabetes mellitus: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;Jul 11; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 148. | Jirkovská A, Boucek P, Wu S, Hosová J, Bém R, Fejfarova V, Skibová J. Power spectral analysis of heart rate variability in patients with Charcot’s neuroarthropathy. J Am Podiatr Med Assoc. 2006;96:1-8. [PubMed] |
| 149. | Stevens MJ, Edmonds ME, Foster AV, Watkins PJ. Selective neuropathy and preserved vascular responses in the diabetic Charcot foot. Diabetologia. 1992;35:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 150. | Ewing DJ, Campbell IW, Clarke BF. Assessment of cardiovascular effects in diabetic autonomic neuropathy and prognostic implications. Ann Intern Med. 1980;92:308-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 306] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 151. | Pfeifer MA, Cook D, Brodsky J, Tice D, Reenan A, Swedine S, Halter JB, Porte D. Quantitative evaluation of cardiac parasympathetic activity in normal and diabetic man. Diabetes. 1982;31:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 152. | Ewing DJ, Campbell IW, Murray A, Neilson JM, Clarke BF. Immediate heart-rate response to standing: simple test for autonomic neuropathy in diabetes. Br Med J. 1978;1:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 273] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 153. | Sandroni P, Benarroch EE, Low PA. Pharmacological dissection of components of the Valsalva maneuver in adrenergic failure. J Appl Physiol (1985). 1991;71:1563-1567. [PubMed] |
| 154. | England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, Asbury AK, Szigeti K, Lupski JR, Latov N. Evaluation of distal symmetric polyneuropathy: the role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Muscle Nerve. 2009;39:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 155. | Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O’Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20:1561-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 156. | Proceedings of a consensus development conference on standardized measures in diabetic neuropathy. Summary and recommendations. Diabetes Care. 1992;15:1104-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 157. | Niskanen JP, Tarvainen MP, Ranta-Aho PO, Karjalainen PA. Software for advanced HRV analysis. Comput Methods Programs Biomed. 2004;76:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 466] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 158. | Bernardi L, Spallone V, Stevens M, Hilsted J, Frontoni S, Pop-Busui R, Ziegler D, Kempler P, Freeman R, Low P, Tesfaye S, Valensi P; on behalf of the Toronto Consensus Panel on Diabetic Neuropathy*. Investigation methods for cardiac autonomic function in human research studies. Diabetes Metab Res Rev. 2011;Jun 21; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 159. | Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9482] [Cited by in RCA: 9268] [Article Influence: 308.9] [Reference Citation Analysis (0)] |
| 160. | Appel ML, Berger RD, Saul JP, Smith JM, Cohen RJ. Beat to beat variability in cardiovascular variables: noise or music. J Am Coll Cardiol. 1989;14:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 342] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 161. | Assessment: Clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996;46:873-880. [PubMed] |
| 162. | La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2192] [Cited by in RCA: 2212] [Article Influence: 79.0] [Reference Citation Analysis (4)] |
| 163. | Gasch J, Reimann M, Reichmann H, Rüdiger H, Ziemssen T. Determination of baroreflex sensitivity during the modified Oxford maneuver by trigonometric regressive spectral analysis. PLoS One. 2011;6:e18061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 164. | Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, Heethaar RM, Stehouwer CD. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care. 2001;24:1793-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 319] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 165. | La Rovere MT, Pinna GD, Maestri R, Robbi E, Caporotondi A, Guazzotti G, Sleight P, Febo O. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. J Am Coll Cardiol. 2009;53:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 166. | Raffel DM, Wieland DM. Assessment of cardiac sympathetic nerve integrity with positron emission tomography. Nucl Med Biol. 2001;28:541-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 167. | Stevens MJ, Raffel DM, Allman KC, Schwaiger M, Wieland DM. Regression and progression of cardiac sympathetic dysinnervation complicating diabetes: an assessment by C-11 hydroxyephedrine and positron emission tomography. Metabolism. 1999;48:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 168. | Bombardieri E, Giammarile F, Aktolun C, Baum RP, Bischof Delaloye A, Maffioli L, Moncayo R, Mortelmans L, Pepe G, Reske SN. 131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2010;37:2436-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 169. | Franzius C, Hermann K, Weckesser M, Kopka K, Juergens KU, Vormoor J, Schober O. Whole-body PET/CT with 11C-meta-hydroxyephedrine in tumors of the sympathetic nervous system: feasibility study and comparison with 123I-MIBG SPECT/CT. J Nucl Med. 2006;47:1635-1642. [PubMed] |
| 170. | Shulkin BL, Wieland DM, Schwaiger M, Thompson NW, Francis IR, Haka MS, Rosenspire KC, Shapiro B, Sisson JC, Kuhl DE. PET scanning with hydroxyephedrine: an approach to the localization of pheochromocytoma. J Nucl Med. 1992;33:1125-1131. [PubMed] |
| 171. | Hamner JW, Taylor JA. Automated quantification of sympathetic beat-by-beat activity, independent of signal quality. J Appl Physiol (1985). 2001;91:1199-1206. [PubMed] |
| 172. | Scheen AJ, Marchand M, Philips JC. [Relationships between baroreflex gain and pulsatile stress in type 1 diabetic patients]. Ann Cardiol Angeiol (Paris). 2012;61:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 173. | Liatis S, Alexiadou K, Tsiakou A, Makrilakis K, Katsilambros N, Tentolouris N. Cardiac autonomic function correlates with arterial stiffness in the early stage of type 1 diabetes. Exp Diabetes Res. 2011;2011:957901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 174. | Turner J, Belch JJ, Khan F. Current concepts in assessment of microvascular endothelial function using laser Doppler imaging and iontophoresis. Trends Cardiovasc Med. 2008;18:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 175. | Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev. 2009;25:604-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 176. | Caselli A, Rich J, Hanane T, Uccioli L, Veves A. Role of C-nociceptive fibers in the nerve axon reflex-related vasodilation in diabetes. Neurology. 2003;60:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 177. | Tahrani AA, Zeng W, Shakher J, Piya MK, Hughes S, Dubb K, Stevens MJ. Cutaneous structural and biochemical correlates of foot complications in high-risk diabetes. Diabetes Care. 2012;35:1913-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 178. | Malik R, Veves A, Tesfaye S, Smith G, Cameron N, Zochodne D, Lauria G; on behalf of the Toronto Consensus Panel on Diabetic Neuropathy*. Small Fiber Neuropathy: Role in the diagnosis of Diabetic Sensorimotor Polyneuropathy. Diabetes Metab Res Rev. 2011;Jun 22; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 179. | Eranki VG, Santosh R, Rajitha K, Pillai A, Sowmya P, Dupin J, Calvet JH. Sudomotor function assessment as a screening tool for microvascular complications in type 2 diabetes. Diabetes Res Clin Pract. 2013;101:e11-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 180. | Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1948] [Cited by in RCA: 1823] [Article Influence: 113.9] [Reference Citation Analysis (0)] |
| 181. | Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 512] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 182. | Kadoi Y. Anesthetic considerations in diabetic patients. Part I: preoperative considerations of patients with diabetes mellitus. J Anesth. 2010;24:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 183. | Carnethon MR, Prineas RJ, Temprosa M, Zhang ZM, Uwaifo G, Molitch ME. The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care. 2006;29:914-919. [PubMed] |
| 184. | Maser RE, Lenhard MJ. An overview of the effect of weight loss on cardiovascular autonomic function. Curr Diabetes Rev. 2007;3:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 185. | Voulgari C, Pagoni S, Vinik A, Poirier P. Exercise improves cardiac autonomic function in obesity and diabetes. Metabolism. 2013;62:609-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 186. | LeRoith D, Fonseca V, Vinik A. Metabolic memory in diabetes--focus on insulin. Diabetes Metab Res Rev. 2005;21:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 187. | Ceriello A, Esposito K, Ihnat M, Thorpe J, Giugliano D. Long-term glycemic control influences the long-lasting effect of hyperglycemia on endothelial function in type 1 diabetes. J Clin Endocrinol Metab. 2009;94:2751-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 188. | Jaiswal M, Urbina EM, Wadwa RP, Talton JW, D’Agostino RB, Hamman RF, Fingerlin TE, Daniels S, Marcovina SM, Dolan LM. Reduced heart rate variability among youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36:157-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 189. | Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3361] [Article Influence: 197.7] [Reference Citation Analysis (0)] |
| 190. | Azad N, Emanuele NV, Abraira C, Henderson WG, Colwell J, Levin SR, Nuttall FQ, Comstock JP, Sawin CT, Silbert C. The effects of intensive glycemic control on neuropathy in the VA cooperative study on type II diabetes mellitus (VA CSDM). J Diabetes Complications. 1999;13:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 191. | Ziegler D, Schatz H, Conrad F, Gries FA, Ulrich H, Reichel G. Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN Study). Deutsche Kardiale Autonome Neuropathie. Diabetes Care. 1997;20:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 176] [Article Influence: 6.1] [Reference Citation Analysis (3)] |
| 192. | Drel VR, Pacher P, Vareniuk I, Pavlov I, Ilnytska O, Lyzogubov VV, Tibrewala J, Groves JT, Obrosova IG. A peroxynitrite decomposition catalyst counteracts sensory neuropathy in streptozotocin-diabetic mice. Eur J Pharmacol. 2007;569:48-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 193. | Drel VR, Pacher P, Vareniuk I, Pavlov IA, Ilnytska O, Lyzogubov VV, Bell SR, Groves JT, Obrosova IG. Evaluation of the peroxynitrite decomposition catalyst Fe(III) tetra-mesitylporphyrin octasulfonate on peripheral neuropathy in a mouse model of type 1 diabetes. Int J Mol Med. 2007;20:783-792. [PubMed] |
| 194. | Arora M, Kumar A, Kaundal RK, Sharma SS. Amelioration of neurological and biochemical deficits by peroxynitrite decomposition catalysts in experimental diabetic neuropathy. Eur J Pharmacol. 2008;596:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 195. | Anagnostis P, Athyros VG, Adamidou F, Panagiotou A, Kita M, Karagiannis A, Mikhailidis DP. Glucagon-like peptide-1-based therapies and cardiovascular disease: looking beyond glycaemic control. Diabetes Obes Metab. 2011;13:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 196. | Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation. 2008;5:19. [PubMed] |
| 197. | Johnson BF, Nesto RW, Pfeifer MA, Slater WR, Vinik AI, Chyun DA, Law G, Wackers FJ, Young LH. Cardiac abnormalities in diabetic patients with neuropathy: effects of aldose reductase inhibitor administration. Diabetes Care. 2004;27:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 198. | Manzella D, Barbieri M, Ragno E, Paolisso G. Chronic administration of pharmacologic doses of vitamin E improves the cardiac autonomic nervous system in patients with type 2 diabetes. Am J Clin Nutr. 2001;73:1052-1057. [PubMed] |
| 199. | Pop-Busui R, Stevens MJ, Raffel DM, White EA, Mehta M, Plunkett CD, Brown MB, Feldman EL. Effects of triple antioxidant therapy on measures of cardiovascular autonomic neuropathy and on myocardial blood flow in type 1 diabetes: a randomised controlled trial. Diabetologia. 2013;56:1835-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 200. | Pousset F, Copie X, Lechat P, Jaillon P, Boissel JP, Hetzel M, Fillette F, Remme W, Guize L, Le Heuzey JY. Effects of bisoprolol on heart rate variability in heart failure. Am J Cardiol. 1996;77:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 201. | Korkmaz ME, Müderrisoğlu H, Uluçam M, Ozin B. Effects of spironolactone on heart rate variability and left ventricular systolic function in severe ischemic heart failure. Am J Cardiol. 2000;86:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 202. | Kontopoulos AG, Athyros VG, Didangelos TP, Papageorgiou AA, Avramidis MJ, Mayroudi MC, Karamitsos DT. Effect of chronic quinapril administration on heart rate variability in patients with diabetic autonomic neuropathy. Diabetes Care. 1997;20:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 203. | Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273:1450-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 185] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 204. | Hamroff G, Katz SD, Mancini D, Blaufarb I, Bijou R, Patel R, Jondeau G, Olivari MT, Thomas S, Le Jemtel TH. Addition of angiotensin II receptor blockade to maximal angiotensin-converting enzyme inhibition improves exercise capacity in patients with severe congestive heart failure. Circulation. 1999;99:990-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 138] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 205. | Baruch L, Anand I, Cohen IS, Ziesche S, Judd D, Cohn JN. Augmented short- and long-term hemodynamic and hormonal effects of an angiotensin receptor blocker added to angiotensin converting enzyme inhibitor therapy in patients with heart failure. Vasodilator Heart Failure Trial (V-HeFT) Study Group. Circulation. 1999;99:2658-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 129] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 206. | McKelvie RS, Yusuf S, Pericak D, Avezum A, Burns RJ, Probstfield J, Tsuyuki RT, White M, Rouleau J, Latini R. Comparison of candesartan, enalapril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. The RESOLVD Pilot Study Investigators. Circulation. 1999;100:1056-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 622] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 207. | Gainer JV, Morrow JD, Loveland A, King DJ, Brown NJ. Effect of bradykinin-receptor blockade on the response to angiotensin-converting-enzyme inhibitor in normotensive and hypertensive subjects. N Engl J Med. 1998;339:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 325] [Article Influence: 11.6] [Reference Citation Analysis (2)] |
| 208. | Didangelos TP, Arsos GA, Karamitsos DT, Athyros VG, Georga SD, Karatzas ND. Effect of quinapril or losartan alone and in combination on left ventricular systolic and diastolic functions in asymptomatic patients with diabetic autonomic neuropathy. J Diabetes Complications. 2006;20:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 209. | Freeman R. Autonomic peripheral neuropathy. Lancet. 2005;365:1259-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 210. | Lahrmann H, Cortelli P, Hilz M, Mathias CJ, Struhal W, Tassinari M. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 211. | Aronson D. Pharmacologic modulation of autonomic tone: implications for the diabetic patient. Diabetologia. 1997;40:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 212. | Freeman R. Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med. 2008;358:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 213. | Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA. 1997;277:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 278] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 214. | Jankovic J, Gilden JL, Hiner BC, Kaufmann H, Brown DC, Coghlan CH, Rubin M, Fouad-Tarazi FM. Neurogenic orthostatic hypotension: a double-blind, placebo-controlled study with midodrine. Am J Med. 1993;95:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 249] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 215. | Mitka M. Trials to address efficacy of midodrine 18 years after it gains FDA approval. JAMA. 2012;307:1124, 1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 216. | Campbell IW, Ewing DJ, Clarke BF. 9-Alpha-fluorohydrocortisone in the treatment of postural hypotension in diabetic autonomic neuropathy. Diabetes. 1975;24:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 217. | Campbell IW, Ewing DJ, Clarke BF. Therapeutic experience with fludrocortisone in diabetic postural hypotension. Br Med J. 1976;1:872-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 218. | Hoeldtke RD, O’Dorisio TM, Boden G. Treatment of autonomic neuropathy with a somatostatin analogue SMS-201-995. Lancet. 1986;2:602-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 219. | Hoeldtke RD, Bryner KD, Hoeldtke ME, Hobbs G. Treatment of autonomic neuropathy, postural tachycardia and orthostatic syncope with octreotide LAR. Clin Auton Res. 2007;17:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 220. | Alam M, Smith G, Bleasdale-Barr K, Pavitt DV, Mathias CJ. Effects of the peptide release inhibitor, octreotide, on daytime hypotension and on nocturnal hypertension in primary autonomic failure. J Hypertens. 1995;13:1664-1669. [PubMed] |
| 221. | Low PA, Singer W. Management of neurogenic orthostatic hypotension: an update. Lancet Neurol. 2008;7:451-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 222. | Sasaki E, Goda K, Nagata K, Kitaoka H, Ohsawa N, Hanafusa T. Acarbose improved severe postprandial hypotension in a patient with diabetes mellitus. J Diabetes Complications. 1995;15:158-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 223. | Ziegler D, Gries FA, Spüler M, Lessmann F. The epidemiology of diabetic neuropathy. Diabetic Cardiovascular Autonomic Neuropathy Multicenter Study Group. J Diabetes Complications. 1992;6:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 164] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 224. | Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 609] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 225. | Armstrong FM, Bradbury JE, Ellis SH, Owens DR, Rosen I, Sonksen P, Sundkvist G. A study of peripheral diabetic neuropathy. The application of age-related reference values. Diabet Med. 1991;8 Spec No:S94-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 226. | Veglio M, Sivieri R, Chinaglia A, Scaglione L, Cavallo-Perin P. QT interval prolongation and mortality in type 1 diabetic patients: a 5-year cohort prospective study. Neuropathy Study Group of the Italian Society of the Study of Diabetes, Piemonte Affiliate. Diabetes Care. 2000;23:1381-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 227. | Chen HS, Hwu CM, Kuo BI, Chiang SC, Kwok CF, Lee SH, Lee YS, Weih MJ, Hsiao LC, Lin SH. Abnormal cardiovascular reflex tests are predictors of mortality in Type 2 diabetes mellitus. Diabet Med. 2001;18:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 228. | Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract. 2002;58:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 229. | Astrup AS, Tarnow L, Rossing P, Hansen BV, Hilsted J, Parving HH. Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006;29:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 230. | Astrup AS, Nielsen FS, Rossing P, Ali S, Kastrup J, Smidt UM, Parving HH. Predictors of mortality in patients with type 2 diabetes with or without diabetic nephropathy: a follow-up study. J Hypertens. 2007;25:2479-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 231. | Lykke JA, Tarnow L, Parving HH, Hilsted J. A combined abnormality in heart rate variation and QT corrected interval is a strong predictor of cardiovascular death in type 1 diabetes. Scand J Clin Lab Invest. 2008;68:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 232. | Ziegler D, Zentai CP, Perz S, Rathmann W, Haastert B, Döring A, Meisinger C. Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care. 2008;31:556-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 233. | Beijers HJ, Ferreira I, Bravenboer B, Dekker JM, Nijpels G, Heine RJ, Stehouwer CD. Microalbuminuria and cardiovascular autonomic dysfunction are independently associated with cardiovascular mortality: evidence for distinct pathways: the Hoorn Study. Diabetes Care. 2009;32:1698-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
P- Reviewers: Mitra A, Padwal R S- Editor: Cui XM L- Editor: A E- Editor: Liu SQ