INTRODUCTION
Diabetes mellitus affected more than 371 million people worldwide and the global expenditure for healthcare of diabetes in the year 2012 alone was more than 471 billion United States Dollars[1]. The disease affects almost every tissue in the body and causes significant organ dysfunction that results in diabetes-related morbidity and mortality. Cardiovascular diseases account for about 65% of diabetes-related mortality and therefore, the American Heart Association (AHA) accepted diabetes as coronary heart disease equivalent towards the turn of the 20th century[2].
Diabetes affects the heart in 3 ways: (1) coronary artery disease (CAD) due to accelerated atherosclerosis; (2) cardiac autonomic neuropathy (CAN); and (3) diabetic cardiomyopathy (DbCM). Although there is high awareness among clinicians about the first two disease entities, DbCM is poorly recognized by most physicians and diabetologists. The purpose of this review is to elaborate the pathophysiology, diagnostic evaluation and management options and to highlight the importance of early identification of DbCM to optimize the care of patients with diabetes.
DbCM was first described by Rubler et al[3] in 1972. DbCM is defined as myocardial dysfunction occurring in patients with diabetes in the absence of CAD, hypertension, or valvular heart disease[3,4]. Diabetes is a well-known risk factor for the development of heart failure and the Framingham Heart Study showed that the frequency of heart failure is double in diabetic men and five times in diabetic women compared to age-matched control subjects[5]. Heart failure reduces the quality of life of the affected individual and complicates the management of diabetes by alterations in the pharmacokinetics of anti-diabetic medications. Therefore, early diagnosis and prompt management of these patients are of utmost importance.
Epidemiology
The prevalence of different degrees of heart failure among diabetic subjects was as high as 19%-26% in different major clinical trials[6-8]. The actual prevalence of DbCM is not yet established, because of the lack of large study data from different populations with diabetes. The prevalence of diastolic dysfunction in patients with type 2 diabetes mellitus (T2DM) was shown to be up to 30% in some studies[9,10]. However, there are other studies which reported a prevalence as high as 40%-60%[11-13]. The small numbers of participants in all these studies limit their utility to estimate the true prevalence of the disease in a common disease like diabetes.
A recent major prospective study examining the prevalence of myocardial dysfunction (MD) and heart failure (HF) in patients with longstanding (≥ 10 years) type 1 diabetes mellitus (T1DM) showed a prevalence of 14.5% and 3.7% respectively at the end of a seven year follow up[14]. The annual incidence of MD and HF were 0.1% and 0.02% respectively. At baseline, diastolic HF constituted 85% of the cases with HF. Different patient selection criteria and various techniques of imaging used for diagnosis may explain the disparity in the reported prevalence of DbCM.
Pathogenesis and pathophysiology
The pathogenesis and pathophysiology of DbCM is not yet fully defined. The development of diabetic cardiomyopathy is multi-factorial. Various proposed mechanisms include metabolic disturbances, insulin resistance, microvascular disease, alterations in the renin-angiotensin system (RAS), cardiac autonomic dysfunction and myocardial fibrosis[15]. Chronic hyperglycemia is thought to play a central role in the development of DbCM, although multiple complex mechanisms and interplay of many molecular and metabolic events within the myocardium and plasma contribute to the pathogenesis.
The main metabolic abnormalities in diabetes are hyperglycemia, hyperlipidemia and inflammation, all of which stimulate generation of reactive oxygen or nitrogen species that cause most of the diabetic complications, including diabetic nephropathy and cardiomyopathy[16,17]. Several adaptive responses caused by these metabolic abnormalities finally result in cardiac dysfunction and heart failure.
HYPERGLYCEMIA AND THE HEART
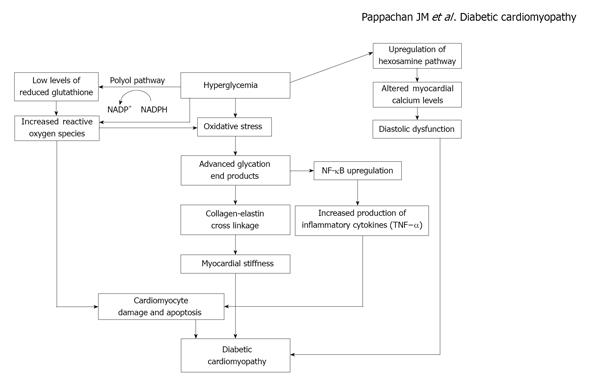
Chronic hyperglycemia results in a number of metabolic and molecular changes in the myocardial cells. Increased glucose metabolism due to hyperglycemia leads to an increase in oxidative stress by generation of reactive oxygen species (ROS) from mitochondria[18]. Overproduction of superoxide by the mitochondrial respiratory chain and the consequent oxidative stress result in reduction of myocardial contractility and eventually myocyte fibrosis[19]. ROS and oxidative stress can cause cellular DNA damage and acceleration of cardiomyocyte apoptosis.
DNA damage induced by oxidative stress also activates poly ADP ribose polymerase (PARP), a DNA reparative enzyme[20]. PARP diverts glucose metabolism from its usual glycolytic pathway (through inhibition of glyceraldehyde phosphate dehydrogenase) into alternative biochemical pathways that result in generation of various mediators which causes hyperglycemia induced cellular injury. These include advanced glycation end products (AGEs), increased flux of hexosamine and polyol, and activation of the enzyme protein kinase C.
Oxidative stress induced by chronic hyperglycemia has been shown to increase the AGEs in diabetic subjects[19]. AGEs can covalently crosslink various intra and extracellular proteins that is thought to be a pivotal factor in diabetic complications. The crosslink in collagen and elastin results in increased myocardial stiffness and impaired cardiac relaxation. AGEs are found to induce myocardial damage in both animals[21] and human beings[22].
AGEs also indirectly exert their detrimental effect on the myocardium by interacting and up-regulating their receptors, including receptors of AGE and galectin-3[21]. This results in activation of transcription factors, such as nuclear factor-κB (NF-κB). NF-κB dependent genes in turn trigger several pathways that induce production of pro-inflammatory cytokines such as Tumour necrosis factor-α and cause myocardial damage[23]. NF-κB blockers were found to attenuate mitochondrial oxidative stress and protect against cardiac dysfunction in diabetic mice[24].
Chronic hyperglycemia can lead to increased flux of glucose into the alternate metabolic pathway known as hexosamine pathway that is implicated in many adverse consequences of diabetes. Increased glucose metabolism in the hexosamine pathway is associated with disruption of normal cardiomyocyte calcium flux linked to reduced sequestration of calcium in the sarcoplasmic reticulum[25]. This results in reduction in myocardial performance and impaired diastolic relaxation, a possible mechanism for DbCM.
Polyol pathway is also activated by chronic hyperglycemia and glucose is converted to sorbitol by the action of the enzyme aldose reductase in the presence of nicotinic acid adenine dinucleotide phosphate (NADPH) that is oxidized to NADP+. NADPH is a co-factor essential for regeneration of reduced glutathione, an important scavenger of ROS in the body, and increased utilization of NADPH in the polyol pathway disturbs the redox balance of cells. The consequent increase in oxidative stress can lead on to DNA damage and cardiomyocyte apoptosis[26]. Sorbitol can also glycate proteins that results in formation of AGEs, which are mediators of tissue injury in diabetes[27,28]. A diagrammatic representation of cardiac damage resulting from hyperglycemia is shown in the Figure 1.
Figure 1 Mechanism of myocardial damage resulting from hyperglycemia.
NADPH: nicotinic acid adenine dinucleotide phosphate; NF-κB: Nuclear factor-κB; TNF-α: Tumour necrosis factor-α.
LIPID METABOLISM AND THE MYOCARDIUM
Overstrain of cellular oxidation capacity in diabetes leads on to ectopic lipid deposition in non-adipose tissues such as skeletal muscle, liver and heart. Cardiac steatosis (increased myocardial lipid content), resulting from disturbed myocardial substrate metabolism, has been proposed as an important cause for DbCM recently[29-31]. Hyperinsulinemia, hyperglycemia and elevated levels of plasma free fatty acids (FFA) are the classical metabolic abnormalities in T2DM that lead on to cardiac steatosis. Patients with diabetes, obesity and impaired glucose tolerance were found to have cardiac steatosis of varying degrees[29,30,32].
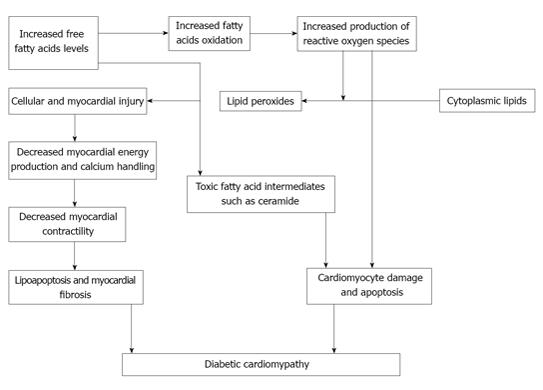
The contribution of glucose oxidation to cardiac energetics is less than normal among patients with obesity and T2DM and fatty acid metabolism is enhanced to meet the myocardial energy needs[33,34]. Increased plasma FFA levels in patients with T2DM and obesity, result in increased cardiac fatty acid (FA) uptake and triglyceride accumulation. Excessive FA delivery and uptake by cardiomyocytes in this setting is likely to exceed mitochondrial oxidative capacity and consequently leads on to lipotoxic cardiac injury. Part of the excess FA enters nonoxidative pathways, giving rise to toxic FA intermediates such as ceramide. These toxic substances in turn disrupt normal cellular signaling and cause mitochondrial dysfunction, cellular damage, apoptosis, and eventually myocardial fibrosis and contractile dysfunction.
Although some earlier studies showed predominantly diastolic dysfunction in diabetic patients with cardiac steatosis[29,30], recent evidence demonstrated biventricular systolic and diastolic dysfunction in patients with high myocardial triglyceride levels[35]. Intracellular accumulation of triglyceride alone is unlikely to be the cause of cardiac injury as it is relatively inert. However, the intermediate metabolites derived from nonoxidative pathways of intracellular lipid handling are probably responsible for lipotoxic tissue injury and eventual cellular apoptosis[36].
Increased FA oxidation in the mitochondria is associated with an increase in generation of ROS that oxidizes cytoplasmic lipids into lipid peroxides. ROS and lipid peroxides in turn cause cellular and mitochondrial damage and uncoupling of mitochondrial oxidative metabolism[37]. Consequently, impaired myocardial generation of energy and reduced cardiac contractility results. Decreased production of energy also leads to an impaired mitochondrial calcium handling that causes cardiac dysfunction[38].
Cell apoptosis that results from lipotoxicity is commonly referred to as lipoapoptosis. Different mechanisms such as palmitate toxicity, ceramide and diacylglycerol formation, endoplasmic reticulum stress, membrane destabilization and inflammation may result in lipoapoptosis[37]. Structural damage and myocardial fibrosis are the results of lipoapoptosis that compromise the cardiac function. Figure 2 shows a model of lipotoxic cardiac injury.
Figure 2 lipotoxic cardiac injury in diabetic cardiomyopathy.
Elevated levels of plasma FFA also induce cellular insulin resistance by various mechanisms[39]. These include activation of protein kinase C (PKC), and peroxisome proliferator-activated receptor-gamma and α (PPAR-γ and PPAR-α). PKC is a family of several isoenzymes that regulates various complex cellular metabolic pathways, and is thought to play a key role in generation of insulin resistance. Similarly, the activation of PPAR-γ and PPAR-α also results in hyperinsulinemia and insulin resistance, mediated through different complex mechanisms.
ROLE OF HYPERINSULINEMIA AND INSULIN RESISTANCE
Hyperinsulinemia and insulin resistance are the characteristic pathological abnormalities in T2DM and prediabetic states. Hyperinsulinemia results in cardiomyocyte hypertrophy by various mechanisms. Brain natriuretic peptide (BNP), a biomolecule released from the ventricles in response to myocardial stretch, has been found to be increased in patients with heart failure. BNP is also an important molecular marker of cardiac hypertrophy. BNP gene expression was found to be significantly higher among animal models of hyperinsulinemia and insulin resistance[40]. Left ventricular hypertrophy and increased left ventricular weight were also found in these animal models. Recently, BNP has emerged as a useful biomarker for screening subclinical ventricular diastolic dysfunction in patients with uncontrolled diabetes[40,41].
Hypertrophy of cardiac myocytes in diabetes was found to be regulated at the transcriptional level[42]. Various genetic and epigenetic alterations resulting from hyperinsulinemia, leads on to activation of multiple transcription factors that modulate cellular and extracellular protein expression. Activation of such transcription factors have been shown to result in cardiomyocyte hypertrophy and deposition of extracellular matrix proteins causing focal cardiac fibrosis in diabetes[42,43].
CONTRIBUTION FROM MICROVASCULAR ISCHEMIA
The pathological hallmark of diabetes-related vascular complications is damage to the microvasculature throughout the body. Classical examples of microvascular complications are diabetic retinopathy, nephropathy and neuropathy. Hyperglycemia confounded by other factors such as hypertension, lipid abnormalities and smoking impose oxidative stress on the vascular endothelium that leads on to endothelial dysfunction, the earliest abnormality in patients with diabetes. Nitric oxide (an endothelium-derived vasodilatory factor) production in relation to vascular stretch is also reduced due to down regulation of endothelial nitric oxide synthase enzyme in diabetes[44,45].
Hyaline change (amorphous, ground-glass appearance resulting from breakdown of structural proteins like collagen) of the medial layers of arterioles and reduction of capillary length density throughout the cardiac circulation is seen in diabetics[15,46]. The reduced blood supply resulting from microvascular disease affecting the vasa vasorum in diabetes, further damages the small and medium arterioles of the diabetic heart. Thickening of the capillary basement membrane, formation of microaneurysms in small vessels, perivascular fibrosis and interstitial changes are the other vascular abnormalities causing cardiac microvascular ischemia in diabetes. Ischemia contributes to myocardial stiffness, fibrosis and cardiac dysfunction in DbCM.
ROLE OF RAS
Recent evidence from animal and human experiments have demonstrated significant role of RAS in diabetes-induced cardiac dysfunction[47-49]. All major components of the classical RAS, i.e., renin, angiotensinogen, angiotensin converting enzyme (ACE), angiotensin II (AGT II) receptors are expressed in the heart[48]. Hyperglycemia activates intra-cardiac RAS that has various effects on the myocardial cells. Intracellular AGT II levels were found to be 3.4-fold higher in the cardiomyocytes of diabetic patients compared to nondiabetics[50].
Cytoplasmic AGT II has been shown to induce cell growth in animal models. AGT II has a direct effect on cell signaling that results in hypertrophy in cardiac myocytes and proliferation of cardiac fibroblasts[48]. Other factors, such as oxidative stress, inflammation and aldosterone, may contribute to the deleterious effects of AGT II on the heart producing myocardial damage in diabetes[49].
CARDIAC AUTONOMIC NEUROPATHY AND DBCM
Cardiac autonomic neuropathy (CAN) is a common complication of longstanding diabetes that causes abnormalities in heart rate control and vascular hemodynamics. The prevalence of varying degrees of CAN may be as high as 60% in individuals with prolonged history of diabetes[51]. CAN affects blood flow in the coronary vasculature and also alters the contractile function of the myocardium. Patients with CAN were found to have a reduction in the vascular elasticity and an increase of peripheral vascular resistance due to abnormal sympathetic tone[52]. Reduction in myocardial perfusion reserve also was shown by other investigators[53]. This may partly explain the ventricular dysfunction associated with diabetic CAN.
Ventricular dysfunction was found to be common in diabetic patients with CAN[54,55]. Correlation between the severity of CAN and the prevalence of diastolic dysfunction also have been demonstrated[55]. Alterations in the myocardial contractility responses in relation to stress is seen in patients with diabetic CAN, and even in those with normal ventricular function at rest, exercise-induced myocardial dysfunction have been demonstrated[56,57].
STRUCTURAL AND FUNCTIONAL ALTERATIONS IN DBCM
Significant changes in the anatomy and the function of myocardium occur as a result of DbCM that cause the clinico-pathological consequences of the disease. Pathological alterations occur mainly in the myocardial interstitium (formation of AGEs, impaired compliance and ischemia from the disease in the vasa vasorum) in the early stages and myocardial contractile dysfunction results as a consequence of the above changes[58]. Ventricular myocardial hypertrophy, interstitial and perivascular fibrosis and cardiac microvascular abnormalities ensue later.
Impaired diastolic function is the earliest abnormality in DbCM and systolic dysfunction supervenes only at later stages of the disease[40,41,58,59]. Diastolic dysfunction is characterized by impaired relaxation of the ventricular musculature during diastole of cardiac cycle and the resultant increase in ventricular filling pressure and diastolic heart failure. Ventricular hypertrophy and fibrosis caused by DbCM are the main reasons for diastolic dysfunction. When systolic dysfunction supervenes the cardiac output diminishes progressively with the severity of disease. LV systolic ejection fraction gives a good reflection of the severity of systolic dysfunction and heart failure.
Interaction with coexistent hypertension and CAD
DbCM is diagnosed only when hypertension and CAD are excluded. However, when these diseases superimpose on existing DbCM, rapid progression to advanced heart failure may result. It is difficult to identify the role of these diseases in the development and progression of DbCM from a clinical perspective. Clinically silent CAD may further complicate the diagnostic evaluation.
Coexistent hypertension was found in approximately 30% of patients with T1DM and in 50% to 80% of patients with T2DM in the United States[60]. Cardiac dysfunction was shown to be worsened by hypertension in animal models of DbCM[61]. Presence of hypertension has also been shown to be independently associated with diastolic dysfunction in diabetic patients[62]. Similarly, CAD was found to cause myocardial structural abnormalities in diabetic patients[46]. Patients with coexistent diabetes and hypertension have a higher incidence of CAD that may worsen myocardial dysfunction.
Cardiac remodeling in DbCM
DbCM results from the structural, functional and regulatory remodeling of the heart induced by diabetes mellitus. Different stages of remodeling has been proposed: the early stage, middle stage and the late stage[39]. The early stage is usually asymptomatic with myocardial changes mostly at the molecular level. Ventricular hypertrophy and diastolic dysfunction with normal left ventricular ejection fraction are the only gross abnormalities demonstrable at this stage.
The middle stage of DbCM is characterized by progressive cardiomyocyte hypertrophy and myocyte fibrosis. Increasing ventricular wall thickness and muscle mass at this stage result in worsening of the diastolic dysfunction and the development of mild systolic dysfunction. Further progression of the disease in the late stage is associated with abnormalities like CAN, microvascular/ macrovascular CAD, hypertension, and overt diastolic and systolic dysfunction.
Diagnostic evaluation of DbCM
A majority of the cases of DbCM are subclinical and the patients may not have any overt symptoms or signs of the disease. In the early stages, there are only substructural changes in the cardiomyocytes, and the detection is possible only by very sensitive methods such as strain, strain rate and myocardial tissue velocity[15]. Later on, myocyte hypertrophy and fibrosis develop, that may be associated with structural changes like LV hypertrophy and increased muscle mass (the middle stage of DbCM). Conventional diagnostic methods such as echocardiography may detect diastolic and/or systolic dysfunction at this stage. Significant microvascular changes and fibrosis occurs in the myocardium in advanced stages of DbCM and this stage is usually associated with hypertension, overt heart failure and ischemic heart disease[15].
ECHOCARDIOGRAPHY
Echocardiography is a relatively inexpensive diagnostic tool for detecting structural and functional cardiac abnormalities. Transmitral Doppler (Mitral valve blood flow measured by pulsed wave Doppler) is the usual technique for assessment of the ventricular diastolic function[63]. The variables measured by transmitral Doppler are: the early ventricular filling wave (E-wave) and the late ventricular filling wave (A-wave), which can be reported as the E/A ratio, the isovolumetric relaxation time (IVRT), E-wave peak velocity (E), E-wave deceleration time (EDT) and A-wave duration (A-dur). Based on the above study results, diastolic function can be categorized as: (1) normal pattern; (2) grade I (impaired relaxation); (3) grade II (pseudonormal pattern); and (4) grade III (restrictive pattern)[64].
Patients with grade I diastolic dysfunction (impaired relaxation) show an E/A ratio < 1 that results from a decreased early and increased late diastolic flows[65]. An increase in the IVRT and EDT are seen in these subjects[66]. In those with grade II diastolic dysfunction (pseudonormal pattern) an E/A ratio > 1 resulting from an increase in left atrial pressure is seen due to defective LV relaxation[64]. An increase in filling pressures in order to maintain normal cardiac output is the end result of impaired LV relaxation[65,67]. An E/A ratio > 2 is characteristic of grade III (restrictive pattern) diastolic dysfunction, the advanced diastolic heart failure[64]. Color M-mode Doppler echocardiography also may be useful to evaluate LV relaxation[68]. However, low sensitivity and specificity limits the diagnostic utility of Doppler and M-mode echocardiography in DbCM.
Tissue Doppler imaging (TDI) measures myocardial tissue velocities during the cardiac cycle and can be used to quantitatively assess global and regional systolic and diastolic functions of the myocardium[69,70]. TDI is a more sensitive and specific tool for the diagnosis of DbCM compared to the transmitral Doppler technique[71]. Newer echocardiographic imaging techniques are evolving with better sensitivities and specificities than these modalities of imaging.
MAGNETIC RESONANCE IMAGING
Cardiac magnetic resonance imaging (MRI) has recently emerged as a very good imaging tool for the diagnosis of various structural and functional disorders of the myocardium[72,73]. Gadolinium-enhanced cardiac MRI have been found to be useful to predict major adverse cardiac events, such as acute myocardial infarction, development of heart failure and ventricular arrhythmias in diabetic patients without previous history of ischemic heart disease[74]. Cardiac MRI is also useful to detect diastolic dysfunction and myocardial steatosis[30]. Cardiac MRI using different radionuclides and positron emission tomography (PET) can detect myocardial metabolic abnormalities and are the newer imaging techniques that may be useful in the diagnosis of DbCM.
CARDIAC CATHETERIZATION AND CORONARY ANGIOGRAPHY
Cardiac catheterization is the best method to assess the hemodynamic events within the heart chambers. Diastolic dysfunction documented invasively through catheterization continues to be the most definitive evidence of diastolic heart failure[75]. Left ventricular end-diastolic pressure of > 16 mmHg or mean pulmonary capillary wedge pressure > 12 mmHg, determined invasively by catheterization, are the most diagnostic features of diastolic dysfunction[75]. However, catheter-based diagnosis of DbCM is rarely used at present because of the availability of noninvasive techniques with high sensitivity and specificity. Coronary angiography is useful for the diagnosis of CAD that may coexist/complicate DbCM. Microvascular CAD is also diagnosed by angiography wherein, the patient presents with symptoms of CAD with normal angiogram. Newer radionuclide-based techniques and CT scan are the noninvasive techniques for coronary evaluation that can be used to diagnose CAD in patients with DbCM.
SEROLOGIC CARDIAC MARKERS
Changes in the levels of various plasma/serum cardiac biomarkers may reflect some of the myocardial metabolic and structural functions. Strong correlation between turnover of extracellular matrix proteins and ongoing cardiac remodeling has been identified in different studies[76,77]. Matrix metalloproteinases (MMPs) are the enzymes that degrade extracellular matrix, increase matrix turn over and alter the expression of several micro-Ribonucleic Acids (mi-RNAs) that lead to contractile dysfunction of the myocardium[39]. Elevated levels of MMPs especially MMP9, and reduced levels of the tissue inhibitors of MMPs are seen in myocardial fibrosis. The clinical utility of these novel biomolecules for diagnosis of DbCM is under investigation.
Serum aminoterminal propeptide of type III (PIIINP), an indicator of type III collagen turnover in the body, was suggested to be an early indicator of LV dysfunction in obese subjects with insulin resistance[78]. The role of BNP has already been discussed. Epshteyn et al[79] showed a high positive predictive value of 96% for plasma BNP levels (> 90 pg/mL) in diabetic subjects for the detection of LV dysfunction with echocardiographic correlation.
Cardiac troponins (T, N and I) are the molecules released to circulation from the injured myocardium from ischemia or inflammatory disease. Elevated troponin T levels were found in infants with cardiac dysfunction and cardiomyopathy born to diabetic mothers[80]. However, the role of troponins for evaluation of adult patients with DbCM is not yet clear.
miRNAs are small non-coding RNA molecules that modulate cellular gene expression. The dysregulation of miRNA has been linked to diabetes and many of its complications. Altered levels of miRNAs were observed in the cardiomyocytes of experimental diabetes models[81]. These novel molecules may emerge as diagnostic and prognostic tools in the future for patients with DbCM.
Therapeutic strategies for the management of DbCM
Better understanding of the disease pathogenesis and pathophysiology in the recent years provides us with improved management options for patients with DbCM. These include changes in lifestyle, improving diabetic control, lipid lowering therapy, management of coexistent hypertension and CAD if present, and management of heart failure.
LIFESTYLE MEASURES
Regular physical activity and healthy eating habits are two cornerstones of the management of diabetes, especially in the background of the global epidemic of obesity and overweight. Physical activity was associated with significant reduction of all-cause mortality and cardiovascular disease in patients with diabetes in many clinical studies[82]. Exercise training has been shown to be beneficial in reducing the incidence of DbCM in both animal models and human subjects[83-85]. It is difficult to predict the benefit of physical activity in established cases of the disease in the absence of controlled clinical trials. However, better diabetic control with regular exercise would have beneficial effect on the disease outcome. Healthy eating pattern appropriate for the diabetic individual is also expected to provide similar beneficial effects.
MANAGEMENT OF DIABETES
Improvement of glycemic control (with HbA1c between 42-53 mmol/mol) has been shown to be associated with better outcomes in diabetic microvascular complications in many clinical trials. However, beneficial effects of strict glycemic control on macrovascular outcomes are still not very clear. Because microvascular disease has important pathogenic role in the development of DbCM, better glycemic control would be expected to benefit patients.
Poor glycemic control in diabetes is associated with increased plasma levels of FFA that cause worsening of the oxidative stress, synthesis of various growth factors and derangement of lipid metabolism, and creates a favorable metabolic and biochemical environment in the body for the development of DbCM. Therefore, optimal diabetic control might be the best and most important strategy for the prevention and treatment of the disease. Better glycemic control has shown to retard DbCM in animal models[86]. Tight glycemic control has been shown to improve stress-induced ventricular dysfunction without CAD (possibly DbCM) in poorly controlled diabetic patients in a large prospective study[87]. Another case-controlled study of cardiac MRI of patients with T1DM showed that strict glycemic control was associated with better parameters of outcome in DbCM[88]. Diabetes management has also been shown to be beneficial in reducing myocardial steatosis[89].
Insulin
Insulin administration is the cornerstone of management of patients with T1DM, and those with advanced T2DM when other medications fail to control hyperglycemia. Prompt administration of insulin, targeting optimal glycemic control, may ameliorate progression of DbCM in established cases.
Metformin
Metformin is believed to be the most widely prescribed anti-diabetic medication in the world. It improves peripheral insulin sensitivity and reduces hepatic glucose output, and thus helps in controlling hyperglycemia. Metformin has shown to upregulate cardiomyocyte autophagy that has role in prevention of diabetic cardiomyopathy in animal models[90]. However, there is no data on its role in human beings with DbCM.
Pioglitazone
Pioglitazone increases insulin sensitivity and is used as a hypoglycemic agent in patients with T2DM for nearly 2 decades. The drug is generally not recommended in patients with heart failure because of its propensity to cause fluid retention. Pioglitazone was found to have anti-inflammatory effects that ameliorate cardiac fibrosis in animal models and the drug may prevent the development of DbCM[91].
Glucagon-like Peptide-1 mimetics
Glucagon-like Peptide-1 (GLP-1) is a peptide hormone, secreted by the L-cells of jejunum and ileum of the small intestine, that stimulates meal-related endogenous insulin secretion. Natural GLP-1 has a very short biological half life. Synthetic GLP-1 mimetic agents with longer half-lives such as Exenatide and liraglutide are the new anti-diabetic agents widely used now. Their use in obese T2DM patients is associated with significant improvement in glycemic control and weight loss. GLP-1 agonists has shown to attenuate cardiomyocyte apoptosis in rat models[92]. This novel group of drugs may emerge as a promising treatment option in obese T2DM patients with DbCM.
Dipeptidyl peptidase-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) is an enzyme that metabolizes endogenous GLP-1. DPP-4 inhibitors were shown to prolong the effect of natural GLP-1 produced in the body. Agents in this class, such as sitagliptin, linagliptin, saxagliptin and vildagliptin, are used as effective anti-diabetic medications now. They are weight-neutral and are useful especially in overweight and obese diabetics in combination with conventional anti-diabetic agents like metformin. Use of sitagliptin has been shown to improve myocardial glucose uptake in patients with non-ischemic cardiomyopathy[93]. The possible therapeutic role of DPP-4 inhibitors in patients with DbCM is yet to be defined.
Amylin analogues
These novel agents, when administered with insulin, have shown to reduce body weight, HbA1c values and even the insulin requirement[94]. Their role in controlling hyperglycemia may benefit patients with DbCM.
Various anti-diabetic combinations
Achievement of optimal long-term glycemic control with a single anti-diabetic agent is rarely possible in patients with T2DM. Different drug combinations like metformin plus DPP-4 inhibitors/GLP-1 mimetics, metformin plus pioglitazone, metformin plus sulphonylurea, and metformin plus insulin may be necessary for optimal glycemic control. Therefore, appropriate combination anti-diabetic therapy should be chosen, according to the clinical situation, for managing the patients with DbCM.
LIPID LOWERING THERAPY
Lipid abnormalities are more harmful in diabetics than in non-diabetic individuals because of their higher atherogenic potential. The particle size of low-density lipoprotein (LDL) cholesterol is smaller in diabetic individuals and this is more atherogenic even with near normal plasma levels. Statin treatment has shown to reduce cardiovascular events and mortality in patients with diabetes and vascular risk factors in multiple clinical trials[95], and are useful even for primary prevention in patients without established cardiovascular disease[96]. The vascular remodeling capacity of statins is referred to as the pleiotropic effect.
Atorvastatin, independent of its LDL cholesterol-lowering capacity, has shown to reduce intramyocardial inflammation and myocardial fibrosis, and improve LV function in rat models of experimental DbCM[97]. Similarly, fluvastatin also has been shown to be beneficial in attenuating myocardial interstitial fibrosis and cardiac dysfunction in rat models of the disease[98]. Although there are no clinical trials investigating the role of lipid lowering therapy in human subjects with established DbCM, beneficial effects of the treatment of dyslipidemia can be anticipated in these patients along with a role in the primary prevention of the disease.
ROLE OF VASOACTIVE DRUGS
Various vasoactive medications have been tried in both animal models and human subjects with DbCM with variable results. The most studied ones were those active on the renin-angiotensin systems. Production of angiotensin II within the myocardium has been proposed as a mechanism for the development of DbCM. Aliskiren (inhibitor of renin), benazeprilat (angiotensin convertase enzyme inhibitor; ACEI) and valsartan (angiotensin II receptor blocker; ARB) have all been shown to be protective against DbCM in rat models recently[99]. ACEI’s and ARB’s were also found to be beneficial in both animal and human models of DbCM[100,101].
Beta adreno-receptor blockers were found to be effective in experimental models of DbCM[102-104]. Because of the proven beneficial effects of beta-blockers in chronic heart failure, this group of drugs should be considered for treatment of DbCM, although there are no reported randomized clinical trials examining the benefit of the same. They can be used as effective antihypertensive agents in DbCM cases with high blood pressure. Similarly, calcium channel antagonists were also found to be beneficial in animal models of DbCM[105-107]. However, data on human subjects is lacking, to make evidence-based recommendations for the use of these agents in the management of DbCM, especially in the absence of coexistent hypertension.
Sildenafil, a selective phosphodiesterase type 5 inhibitor, has recently been shown to improve cardiac remodeling, myocardial function and few circulatory markers of cardiac inflammation in patients with DbCM[108]. Larger clinical trials in the future may prove if this novel agent can be recommended routinely in patients with the disease.
ROLE OF ANTIOXIDANTS
Trimetazidine is an atypical anti-anginal agent with antioxidant properties that shifts cardiac energy metabolism from free fatty acid oxidation to glucose oxidation. The drug has shown promising beneficial effect on heart failure in diabetic patients with both ischemic and idiopathic dilated cardiomyopathy[109,110]. Animal model has shown that trimetazidine improved myocardial function by attenuating lipotoxicity and augmented oxidation status of the heart and might suppress the development of DbCM[111]. Human trials are needed to investigate the beneficial effects of this well-tolerated drug on treatment and prevention of DbCM.
Although experimental models have shown beneficial effects of alpha-lipoic acid on cardiac redox homeostasis and suppression of cardiac fibrosis[112], human data is not yet available. Other investigational agents, found to be useful recently in animal models are, riboflavin[113], luteolin[114], sodium ferulate[115] and resveratrol[116]. Many other agents that have been tried in lab animals are not discussed here as they are beyond the scope of this review.
EMERGING TREATMENT MODALITIES
The dysregulation of miRNA function being an important pathogenic mechanism of diabetes and its complications like DbCM, artificial restoration of normal function can be a potential therapeutic target. Specific miRNA targets are found useful in the treatment of structural heart disease in mice[117]. Similarly, transplantation of bone marrow-derived endothelial progenitor cells (stem cell therapy) has shown to ameliorate DbCM in rat models[118]. Ongoing research may help us to translate the success in these experimental models to clinical practice in the future.
MANAGEMENT OF COEXISTENT HYPERTENSION, CAD AND HEART FAILURE
There are no formal guidelines on the management of coexistent hypertension and cardiac ischemia in patients with DbCM. However, when these diseases coexist, they accelerate the progression of DbCM because of their detrimental effects on ventricular function and structure. Optimal treatment of hypertension and CAD would be expected to ameliorate the disease progression and even slow it down. Coronary intervention in appropriate cases with significant CAD may improve the symptoms and clinical outcomes. Management of heart failure depends on the type (diastolic or systolic), severity and associated conditions like hypertension and CAD.
CONCLUSION
DbCM is an important but less well-recognized complication of longstanding diabetes that is associated with significant cardiac morbidity and mortality. The wide disparity in the reported prevalence of the disease may be related to differences in the types of diagnostic tests used by various investigators. The pathogenesis and pathophysiology of DbCM are still not fully elucidated, although suggested pathogenic mechanisms include chronic hyperglycemia-associated oxidative and metabolic stress, lipotoxicity, insulin resistance, microvascular disease, CAN and coexistent hypertension and CAD. The disease manifestations can vary from subclinical ventricular dysfunction to overt heart failure. Echocardiography is the standard clinical diagnostic tool for DbCM at present. Newer investigative modalities like cardiac MRI, radionuclide scans, PET imaging and various plasma markers are emerging in the diagnostic armamentarium. The management of DbCM includes changes in lifestyle, good glycemic control, treatment of dyslipidemia, coexistent hypertension, CAD and medications for heart failure.