Published online Dec 15, 2013. doi: 10.4239/wjd.v4.i6.358
Revised: September 20, 2013
Accepted: October 17, 2013
Published online: December 15, 2013
Processing time: 197 Days and 0.3 Hours
AIM: To compare the use of vildagliptin and sulfonylurea with or without metformin in Indian Muslim patients with type 2 diabetes mellitus, fasting during Ramadan.
METHODS: This was a 4-wk, multicenter, non-interventional, open-label, observational study. Incidence of hypoglycemic events (HEs), adverse events, and changes in glycosylated hemoglobin A1c (HbA1c), fasting plasma glucose, postprandial plasma glucose and body weight were measured pre- and post-Ramadan.
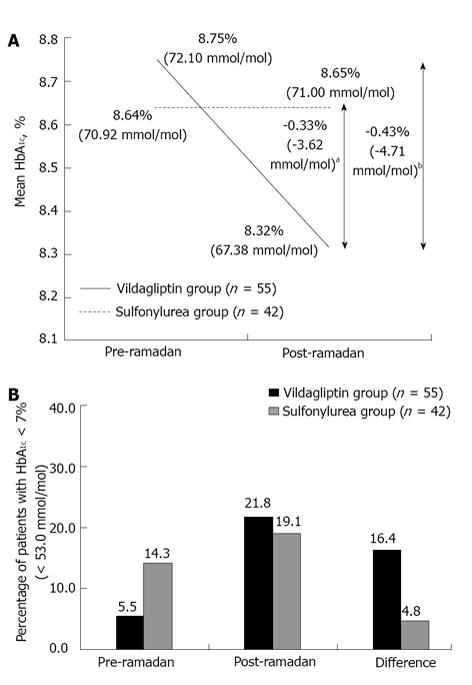
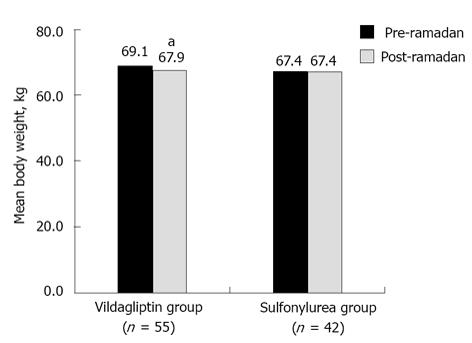
RESULTS: Totally, 97 patients were recruited and all completed the study (vildagliptin group, n = 55; sulfonylurea group, n = 42). HEs were reported in low frequencies in both the vildagliptin and the sulfonylurea groups [0 vs 2 (4.8%) patients, respectively]. Interestingly, HbA1c reduced by -0.43% (-4.71 mmol/mol) in the vildagliptin group [8.75% (72.10 mmol/mol) to 8.32% (67.38 mmol/mol), P = 0.009] while in the sulfonylurea group there was a small increase by 0.01% [0.08 mmol/mol; 8.64% (70.92 mmol/mol) to 8.65% (71.00 mmol/mol), P = 0.958]. Higher percentage of vildagliptin-treated patients achieved HbA1c < 7.0% (< 53 mmol/mol) compared with sulfonylurea (16.4% vs 4.8%). Mean decrease in the body weight was 1.2 kg and 0.03 kg, respectively (P < 0.001). Both treatment groups were well tolerated during Ramadan.
CONCLUSION: Vildagliptin is an attractive treatment option for Indian patients with type 2 diabetes mellitus who are fasting during Ramadan.
Core tip: Management of glycemic control in diabetes patients fasting during Ramadan has been recognized as a critically important health challenge worldwide. India has the world’s second largest diabetes population and caters large Muslim community; however, there is limited data available exploring the effect of treatments in these fasting diabetes patients. This non-interventional, multicenter, double-arm study compared the effect of vildagliptin with sulfonylureas on hypoglycemic events, HbA1c, blood glucose levels, and response rate in 97 fasting diabetic patients during Ramadan in real-world setting. Vildagliptin appears to be an attractive treatment option for diabetes patients fasting during Ramadan.
-
Citation: Shete A, Shaikh A, Nayeem KJ, Rodrigues L, Ali MSS, Shah P, Khanna R, Majid S, Rasheed SA, Shaikh S, Rahman T. Vildagliptin
vs sulfonylurea in Indian Muslim diabetes patients fasting during Ramadan. World J Diabetes 2013; 4(6): 358-364 - URL: https://www.wjgnet.com/1948-9358/full/v4/i6/358.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i6.358
Worldwide, about 40-50 million Muslim patients with type 2 diabetes mellitus observe fasting during the holy month of Ramadan[1]. India has the world’s largest diabetes population, with an estimated 51 million affected individuals[2]. The prevalence of diabetes is rising by 10% annually in countries with large Muslim populations, similar to the rates noted in Western countries, as an upshot of urbanization and socioeconomic development[3].
Muslims, on average, fast for 12 h per day during Ramadan, which varies from country to country and from season to season and lasts for up to 30 d[4]. Although Islamic rules exempt patients with illnesses from fasting, almost 79% of patients with type 2 diabetes mellitus prefer to fast; therefore, it is essential to make fasting as safe as possible[5]. As the fast is absolute in nature, that is, without water and food from dawn to dusk, hypoglycemia presents a serious risk[1]. Prolonged fasting in the absence of adequate insulin can cause excessive gluconeogenesis leading to hyperglycemia. There is also a risk of severe post-prandial hyperglycemia in those who tend to overeat after breaking the fast[5]. The Epidemiology of Diabetes and Ramadan study conducted in 13 Islamic countries reported that in patients with type 2 diabetes mellitus, fasting during Ramadan increased the risk of severe hypoglycemia by 7.5-fold and severe hyperglycemia by 5-fold. During Ramadan, up to 2% of fasting patients with type 2 diabetes mellitus may experience at least one episode of severe hypoglycemia requiring hospitalization[5].
Improved diabetes management during Ramadan can be achieved by appropriate treatment adjustments such as timing and dosage of the drug[5]. The American Diabetes Association emphasizes the importance of monitoring blood glucose levels and providing nutritional advice and Ramadan-focused structured education in patients with type 2 diabetes mellitus[5].
Improved glycemic control without hypoglycemia and weight gain is the key goal in the management of diabetes; however, achieving this goal is challenging in patients with diabetes, especially who are fasting. Therapies that affect the incretin system like dipeptidyl-peptidase-4 (DPP-4) inhibitors, which could maintain glycemic control in a glucose-dependent manner, might provide a safe alternative therapeutic option during Ramadan[5]. Vildagliptin, a potent and selective oral DPP-4 inhibitor, improves glycemic control in patients with type 2 diabetes mellitus by improving both α- and β-cell responsiveness to glucose[6-8]. A long-term randomized clinical study with vildagliptin demonstrated significantly lower hypoglycemic risk compared with sulfonylureas in patients with type 2 diabetes mellitus[9,10]. A real-world observational and non-interventional study, VECTOR, conducted in United Kingdom Muslim patients with type 2 diabetes mellitus fasting during Ramadan demonstrated no hypoglycemic events (HEs) and better glycemic control with vildagliptin compared with gliclazide[11]. In a similar study by Devendra et al[12] conducted in North West London, both vildagliptin and gliclazide were associated with similar reductions in mean glycosylated haemoglobin A1c (HbA1c).
Although there is data available for vildagliptin in fasting Ramadan patients, the present study was conducted to assess whether similar efficacy and safety results are also seen in Indian patients with type 2 diabetes mellitus fasting during Ramadan.
This was an 8- to 10-wk, multicenter, non-interventional, prospective, open-label, observational study conducted across 10 centers in India in 2010. Patients with type 2 diabetes mellitus, aged 18-80 years, fasting during Ramadan and taking vildagliptin with or without metformin (hereafter called the vildagliptin group) or sulfonylureas with or without metformin (hereafter called the sulfonylurea group) were included in the study. Patients were given Ramadan-focused advice about diabetes management. Dosages were given according to the approved package labels. Patients receiving insulin and/or having contraindications as described in the summary of product characteristics for vildagliptin (with or without metformin; Galvus® or GalvusMet®) (pregnancy and hypersensitivity) were not recruited in the study.
Following a screening visit during the 2- to 3-wk pre-Ramadan period, eligible patients were observed for 4 wk (29 d) during the Ramadan period, followed by a second visit within 10 d post-Ramadan.
This study was conducted and reported in accordance with the International Conference on Harmonization for Good Clinical Practice, with applicable local regulations and with the ethical principles laid down in the Declaration of Helsinki. Protocol and any amendments were reviewed by the Independent Ethics Committee. Written informed consent was obtained from each patient or by a legally acceptable representative before inclusion in the study.
The primary assessment was incidence of HEs during the study period. Patients were provided with a questionnaire for self-assessment of HEs based on symptoms, which were confirmed by blood glucose levels < 70 mg/dL either self-measured with a glucometer (Accu-Chek Active®, Roche) and recorded in patients’ diaries or measured at any laboratory. Secondary assessments included mean changes in HbA1c, body weight, fasting plasma glucose (FPG) and postprandial plasma glucose (PPG, glucose levels following breakfast) from pre-Ramadan (baseline) to post-Ramadan (endpoint) period. The proportion of patients reaching the HbA1c goal < 7.0% (< 53 mmol/mol) during the study period was also determined. Safety assessments included monitoring and recording of all adverse events (AEs), serious AEs (SAEs) and laboratory tests.
A sample size of 72 patients (minimum 36 patients in each treatment group) with type 2 diabetes mellitus was estimated to detect the difference between the two groups at 95%CI and a study power of 80%. This was based on data from previous international clinical trials, which reported that one-third of the patients in the sulfonylurea group and 5% of the patients in the vildagliptin group developed hypoglycemia during Ramadan fasting.
The null hypothesis, which was proportion of patients experiencing at least one HE was the same in each cohort, was tested using a 2-group Fisher’s exact test. The proportion of patients experiencing an AE was described in terms of their frequency and percentage for each event (the basis of percentage being the number of patients who provided data). Descriptive statistics were used to analyze quantitative variables such as change in body weight, FPG, PPG and HbA1c levels. Paired t-test was performed to assess the statistical significance of the differences between pre- and post-Ramadan periods and unpaired t test for the between-group differences at 95%CI.
A total of 97 patients (55 in the vildagliptin group and 42 in the sulfonylurea group) were enrolled and all patients (100%) completed the study within the stipulated 10 d period post Ramadan. All patients observed fasting for a period of 4 wk (29 d). The demographics and baseline characteristics are summarized in Table 1.
| Vildagliptin group(n = 55) | Sulfonylureagroup (n = 42) | |
| Age (yr) | 51.0 ± 8.8 | 50.9 ± 9.1 |
| ≥ 65 yr | 4 (7.3) | 4 (9.5) |
| Weight, kg | 69.1 ± 10.9 | 67.4 ± 10.0 |
| Height, cm | 159.7 ± 8.1 | 158.4 ± 9.8 |
| HbA1c, % (mmol/mol) | 8.75 ± 1.27 (72.10 ± 13.90) | 8.64 ± 1.57 (70.92 ± 17.14) |
| HbA1c < 7.0% (< 53.0 mmol/mol) | 3 (5.5) | 6 (14.3) |
| FPG, mg/dL | 140.2 ± 42.0 | 162.1 ± 48.2 |
| PPG, mg/dL | 212.2 ± 52.2 | 220.6 ± 54.0 |
| Patients receiving metformin, n (%) | 38 (69.1) | 30 (71.4) |
| Mean doses of sulfonylureas, mg/d | ||
| Glibenclamide (n = 7) | - | 10 |
| Gliclazide (n = 10) | - | 106 |
| Glimepiride (n = 23) | - | 4.2 |
| Glipizide (n = 2) | - | 10 |
HEs were reported in low frequencies in both the vildagliptin group and the sulfonylurea group [0 vs 2 (4.8%) patients, respectively]. In the sulfonylurea group one HE was reported by patient who was taking glipizide 5 mg twice a day and the other HE was reported by patient who was taking glibenclamide 5 mg twice a day during the study period. The difference in the proportion of patients who experienced at least one HE during the study period was not significant (P = 0.104) between the treatment groups.
The reduction in HbA1c from pre- to post-Ramadan was statistically significant in the vildagliptin group [0.43% (4.71 mmol/mol), P = 0.009], whereas there was a negligible increase in HbA1c in the sulfonylurea group [0.01% (0.08 mmol/mol), P = 0.958]. The mean between-group difference for the change in HbA1c from pre- to post-Ramadan was statistically significant (P < 0.05) in favor of the vildagliptin group (Figure 1A). In patients who had HbA1c≥ 7.0% (≥ 53 mmol/mol) at baseline, nine patients (16.4%) achieved the target HbA1c < 7.0% (< 53 mmol/mol) in the vildagliptin group compared with only 2 patients (4.8%) in the sulfonylurea group (P = 0.055) (Figure 1B).
The vildagliptin group showed a significant reduction in mean body weight (P < 0.001), whereas the sulfonylurea group had negligible mean weight reduction from pre- to post-Ramadan period. The between-group difference for mean body weight reduction was statistically significant (1.2 kg vs 0.03 kg; P < 0.001) in favor of vildagliptin (Figure 2).
Mean FPG and PPG decreased significantly in both the groups from pre- to post-Ramadan period. The reduction in mean FPG was 14.7 mg/dL (pre-Ramadan vs post-Ramadan; 140.2 mg/dL vs 125.5 mg/dL; P = 0.015) and 20.0 mg/dL (162.1 mg/dL vs 142.1 mg/dL; P = 0.015) in the vildagliptin and sulfonylurea groups, respectively. Similarly, the drop in mean PPG was 26.2 mg/dL (212.2 mg/dL vs 186.0 mg/dL; P = 0.001) and 33.1 mg/dL (220.6 mg/dL vs 187.5 mg/dL; P = 0.001) in the vildagliptin and sulfonylurea groups, respectively. The between-group differences for reductions in FPG and PPG were not statistically significant.
The overall incidence of AEs was 5.5% in the vildagliptin group and 9.5% in the sulfonylurea group (largely driven by the 2 HEs). The AEs reported in the vildagliptin group and the sulfonylurea group are listed in Table 2. No SAEs, drug-related AEs or discontinuations due to AEs were reported in either group.
| Adverse events | Vildagliptin group(n = 55) | Sulfonylurea group(n = 42) |
| Number of patients with adverse event(s) | 3 (5.5) | 4 (9.5) |
| Palpitation | 2 (3.6) | 1 (2.4) |
| Gastrointestinal disturbances | 2 (3.6) | 0 (0.0) |
| Hypoglycemia | 0 (0.0) | 2 (4.8) |
| Headache | 0 (0.0) | 1 (2.4) |
| Nausea | 0 (0.0) | 1 (2.4) |
This is the first study to compare the efficacy and safety of vildagliptin with sulfonylureas in fasting Indian patients with type 2 diabetes during Ramadan. This 8-10 wk, real-world, observational study in Indian Muslim patients with type 2 diabetes mellitus, fasting during Ramadan reported no significant differences in HEs between the vildagliptin and sulfonylurea groups, with only 0 and 2 HEs in those two groups, respectively. The vildagliptin group showed greater reductions in HbA1c compared with the sulfonylurea group, although the mean FPG and PPG levels decreased significantly in both the groups. The percentage of patients reaching target HbA1c < 7.0% (< 53 mmol/mol) was 3-fold higher in the vildagliptin group compared with the sulfonylurea group. The vildagliptin group also showed a significant but modest reduction in the body weight compared with the sulfonylurea group. The overall incidence of AEs was lower in the vildagliptin group compared with the sulfonylurea group, and there were no drug-related SAEs or discontinuations due to AEs in either group.
Achieving glycemic control without hypoglycemia during fasting, especially during Ramadan, which spans for a period of 4 wk, is challenging in patients with type 2 diabetes mellitus. Incretin therapies like DPP-4 inhibitors are potentially safe during Ramadan[5]. Vildagliptin, a potent and selective oral DPP-4 inhibitor, has been shown to improve insulin secretion in response to glucose levels[13] and suppress inappropriate glucagon response to glucose[14,15], thus maintaining glycemic control in a glucose-dependent manner. In the present study, vildagliptin reduced HbA1c without any incidence of HE, which indicates a regulated glycemic control during Ramadan fasting, while there was no mean HbA1c drop with a few HEs in the sulfonylurea group. The HbA1c differences may be driven by the meals and/or compliance to medication, which were not assessed. However, the results need a cautious interpretation, given that Ramadan lasted only a month and the fact that at least 3 mo may be required for a given change in blood glucose levels to translate to a stable and maximal HbA1c change is likely to misestimate the true lowering of average blood glucose levels during Ramadan.
The low incidence of HEs with sulfonylureas may be explained by the high baseline HbA1c of > 8.5% where hypoglycemic rates are lower and by the fear of hypoglycemia associated with sulfonylureas leading to non-compliance. For instance, the relatively low number of HEs in the sulfonylurea group is consistent with the observation in an earlier study in which the investigators chose not to escalate the dose in the first 16 wk of the study to avoid hypoglycemia[16]. This may also explain the lack of decrease in the mean HbA1c in the sulfonylurea group over 10 wk in this study. Failure to lower HbA1c is not likely explained by over-eating, due to the specific pattern of food intake including Ramadan fasting from dawn to dusk and feasting before and after the fast, since there was no increase in weight. The lack of weight gain in the sulfonylurea group appears to rule out defensive eating as an alternative mechanism to explain the low incidence of HEs.
Efficacy and safety of vildagliptin as monotherapy or in combination with other oral anti-diabetic drugs has been reported extensively[6,17-25]. A long-term study with vildagliptin as add-on to metformin compared with glimepiride demonstrated a lower proportion of patients experiencing HEs over 1 year (1.7% vs 16.2%) and 2 years (2.3% vs 18.2%) in a randomized clinical trial in patients with type 2 diabetes mellitus[9,10]. In Muslim patients with type 2 diabetes mellitus who were fasting during Ramadan, in whom hypoglycemia remains a major risk, vildagliptin, compared with sulfonylureas, showed no or lower incidence of HEs[11,12]. In one observational study, compared with gliclazide, vildagliptin demonstrated a significantly lower number of HEs (2 vs 24) and lower proportion of patients experiencing HEs (7.7% vs 61.5%; P < 0.001) in Muslim type 2 diabetes mellitus patients fasting during Ramadan. Moreover, shifting from sulfonylurea to vildagliptin was associated with a reduction in HEs during Ramadan, while continuation with sulfonylurea treatment showed an increase in the number of HEs[12].
Furthermore, in the VECTOR study conducted in United Kingdom Muslim patients with type 2 diabetes mellitus fasting during Ramadan, vildagliptin demonstrated significantly better glycemic control than gliclazide (mean difference between treatments for change in HbA1c was 0.5%, P < 0.0262) and accompanied with no reports of HEs or severe HEs, while 34 HEs in 15 patients and one severe HE (grade 2) were reported with gliclazide[11]. The incidence of hypoglycemia with vildagliptin in the present study is in line with the VECTOR study, while sulfonylurea is associated with a lower hypoglycemic incidence than in the VECTOR study. These differences could be partly explained by the higher baseline of > 8.5% in both treatment arms of this study and the fact that our study did not specifically look at treatment compliance during Ramadan.
Vildagliptin is known to maintain weight neutrality[26,27], whereas a trend towards weight gain is known with sulfonylurea[11]. However, in this study, a reduction in body weight with vildagliptin was observed in Muslim patients with type 2 diabetes fasting during Ramadan, whereas there was a negligible change with sulfonylurea. Although not investigated, a lesser food or carbohydrate intake associated with fasting and physical activity could be a reason for differential effect of both treatments on weight compared with earlier studies. Noncompliance to treatment could be another possible reason for weight neutrality in the sulphonylurea group in this study.
A few limitations of this study include a small sample size, lack of data on treatment adherence, diet, eating pattern and exercise. Although the treatment adherence was not measured in this study, the VECTOR study showed a significantly better treatment adherence for vildagliptin compared with sulfonylurea, most likely due to the better tolerability and less fear of hypoglycemia[11]. Overall, vildagliptin compared with sulphonylurea showed an improvement in the glycemic control with no accompanied HEs in this study. This might be the reason for not attaining mean HbA1c drop in the sulfonylurea group over 10 wk in this study.
However, despite all the limitations, there appears to be a trend suggestive of good glycemic control with vildagliptin. This effect is particularly meaningful when looked in the context of no HEs (and at least no weight gain) seen with vildagliptin during fasting. These effects need to be confirmed by larger, randomized, interventional trails assessing the relative value of a vildagliptin compared with sulfonylurea in fasting type 2 diabetes patients during Ramadan.
Vildagliptin was not associated with hypoglycemia and tended towards better glycemic control than sulfonylurea. Vildagliptin appears to be an attractive treatment option for Indian patients with type 2 diabetes mellitus who are fasting during Ramadan.
The authors gratefully acknowledge medONE Pharma Solutions (New Delhi, India) for providing the data management and statistical support. The authors would like to thank Dr. Sreedevi Boggarapu (Novartis Healthcare Private Limited, Hyderabad) for medical writing support, editorial assistance, and collation and incorporation of comments from all authors.
Muslims, on average, fast for 12 h per day during Ramadan, which varies from country to country and from season to season and lasts for up to 30 d. Almost 79% of patients with type 2 diabetes mellitus prefer to fast; therefore, it is essential to make fasting as safe as possible. Hypoglycemia presents a serious risk in these patients and it is necessary to test treatment options in this important population group.
Management of glycemic control in diabetes patients fasting during Ramadan has been recognized as a critically important health challenge worldwide. Therapies that affect the incretin system like dipeptidyl-peptidase-4 inhibitors, which could maintain glycemic control in a glucose-dependent manner, might provide a safe alternative therapeutic option during Ramadan. India has the world’s second largest diabetes population and caters large Muslim community; however, there is limited data available exploring the effect of treatments in these fasting diabetes patients.
The current study assesses the relative value for vildagliptin compared to sulfonylurea in fasting type 2 diabetes Muslim patients in a real-world Indian setting and adds useful evidence to the currently limited evidence in this setting.
This study could guide the physicians in selecting suitable antidiabetic agent to manage glycemic control in Indian Muslims patients with diabetes who tend to fast from dawn to dusk during the month of Ramadan.
Hypoglycemia was the primary assessment during the study period. Patients were provided with a questionnaire for self-assessment of hypoglycemic event based on symptoms, which were confirmed by blood glucose levels < 70 mg/dL either self-measured with a glucometer (Accu-Chek Active®, Roche) and recorded in patients’ diaries or measured at any laboratory and were also assessed by the treating physicians.
The primary aim of this study is to compare the effect of vildagliptin and sulfonylureas on the incidence of hypoglycemia events, adverse events, glycemic control, and body mass in Indian Muslim patients during Ramadan. The authors should raise the issue that the use of glycosylated haemoglobin A1c levels as a means to assess glycemic control is likely to misestimate the true lowering of average blood glucose levels during Ramadan.
| 1. | Ahmed MH, Abdu TA. Diabetes and Ramadan: an update on use of glycemic therapies during fasting. Ann Saudi Med. 2011;31:402-406. |
| 2. | Anjana RM, Ali MK, Pradeepa R, Deepa M, Datta M, Unnikrishnan R, Rema M, Mohan V. The need for obtaining accurate nationwide estimates of diabetes prevalence in India - rationale for a national study on diabetes. Indian J Med Res. 2011;133:369-380. |
| 3. | Salti I, Bénard E, Detournay B, Bianchi-Biscay M, Le Brigand C, Voinet C, Jabbar A. A population-based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care. 2004;27:2306-2311. |
| 5. | Al-Arouj M, Assaad-Khalil S, Buse J, Fahdil I, Fahmy M, Hafez S, Hassanein M, Ibrahim MA, Kendall D, Kishawi S. Recommendations for management of diabetes during Ramadan: update 2010. Diabetes Care. 2010;33:1895-1902. |
| 6. | Ahrén B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2874-2880. |
| 7. | Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078-2084. |
| 8. | Ahrén B, Schweizer A, Dejager S, Dunning BE, Nilsson PM, Persson M, Foley JE. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:1236-1243. |
| 9. | Ferrannini E, Fonseca V, Zinman B, Matthews D, Ahrén B, Byiers S, Shao Q, Dejager S. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11:157-166. |
| 10. | Matthews DR, Dejager S, Ahren B, Fonseca V, Ferrannini E, Couturier A, Foley JE, Zinman B. Vildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2-year study. Diabetes Obes Metab. 2010;12:780-789. |
| 11. | Hassanein M, Hanif W, Malik W, Kamal A, Geransar P, Lister N, Andrews C, Barnett A. Comparison of the dipeptidyl peptidase-4 inhibitor vildagliptin and the sulphonylurea gliclazide in combination with metformin, in Muslim patients with type 2 diabetes mellitus fasting during Ramadan: results of the VECTOR study. Curr Med Res Opin. 2011;27:1367-1374. |
| 12. | Devendra D, Gohel B, Bravis V, Hui E, Salih S, Mehar S, Hassanein M. Vildagliptin therapy and hypoglycaemia in Muslim type 2 diabetes patients during Ramadan. Int J Clin Pract. 2009;63:1446-1450. |
| 13. | Vardarli I, Nauck MA, Köthe LD, Deacon CF, Holst JJ, Schweizer A, Foley JE. Inhibition of DPP-4 with vildagliptin improved insulin secretion in response to oral as well as “isoglycemic” intravenous glucose without numerically changing the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:945-954. |
| 14. | Ahrén B, Foley JE, Ferrannini E, Matthews DR, Zinman B, Dejager S, Fonseca VA. Changes in prandial glucagon levels after a 2-year treatment with vildagliptin or glimepiride in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care. 2010;33:730-732. |
| 15. | He YL, Wang Y, Bullock JM, Deacon CF, Holst JJ, Dunning BE, Ligueros-Saylan M, Foley JE. Pharmacodynamics of vildagliptin in patients with type 2 diabetes during OGTT. J Clin Pharmacol. 2007;47:633-641. |
| 16. | Foley JE, Sreenan S. Efficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naïve patients with type 2 diabetes. Horm Metab Res. 2009;41:905-909. |
| 17. | Ahrén B. Novel combination treatment of type 2 diabetes DPP-4 inhibition + metformin. Vasc Health Risk Manag. 2008;4:383-394. |
| 18. | Bolli G, Dotta F, Rochotte E, Cohen SE. Efficacy and tolerability of vildagliptin vs. pioglitazone when added to metformin: a 24-week, randomized, double-blind study. Diabetes Obes Metab. 2008;10:82-90. |
| 19. | Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890-895. |
| 20. | Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naïve patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res. 2007;39:218-223. |
| 21. | Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132-138. |
| 22. | Rosenstock J, Kim SW, Baron MA, Camisasca RP, Cressier F, Couturier A, Dejager S. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:175-185. |
| 23. | Scherbaum WA, Schweizer A, Mari A, Nilsson PM, Lalanne G, Jauffret S, Foley JE. Efficacy and tolerability of vildagliptin in drug-naïve patients with type 2 diabetes and mild hyperglycaemia*. Diabetes Obes Metab. 2008;10:675-682. |
| 24. | Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naïve patients with Type 2 diabetes. Diabet Med. 2007;24:955-961. |
| 25. | Schweizer A, Dejager S, Bosi E. Comparison of vildagliptin and metformin monotherapy in elderly patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Obes Metab. 2009;11:804-812. |
| 26. | Foley JE, Jordan J. Weight neutrality with the DPP-4 inhibitor, vildagliptin: mechanistic basis and clinical experience. Vasc Health Risk Manag. 2010;6:541-548. |
P- Reviewer: Fournier PA S- Editor: Zhai HH L- Editor: A E- Editor: Wu HL