INVOLVEMENT OF OXIDATIVE STRESS IN THE DETERIORATION OF β-CELL FUNCTION FOUND IN TYPE 2 DIABETES
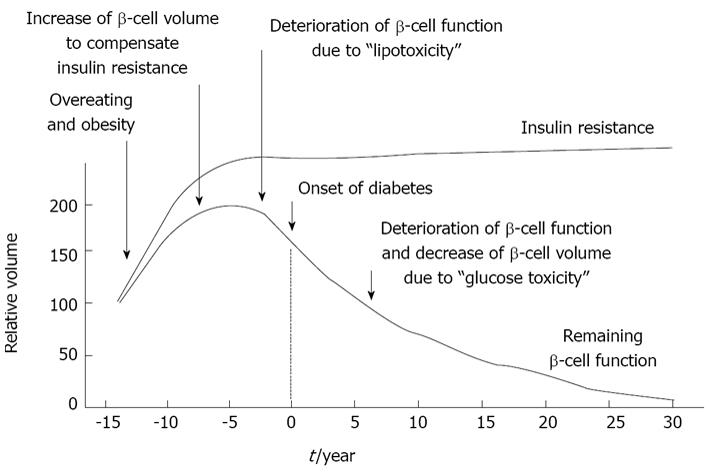
The development of type 2 diabetes is associated with pancreatic β-cell dysfunction and insulin resistance. First, overeating and/or obesity lead to the development of insulin resistance and normal β-cells secrete a larger amount of insulin to compensate for the increased insulin resistance. Next, large adipocytes secrete a larger amount of free fatty acids (FFAs) and/or various inflammatory cytokines which gradually deteriorate β-cell function and finally lead to the onset of diabetes. This process is known as “β-cell lipotoxicity”. Indeed, it has been reported that when islets or β-cell-derived cell line are exposed to FFAs, oxidative stress is induced, which leads to the reduction of insulin secretion[1-5]. It has also been reported that FFA-mediated induction of inducible nitric oxide synthase (iNOS) and excess nitric oxide (NO) generation are involved in the progression of β-cell dysfunction[6]. Once hyperglycemia becomes apparent, β-cell function such as insulin biosynthesis and secretion progressively deteriorates. This process is known as “β-cell glucose toxicity” which is often observed under diabetic conditions. In the diabetic state, hyperglycemia per se and subsequent induction of oxidative stress decrease insulin biosynthesis and secretion and finally bring about apoptosis[7-28] (Figure 1).
Figure 1 Typical progress of type 2 diabetes.
The development of type 2 diabetes is associated with pancreatic β-cell dysfunction and insulin resistance. First, overeating and/or obesity lead to the development of insulin resistance and normal β-cells secrete a larger amount of insulin to compensate for the increased insulin resistance. Next, large adipocytes secrete a larger amount of free fatty acids and/or various inflammatory cytokines which gradually deteriorate β-cell function and finally lead to the onset of diabetes. This process is known as “β-cell lipotoxicity”. Once hyperglycemia becomes apparent, β-cell function progressively deteriorates; insulin biosynthesis and secretion are reduced. This process is known as “β-cell glucose toxicity” which is often observed in type 2 diabetes.
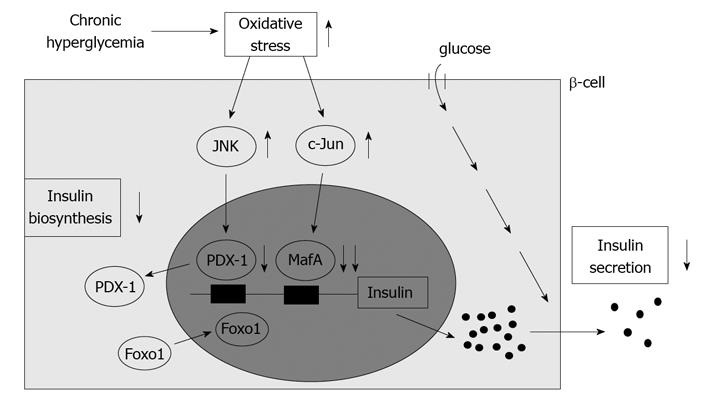
Under diabetic conditions, oxidative stress is induced and involved in the β-cell glucose toxicity[22-36]. β-cells express GLUT2, a high-Km glucose transporter, and thereby display highly efficient glucose uptake when exposed to a high glucose concentration. Indeed, it was shown that expression levels of oxidative stress markers such as 8-hydroxy-2'-deoxyguanosine (8-OHdG) and 4-hydroxy-2, 3-nonenal (4-HNE) were increased in islets under diabetic conditions[14,16]. In addition, β-cells are rather vulnerable to oxidative stress due to the relatively low expression of antioxidant enzymes such as catalase and glutathione peroxidase. Therefore, it is likely that oxidative stress is involved in the deterioration of β-cell function found in diabetes. It was shown that when β-cell-derived cell lines or isolated islets were exposed to oxidative stress, insulin gene promoter activity and mRNA expression were suppressed[19-26]. In addition, when they were exposed to oxidative stress, bindings of pancreatic transcription factors PDX-1 and/or MafA to the insulin gene promoter were reduced. It is noted here that PDX-1 plays a crucial role in pancreas development, β-cell differentiation, induction of surrogate β-cells, and maintenance of mature β-cell function[29-41] and that MafA is a β-cell-specific transcription factor and functions as a potent activator of insulin gene transcription[42-47]. Furthermore, it was shown that the decrease of insulin gene expression after chronic exposure to a high glucose concentration was prevented by treatment with antioxidants[16,19,25,26]. Reduction of expression and/or DNA binding activities of PDX-1 and/or MafA by chronic exposure to high glucose was also prevented by an antioxidant treatment. These results suggest that chronic hyperglycemia suppresses insulin biosynthesis and secretion by increasing oxidative stress, accompanied by reduction of expression and/or DNA binding activities of two important pancreatic transcription factors, PDX-1 and MafA. Therefore, it is likely that the alteration of such transcription factors explains, at least in part, the suppression of insulin biosynthesis and secretion, and thereby is involved in β-cell glucose toxicity (Figure 2).
Figure 2 Possible molecular mechanism for suppression of insulin biosynthesis in type 2 diabetes.
Under diabetic conditions, hyperglycemia induces oxidative stress and thereby leads to suppression of insulin biosynthesis and secretion which is accompanied by reduction of nuclear pancreatic duodenal homeobox 1 (PDX-1) and MafA expression. Oxidative stress and subsequent activation of the JNK pathway translocate Foxo1 from cytoplasm to nuclei, leading to translocation of PDX-1 from nuclei to cytoplasm in β-cells. In addition, oxidative stress and subsequent induction of c-Jun expression suppress nuclear expression of MafA in β-cells. Therefore, it is likely that induction of oxidative stress and suppression of nuclear PDX-1 and MafA expression are involved in β-cell glucose toxicity found in type 2 diabetes.
MOLECULAR MECHANISM FOR DOWN-REGULATION OF NUCLEAR PDX-1 EXPRESSION UNDER DIABETIC CONDITIONS: POSSIBLE INVOLVEMENT OF OXIDATIVE STRESS AND SUBSEQUENT ACTIVATION OF THE JNK PATHWAY
It has been suggested that activation of the c-Jun N-terminal kinase (JNK) pathway is involved in pancreatic β-cell dysfunction found in type 2 diabetes. It was reported that activation of the JNK pathway is involved in reduction of insulin gene expression by oxidative stress and that suppression of the JNK pathway can protect β-cells from oxidative stress[48]. When isolated islets were exposed to oxidative stress, the JNK pathway was activated, preceding the decrease of insulin gene expression. Adenoviral overexpression of dominant-negative type JNK1 (DN-JNK) protected insulin gene expression and secretion from oxidative stress. These results were correlated with change in the binding of PDX-1 to insulin gene promoter. Adenoviral overexpression of DN-JNK preserved PDX-1 DNA binding activity in the face of oxidative stress, while wild type JNK (WT-JNK) overexpression decreased PDX-1 DNA binding activity[48]. Taken together, it is likely that activation of the JNK pathway leads to decreased PDX-1 activity and consequent suppression of insulin gene transcription found in the diabetic state. Also, it was shown that PDX-1 was transported from the nuclei to the cytoplasm in response to oxidative stress. When β-cell-derived HIT cells were exposed to oxidative stress, both intrinsically expressed PDX-1 and exogenously introduced PDX-1 moved from the nuclei to the cytoplasm[49]. DN-JNK overexpression inhibited the oxidative stress-induced PDX-1 translocation, suggesting that activation of the JNK pathway is involved in PDX-1 translocation by oxidative stress. Furthermore, leptomycin B, a specific inhibitor of the classical, leucine-rich nuclear export signal (NES), inhibited nucleo-cytoplasmic translocation of PDX-1 induced by oxidative stress[49]. Taken together, it is likely that oxidative stress induces nucleo-cytoplasmic translocation of PDX-1 through activation of the JNK pathway, which leads to reduction of its DNA binding activity and suppression of insulin biosynthesis (Figure 2).
The forkhead transcription factor Foxo1 is known as one of the important fundamental transcription factors playing a key role in the process of apoptosis, cellular proliferation and differentiation, and glucose metabolism through regulating the transcription of various target genes[50,51]. Foxo1 regulates hepatic gluconeogenesis and thus contributes to insulin resistance[52]. Insulin inhibits the function of Foxo1 through Akt/PKB-mediated phosphorylation and nuclear exclusion[53], and thereby suppresses hepatic gluconeogenesis. In addition, Foxo1 exhibits a counter localization to PDX-1 in β-cells[54], suggesting that it is involved in the deterioration of β-cell function. Moreover, Foxo1 plays a role as a mediator between the JNK pathway and PDX-1[55]. In β-cell-derived cell line HIT cells, Foxo1 changed its intracellular localization from the cytoplasm to the nucleus after exposure to oxidative stress. In contrast to Foxo1, the nuclear expression of PDX-1 was decreased and its cytoplasmic distribution was increased by oxidative stress. Activation of the JNK pathway also induced the nuclear localization of Foxo1, whereas suppression of the JNK pathway reduced the oxidative stress-induced nuclear localization of Foxo1, suggesting an involvement of the JNK pathway in Foxo1 translocation[55]. In addition, oxidative stress or activation of the JNK pathway decreased Akt phosphorylation in HIT cells, leading to the decreased phosphorylation of Foxo1 following nuclear localization. Furthermore, adenoviral Foxo1 overexpression reduced the nuclear expression of PDX-1, whereas suppression of Foxo1 by Foxo1-specific small interfering RNA retained the nuclear expression of PDX-1[55]. Taken together, oxidative stress and subsequent activation of the JNK pathway induce nuclear translocation of Foxo1 through the modification of the insulin signaling in β-cells, which leads to the nucleo-cytoplasmic translocation of PDX-1 and reduction of its DNA binding activity (Figure 2).
MOLECULAR MECHANISM FOR DOWN-REGULATION OF NUCLEAR MAFA EXPRESSION UNDER DIABETIC CONDITIONS: POSSIBLE INVOLVEMENT OF OXIDATIVE STRESS AND SUBSEQUENT INDUCTION OF C-JUN EXPRESSION
It is known that c-Jun protein level and activity are increased in response to oxidative stress in various cells[56,57]. We recently reported that c-Jun expression was not clearly detected in islets of control m/m mice and young diabetic db/db mice, but that the number of c-Jun-positive cells gradually increased with age in the islets of diabetic db/db mice[58]. This expression pattern of c-Jun paralleled the loss of insulin gene transcription factor MafA expression. Quantitative real-time PCR analysis using freshly isolated islets from db/db mice clearly showed that c-Jun mRNA level was significantly increased but that both MafA and insulin mRNA levels were markedly decreased with age[58]. These results imply that the increased level of c-Jun caused a decrease in MafA and insulin gene expression in old diabetic mice. Furthermore, in immunostaining, in db/db mice nuclear MafA expression in pancreatic islets was markedly decreased with age and was not clearly detected in old mice, whereas in control m/m mice MafA expression retained up to old age[58]. In db/db mice insulin expression was also decreased in some β-cells in which MafA was undetectable or weakly expressed. Furthermore, MafA and insulin expression was suppressed in most c-Jun-positive β-cells. Similarly, in islets of diabetic KKAy mice, the number of c-Jun-positive β-cells was increased with marked hyperglycemia, and both MafA and insulin protein levels were decreased in those cells[58]. These findings suggest that c-Jun is involved in the suppression of MafA and insulin expression under diabetic conditions. In addition, c-Jun overexpression markedly decreased insulin promoter activity, which was consistent with previous reports[59,60] (Figure 2).
Although c-Jun protein expression was almost undetectable in MIN6 cells, adenoviral c-Jun overexpression markedly suppressed MafA protein level and its DNA-binding activity in MIN6 cells[58]. Northern blotting and real-time PCR analysis also showed that c-Jun overexpression significantly suppressed MafA mRNA level. Adenoviral overexpression of c-Jun in isolated mouse islets also markedly suppressed MafA mRNA and protein levels. Consistent with these results, mRNA levels of insulin 1 and 2 and insulin content were suppressed by c-Jun overexpression in both MIN6 cells and islets[58]. These findings directly demonstrate that c-Jun suppresses the expression of both MafA and insulin. In addition, since MafA appears to not only regulate insulin expression but also to be involved in insulin secretion[61,62], it is likely that the suppression of MafA protein levels by c-Jun leads to insulin secretory defects that are often observed under diabetic conditions. In conclusion, the augmented expression of c-Jun in diabetic islets decreases MafA activity followed by reduced insulin biosynthesis and secretion, and thereby explains, at least in part, the molecular mechanism for β-cell glucose toxicity that is often observed in type 2 diabetes (Figure 2).
MOLECULAR MECHANISM FOR DOWN-REGULATION OF INCRETIN RECEPTOR EXPRESSION IN β-CELLS UNDER DIABETIC CONDITIONS: POSSIBLE INVOLVEMENT OF HYPERGLYCEMIA AND SUBSEQUENT REDUCTION OF TCF7L2 EXPRESSION
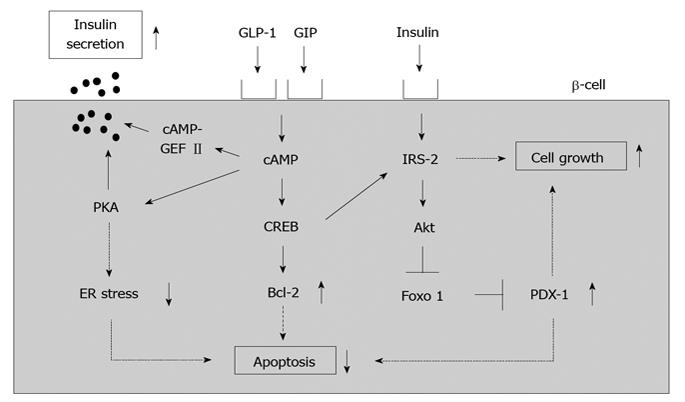
The incretin effect causes more insulin to be secreted when glucose is orally taken compared to when given intravenously, even when blood glucose levels have the same profile. This effect is thought to be very important for maximizing insulin response during meals, thereby limiting postprandial glucose excursions. Two incretins have been identified: glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). It is thought that such incretins play an important role in glucose homeostasis by promoting insulin secretion immediately on meal ingestion. It is well known that incretins (GLP-1 and GIP) bind their incretin receptors (GLP-1 and GIP receptors) in β-cells and increase intracellular cAMP levels, leading to stimulation of insulin secretion, suppression of β-cell apoptosis and increase of β-cell growth (Figure 3).
Figure 3 A role of incretin signaling in pancreatic β-cells.
Incretins (GLP-1 and GIP) bind their incretin receptors (GLP-1 and GIP receptors) in β-cells and increase intracellular cAMP levels, leading to stimulation of insulin secretion, suppression of β-cell apoptosis and increase of β-cell growth.
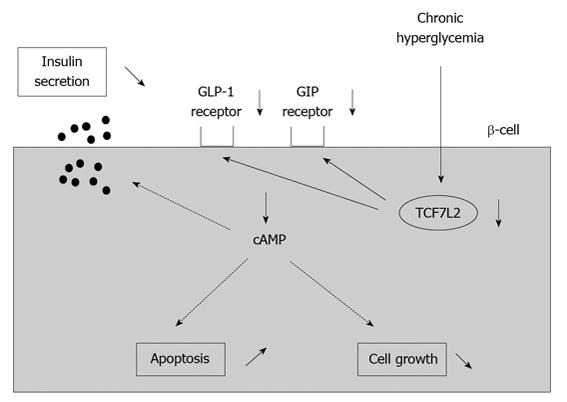
Although plasma GLP-1 and GIP levels after meals are almost normal in type 2 diabetes, striking abnormalities are observed in the action of incretin hormones in type 2 diabetes[63]. It was reported that GLP-1 and GIP receptor expression was decreased in a glucose-dependent manner in islets isolated from 90% pancreatectomized diabetic (Px) rats[64]. Such decrease was not observed after normalization of blood glucose levels with phlorizin which is known to lower blood glucose levels by preventing glucose reabsorption from the glomerular filtrate in the kidney. These results suggest that hyperglycemia per se leads to down-regulation of GLP-1 and GIP receptor expression. Furthermore, insulin response to GLP-1 or GIP was markedly reduced in islets isolated from diabetic rats compared to those from control rats[65]. These results indicate that down-regulation of GLP-1 and GIP receptor expression leads to the deterioration of β-cell function. Similar results were reported in obese type 2 diabetic db/db mice; incretin receptor expression in islets was markedly decreased at 16 wk of age in db/db mice but was preserved by normalization of blood glucose levels with insulin therapy[66]. These results strengthen the hypothesis that hyperglycemia per se leads to down-regulation of GLP-1 and GIP receptor expression (Figure 4).
Figure 4 Down-regulation of incretin receptors in pancreatic β-cells under diabetic conditions.
Under diabetic conditions, expression of incretin receptors (GLP-1 and GIP receptors) in β-cells are down-regulated, leading to decrease of insulin secretion, increase of β-cell apoptosis and decrease of β-cell growth.
Furthermore, down-regulation of GLP-1 and GIP receptor expression was observed in type 2 diabetic subjects, as observed in diabetic rodents[66]. In addition, it was shown that transcription factor TCF7L2 is involved in down-regulation of GLP-1 and GIP receptor expression found under diabetic conditions[66]. Recent human genetics studies have revealed that common variants of the TCF7L2 gene are strongly associated with type 2 diabetes mellitus. It was shown that expression levels of GLP-1 and GIP receptors were lower in islets of type 2 diabetic subjects as well as in isolated human islets treated with siRNA to TCF7L2 (siTCF7L2). Insulin secretion stimulated by glucose, GLP-1 or GIP was also impaired in siTCF7L2-treated isolated human islets. In conclusion, we think that the down-regulation of incretin receptors by hyperglycemia is largely responsible for the impaired incretin effects and thus, at least in part, explains the molecular mechanism for β-cell dysfunction found in diabetes (Figure 4).
P- Reviewers: Bilotta F, Hsieh PS, Hsu YH, Salles GF S- Editor: Cui XM L- Editor: Roemmele A E- Editor: Liu XM