Published online Dec 15, 2013. doi: 10.4239/wjd.v4.i6.295
Revised: July 17, 2013
Accepted: August 4, 2013
Published online: December 15, 2013
Processing time: 147 Days and 2.8 Hours
Diabetic macular edema (DME) is a common cause of visual impairment in diabetic patients. It is caused by an increase in the permeability of the perifoveal capillaries and a disruption of the blood retinal-barrier. The pathogenesis of DME is multifactorial. Several therapeutic modalities have been proposed for the treatment of DME. Corticosteroid treatments have emerged as an alternative therapy for persistent DME or refractory to conventional laser photocoagulation and other modalities, due to anti-inflammatory, anti-vascular endothelial growth factor and anti-proliferative effects. Many studies have demonstrated the beneficial therapeutic effect of corticosteroids with improvement to both retinal thickness and visual acuity in short-term on the treatment of DME. Peribulbar and intravitreal injections have been used to deliver steroids for DME with frequent injections due to the chronic and recurrent nature of the disease. Steroid-related side effects include elevated intraocular pressure, cataract, and injection related complications such as endophthalmitis, vitreous hemorrhage, and retinal detachment particularly with intravitreal steroid injections. In order to reduce the risks, complications and frequent dosing of intravitreal steroids, intravitreal implants have been developed recently to provide sustained release of corticosteroids and reduce repeated intravitreal injections for the management of DME.
Core tip: Despite the documented ocular side effects of corticosteroids by the time being they are still considered as one of the essential effective adjunct modalities for the treatment of diabetic macular edema especially in refractory and persistent cases.
- Citation: Al Dhibi HA, Arevalo JF. Clinical trials on corticosteroids for diabetic macular edema. World J Diabetes 2013; 4(6): 295-302
- URL: https://www.wjgnet.com/1948-9358/full/v4/i6/295.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i6.295
Diabetic macular edema (DME) is considered to be a process of low-grade inflammation in which numerous inflammatory cells, mediators and cytokines are involved and subsequently lead to increase in vascular permeability[1-6].
Corticosteroids are anti-inflammatory agents that stabilize retinal capillaries and tend to reduce their permeability by enhancing the activity or density of the tight junctions in the retinal capillary endothelium[6-8] as well inhibit and downregulate the metabolic pathway of the vascular endothelial growth factor[9-12] in order to decrease the leakage of plasma proteins into the interstitial tissue compartment and help to restore the osmotic gradient between blood and tissue compartments, and eventually will resolve edema formation[7,13,14].
Corticosteroid has emerged as an alternative therapy for persistent DME or refractory to conventional laser photocoagulation and other modalities[15-23]. Many studies have demonstrated the beneficial therapeutic effect of corticosteroids such as triamcinolone and dexamethasone on the treatment of diabetic macular edema[15-27]. Various routes have been used to deliver steroid for DME treatment including peribulbar injection, intravitreal injection and intravitreal implants.
Peribulbar or subtenon’s steroid injections have been used to treat diabetic macular edema either as monotherapy or as adjunctive therapy to laser[16,17,24,28]. Although they are not considered ideal to obtain a therapeutic dose of cortisone at the level of the retina[29,30], short-term efficacy has been demonstrated with transient improvement to both retinal thickness and visual acuity but less effective than intravitreal therapy[16,17,30]. However, a phase II study sponsored by the national eye institute, showed no benefit in reducing retinal thickness by adding peribulbar steroids to focal laser treatment for eyes with mild DME and good visual acuity[6,24]. Although, subtenon’s approach is clearly less invasive than the intravitreal one[31], it is not free of potential complications such as accidental injection directly into the choroidal or retinal circulation, perforation of the ocular bulb, occlusion of the central retinal artery and cataract[31]. Other complications reported are blepharoptosis, orbital fat atrophy, strabismus and conjunctival necrosis[31,32]. It is important to add, that intra-ocular pressure (IOP) is not increased by the use of the posterior sub-Tenon’s approach in comparison to anterior sub-Tenon or intravitreal triamcinolone acetonide (IVTA) injections with the exception of steroid responder patients, the risk of IOP elevation is 44% steroid responders compared to 13% in non steroid responders[28,31-34].
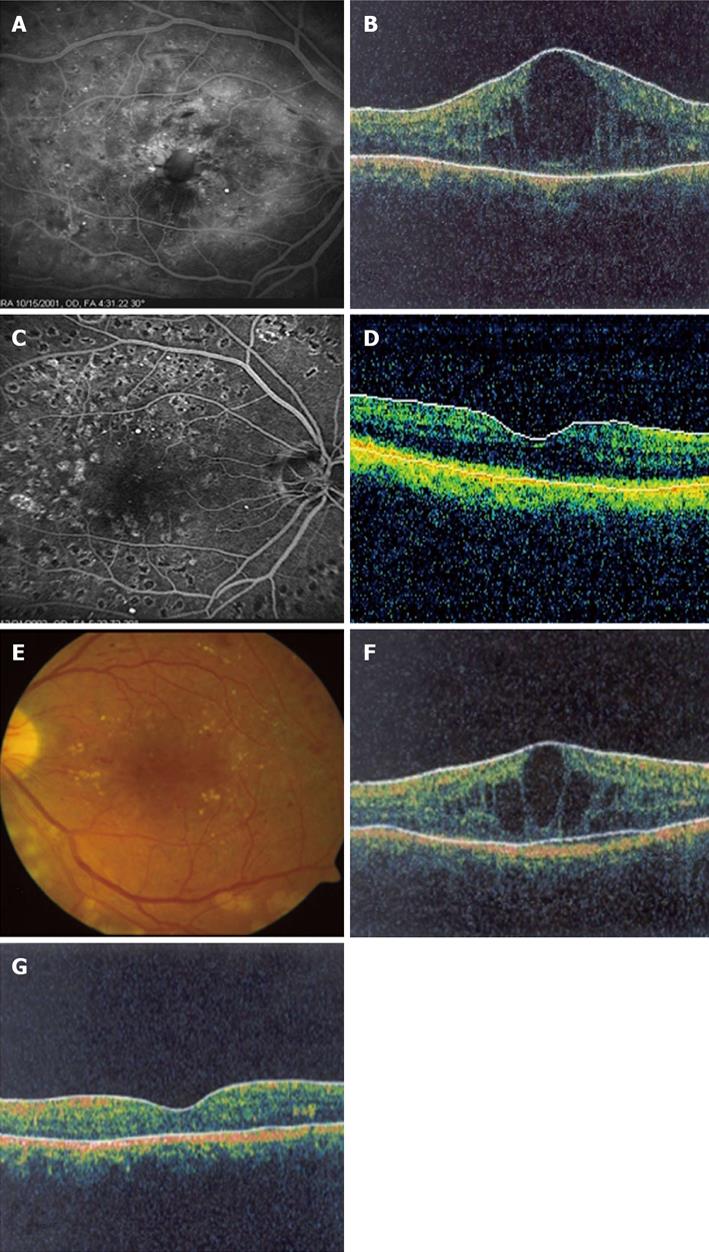
Intravitreal triamcinolone acetonide (IVTA) injection has been widely used to treat DME particularly diffuse macular edema that persist after appropriate laser treatment (Figures 1 and 2). Many clinical trials have been conducted to evaluate the efficacy of IVTA therapy for DME[18-23,35-52]. IVTA has shown significant improvements in diabetic macular edema and visual acuity in the short term and found to be transient. The therapeutic effect is typically seen within 1 wk and the duration of the effect increased with increasing dosage. However, in many patients re-injections are needed every three to six months as the effect diminishes[18,19,42,44,53-57].
The Diabetic Retinopathy Clinical Research Network (DRCR) compared 2 doses of IVTA as monotherapy to focal/grid laser photocoagulation in 840 eyes with DME. The 4-mg IVTA group had better visual acuity at four months; however, at 16 mo, two years and three years, the laser group had better visual acuity than either IVTA groups. In addition, the laser group had fewer incidences of cataract and glaucoma[27,58,59].
In the second randomized controlled trial by the DRCR network, focal/grid laser alone was compared to 4 mg of intravitreal triamcinolone plus laser. Two additional arms utilized intravitreal ranibizumab. Similar to the previous study, the triamcinolone plus laser showed superiority compared to laser alone in terms of visual acuity at 24 wk follow-up. However, at one and two years, the treatments appeared equivalent in terms of visual acuity outcome, but with increased rates of cataract and elevated intraocular pressure in the triamcinolone plus laser group[60,61]. In the subgroup analysis of patients who were pseudophakic at baseline, the triamcinolone plus laser group appeared superior to the laser alone treatment and equivalent to the treatment arms utilizing ranibizumab[60,61].
Recently, Gillies et al[62] reported the 24-mo results of a randomized controlled trial of intravitreal triamcinolone plus laser versus laser treatment only for diabetic macular edema which showed that treatment with IVTA plus macular laser resulted in a doubling of improvement in vision compared with laser only over 2 years in eyes with DME, but associated with cataract and raised intraocular pressure.
The role of IVTA as adjunctive treatment to panretinal photocoagulation (PRP) for patients with proliferative diabetic retinopathy (PDR) and DME is being evaluated, and clinical studies have demonstrated the effectiveness of combination in preventing exacerbations of macular edema with improvement in visual acuity and macular thickness in patients having PDR and DME[49-51,63,64]. However, a recent study demonstrated no beneficial effect of combined IVTA plus PRP and macular photocoagulation in coexisting high-risk PDR and DME in terms of visual acuity and macular thickness compared with standard treatment[65].
Nevertheless, the utilization of IVTA is not free of risks. The most common side-effects of IVTA are steroid-induced elevation of IOP varying from 20% to 70%, cataract in about 15%-20%, and crystalline maculopathy[18,19,21,22,52,66-71]. Other complications include endophthalmitis, intraocular hemorrhages, detachment of the retina[52,70,71-74]. In order to reduce the risks , complications and frequent dosing of intravitreal steroid , intravitreal steroid sustained-release implants have been developed.
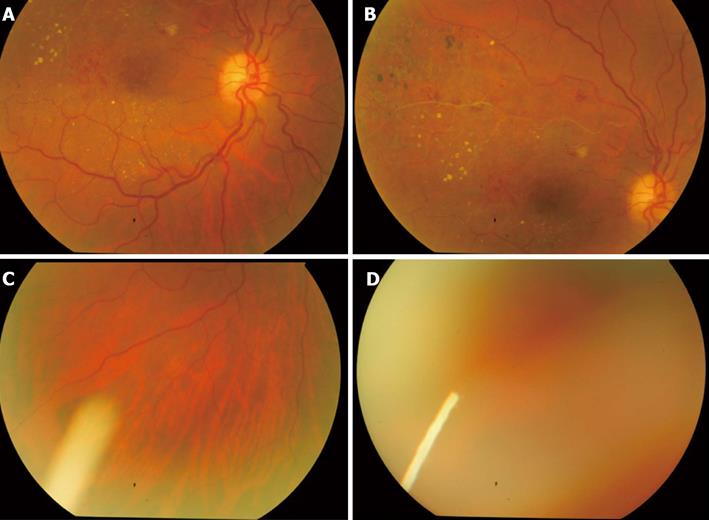
Ozurdex (Allergan, Irvine, CA, United States) is a biodegradable dexamethasone injectable intravitreal implant approved by the FDA to treat patients with macular edema due to retinal vein occlusion, as well as noninfectious posterior uveitis (Figure 3). Studies have shown the benefit of Ozurdex in treating DME with improvement in visual acuity and macular thickness[75,76]. Analysis of 171 eyes with persistent DME that were treated with either 0.7 mg or 0.35 mg of Ozurdex, a best corrected visual acuity (BCVA) improvement of 10 letters or more was seen in more eyes in the 0.7 mg group (33.3%) and 0.35 mg group (21.1%) than the observation group (12.3%; P = 0.007) with decreased central foveal thickness and leakage on fluorescein angiogram at 90 d compared to observation[75]. However, at 180 d, no significant difference was found between Ozurdex groups and the observation group. There was no significant difference in the number of patients with cataract among the study groups. Both the 0.7-mg and 0.35-mg group had IOP elevation, 15% of patients who had received Ozurdex implants had an IOP increase of 10 mmHg or more from baseline, compared with 2% among patients from the observation group. All cases were successfully managed by observation or with topical IOP lowering medications. However, the rate of IOP elevation was lower than what has been reported for IVTA[75]. In addition, 0.7 mg of Ozurdex for treatment-resistant DME in vitrectomized eyes has been evaluated prospectively and showed significant improvement in both vision and vascular leakage from diabetic macular edema at 26 wk compared to baseline and may have a role in management of difficult to treat DME in vitrectomized eyes with acceptable safety profile[75,76].
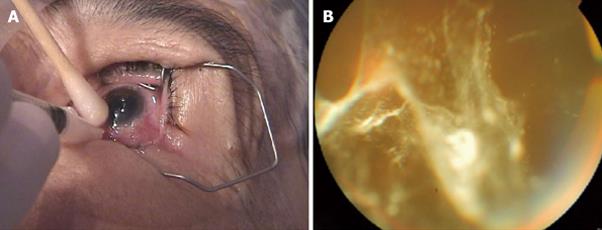
Retisert (Bausch and Lomb, Rochester, NY, United States) is another non-biodegradable sustained-release fluocinolone acetonide intravitreal implants that is approved by the FDA for the treatment of non-infectious uveitis. It is designed to release 0.59 mg/d of drug for about two-and-a-half years. Recently, the 3-year efficacy and safety results of a 4-year study evaluating fluocinolone acetonide (FA) intravitreal implants in eyes with persistent or recurrent DME has been published. They included 196 eyes with refractory DME. Patients were randomized 2:1 to receive 0.59-mg FA implant (n = 127) or standard of care (SOC additional laser or observation; n = 69). The implant was inserted through a pars plana incision. Overall, VA improved 3 lines in 16.8% of implanted eyes at 6 mo (P = 0.0012; SOC, 1.4%); in 16.4% at 1 year (P = 0.1191; SOC, 8.1%); in 31.8% at 2 years (P = 0.0016; SOC, 9.3%); and in 31.1% at 3 years (P = 0.1566; SOC, 20.0%). The number of implanted eyes with no evidence of retinal thickening at the center of the macula was higher than SOC eyes at 6 mo (P < 0.0001), 1 year (P < 0.0001; 72% vs 22%), 2 years (P = 0.016), and 3 years (P = 0.861). The most common adverse events included cataract progression in 91% of phakic eyes, and about 61% of implanted eyes had an IOP of ≥ 30 mmHg at any time and 33.8% required glaucoma surgery by 4 years. Despite cataract progression and elevated IOP, the 0.59-mg FA intravitreal implant significantly improved VA, Diabetic Retinopathy Severity Score, reduced central macular thickening, and may be used as an effective treatment for eyes with persistent or recurrent DME[77].
Iluvien (Alimera Sciences, Alpharetta, GA, United States) is another FA implant, which is biodegradable and releases FA at a rate of either 0.2 or 0.5 μg per day for up to three years. The Fluocinolone Acetonide for Macular Edema (FAME) studies conducted over a 36-mo period included a total of 956 patients with DME. The study consisted of two separate prospective, randomized, controlled, double-masked, multicenter clinical trials conducted to assess the efficacy and safety of low dose (releasing 0.2 μg/d) and high dose (0.5 μg/d) intravitreal FA (Iluvien) in patients with DME. At 24 mo, 28.7% of the low-dose and 28.6% of the high-dose group had 15 or more letter improvement, compared to 16.2% in the sham group (P = 0.002 for each)[78]. At 24 mo, 3.7% of the low-dose, 7.6% of the high-dose, and 0.5% of the sham group required incisional glaucoma surgery. Cataract developed more frequently in the treatment group, with about 75% of the initially phakic patients undergoing cataract surgery at 24 mo[78]. At month 36, the percentage of patients who gained ≥ 15 in letter was 28.7% (low dose) and 27.8% (high dose) in the FA insert groups compared with 18.9% (P = 0.018) in the sham group[79]. Preplanned subgroup analysis demonstrated a doubling of benefit compared with sham injections in patients who reported duration of DME ≥ 3 years at baseline; the percentage who gained ≥ 15 in letter score at month 36 was 34.0% (low dose; P < 0.001) or 28.8% (high dose; P = 0.002) compared with 13.4% (sham). At 36 mo, almost all phakic patients in the FA insert groups developed cataract, but their visual benefit after cataract surgery was similar to that in pseudophakic patients. The incidence of incisional glaucoma surgery at month 36 was 4.8% in the low-dose group and 8.1% in the high-dose insert group[80].
Novel delivery approaches using sustained release approaches are likely to provide much needed benefit for patients with refractory DME resistant to more conventional and conservative therapy, though long-term data is so far not known. The need to perform initial surgery as well as the need to remove the empty device could be considered a hurdle to use non-biodegradable delivery systems releasing corticosteroids, also with many concerns about the side effects. Compared to other steroid systems, the biodegradable dexamethasone delivery system is easier to apply and does not need to be removed. The safety and performance of an applicator-inserted dexamethasone drug delivery system was evaluated and also compared to the incisional placement. The results have revealed that the applicator-inserted dexamethasone drug delivery system performed well and effectively, and provided safe sutureless intravitreal placement of dexamethasone delivery system[79].
Despite the documented ocular side effects of corticosteroids, they are still considered as one of the effective adjunct modalities for the treatment of DME especially for refractory and persistent cases that failed to respond to standard conventional laser photocoagulation or anti-angiogenics. Corticosteroids have the advantage of repeatability, as long as the IOP and cataract side effects are not significant, due to their potent anti-inflammatory and anti-vascular endothelial growth factor effect that maintain and help in reducing blood retinal barrier disruption in diabetic patients. However recently, in order to reduce the risks, minimized the side effects and frequent dosing of intravitreal steroid injections, intravitreal steroid sustained-release implants have been developed.
| 1. | Kaji Y, Usui T, Ishida S, Yamashiro K, Moore TC, Moore J, Yamamoto Y, Yamamoto H, Adamis AP. Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Invest Ophthalmol Vis Sci. 2007;48:858-865. |
| 2. | Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450-1452. |
| 3. | Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133:70-77. |
| 4. | Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408-2413. |
| 5. | Murata T, Ishibashi T, Khalil A, Hata Y, Yoshikawa H, Inomata H. Vascular endothelial growth factor plays a role in hyperpermeability of diabetic retinal vessels. Ophthalmic Res. 1995;27:48-52. |
| 6. | Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54:1-32. |
| 7. | Stefa’nsson E. Diabetic Macular Edema. Saudi Journal of Ophthalmology. 2009;23:1483-1488. |
| 8. | Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RC. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002;80:667-677. |
| 9. | Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005;80:249-258. |
| 10. | Fischer S, Renz D, Schaper W, Karliczek GF. In vitro effects of dexamethasone on hypoxia-induced hyperpermeability and expression of vascular endothelial growth factor. Eur J Pharmacol. 2001;411:231-243. |
| 11. | Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341:309-315. |
| 12. | Nauck M, Roth M, Tamm M, Eickelberg O, Wieland H, Stulz P, Perruchoud AP. Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. Am J Respir Cell Mol Biol. 1997;16:398-406. |
| 13. | Sørensen TL, Haamann P, Villumsen J, Larsen M. Intravitreal triamcinolone for macular oedema: efficacy in relation to aetiology. Acta Ophthalmol Scand. 2005;83:67-70. |
| 14. | Sivaprasad S, McCluskey P, Lightman S. Intravitreal steroids in the management of macular oedema. Acta Ophthalmol Scand. 2006;84:722-733. |
| 15. | Ip MS. Intravitreal injection of triamcinolone: an emerging treatment for diabetic macular edema. Diabetes Care. 2004;27:1794-1797. |
| 16. | Bakri SJ, Kaiser PK. Posterior subtenon triamcinolone acetonide for refractory diabetic macular edema. Am J Ophthalmol. 2005;139:290-294. |
| 17. | Entezari M, Ahmadieh H, Dehghan MH, Ramezani A, Bassirnia N, Anissian A. Posterior sub-tenon triamcinolone for refractory diabetic macular edema: a randomized clinical trial. Eur J Ophthalmol. 2005;15:746-750. |
| 18. | Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R, Caulin C, Gaudric A. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218-224; discussion 224-225. |
| 19. | Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533-1538. |
| 20. | Ciardella AP, Klancnik J, Schiff W, Barile G, Langton K, Chang S. Intravitreal triamcinolone for the treatment of refractory diabetic macular oedema with hard exudates: an optical coherence tomography study. Br J Ophthalmol. 2004;88:1131-1136. |
| 21. | Jonas JB, Kreissig I, Söfker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003;121:57-61. |
| 22. | Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E, Baumal C. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920-927. |
| 23. | Ramezani A, Ahmadieh H, Tabatabaei H. Intravitreal triamcinolone reinjection for refractory diabetic macular edema. Korean J Ophthalmol. 2006;20:156-161. |
| 24. | Chew E, Strauber S, Beck R, Aiello LP, Antoszyk A, Bressler N, Browning D, Danis R, Fan J, Flaxel C. Randomized trial of peribulbar triamcinolone acetonide with and without focal photocoagulation for mild diabetic macular edema: a pilot study. Ophthalmology. 2007;114:1190-1196. |
| 25. | Jonas JB, Söfker A. Intraocular injection of crystalline cortisone as adjunctive treatment of diabetic macular edema. Am J Ophthalmol. 2001;132:425-427. |
| 26. | Jonas JB, Akkoyun I, Kreissig I, Degenring RF. Diffuse diabetic macular oedema treated by intravitreal triamcinolone acetonide: a comparative, non-randomised study. Br J Ophthalmol. 2005;89:321-326. |
| 27. | A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447-1449. |
| 28. | Yalcinbayir O, Gelisken O, Kaderli B, Avci R. Intravitreal versus sub-tenon posterior triamcinolone injection in bilateral diffuse diabetic macular edema. Ophthalmologica. 2011;225:222-227. |
| 29. | Inoue M, Takeda K, Morita K, Yamada M, Tanigawara Y, Oguchi Y. Vitreous concentrations of triamcinolone acetonide in human eyes after intravitreal or subtenon injection. Am J Ophthalmol. 2004;138:1046-1048. |
| 30. | Cardillo JA, Melo LA, Costa RA, Skaf M, Belfort R, Souza-Filho AA, Farah ME, Kuppermann BD. Comparison of intravitreal versus posterior sub-Tenon’s capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology. 2005;112:1557-1563. |
| 31. | Mueller AJ, Jian G, Banker AS, Rahhal FM, Capparelli E, Freeman WR. The effect of deep posterior subtenon injection of corticosteroids on intraocular pressure. Am J Ophthalmol. 1998;125:158-163. |
| 32. | Agrawal S, Agrawal J, Agrawal TP. Conjunctival ulceration following triamcinolone injection. Am J Ophthalmol. 2003;136:539-540. |
| 33. | Choi YJ, Oh IK, Oh JR, Huh K. Intravitreal versus posterior subtenon injection of triamcinolone acetonide for diabetic macular edema. Korean J Ophthalmol. 2006;20:205-209. |
| 34. | Levin DS, Han DP, Dev S, Wirostko WJ, Mieler WF, Connor TB, George V, Eastwood D. Subtenon’s depot corticosteroid injections in patients with a history of corticosteroid-induced intraocular pressure elevation. Am J Ophthalmol. 2002;133:196-202. |
| 35. | Jonas JB, Kamppeter BA, Harder B, Vossmerbaeumer U, Sauder G, Spandau UH. Intravitreal triamcinolone acetonide for diabetic macular edema: a prospective, randomized study. J Ocul Pharmacol Ther. 2006;22:200-207. |
| 36. | Avci R, Kaderli B, Akalp FD. Intravitreal triamcinolone injection for chronic diffuse diabetic macular oedema. Clin Experiment Ophthalmol. 2006;34:27-32. |
| 37. | Desatnik H, Habot-Wilner Z, Alhalel A, Moroz I, Glovinsky J, Moisseiev J. The transient efficacy of a single intravitreal triamcinolone acetonide injection for diabetic macular edema. Isr Med Assoc J. 2006;8:383-387. |
| 38. | Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trial. Ophthalmology. 2004;111:2044-2049. |
| 39. | Micelli Ferrari T, Sborgia L, Furino C, Cardascia N, Ferreri P, Besozzi G, Sborgia C. Intravitreal triamcinolone acetonide: valuation of retinal thickness changes measured by optical coherence tomography in diffuse diabetic macular edema. Eur J Ophthalmol. 2004;14:321-324. |
| 40. | Jonas JB, Degenring RF, Kamppeter BA, Kreissig I, Akkoyun I. Duration of the effect of intravitreal triamcinolone acetonide as treatment for diffuse diabetic macular edema. Am J Ophthalmol. 2004;138:158-160. |
| 41. | Jonas JB, Harder B, Kamppeter BA. Inter-eye difference in diabetic macular edema after unilateral intravitreal injection of triamcinolone acetonide. Am J Ophthalmol. 2004;138:970-977. |
| 42. | Avitabile T, Longo A, Reibaldi A. Intravitreal triamcinolone compared with macular laser grid photocoagulation for the treatment of cystoid macular edema. Am J Ophthalmol. 2005;140:695-702. |
| 43. | Lam DS, Chan CK, Mohamed S, Lai TY, Li KK, Li PS, Tsang CW, Chan WM, Shanmugam MP. A prospective randomised trial of different doses of intravitreal triamcinolone for diabetic macular oedema. Br J Ophthalmol. 2007;91:199-203. |
| 44. | Audren F, Lecleire-Collet A, Erginay A, Haouchine B, Benosman R, Bergmann JF, Gaudric A, Massin P. Intravitreal triamcinolone acetonide for diffuse diabetic macular edema: phase 2 trial comparing 4 mg vs 2 mg. Am J Ophthalmol. 2006;142:794-799. |
| 45. | Spandau UH, Derse M, Schmitz-Valckenberg P, Papoulis C, Jonas JB. Dosage dependency of intravitreal triamcinolone acetonide as treatment for diabetic macular oedema. Br J Ophthalmol. 2005;89:999-1003. |
| 46. | Chan CK, Mohamed S, Shanmugam MP, Tsang CW, Lai TY, Lam DS. Decreasing efficacy of repeated intravitreal triamcinolone injections in diabetic macular oedema. Br J Ophthalmol. 2006;90:1137-1141. |
| 47. | Jonas JB, Spandau UH, Kamppeter BA, Vossmerbaeumer U, Harder B, Sauder G. Repeated intravitreal high-dosage injections of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology. 2006;113:800-804. |
| 48. | Jonas JB, Kreissig I, Degenring RF, Kamppeter BA. Repeated intravitreal injection of triamcinolone acetonide for diffuse diabetic macular oedema. Br J Ophthalmol. 2005;89:122. |
| 49. | Zein WM, Noureddin BN, Jurdi FA, Schakal A, Bashshur ZF. Panretinal photocoagulation and intravitreal triamcinolone acetonide for the management of proliferative diabetic retinopathy with macular edema. Retina. 2006;26:137-142. |
| 50. | Bandello F, Polito A, Pognuz DR, Monaco P, Dimastrogiovanni A, Paissios J. Triamcinolone as adjunctive treatment to laser panretinal photocoagulation for proliferative diabetic retinopathy. Arch Ophthalmol. 2006;124:643-650. |
| 51. | Zacks DN, Johnson MW. Combined intravitreal injection of triamcinolone acetonide and panretinal photocoagulation for concomitant diabetic macular edema and proliferative diabetic retinopathy. Retina. 2005;25:135-140. |
| 52. | Jonas JB. Intravitreal triamcinolone acetonide for diabetic retinopathy. Dev Ophthalmol. 2007;39:96-110. |
| 53. | Dehghan MH, Ahmadieh H, Ramezani A, Entezari M, Anisian A. A randomized, placebo-controlled clinical trial of intravitreal triamcinolone for refractory diabetic macular edema. Int Ophthalmol. 2008;28:7-17. |
| 54. | Yilmaz T, Weaver CD, Gallagher MJ, Cordero-Coma M, Cervantes-Castaneda RA, Klisovic D, Lavaque AJ, Larson RJ. Intravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: a systematic review. Ophthalmology. 2009;116:902-911; quiz 912-913. |
| 55. | Rudnisky CJ, Lavergne V, Katz D. Visual acuity after intravitreal triamcinolone for diabetic macular edema refractory to laser treatment: a meta-analysis. Can J Ophthalmol. 2009;44:587-593. |
| 56. | Takata C, Messias A, Folgosa MS, Lucena LR, Lucena DR, Scott IU, Jorge R. Intravitreal injection versus subtenon infusion of triamcinolone acetonide during cataract surgery in patients with refractory diabetic macular edema. Retina. 2010;30:562-569. |
| 57. | Isaac DL, Abud MB, Frantz KA, Rassi AR, Avila M. Comparing intravitreal triamcinolone acetonide and bevacizumab injections for the treatment of diabetic macular oedema: a randomized double-blind study. Acta Ophthalmol. 2012;90:56-60. |
| 58. | Ip MS, Bressler SB, Antoszyk AN, Flaxel CJ, Kim JE, Friedman SM, Qin H. A randomized trial comparing intravitreal triamcinolone and focal/grid photocoagulation for diabetic macular edema: baseline features. Retina. 2008;28:919-930. |
| 59. | Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, Hartnett E, Ip MS, Kim JE, Kollman C. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245-251. |
| 60. | Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL, Friedman SM, Glassman AR, Miller KM. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064-1077.e35. |
| 61. | Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL, Friedman SM, Glassman AR, Scott IU, Stockdale CR, Sun JK. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609-614. |
| 62. | Gillies MC, McAllister IL, Zhu M, Wong W, Louis D, Arnold JJ, Wong TY. Intravitreal triamcinolone prior to laser treatment of diabetic macular edema: 24-month results of a randomized controlled trial. Ophthalmology. 2011;118:866-872. |
| 63. | Bandello F, Pognuz DR, Pirracchio A, Polito A. Intravitreal triamcinolone acetonide for florid proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2004;242:1024-1027. |
| 64. | Maia OO, Takahashi BS, Costa RA, Scott IU, Takahashi WY. Combined laser and intravitreal triamcinolone for proliferative diabetic retinopathy and macular edema: one-year results of a randomized clinical trial. Am J Ophthalmol. 2009;147:291-297.e2. |
| 65. | Mirshahi A, Shenazandi H, Lashay A, Faghihi H, Alimahmoudi A, Dianat S. Intravitreal triamcinolone as an adjunct to standard laser therapy in coexisting high-risk proliferative diabetic retinopathy and clinically significant macular edema. Retina. 2010;30:254-259. |
| 66. | Jonas JB, Kreissig I, Degenring R. Secondary chronic open-angle glaucoma after intravitreal triamcinolone acetonide. Arch Ophthalmol. 2003;121:729-730. |
| 67. | Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003;87:24-27. |
| 68. | Wingate RJ, Beaumont PE. Intravitreal triamcinolone and elevated intraocular pressure. Aust NZJ Ophthalmol. 1999;27:431-432. |
| 69. | Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004;138:740-743. |
| 70. | Moshfeghi AA, Flynn HW Jr: Complications of intravitreal triamcinolone injection. Rev Ophthalmol. 2004;4:64-67. |
| 71. | Sarraf D, Vyas N, Jain A, Bui A, Kertes PJ, Freund KB, Chan C. Triamcinolone-associated crystalline maculopathy. Arch Ophthalmol. 2010;128:685-690. |
| 72. | Moshfeghi DM, Kaiser PK, Scott IU, Sears JE, Benz M, Sinesterra JP, Kaiser RS, Bakri SJ, Maturi RK, Belmont J. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003;136:791-796. |
| 73. | Nelson ML, Tennant MT, Sivalingam A, Regillo CD, Belmont JB, Martidis A. Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina. 2003;23:686-691. |
| 74. | Moshfeghi AA, Scott IU, Flynn HW, Puliafito CA. Pseudohypopyon after intravitreal triamcinolone acetonide injection for cystoid macular edema. Am J Ophthalmol. 2004;138:489-492. |
| 75. | Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA, Weinberg DV, Chou C, Whitcup SM. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128:289-296. |
| 76. | Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li XY, Liu CC, Lou J, Whitcup SM. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31:915-923. |
| 77. | Pearson PA, Comstock TL, Ip M, Callanan D, Morse LS, Ashton P, Levy B, Mann ES, Eliott D. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology. 2011;118:1580-1587. |
| 78. | Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, Tolentino M, Gupta A, Duarte L, Madreperla S. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626-635.e2. |
| 79. | Haller JA, Dugel P, Weinberg DV, Chou C, Whitcup SM. Evaluation of the safety and performance of an applicator for a novel intravitreal dexamethasone drug delivery system for the treatment of macular edema. Retina. 2009;29:46-51. |
| 80. | Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, Garretson B, Gupta A, Hariprasad SM, Bailey C. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125-2132. |
P- Reviewer: List JF S- Editor: Wen LL L- Editor: A E- Editor: Wu HL