INTRODUCTION
Diabetic macular edema (DME) is a common manifestation of diabetic retinopathy that causes loss of central vision[1]. Data from the Wisconsin Epidemiologic Study of Diabetic Retinopathy stated that the 10-year cumulative incidence of macular edema was 20.1% among those with type 1 diabetes and 25.4% among people with type 2 diabetes treated with insulin[2]. The ultimate treatment for DME involves strict glycemic and blood pressure control, which was demonstrated by the Diabetes Control and Complications Trial and the United Kingdom Prospective Diabetes Study[3-5]. Additionally, laser treatment for clinically significant macular edema (CSME) has been the mainstay form of treatment after the recommendations by the Early Treatment Diabetic Retinopathy Study (ETDRS) study that demonstrated a 50% reduction in moderate visual loss following focal laser photocoagulation[6,7] with only 3% of patients improving > 3 lines of visual acuity (VA) 36 mo after treatment. Therefore, a variety of other therapies have been studied with the aim of improving vision in more patients as well as preventing deterioration of VA in most. These include surgical options, intravitreal corticosteroids, novel intravitreal vascular endothelial growth factor (VEGF) inhibitors and other various new pharmacotherapies that are currently being investigated. Results from new clinical trials are constantly being published, challenging frequent updates on the most recent therapeutic options for this complex disease. In this report, we discuss the evolution of DME treatment over the past few years and give general guidelines for treatment based on available evidence to date.
LASER PHOTOCOAGULATION
Until the advent of intravitreal anti-VEGF agents, laser photocoagulation has been unequivocally the standard of care for treatment of DME[1]. The efficacy of focal laser treatment may in part be due to occlusion of leaking microaneurysms, but the exact mechanisms by which focal photocoagulation reduces DME is unknown. Histopathological studies show changes located in the retina and retinal pigment epithelium (RPE)[8,9]. Some researchers have suggested that with reduced retinal tissue following photocoagulation, autoregulation results in decreased retinal blood flow to the macula with lower fluid flow resulting in decreased edema[10]. Others have hypothesized that the reduced retinal blood flow is due to improved oxygenation following photocoagulation. Biochemical and physiological studies propose that resolution of the edema may also result from changes in the biochemical processes within the RPE[11-17]. An indirect effect of retinal photocoagulation on macular edema has also been supported by the effectiveness of grid treatment alone without direct focal treatment of microaneurysms[18,19].
The ETDRS trial (1985)[6], was the first properly performed multicenter, randomized trial to determine the benefits of laser for DME. Three years after randomization, patients treated with focal photocoagulation for CSME had a 50% reduction in the risk of moderate visual loss compared to controls (from 24% to 12%). However a gain of 3 or more lines during the same period was only reported in three percent of the patients.
The suggested guidelines for laser photocoagulation for DME as provided by the ETDRS[6] is a direct treatment to leaking microaneurysms and grid treatment to diffuse macular leakage and areas of non-perfusion in thickened retina in cases of non-proliferative diabetic retinopathy (NPDR). Because initial pan-retinal photocoagulation (PRP) may worsen macular edema by increasing the inflammatory component and/or central retinal blood flow[20,21], the ETDRS recommended combination of PRP and focal laser photocoagulation for DME in general for selected cases of severe NPDR and early proliferative diabetic retinopathy (PDR). The ETDRS also suggested that if the retinopathy severity allows, eyes with CSME that need PRP may benefit from treatment of the macular edema first followed by PRP 2-4 wk later. Eyes with high risk PDR and CSME on the other hand may be better managed by concomitant treatment of the macular edema in addition to PRP in the same setting because such eyes are at higher risk of bleeding and severe visual loss.
Although effective, the conventional ETDRS macular photocoagulation protocol causes visible laser scars that can enlarge following treatment and encroach towards fixation[22-24]. In addition, the thermal effects of laser photocoagulation for DME can lead to complications, such as choroidal neovascularisation (CNV)[25,26], subretinal fibrosis[27,28] and visual field loss (i.e., central and paracentral scotomata)[29]. Consequently, this intrinsic damage from visible end point laser photocoagulation has prompted interest of many retinal specialists to treat by reducing laser exposure duration and using a sub-visible clinical end point than originally specified in the ETDRS[30-32]. However, exact evidence to support such practice is still deficient. Recently, the outcomes following modified ETDRS laser protocol and mild macular grid (MMG) laser photocoagulation strategies were evaluated in a randomized controlled trial including 263 eyes with previously untreated DME that were followed for over 12 mo period. The MMG consisted of sub-threshold (non-visible) laser applications in the form of a grid treatment. Though, there was a significantly greater reduction in macular thickness in the modified ETDRS laser protocol group, there was no difference in the mean change in best-corrected VA (0 letters in ETDRS and -2 letters in the MMG group, P = 0.10). The conclusion of this trial suggested that modified ETDRS focal photocoagulation should continue to be the standard approach for treating DME[33].
INTRAVITREAL TRIAMCINOLONE ACETONIDE
In recent years, several studies[34-37] have demonstrated the possible benefits of intravitreal injection of triamcinolone acetonide (IVTA) in the management of refractory DME; especially that IVTA does not appear to have a retinotoxic effect[38]. Triamcinolone acetonide (TA) is a synthetic steroid of the glucocorticoid family with a molecular weight of 434.50. Its decreased water solubility accounts for its prolonged duration of action[39]. The elimination half-life of the commercial preparation of TA in the vitreous humor of rats was recently reported by Oishi et al[40] as 6.08 d. In nonvitrectomized patient eyes, the mean elimination half-life was 18.6 d, while in 1 post vitrectomy patient it decreased to 3.2 d [41].
The precise mechanism of action of corticosteroids is still unknown, however, the rationale could be found in their ability to inhibit arachidonic acid pathway, of which prostaglandin is a product.
In addition to the anti-inflammatory effect, TA increases the levels of tight-junctions in between endothelial cells[42] and thus lessens vessel leakage. It also has an angiostatic action through VEGF inhibition[43] and therefore may have a useful effect on DME. IVTA has been increasingly used in refractory DME and even as an alternative to macular photocoagulation, although its use is technically off-label for DME.
In recent years many authors have investigated the possible benefits of intravitreal TA for treating DME. The most considerable evidence reported by Sutter et al[36], in a prospective, double-masked, and randomized trial comparing 4-mg intravitreal TA with placebo. This study reported that 55% of 33 eyes treated with 4 mg of IVTA improved by 5 or more letters of best-corrected visual acuity (BCVA) at 3 mo compared with 16% of 32 eyes treated with placebo (P = 0.002). Macular edema was decreased by 1 or more grades as determined by masked semi-quantitative contact lens examination in 25 of 33 treated eyes (75%) vs 5 of 32 untreated eyes (16%; P < 0.0001).
Several studies were performed to compare the efficacy and safety between IVTA and other modalities of treatment (e.g., focal/grid photocoagulation[44-47], vitrectomy with internal limiting membrane peeling[48] and intravitreal bevacizumab[49]). The Diabetic Retinopathy Clinical Research network (DRCRnet)[44] group designed a two year multicenter randomized clinical trial to compare IVTA (1- and 4-mg doses) with modified ETDRS focal/grid photocoagulation for the treatment of DME. The mean VA was better in the 4-mg IVTA group at 4 mo than in either the laser or the 1-mg IVTA groups; though this beneficial effect was not maintained. At 2 years after starting the treatment, the mean VA was statistically better in the laser group compared to the steroid-injected groups. Optical coherence tomography (OCT) results generally paralleled the VA results. The 3-year visual outcome results of the DRCRnet were consistent with the previously published 2-year results. Furthermore, the cumulative probability of cataract surgery by 3 years was most frequent in the 4-mg IVTA group (83%) vs the 1-mg IVTA group (46%) and the laser group (31%). The intraocular pressure (IOP) increased by more than 10 mmHg at any visit in 4%, 18% and 33% of the eyes, in the laser, 1 mg IVTA and 4 mg IVTA groups respectively. This randomized study pointed out clearly that focal/grid photocoagulation is a better treatment option than IVTA in eyes with DME involving the center of the macula with VA between 20/40 and 20/320. The DRCR net[50] reported another randomized clinical trial evaluating the efficacy of intravitreal ranibizumab (RBZ) with prompt or deferred laser treatment, the combination of IVTA with prompt laser photocoagulation, and laser treatment alone. The 1-year mean change (± standard deviation) in the VA letter score from baseline was significantly greater in both RBZ groups but not in the IVTA + prompt laser group (+4 ± 13, P = 0.31) compared with the laser group (+3 ± 13). Nevertheless, among a subgroup of 62 pseudophakic eyes, VA results were considerably better than for phakic eyes at baseline in the IVTA + prompt laser group such that the degree of improvement appeared comparable to that of the pseudophakic eyes in the RBZ groups and superior to that of the pseudophakic eyes in the sham + prompt laser group at 1 year and 2 years.
The appropriate dose of IVTA for DME is a controversial topic. As reported by Hauser et al[51] and Audren et al[52] the use of the higher 4-mg dose of IVTA does not have enough advantages over the lower 1- or 2-mg doses. However, Lam et al[53] showed that the higher dose (8 mg vs 4 mg) had a more persistent effect on both VA and central macular thickness (CMT) though associated with more ocular hypertensive responses.
Despite having the potential for benefit in selected patients (Figure 1), IVTA does carry substantial risks which are related to both the injection itself and the potential toxicity of corticosteroids. Injection-related events include endophthalmitis, vitreous hemorrhage, and retinal detachment. The potential toxicity of corticosteroids includes the development of cataracts[54] and glaucoma[55] Moreover, the treatment effect typically wanes, and patients may require repeated injections that increases the chances for the above-mentioned risks, and therefore, the benefits of its use have to be weighed against the risks.
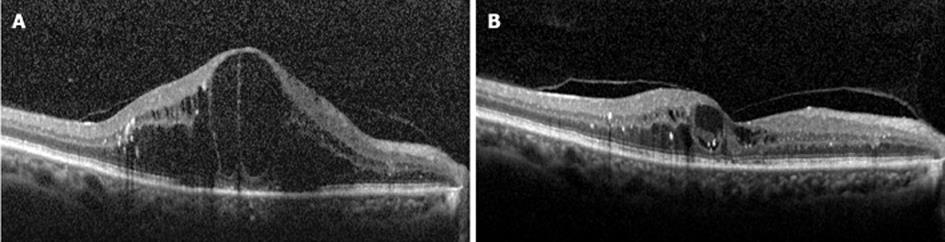
Figure 1 Horizontal spectral-domain optical coherence tomography scans of the macula before (A) and after (B) 1 mg of intravitreal triamcinolone therapy in a patient with diabetic macular edema in the right eye.
Four months following treatment, the macular edema almost completely resolved (B). The Snellen visual acuity improved from 20/200 to 20/60.
VEGF ANTAGONISTS
VEGF levels are elevated in the retina and the vitreous of eyes with diabetic retinopathy[56]. Also, when VEGF was injected in mouse eyes it was found to cause breakdown of the inner blood retinal barrier[57]. Therefore, VEGF seemed a reasonable target in the treatment of DME and recently, these agents have quickly become the first line of therapy in patients with center-involved DME. There are four currently available anti-VEGF agents.
Pegaptanib (Macugen; OSI pharmaceuticals, Melville, NY)
Pegaptanib was the first anti-VEGF agent to be approved by food and drug administration (FDA) for intravitreal injection in patients with wet age-related macular degeneration (AMD). A study of pegaptanib in DME showed a better VA at 36 wk following treatment with 0.3 mg pegaptanib as compared to sham. Mean central thickness decreased 68 micron with pegaptanib vs an increase of 4 micron with sham (P = 0.02). Laser photocoagulation was needed in fewer patients using pegaptanib as compared to sham (25% vs 48%). In a recent study of pegaptanib for patients with DME, VA improved ≥ 10 letters in 36.8% in the pegaptanib group and 19.7% in the sham group and fewer laser treatments were needed in the pegaptanib group[58].
Ranibizumab (Lucentis; Genentech, San Francisco, CA)
Ranibizumab (RBZ) is an intravitreal anti-VEGF agent that is FDA approved for the treatment of wet AMD, and has been used for the treatment of DME[59]. Several studies have reported the superiority of RBZ as compared to laser treatment. The RESOLVE study[60], a randomized multicenter study, showed that RBZ is more effective in improving vision when compared to sham treatment (with rescue laser) over 12 mo (mean BCVA improvement +10.3 for RBZ vs -1.4 for sham, P = 0.0001). READ 2 study[61] showed that patients who received RBZ alone (group 1) gained an average of 7.4 letters at 6 mo as compared to a 0.5 letter loss in patients receiving macular laser therapy only (group 2) and a 3.8 letters gain in patients received both laser treatment and RBZ (group 3). At 24 mo, and after allowing for RBZ to groups 2 and 3 starting at 6 mo, the mean improvement in BCVA was 7.7, 5.1 and 6.8 letters in groups 1, 2 and 3 respectively[50]. The OCT findings, however, did not parallel the visual outcome. The mean foveal thickness at 24 mo was 340 μm, 286 μm and 258 μm for groups 1, 2 and 3 respectively.
The RESTORE study[62] was a phase 3 study in Australia that showed that RBZ alone or combined with laser was superior to laser monotherapy in improving BCVA (+6.1 letters for RBZ alone, +5.9 letters for the combination group and +0.8 for the laser group, P < 0.0001). The mean central macular thickness was significantly decreased from baseline (-118.7 μm, -128.3 μm and -61.3 μm for RBZ, RBZ + laser and laser alone respectively). Another phase 3 trial from the DRCRnet[50] showed that RBZ injection with laser (prompt or deferred ) was significantly more effective than laser alone in improving VA in patients with center involved DME after 1 year of treatment (+9 vs +3 letters gain; P < 0.001). Approximately 30% of the patients gained ≥ 15 letters compared to 15% of the laser alone group. Moreover, RBZ was found to significantly decrease the risk of progression of DR. The RISE and RIDE[63] were also phase 3 randomized multicenter studies that addressed the efficacy of RBZ in the treatment of DME. Patients were randomized to 3 groups (sham injection, 0.3 mg RBZ and 0.5 mg RBZ). At 24 mo, 18.1% of the sham group of eyes gained ≥ 15 letters vs 44.8% of the 0.3 mg and 39.2% of the 0.5 mg treated eyes in the RISE study. Similarly, significantly more RBZ treated patients gained ≥ 15 letters in the RIDE study, (12.3% of sham group vs 33.6% of the 0.3 mg and 45.7% of the 0.5 mg RBZ patients). RBZ treated eyes underwent significantly fewer macular laser treatments. The above studies confirm that RBZ rapidly and sustainably improves edema and vision, and reduces the risk of further vision loss and progression of DR in patients with DME.
Bevacizumab (Avastin; Genentech, San Francisco,CA)
Bevacizumab was approved by the FDA for the treatment of colorectal cancer. It has been used off label in the treatment of wet AMD and other ocular diseases including DME[64]. Multiple studies showed the beneficial effect of bevacizumab in the treatment of DME[65-68] (Figure 2).
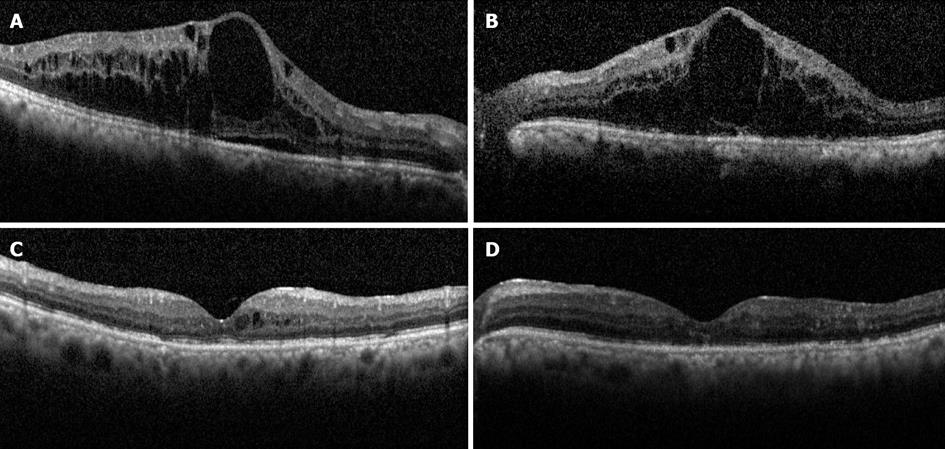
Figure 2 Horizontal spectral-domain optical coherence tomography scans of the macula before (A and B) and after (C and D) intravitreal bevacizumab therapy in a patient with diabetic macular edema.
Note extensive cystoid macular edema in both eyes (A and B) and subretinal fluid in the right (A). Six months following treatment with 3 injections of bevacizumab, the macular edema almost completely resolved in both eyes (C and D). The Snellen visual acuity improved from 20/125 to 20/70 in the right eye, but did not change significantly in the left, likely due to atrophic changes in the outer retina as seen on optical coherence tomography.
In addition, a prospective randomized controlled clinical trial (the BOLT study)[69], found that bevacizumab has a greater treatment effect than macular laser treatment in patients with center involving persistent CSME. At 12 mo, there was a statistical significant difference in the mean ETDRS BCVA (P = 0.0006). At 2 years, the mean ETDRS BCVA was 64.4 (ETDRS equivalent Snellen fraction: 20/50) in the bevacizumab group and 54.8 (20/80) in the macular laser therapy group (P = 0.005). Also, there was a mean gain of 8.6 letters in the bevacizumab group vs a loss of 0.5 letters for the macular laser group. Fourty-nine percent of patients gained ≥ 10 letters (P = 0.001) and 32% gained at least 15 letters (P = 0.004) in the bevacizumab group vs 7% and 4% in the macular laser group.
Aflibercept (VEGF Trap-Eye, Regeneron Pharmaceutical, Inc, Tarrytown, NY, United States)
VEGF Trap-Eye (VTE) is the most recent anti-VEGF available for clinical use. Unlike RBZ and bevacizumab, the VTE incorporates the second binding domain of the VEGFR-1 receptor and the third domain of VEGFR-2 receptor to the FC segment of human immunoglobulin G. It has been investigated in the treatment of wet AMD, DME and retinal vein occlusion. The DA VINCI study[70], a randomized multicenter phase 2 clinical trial compared different doses and dosing regimens of VTE with laser photocoagulation in patients with DME. Patients were assigned randomly to 1 of 5 treatment regimens. VTE 0.5 mg every 4 wk, 2 mg ever 4 wk, 2 mg every 8 wk, 2 mg as needed after 3 initial monthly injections or macular laser treatment. The mean improvement in BCVA in the VTE groups at 24 wk ranged from 8.5 to 11.4 letters vs 2.5 letters for the laser group. At 52 wk, the mean improvement ranged from 9.7 to 12 letters in the VTE groups vs -1.3 for laser group .The mean reduction in central retinal thickness in the VTE groups ranged from -165.4 to 227.4 vs -58.4 for the laser group. However, phase 3 studies are still pending.
Side effects of anti VEGF
The anti-VEGF agents, particularly RBZ and bevacizumab, are currently widely used in the treatment of center involving DME and are usually well tolerated. There are some potential systemic and ocular side effects that are well recognized. The ocular side effects include inflammation, traumatic injury to the lens, retinal detachment, vitreous hemorrhage and endophthalmitis. Fortunately, these occur at relatively infrequent rates. The potential systemic side effects include cardiovascular and cerebrovascular accidents such as myocardial infarction, pulmonary embolism, hypertension, hemorrhage. Although such systemic side effects have been clearly associated with the systemic use of bevacizumab, their relationship to the intravitreal use of anti-VEGF agents is less well understood, and the data so far suggests that no such cause effect relationship exists[56].
VITRECTOMY
The role of the vitreous in the development and progression of DME has been recognized through several mechanical and physiologic mechanisms, all of which are presumed to lead to increased vascular permeability[71-78]. The attachment of the posterior hyaloid is a vital observation in eyes with DME which have a lower rate of posterior vitreous detachment than those without[79], and spontaneous posterior vitreous separation is associated with improvement in DME[79,80]. Schepens[81] was the first to propose the possible role of vitreous traction in the development of cystoid macular edema associated with retinitis pigmentosa, aphakia, and uveitis. Nasrallah et al[71] reported the first data to suggest that the vitreous can play a role in the pathogenesis of DME. Among their study population with diabetic retinopathy, DME occurred only in 20% of patients with a posterior vitreous detachment (PVD) compared to 55% in those with an attached hyaloid. Furthermore, Hikichi et al[79] reported that over a period of 6 mo, DME resolved spontaneously in 55% of the cases with vitreomacular detachment compared to 25% of the cases with vitreomacular adhesion. The efficacy of pars plana vitrectomy (PPV) has been advocated for the treatment of diffuse DME with taut posterior hyaloid. In 1992, Lewis et al[82] described that PPV was effective for treating DME with thickened and taut posterior hyaloid that did not respond to laser treatment whereby 8 out of 10 patients experience VA and macular thickening improvement. Since then many studies showed that vitrectomy resulted in a decrease of macular edema (Figure 3) and frequently improvement of VA.
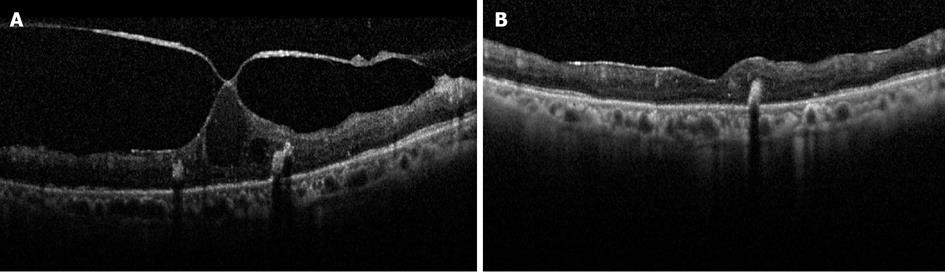
Figure 3 Spectral-domain optical coherence tomography scans of the macula before (A) and after (B) pars plana vitrectomy in a patient with diabetic macular edema in the left eye.
Note the focal vitreomacular adhesion with associated cystoid macular edema and intraretinal hard exudates. Seven months following surgery, the macular edema completely resolved (B), but the Snellen visual acuity was not changed and measured 20/400 likely due to atrophic changes in the outer retina as seen on optical coherence tomography.
Various mechanisms have been assumed to elucidate the beneficial effects of vitrectomy in DME. The vitreomacular traction in both the antero-posterior and tangential dimensions may be important in the pathogenesis of ME which has been well-demonstrated by optical coherence tomography[83]. On the other hand, Gandorfer et al[76], Ikeda et al[84] and La Heij et al[85] showed that vitrectomy was effective even when there is no evidence of macular traction. One possible explanation is that vitrectomy appears to increase oxygenation of the macula. Kadonosono et al[86] demonstrated increased perifoveal capillary blood flow after vitrectomy in their series. Stefansson[87,88] reported that vitrectomy with lensectomy allows aqueous to provide increased levels of oxygen to the inner retina and that movement of oxygen trans-corneally to the vitreous has been shown to be more effective in vitrectomized eyes[89]. In addition, the removal of the posterior hyaloid also helps in reducing the load of toxic substances and cytokines, such as histamine, free radical scavengers, and VEGF, which may be harbored in the preretinal space in such eyes[90].
Recently, internal limiting membrane (ILM) peeling has been added to vitrectomy for ME though its effect is not yet certain. The ILM in diabetic eyes contains increased levels of fibronectin, laminin and collagen[91,92]. Histopathologically, peeled ILM specimens from DME eyes have been shown to be several times thicker than normal ILM, possibly contributing to altered fluid shifts between the vitreous and retina[93]. Moreover, Funatsu et al[94-96] and Aiello et al[56] independently stated a hypothesis that in the vitreous body of diabetic patients there are humoral substances such as VEGF and interleukin 6, generated in the cells of epiretinal membranes, which impair the oculovascular barrier and contribute to the pathogenesis of DME. Similarly, Antonetti and associates demonstrated that increased levels of VEGF in the vitreous decrease levels of occludin, a membrane spanning tight junction protein, which could change the structure of the retinal endothelial junction and may account for the increased vasopermeability in patients with DME[97].
The addition of ILM peel to vitrectomy was suggested to yield a better anatomical as well as functional results[76,98], and its effectiveness was sustained long term with less recurrence[99]. The mechanism for this was hypothesized to be removal of the scaffold used by astrocytes to proliferate on the retinal surface and the elimination of all tractional forces at the vitreoretinal interface. In addition, it allows the ME to decompress by facilitating the release of extracellular fluid into the vitreous, which restore normal retinal thickness and intraretinal tissue pressure.
Regression of macular edema after PPV and ILM peel may also be explained by a decrease or elimination of factors enhancing vasopermeability. These factors could be produced by cells within the cortical vitreous and ILM, or they could build up in the vitreous and concentrate in the macular region and provoke or aggravate macular edema. This hypothesis is also supported by the gradual postoperation regression of CSME[100].
Surgical removal of the ILM is challenging and can compromise the already affected retinal tissue. Some of the challenges include poor visibility of the thin transparent membrane, the small dimensions and the sensitivity of the macular tissue, as well as the potential staining dye toxicity during the procedure[101,102]. In a study by Kumagai et al[103] of 135 eyes, 74 of which underwent ILM peeling, although ILM removal accelerated the absorption of ME, the final VA were similar in both groups. Furthermore, Hartley et al[104] found that PPV with ILM peeling improves anatomy in DME patients, but does not necessarily improve VA. In one of the DRCR studies, 87 eyes with moderate visual loss which underwent vitrectomy for DME and associated vitreomacular traction were reviewed. As an adjunct to vitrectomy, epiretinal membrane peeling was done in 61%, ILM peeling in 54%, PRP in 40%, and injection of corticosteroids at the end of the procedure in 64%. At 6 mo, a median improvement in VA of 3 letters was reported, with VA improving by ≥ 10 letters in 38% (95%CI: 28%-49%) and worsening by ≥ 10 letters in 22% (95%CI: 13%-31%). Greater VA improvement was reported in eyes with worse baseline acuity (P < 0.001) and in eyes that had epiretinal membrane removal (P = 0.006)[105]. In this study, ILM removal was not one of the factors associated with VA improvement following vitrectomy although it was associated with a better anatomical outcome.
In addition to its controversial role in DME, vitrectomy may be associated with several serious and sight threatening potential complications such as ocular haemorrhage, endophthalmitis, retinal tears and detachment, cataract formation, and glaucoma. Aside from cataract, the rate of these is relatively low fortunately. For example, in one study[106] post-vitrectomy complications in eyes with DME were as follows: retinal tears (9.6%), epiretinal membrane (9%), retinal detachment, neovascular glaucoma and rubeosis iridis (each 1.9%). Likewise, the DRCRnet study group[107] reported postoperative complications at 6 mo as vitreous hemorrhage (5 eyes), elevated intraocular pressure requiring treatment (7 eyes), retinal detachment (3 eyes), and endophthalmitis (1 eye). Few changes in results were observed between 6 mo and 1 year follow up.
STEROID IMPLANTS
Chronic diseases, like DME, may be best controlled by treatment modalities capable of providing a therapeutic effect for a prolonged period. Moreover, new formats of drug delivery like long-acting intravitreal drug delivery implants can overcome many of the limitations of other therapeutic approaches, such as frequent intravitreal injections or systemic side effects, by providing long-term localized delivery of therapeutic agents to the posterior segment at sufficiently high local dosages necessary to elicit the desired therapeutic effects.
The Retisert implant [fluocinolone acetonide(FA) intravitreal implant 0.59 mg; Bausch and Lomb, Rochester, NY] is a first-generation device that has been FDA-approved for the treatment of chronic macular edema due to non-infectious uveitis[108]. It is composed of a non-biodegradable intravitreal implant that is surgically inserted through a 3.5 mm incision and sutured to the eye wall, where it provides a relatively consistent delivery for about two and a half years. The initial dose is 0.6 μg a day, which decreases over the first month to about 0.3-0.4 μg a day[109]. In a multicenter clinical trial Pearson et al[110] reported a group of 197 patients randomized to receive either sustained release FA intravitreal implant (Retisert) or modified ETDRS laser for DME. Over the 3-year study period, 58% of the eyes in the Retisert group had no evidence of DME, while only 30% of the laser-treated eyes had resolution of the edema (P < 0.001). VA improvement of > 3 lines was achieved in 28% of eyes in the Retisert group vs 15% in the laser group (P < 0.05). Loss of > 3 lines VA was similar in both groups (19% with Retisert vs 16% with laser). There were significant adverse effects among the Retisert group which included IOP rise in 35%, the rate of filtration surgery was 28%, and in 5% of eyes the device had to be explanted to manage the IOP. The other major obstacles that restricted the widespread use of the this implant for DME include the need for initial surgical implantation, the potential need to explant empty devices and re-implant loaded ones in the operating room for maintenance of therapy, as well as cost.
Iluvien (Alimera Sciences, Alpharetta, United States), is an injectable second-generation version of the Retisert FA implant that can be delivered in an office setting without surgery using a 25-gauge injector. It can be designed to release either 0.5 μg of FA a day for 18 mo, or 0.2 μg a day for three years. A pharmacokinetic study revealed that each supplied excellent sustained delivery of FA in the eye for at least 1 year and reduced DME[111].
Based on the clinical results and safety profile perceived in the Retisert studies, the Iluvien insert has entered phase 3 testing in DME. The Fluocinolone Acetonide in Diabetic Macular Edema (FAME) study was a randomized, double-masked, multicenter clinical trial that compares the 0.5 μg/d and 0.2 μg/d injectable Iluvien inserts with sham injection among patients with persistent DME despite focal laser. Over 24 mo of follow-up, 28.7% and 28.6% in the low- and high-dose insert groups, respectively, reported an improvement in ETDRS vision of 15 or more letters compared with 16.2% in the sham group (P = 0.002 for each). The mean improvement in BCVA letter score between baseline and month 24 was 4.4 and 5.4 in the low- and high-dose groups, respectively, compared with 1.7 in the sham group (P = 0.02 and P = 0.016). Subjects needing cataract surgery were more frequent in the insert groups, and their visual benefit was similar to that of subjects who were pseudophakic at baseline. Glaucoma requiring incisional surgery reported in 3.7%, 7.6% and 0.5% of the low-dose, high-dose and sham groups, respectively[112].
Another device being studied in the setting of DME is Ozurdex (dexamethasone intravitreal implant), a biodegradable implant that can be placed in the vitreous cavity by a 22-gauge applicator through a small self-sealing puncture. The implant may contain either 350 or 700 mcg of dexamethasone and the drug is released in a biphasic fashion, with very high doses for up to six weeks, followed by low but therapeutic doses out to six months or more. In a randomized, controlled clinical trial to evaluate the safety and efficacy of dexamethasone intravitreous drug delivery system (DDS) among patients with persistent DME, patients were randomized to treatment with 700 mcg or 350 mcg of DDS or observation. At day 90, more eyes in the 700 (33.3%) and 350 (21.1%) mcg groups reported a BCVA improvement of 10 letters or more compared with the observation group (12.3%; P = 0.007 vs 700-microg group). The treatment benefit was less noticeable at 180 d where 30% of eyes in the 700 mcg group, 19% in the 350 mcg group, and 23% in the observation group experienced a BCVA improvement of 10 letters or more (P≥ 0.4 for treated vs observed eyes). Moreover, a statistically significant reduction in both OCT retinal thickness and leakage by fluorescein angiography was also demonstrated in both implanted dosages vs controls (P =0.03; day 90)[113].
A prospective, multicenter, open-label, 26-wk study was designed to evaluate the safety and efficacy of Ozurdex 0.7 mg received in a single dose by 55 patients with treatment-resistant DME (mean duration of 43 mo) and a history of previous PPV. At week 8, the mean change from baseline central retinal thickness (403 μm) was -156 μm (P < 0.001) and -39 μm at week 26 (P = 0.004). The mean increase in BCVA from baseline (54.5 letters) was 6.0 letters at week 8 (P < 0.001) and 3.0 letters at week 26 (P = 0.046). At week 8, a gain of ≥ 10 letters in BCVA were reported in 30.4% of patients[114].
A number of other studies evaluating the Ozurdex implant for the treatment of DME as monotherapy, in combination with laser, and in post-vitrectomized eyes have been carried out as well. The results of the pivotal phase 3 trial (MEAD), comparing two doses of the dexamethasone implant with sham treatment, are not yet available, although recruitment is complete, and full three-year follow-up is expected to be complete by the end of 2012[115]. Another phase 3 trial, PLACID, compared the safety and efficacy of the dexamethasone implant plus laser with sham implant procedure plus laser in the treatment of DME. This one-year trial (results reported at scientific conferences, not yet published) which permitted reinjection of Ozurdex after six months, showed that patients with diffuse DME had better BCVA at 12 mo when treated with Ozurdex plus laser vs laser alone[116]. The trial in postvitrectomized eyes with persistent DME (the CHAMPLAIN study) was a 26-wk open-label single Ozurdex injection trial. The study reported that 30% of eyes had experienced a two-line improvement in BCVA by 13 wk, although this effect diminished by the study endpoint of 26 wk[114].
In terms of adverse events, the dexamethasone implant was well tolerated and most events were mild in severity. High IOP was successfully managed with either observation or topical medications; none required laser or surgical intervention to control the IOP. There was no statistically significant difference between groups in the occurrence of any of the non-ocular adverse events[113].
MISCELLANEOUS
Micorpulse Laser
The value of conventional laser photocoagulation for DME was well established since the ETDRS reported its results and the procedure involves production of visible burns in the retina, which when observed; indicate that the thermal spread has reached the overlying neurosensory retina with a temperature high enough to alter its natural transparency. Enlargement of laser scars after treatment has been reported[23]. A sub-threshold micropulse diode laser (with 810 nm and 577 nm lasers), seems to have a theoretical advantage since the laser burns will selectively affect deeper layers with relative sparing of the inner neurosensory retina, thus reducing the scarring and paracentral scotomas post-treatment[15].
Using a micropulse mode, laser energy is delivered with a train of repetitive short pulses (typically 100 to 300 microseconds “on” and 1700 to 1900 microseconds “off”) within an “envelope” whose width is typically 200 to 300 milliseconds[117]. Micropulse power as low as 10% to 25% of the visible threshold power has been revealed to be sufficient to show consistent RPE-confined photo thermal effect with sparing of the neurosensory retina on light and electron microscopy[118].
Micropulse has been demonstrated to be as effective as conventional argon laser for DME by several authors[15,119-122]. In order to assess the efficacy of subthreshold micropulse diode laser photocoagulation for DME, a prospective, nonrandomized interventional case study was carried out evaluating forty-three eyes (36 patients) with CSME and a central macular thickness (CMT) of < 600 micron on OCT. A significant reduction of CMT was detected within the first 3 mo of the laser treatment, but the changes of BCVA and macular volume were not significant. After 12 mo, 94.7% of the patients had a VA that was either improved or preserved within 0.2 logMAR. Laser scars were not identified in any patient in this study[121].
In another prospective, randomized study using multifocal electroretinography (MfERG) the efficacy of subthreshold micropulse diode laser was compared against double-frequency neodymium YAG (Nd:YAG) laser. Thirty three patients (46 eyes) with CSME were randomized to either SDM (810 nm) laser or the conventional double-frequency Nd:YAG (532 nm) laser. At 6 mo, no statistically significant differences were reported for all of the following: CMT as measured by OCT and macular retinal sensitivity measured using MfERG (as a primary outcome measures), BCVA and contrast sensitivity (as secondary outcome measures), between the two groups. The authors concluded that subthreshold micropulse diode laser photocoagulation showed an equally good effect on VA, contrast sensitivity, and DME as compared to conventional Nd:YAG laser photocoagulation with a potentially better preservation of retinal tissue and electrophysiological indices[122].
In the anti-VEGF era, subthreshold tissue-sparing therapy may play a major role in the management of DME in the future, especially when considering combining it with intravitreal injections. This regimen may prove helpful in reducing the number of injections needed to control the edema. However, future studies are needed in this regard.
Anti-tumor necrosis factor alpha (or tumor necrosis factor antagonist)
Several levels of evidence suggest an inflammatory basis for DME[123]. Moreover, many authors reported data supporting the important role of an altered local expression of TNF in the pathogenesis of DME[124,125] and that low-grade subclinical inflammation is responsible for many of the characteristic vascular lesions of diabetic retinopathy[123]. Along this line, treatment modalities have been tried with variable success.
The monoclonal anti-TNF antibody Infliximab neutralizes TNF actions and has been used for inflammatory arthritic conditions and Crohn’s disease since 1998 with a favorable safety profile[126]. Studies in patients with arthritis have shown that anti-TNF therapy negatively affects vascular permeability and angiogenesis by decreasing VEGF[127], which has been implicated directly in the pathogenesis of DME and diabetic retinopathy[61,128]. Likewise, repeated treatment in one diabetic patient produced a further significant improvement of DME, suggesting that the clinical response to anti-TNF dosing regimens is individualized, as observed in patients with arthritis, or in patients with uveitic macular edema[126].
The efficacy and safety of infliximab in treating patients at risk of vision loss secondary to DME refractory to laser photocoagulation was evaluated by a small phase III prospective, double-blind, randomized, placebo-controlled study . At weeks 0, 2, 6 and 14, eleven patients received infliximab (5 mg/kg) intravenously, followed by placebo at weeks 16, 18, 22 and 30, or vice versa. VA improved from 23.5 + 10.3 (mean + SD letters read) at baseline to 30.4 + 13.4 letters at week 16 in 8 infliximab-treated eyes that were eligible for analysis, and the effect was sustained till the completion of placebo treatment (31.4 + 12.1). A higher proportion of eyes experienced VA improvement in the infliximab-treated eyes (P = 0.017). Infliximab treatment was well tolerated in this study[129].
Further studies examined the use of intravitreal TNF inhibitors but the results were not encouraging. An interventional, retrospective, multicenter study by Wu and associates, evaluated the short-term visual and anatomical outcomes after intravitreal injections of two TNF-α inhibitors (adalimumab 2 mg or infliximab 1 mg or 2 mg) in 39 eyes with refractory DME. No apparent benefit for treatment was reported in any of the study groups. Additionally, no ocular side effects were documented in the eyes injected with either adalimumab or 1 mg of infliximab, while severe uveitis was reported in 42% (8 of 19) of the eyes receiving 2-mg infliximab. All were controlled with topical steroid therapy except three eyes (37.5%) required PPV[130]. Similarly, in a prospective, noncomparative, interventional small case series of four patients, with age-related macular degeneration, half of the cases developed intraocular inflammation with high intraocular pressure 3 and 5 wk after the infliximab injection, respectively. One case was controlled with topical medication, and one case required posterior vitrectomy[131]. Tsilimbaris et al[132], in a pilot study of seven patients with refractory DME, reported no definite benefit to intravitreal etanercept after 2 consecutive intravitreal injections of 2.5 mg (0.1 mL) at a two-week interval. Three months after initiation of therapy, analysis of the data shows a tendency for improvement of VA, a slight worsening of hard exudates and fluorescein leakage, while hemorrhages remained stable, however, no statistical significance for therapy was attained. No adverse reactions or adverse events were observed in any patient. Therefore, the role of TNF inhibition in patients with DME remains to be determined.
PKC beta-isoform inhibitors (or Ruboxistaurin)
Substantial data suggest that the Protein Kinase-C β (PKC-b) may play an important function in regulating endothelial cell permeability and is an important triggering component for VEGF[132]. Inhibition of PKC β-isoform activity was shown to reduce diabetes-induced retinal permeability and ischemia-induced retinal neovascularization[133,134]. Zhu et al[135] also reported that the enhanced endothelin-1 (ET-1) expression associated with the activation of PKC occurs in early diabetes, and PKC inhibitor could reverse the up regulation of ET-1.
Ruboxistaurin (RBX), an orally active PKC-b inhibitor, has revealed inconsistent results against DME. In several animal models, RBX improved hyperglycemia-induced diabetic microvascular complications, including diabetic retinopathy and DME[133]. The PKC-b Inhibitor-Diabetic Retinopathy study 2 (PKC-DRS2)[136], documented that treatment with oral RBX (32 mg/d) decreased moderate visual loss [9.1% of placebo-treated patients vs 5.5% of RBX-treated patients (40% risk reduction, P = 0.034)], macular edema progression (68% vs 50%, P = 0.003), and the need for laser treatment for macular edema (26% less frequent in eyes of RBX-treated patients, P = 0.008), with increasing occurrence of visual improvement in patients with nonproliferative retinopathy. Although RBX was well tolerated and reduced the risk of visual loss, it did not prevent DR progression. On the other hand, the protein kinase C-Beta inhibitor Diabetic Macular Edema study (PKC-DMES)[137] reported some evidence that ruboxistaurin was associated with reduced progression of DME, although this was a secondary endpoint. Ruboxistaurin has not received approval from the United States FDA.
Nicotinic acetylcholine receptor antagonist (Mecamylamine)
In the 1950s, mecamylamine [a nonspecific nicotinic acetylcholine (nACh) receptor antagonist] was approved as an oral antihypertensive agent[138]. In animal models, it could inhibit angiogenesis and vascular permeability by blocking nACh receptors on vascular endothelial cells. Its application using a topical ocular formulation containing 0.1% or 1% mecamylamine 3 times daily in rabbits resulted in detectable levels of mecamylamine in the retina and suppressed CNV in mice[139]. A multicenter phase I/IIclinical trial reviewed the safety and bioactivity of topical mecamylamine in twenty-three patients with chronic DME. Over a period of 12 wk, all eyes were treated twice daily with topical 1% mecamylamine. The mean improvement in BCVA was 2.8, 1.9, 2.4, 0.8 and 3.1 letters at 1, 4, 8, 12 and 16 wk respectively, with little associated change in mean excess foveal thickness. There were no recognized drug-related safety problems[140].
Although these findings are encouraging especially for a topical formulation, further studies are needed to address the efficacy of the medication especially in relation to other available therapies such as the intravitreal anti-VEGF agents.
Pharmacologic vitreolysis (Microplasmin-ThromboGenics)
As discussed above in the section on “vitrectomy”, vitreous traction is one of the causes for persistence of DME in some cases. Inducing a PVD could be one line of management for these cases. Although the surgical vitrectomy instrumentation and techniques have markedly improved in recent years, surgery is still associated with several potential complications[141,142]. As a result, it would be useful to have a biochemical agent that could cleave the vitreoretinal interface selectively with a relatively good safety profile.
Recently, pharmacologic vitreolysis has been explored as a new modality of treatment to manage DME. Microplasmin, which is approved by the United States-FDA, has shown promise in inducing a posterior vitreous detachment when given as a single intravitreal injection. Microplasmin is a truncated form of plasmin, an enzyme that dissolves protein formations that are crucial to blood clot (thrombus) formation; similar protein formations are seen linking the vitreous to the retina in the eye. It has the benefit of reaching a peak of action after 15-30 min of administration and continuing in a plateau for another 90 min then decreases to undetectable levels in 24 h[143]. A phase 2, multicenter, placebo-controlled, double-masked, clinical trial was designed to evaluate the safety and efficacy of a preoperative intravitreous injection of microplasmin in patients (n = 125) scheduled for vitreous surgery. The rate of total PVD at the time of surgery were reported in 10%, 14%, 18%, and 31% in the placebo group, 25-microg, 75-microg and 125-microg microplasmin groups, respectively. The secondary end point of resolution of vitreomacular interface abnormality precluding the need for vitrectomy at the 35-d time point was observed at the rates of 3%, 10%, 15% and 31% respectively. However, a more definitive evaluation of the efficacy of microplasmin in the management of patients with DME in phase 3 clinical trials is warranted[144].
OTHERS THERAPIES
A range of new additional medical and surgical therapies and investigational modalities show potential for the management of refractory DME. Nepafenac, the amide analog of the NSAID amfenac, is hydrolyzed by the uveal tissue and retina[145] where it could probably reduce vascular permeability by inhibiting the inflammatory cascade. A small case series[146] suggested that topical nepafenac might have a beneficial effect in the treatment of DME.
Another example is Sirolimus that has been documented to inhibit the production and activity of many growth factors related to the development of diabetic retinopathy. In a phase 2 clinical study[147], it was shown that repeated subconjunctival sirolimus injections were well-tolerated with no significant drug-related undesirable events. There was an inconsistent treatment effect related to sirolimus, which deserved further assessment by a randomized trial to reveal any possibility of treatment benefits.
In advanced cases of extensive lipid maculopathy where other treatments are usually ineffective and where an increased risk of subretinal fibrosis exists, surgical removal of massive deposition of macular hard exudates was tried by several retina surgeons[148]. Takagi et al[149], Sakuraba et al[150], and Takaya et al[151] have removed macular hard exudates surgically following vitrectomy. Despite good anatomical results, sustained VA improvement was not observed because of atrophic or degenerative changes. At present, more experience is required to understand the long-term benefit and timing of such a modality of treatment.
Lastly, the new ultra-widefield fluorescein angiography (capturing an image up to 200 degrees) offers a significant tool for assessment of the macula as well as the retinal periphery, and may yield additional information as to any potential role for the peripheral retina in the pathogenesis of DME. Freyler et al[152], suggested a likely association between DME and peripheral non-perfusion after a study with a 60-degree wide-angle angiography camera. In 2005, Kimble et al[153] reported that peripheral capillary nonperfusion was detected in 84% of patients with CSME and nonproliferative retinopathy. The RaScaL (Ranibizumab + Scatter Laser) study, a small pilot trial, hypothesized that DME is driven predominantly by peripheral retinal ischemia. The patients were randomized to either conventional treatment (macular laser plus IVTA) or a novel treatment strategy of ultra-widefield fluorescein angiography-directed peripheral laser plus intravitreal RBZ. This study revealed a higher recurrence of DME needing retreatment in macular laser + steroid (4/5) vs RaScal (1/5), and improvement in central foveal thickness in both groups which was better in RaScal at 6 mo. These findings need to be confirmed by larger studies. In addition the differential effect of ranibizumab vs peripheral scatter laser on DME needs to be addressed[154].
In summary, although laser photocoagulation has been the gold standard of therapy for DME, it is being quickly replaced by intravitreal pharmacotherapy as evidenced by three phase 3 clinical trials supporting the superiority of ranibizumab for center involved edema. The role of other agents such as intravitreal triamcinolone injections, steroid implants and aflibercept, as well as that of vitrectomy remains to be determined. While the use of intravitreal triamcinolone and vitrectomy appear to be currently limited to cases that have failed other available therapeutic options, the former may be effective in pseudophakic patients who have no personal or family history of glaucoma, and the latter in cases with evidence of vitreomacular traction. Several new treatment modalities for DME are in the horizon of which topical therapy may play a major role in the future with all the advantages the topical route may offer compared to the intravitreal route.