Published online Aug 15, 2013. doi: 10.4239/wjd.v4.i4.151
Revised: May 11, 2013
Accepted: June 18, 2013
Published online: August 15, 2013
Processing time: 139 Days and 17.7 Hours
AIM: To assess the efficacy and safety of vildagliptin 50 mg bid as add-on therapy to insulin in Asian patients with type 2 diabetes mellitus (T2DM).
METHODS: This was a post hoc analysis of a subgroup of Asian patients from a multicenter, randomized, double-blind, placebo-controlled, parallel-group study in T2DM patients inadequately controlled by stable insulin therapy, with or without metformin. A total of 173 patients were randomized 1:1 to receive treatment with vildagliptin 50 mg bid (n = 87) or placebo (n = 86) for 24 wk. Changes in HbA1c and fasting plasma glucose (FPG), from baseline to study endpoint, were analyzed using an analysis of covariance model. Change from baseline to endpoint in body weight was summarized by treatment. Safety and tolerability of vildagliptin was also evaluated.
RESULTS: After 24 wk, the difference in adjusted mean change in HbA1c between vildagliptin and placebo was 0.82% (8.96 mmol/mol; P < 0.001) in Asian subgroup, 0.85% (9.29 mmol/mol; P < 0.001) in patients also receiving metformin, and 0.73% (7.98 mmol/mol; P < 0.001) in patients without metformin, all in favor of vildagliptin. There was no significant difference in the change in FPG between treatments. Weight was stable in both treatment groups (+ 0.3 kg and -0.2 kg, for vildagliptin and placebo, respectively). Overall, vildagliptin was safe and well tolerated with similarly low incidences of hypoglycemia (8.0% vs 8.1%) and no severe hypoglycemic events were experienced in either group.
CONCLUSION: In Asian patients inadequately controlled with insulin (with or without concomitant metformin), insulin-vildagliptin combination treatment significantly reduced HbA1c compared with placebo, without an increase in risk of hypoglycemia or weight gain.
Core tip: In Asian patients, vildagliptin added to stable dose of insulin, with or without concomitant metformin, significantly improves glycemic control without increase in weight and hypoglycemia incidence.
- Citation: Kozlovski P, Foley J, Shao Q, Lukashevich V, Kothny W. Vildagliptin-insulin combination improves glycemic control in Asians with type 2 diabetes. World J Diabetes 2013; 4(4): 151-156
- URL: https://www.wjgnet.com/1948-9358/full/v4/i4/151.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i4.151
The unfolding diabetes epidemic is projected to affect more than 550 million people worldwide by the year 2030 with approximately 60% of patients coming from Asia[1]. Despite available antidiabetic treatments, glycemic control in most Asian countries is unsatisfactory[2,3]. The progressive nature of type 2 diabetes requires continuous treatment intensification with a combination of antidiabetic agents having different mechanisms of action, and initiation of insulin therapy when beta cell function significantly deteriorates. However, delay in insulin initiation and intensification is a major problem across the world. In Asia, the mean HbA1c at the time of insulin intensification exceeds 9%[4], with fear of hypoglycemia and concern of weight gain identified as the main barriers for early and optimal insulin use[5]. Therefore, antidiabetic agents that can significantly improve glycemic control without increasing the risk of hypoglycemia and weight gain when used in combination with insulin are needed. While the use of insulin in combination with oral antidiabetic drugs (OADs) is increasing in Asia[5], there is little data from randomized controlled trials investigating the efficacy and safety of OAD-insulin combination in Asian patients with type 2 diabetes mellitus (T2DM).
Vildagliptin is a potent and selective inhibitor of dipeptidyl peptidase-4 (DPP-4), which extends the physiological effects of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) resulting in improvement of glycemic control in a glucose-sensitive manner[6-8]. In Asian patients with T2DM, vildagliptin showed significant improvements in HbA1c with low incidence of hypoglycemia when used as monotherapy[9], in combination with metformin[10], or in combination with a sulfonylurea[11].
We recently reported that vildagliptin added to insulin therapy resulted in a robust improvement in glycemic control without increasing the risks of hypoglycemia and weight gain[12]. This study included about 40% patients from Asia (n = 173) allowing for a meaningful analysis of the efficacy and safety data in this population. Asian patients with T2DM could be more susceptible to hypoglycemia than Caucasians due to their lower body weight and increased sensitivity to insulin[13,14] and there is a general lack of data in Asians as discussed above. We, therefore, analyzed the subgroup of Asian patients in this study to characterize the response to vildagliptin when combined with insulin in this growing patient population.
This was a post hoc analysis of a subgroup of Asian patients from a 24 wk, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Eligible patients included 89 men and 84 women aged 18-80 years with T2DM, HbA1c ≥ 7.5% (≥ 58.5 mmol/mol) and
≤ 11.0% (≤ 96.7 mmol/mol), and fasting plasma glucose levels (FPG) < 15 mmol/L, who were being treated with stable insulin doses ≤ 1 U/kg per day (long-acting, intermediate-acting, or premixed, once or twice daily) with or without stable concomitant metformin treatment (≥ 1500 mg or maximally tolerated dose) for at least 12 wk.
After a 2 wk screening period, patients were randomized in a 1:1 ratio to treatment with vildagliptin 50 mg bid or placebo. Randomization was stratified by metformin use and type of insulin used (long-acting vs intermediate acting/premixed). Further details of the study design were reported by Kothny et al[12].
HbA1c, FPG, and body weight were assessed at every visit, scheduled at 4 wk intervals. The efficacy endpoints were the change in HbA1c, FPG, and body weight from baseline to 24 wk or to the final visit. Safety assessments included monitoring and recording of treatment emergent adverse events (AEs), serious AEs (SAEs), biochemistry and hematology laboratory test results, electrocardiogram (ECG) findings, and vital signs.
Hypoglycemia was defined by symptoms suggestive of hypoglycemia and a self-monitored plasma glucose measurement < 3.1 mmol/L. Severe hypoglycemia was defined as an episode that required assistance of another person or hospitalization with or without a plasma glucose measurement < 3.1 mmol/L.
The changes in HbA1c from baseline to week 24 were compared between vildagliptin and placebo using an analysis of covariance with treatment, region, metformin use, and insulin type as classification variables and baseline HbA1c as covariate. This comparison was performed for the overall Asian population and for patients with/without concomitant metformin. Change in FPG was analyzed using the same model as for HbA1c. In addition, responder rates [percentage of patients achieving endpoint HbA1c < 7.0% (53.0 mmol/mol)] were compared between treatments using a chi-squared test. The efficacy analyses were performed on the full analysis set population consisting of all randomized patients who received at least one dose of the study drug and had at least one post-baseline assessment of any efficacy variable.
Efficacy data used in analyses were censored at the start of major changes in insulin background therapy. Major changes in insulin therapy were defined as changes occurring ≥ 7 d in any 30-d period or ≥ 5 d consecutively, including changes in insulin frequency and/or type and/or a ≥ 10% dose increase either as rescue medication or for any other reasons. The last observation carried forward (LOCF) method was used to handle missing data because of early discontinuation or data censoring.
Change in body weight from baseline to endpoint was summarized descriptively. The safety data (AEs, SAEs, including hypoglycemia) were summarized descriptively by treatment on all available data.
This trial was conducted in accordance with the Declaration of Helsinki. An independent ethics committee or institutional review board at each research site reviewed the study protocol. Each patient gave written informed consent before randomization.
A total of 173 Asian patients (38.5% of the overall study population) were randomized: 87 patients to vildagliptin 50 mg bid and 86 patients to placebo. The demographic and baseline characteristics of the randomized patients are summarized in Table 1. The groups were well balanced for all the baseline characteristics. Most patients were from the Indian subcontinent (71%) followed by patients of Chinese ethnicity (27.7%) with a mean age of 54.5 years; just over 12% of patients were ≥ 65 years of age. Mean baseline values of HbA1c and FPG were 8.9% (73.8 mmol/mol) and 8.8 mmol/L, respectively. Mean BMI was 26.4 kg/m2 and the majority (86%) had body mass index (BMI) < 30 kg/m2. The mean duration of T2DM was 11.6 years. Mean duration of insulin usage was 3.6 years, mean daily insulin dose at screening was 39.5 units, and pre-mixed insulin was the most frequent type of insulin used. Overall, 60.1% of patients were treated with metformin. The mean metformin dose at the time of randomization was approximately 2000 mg for both treatment groups.
| Vildagliptin50 mg bid | Placebo | |
| n = 87 | n = 86 | |
| Age, years | 54.0 ± 8.4 | 54.9 ± 10.5 |
| ≥ 65, n (%) | 8 (9.2) | 13 (15.1) |
| Gender, female, n (%) | 45 (51.7) | 39 (45.3) |
| Race, n (%) | ||
| Indian (Indian subcontinent) | 62 (71.3) | 61 (70.9) |
| Chinese | 24 (27.6) | 24 (27.9) |
| Other | 1 (1.1) | 1 (1.2) |
| BMI, kg/m2 | 26.2 ± 3.0 | 26.7 ± 3.7 |
| Body weight, kg | 67.5 ± 9.5 | 68.8 ± 12.1 |
| HbA1c, % (mmol/mol) | 8.9 ± 1.0 | 9.0 ± 1.0 |
| (73.7 ± 10.9) | (74.8 ± 10.9) | |
| FPG, mmol/L | 9.1 ± 2.6 | 8.6 ± 2.5 |
| T2DM duration, years | 11.1 ± 6.4 | 12.1 ± 7.6 |
| GFR, mL/min per 1.73 m2, n (%) | ||
| Normal, > 80 | 43 (49.4) | 52 (60.5) |
| Mild, ≥ 50 to ≤ 80 | 42 (48.3) | 33 (38.4) |
| Moderate, ≥ 30 to < 50 | 2 (2.3) | 1 (1.2) |
| Background antidiabetic therapy | ||
| Insulin use at screening, n (%) | ||
| Intermediate-acting | 21 (24.1) | 20 (23.3) |
| Long-acting | 14 (16.1) | 8 (9.3) |
| Pre-mixed | 52 (59.8) | 58 (67.4) |
| Duration of insulin use, years | 3.3 ± 2.9 | 3.9 ± 4.3 |
| Daily dose of insulin, U | 39.5 ± 15.8 | 39.5 ± 15.3 |
| Daily number of insulin injections | 1.9 ± 0.4 | 1.8 ± 0.4 |
| Metformin use at screening, n (%) | ||
| Yes | 52 (59.8) | 52 (60.5) |
| No | 35 (40.2) | 34 (39.5) |
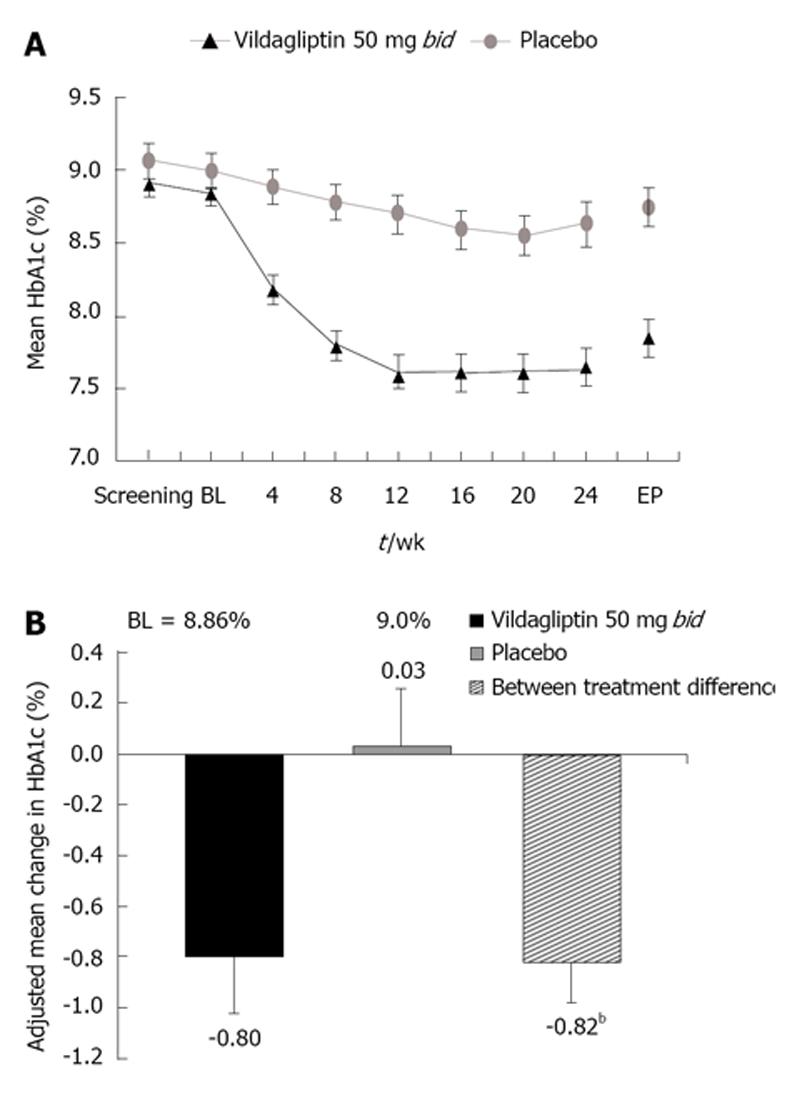
In this Asian population, vildagliptin demonstrated consistent reductions in HbA1c from baseline to week 24 endpoint (Figure 1A). After 24 wk of treatment, HbA1c had decreased by 0.8 ± 0.2% (8.74 ± 2.2 mmol/mol) in patients receiving vildagliptin (n = 85) and HbA1c increased by 0.03 ± 0.2% (0.32 ± 2.2 mmol/mol) in patients receiving placebo (n = 84). The adjusted between-treatment difference (vildagliptin 50 mg bid-placebo) in HbA1c of 0.82 ± 0.1% (8.96 ± 1.1 mmol/mol) was statistically significant (P < 0.001) in favor of vildagliptin (Figure 1B).
Vildagliptin significantly reduced HbA1c in both patients with and without concomitant metformin therapy, with adjusted mean differences vs placebo of 0.85% (9.29 mmol/mol) and 0.73% (7.98 mmol/mol) (P < 0.001 for both groups), respectively, in favor of vildagliptin. In subgroups by ethnicity, reductions in HbA1c from baseline were 0.99% (10.82 mmol/mol) and 1.17% (12.78 mmol/mol) with vildagliptin, and 0.31% (3.38 mmol/mol) and 0.08% (0.87 mmol/mol) with placebo, in Indian and Chinese patients, respectively.
In a responder analysis, significantly more patients receiving vildagliptin achieved the HbA1c target of < 7.0% (53.0 mmol/mol) than those receiving placebo (22.4% and 4.8%, respectively; P = 0.001). FPG did not change significantly in the vildagliptin group (n = 85) with a 0.2 mmol/L
increase at week 24 from baseline of 9.6 mmol/L; a more pronounced change was seen in the placebo group (n = 84) with a 0.7 mmol/L increase at week 24 from baseline of 9.0 mmol/L; mean placebo-subtracted difference was
0.5 mmol/L (P = 0.335) in favor of vildagliptin.
The mean insulin dose at baseline was 39.5 units in both vildagliptin and placebo groups. The mean changes from baseline to study end were reductions of 1.39 and 1.48 units in the vildagliptin group and the placebo group, respectively. Overall, the small changes in the insulin dose in both treatment groups are consistent with the protocol requirement for a stable insulin dose during the study.
Vildagliptin 50 mg bid added to intermediate-acting, long-acting, or premixed insulin, with or without metformin, was generally safe and well tolerated.
The overall incidence of AEs was numerically higher with vildagliptin (62.1%) than with placebo (53.5%). This difference was driven by gastrointestinal disorders (14.9% vs 7.0%), blurred vision (9.2% vs 0%), and upper respiratory tract infections (13.8% vs 8.1%). The latter were assessed by investigators as mild or moderate and not related to study drug. Diarrhea and gastritis were more frequently reported in the vildagliptin group; however, the drug was not discontinued in any of the cases.
The proportion of patients who experienced hypoglycemic events was low and similar in both treatment groups (8.0% and 8.1% in the vildagliptin and placebo groups, respectively). No patient in either treatment group experienced a severe hypoglycemic event. Similar number of patients in the vildagliptin and placebo groups reported hyperhidrosis, dizziness, tremors, and palpitations, which may be symptoms of hypoglycemia. Blurred vision was reported by 8 patients (9.2%). For three of them, blurred vision was identified as hypoglycemia and included in the hypoglycemic events summary since they had accompanying glucose measurements < 3.1 mmol/L. Of the remaining five, four reported, together with the blurred vision, one or more other symptoms suggestive of hypoglycemia (dizziness, weakness, palpitations, tremor or hyperhidrosis); however no blood glucose measurement had been performed to confirm a hypoglycemic event. Six of these eight patients experienced considerable reduction in HbA1c of 1.4% (15.3 mmol/mol) or more during the study; another one had a smaller HbA1c reduction, but reached HbA1c of 6.5% (47.5 mmol/mol). These events of blurred vision could be symptoms of hypoglycemia, or in some cases a reflection of rapidly improving glucose levels.
The rate of serious AEs was very low in this subgroup with only one serious AE reported. This was a case of liver enzyme elevation reported in one vildagliptin-treated patient with history of non-alcoholic steato-hepatitis. This event was associated with respiratory infection and the adjudication committee concluded that it was unrelated to study drug.
Body weight remained stable during the study with an increase of 0.3 kg in the vildagliptin group and a decrease of 0.2 kg in the placebo group. Overall, the safety profile of vildagliptin in the Asian subgroup was consistent with the safety profile in the overall patient population[12], without any clinically relevant differences between treatments.
In Asian patients, the addition of vildagliptin 50 mg bid to stable therapy with basal or pre-mixed insulin, with or without concomitant metformin, demonstrated a robust reduction in HbA1c vs placebo after 24 wk of treatment. Vildagliptin was efficacious in patients both from Indian and Chinese ethnicity with clinically relevant reductions in HbA1c from baseline of about 1.0% (10.93 mmol/mol). Importantly, the addition of vildagliptin to insulin was not associated with an increased risk for hypoglycemia or weight gain. These findings are consistent with the results from the overall study population[12]. Mean baseline HbA1c was similar in both the overall population [8.8% (72.7 mmol/mol)] and in the Asian population [8.9% (73.8 mmol/mol)] and so was the reduction in HbA1c vs placebo after 24 wk of treatment [0.7% (7.6 mmol/mol) and 0.8% (8.7 mmol/mol), respectively].
Asian patients had lower BMI than patients in the overall study population (26.4 kg/m2vs 28.9 kg/m2, respectively) which could make them more sensitive to insulin and, thus, place them at a higher risk of hypoglycemia[15]. However, the incidence of hypoglycemia was similar for vildagliptin and placebo in spite of better glycemic control with vildagliptin indicating that vildagliptin exerts its protective effect against hypoglycemia also in Asian patients. Vildagliptin has demonstrated a protective effect against hypoglycemia at all stages of type 2 diabetes[16] resulting from its ability to increase glucagon levels during hypoglycemia[6].
In this study, adding vildagliptin to a stable insulin dose was weight neutral, which is consistent with the known vildagliptin weight profile when used as monotherapy or in combination with other OADs[17-21]. The weight neutrality of vildagliptin likely results in part from its intrinsically low risk for hypoglycemia and avoidance of “defensive eating” characteristic for antidiabetic agents associated with increased hypoglycemia risk. Other potential mechanisms may include possible inhibition of intestinal fat extraction and fatty acid mobilization and oxidation in the postprandial state, in conjunction with increased sympathetic stimulation[21].
Multinational studies with DPP-4 inhibitors added to insulin included small numbers of Asian patients[22-24] and, therefore, meaningful analysis might have not been possible. However, in a Korean study, addition of sitagliptin to a stable insulin dose resulted in reduction in HbA1c of 0.6% (6.5 mmol/mol) from baseline of 9.2% (77.1 mmol/mol), with one patient experiencing severe hypoglycemia[25]. This is consistent with the findings from a multinational study with sitagliptin, which showed improvement in glycemic control at the expense of increased hypoglycemia incidence compared with placebo[24]. In contrast, addition of vildagliptin to insulin in this study was not associated with an increased risk of hypoglycemia in Asian patients and no events of severe hypoglycemia were reported. This difference between vildagliptin and sitagliptin could be due to vildagliptin’s ability to maintain elevated GIP levels into periods where hypoglycemia is likely to occur, thus resulting in improved glucagon counter-regulation[26].
In conclusion, the presented efficacy and safety data in Asian patients inadequately controlled with a stable insulin dose with or without concomitant metformin showed that vildagliptin can be a suitable add-on treatment leading to improved glycemic control without increased risk of hypoglycemia or weight gain. Despite some differences in diabetes phenotype between Asians and Caucasians as well as potential differences in the pathophysiology of T2DM in these populations, the beneficial effects of vildagliptin when added to insulin are maintained in an Asian population.
The authors gratefully acknowledge the support of all the investigators and medical staff at the participating centers. The authors also thank Sanchika Agarwal for editorial assistance.
The increasing diabetes epidemic by 2030 with majority of patients from Asia is of major concern. The progressive nature of disease requires intensified treatment with multiple antidiabetic agents, and insulin initiation when beta cell function deteriorates. Therefore, agents which improve glycemic control without hypoglycemia and weight gain when used with insulin are needed. However, there is little data from randomized controlled trials investigating the efficacy and safety of oral antidiabetic drugs-insulin combination in Asian patients with type 2 diabetes. The authors recently reported that vildagliptin added to insulin therapy resulted in a robust improvement in glycemic control without increasing the risks of hypoglycemia and weight gain. This study included about 40% patients from Asia and thus the authors analyzed the subgroup of Asian patients to characterize the response to vildagliptin when combined with insulin in a patient population in which diabetes is a growing concern.
Vildagliptin is a selective inhibitor of dipeptidyl peptidase-4 (DPP-4) enzyme, improves glycemic control by increasing the availability of incretins. Considering that Asian patients with type 2 diabetes could be more susceptible to hypoglycemia than Caucasians due to their lower body weight and increased sensitivity to insulin, and due to the general lack of data in Asians, the efficacy and safety of vildagliptin-insulin combination in this population was assessed.
This is the first double-blind placebo controlled study that reports the efficacy and safety of a DPP-4 inhibitor (vildagliptin) as add-on to insulin in an Asian population.
This study demonstrates that vildagliptin in combination with insulin is a safe and efficacious antidiabetic treatment by significantly reducing HbA1c without an increased incidence of hypoglycemia or weight gain.
DPP-4 inhibitors: Dipeptidyl peptidase-4 inhibitors are a class of oral antihyperglycemic agents that inhibit the enzyme DPP-4. They are used to treat type 2 diabetes mellitus. HbA1c: Glycated hemoglobin is a form of hemoglobin that is measured primarily to identify the average plasma glucose concentration over prolonged periods of time. It is formed in a non-enzymatic glycation pathway by hemoglobin’s exposure to plasma glucose. The 2010 American Diabetes Association Standards of Medical Care in Diabetes added the HbA1c ≥ 48 mmol/mol (≥ 6.5%) as another criterion for the diagnosis of diabetes.
This manuscript presents an analysis of the Asian subgroup of a recently published study. It addresses new findings regarding the effects of vildagliptin as add-on therapy to insulin in Asian patients with type 2 diabetes mellitus. The present work contains interesting data and appears timely.
| 1. | International Diabetes Federation. IDF Diabetes Atlas. 5th ed. Brussels, Belgium: International Diabetes Federation 2011; Available from: http: //www.idf.org/diabetesatlas. |
| 2. | Bi Y, Yan JH, Liao ZH, Li YB, Zeng LY, Tang KX, Xue YM, Yang HZ, Li L, Cai DH. Inadequate glycaemic control and antidiabetic therapy among inpatients with type 2 diabetes in Guangdong Province of China. Chin Med J (Engl). 2008;121:677-681. [PubMed] |
| 3. | Chuang LM, Tsai ST, Huang BY, Tai TY. The status of diabetes control in Asia--a cross-sectional survey of 24 317 patients with diabetes mellitus in 1998. Diabet Med. 2002;19:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Home P, Naggar NE, Khamseh M, Gonzalez-Galvez G, Shen C, Chakkarwar P, Wenying Y. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1chieve study. Diabetes Res Clin Pract. 2011;94:352-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Mohamed M. An audit on diabetes management in Asian patients treated by specialists: the Diabcare-Asia 1998 and 2003 studies. Curr Med Res Opin. 2008;24:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Ahrén B, Schweizer A, Dejager S, Dunning BE, Nilsson PM, Persson M, Foley JE. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Ahrén B, Schweizer A, Dejager S, Villhauer EB, Dunning BE, Foley JE. Mechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humans. Diabetes Obes Metab. 2011;13:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Mathieu C. The scientific evidence: vildagliptin and the benefits of islet enhancement. Diabetes Obes Metab. 2009;11 Suppl 2:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Kikuchi M, Abe N, Kato M, Terao S, Mimori N, Tachibana H. Vildagliptin dose-dependently improves glycemic control in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;83:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Pan C, Xing X, Han P, Zheng S, Ma J, Liu J, Lv X, Lu J, Bader G. Efficacy and tolerability of vildagliptin as add-on therapy to metformin in Chinese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Kikuchi M, Haneda M, Koya D, Tobe K, Onishi Y, Couturier A, Mimori N, Inaba Y, Goodman M. Efficacy and tolerability of vildagliptin as an add-on to glimepiride in Japanese patients with Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2010;89:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Kothny W, Foley J, Kozlovski P, Shao Q, Gallwitz B, Lukashevich V. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1511] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 14. | Lüddeke HJ, Sreenan S, Aczel S, Maxeiner S, Yenigun M, Kozlovski P, Gydesen H, Dornhorst A. PREDICTIVE- a global, prospective observational study to evaluate insulin detemir treatment in types 1 and 2 diabetes: baseline characteristics and predictors of hypoglycaemia from the European cohort. Diabetes Obes Metab. 2007;9:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Khunti K, Srinivasan BT, Shutler S, Davies MJ. Effect of insulin glargine on glycaemic control and weight in obese and non-obese people with type 2 diabetes: data from the AT.LANTUS trial. Diabetes Obes Metab. 2010;12:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Dejager S, Schweizer A. Minimizing the risk of hypoglycemia with vildagliptin: Clinical experience, mechanistic basis, and importance in type 2 diabetes management. Diabetes Ther. 2011;2:51-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 18. | Scherbaum WA, Schweizer A, Mari A, Nilsson PM, Lalanne G, Wang Y, Dunning BE, Foley JE. Evidence that vildagliptin attenuates deterioration of glycaemic control during 2-year treatment of patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes Metab. 2008;10:1114-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Care. 2007;30:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Garber AJ, Foley JE, Banerji MA, Ebeling P, Gudbjörnsdottir S, Camisasca RP, Couturier A, Baron MA. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (10)] |
| 21. | Foley JE, Jordan J. Weight neutrality with the DPP-4 inhibitor, vildagliptin: mechanistic basis and clinical experience. Vasc Health Risk Manag. 2010;6:541-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Barnett AH, Charbonnel B, Donovan M, Fleming D, Chen R. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin. 2012;28:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (7)] |
| 23. | Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab. 2009;11:1145-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Vilsbøll T, Rosenstock J, Yki-Järvinen H, Cefalu WT, Chen Y, Luo E, Musser B, Andryuk PJ, Ling Y, Kaufman KD. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 25. | Hong ES, Khang AR, Yoon JW, Kang SM, Choi SH, Park KS, Jang HC, Shin H, Walford GA, Lim S. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Schweizer A, Foley JE, Kothny W, Ahrén B. Clinical evidence and mechanistic basis for vildagliptin’s effect in combination with insulin. Vasc Health Risk Manag. 2013;9:57-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
P- Reviewers GeorgescuA, Sourij H S- Editor Wen LL L- Editor A E- Editor Lu YJ