Published online Jun 15, 2013. doi: 10.4239/wjd.v4.i3.76
Revised: March 26, 2013
Accepted: May 16, 2013
Published online: June 15, 2013
Processing time: 118 Days and 11.1 Hours
AIM: To assess the utility of hemoglobin A1c (HbA1c) in the early postpartum screening of women with gestational diabetes mellitus (GDM).
METHODS: Over a 3 years period, HbA1c estimations were undertaken in addition to and simultaneously with the traditional oral glucose tolerance test (OGTT), in 203 women with GDM as a part of early postpartum screening for dysglycaemia, at 6 wk post-partum. World Health Organization criteria was used for diagnosing diabetes: fasting blood glucose (FBG) ≥ 7.0 mmol/L and/or 2-h postprandial blood glucose (PPBG) ≥ 11.1 mmol/L and/or HbA1c ≥ 48 mmol/mol; and impaired glycaemiastate: impaired fasting glucose 6.1-6.9 mmol/L and/or impaired glucose tolerance 7.8-11.0 mmol/L and/or HbA1c: 42-47 mmol/mol.
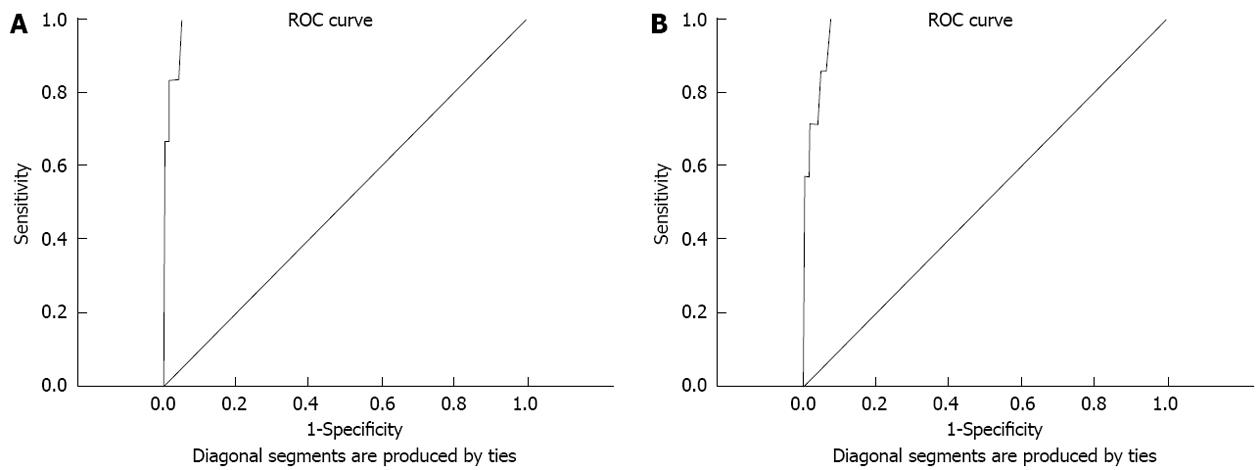
RESULTS: Mean FBG, 2-h PPBG and HbA1c were 4.9 ± 0.7 mmol/L, 5.6 ± 2.0 mmol/L and 38 ± 5 mmol/mol respectively. FBG, 2-h PPBG and HbA1c detected 6 (3%), 7 (3.5%) and 11 (5.4%) cases of diabetes respectively, and 11 (5.4%), 25 (12.3%) and 23 (11.3%) cases of pre-diabetes state respectively. HbA1c values ≥ 48 mmol/mol (≥ 6.5%) showed a diagnostic sensitivity of 71.4% and specificity of 98.5% for diabetes in comparison to OGTT in receiver operating characteristics curve analysis. At HbA1c cut-off 44 mmol/mol, sensitivity and specificity were 100% and 92.3% respectively [area under the curve: 0.98 (95%CI: 0.96-1.00)]. Sensitivity and specificity for detecting high risk “impaired glycaemia” state [HbA1c 42 mmol/mol (6.0%)] were 28% and 80%, respectively.
CONCLUSION: HbA1c level ≥ 48 mmol/mol (≥ 6.5%) has reasonable sensitivity and high specificity in comparison to OGTT for early postpartum screening of diabetes in GDM. At 6th week postpartum screening, if FBG is normal and HbA1c < 44 mmol/mol OGTT is not recommended.
Core tip: Hemoglobin A1c (HbA1c) though accepted as a screening tool for diagnosis of diabetes by professional bodies, its role in early postnatal screening of women with gestational diabetes mellitus is not known, which is explored in this study. Analysing the results of simultaneous oral glucose tolerance test (OGTT) and HbA1c estimations undertaken as a part of postpartum screening at 6 wk, we note that HbA1c has a high negative predictive value and can help in excluding diabetes (but not impaired glycaemia). We thus propose that HbA1c could potentially be used with fasting blood glucose estimation to avoid OGTT in those women with an HbA1c < 44 mmol/mol.
- Citation: Katreddy MV, Pappachan JM, Taylor SE, Nevill AM, Indusekhar R, Nayak AU. Hemoglobin A1c in early postpartum screening of women with gestational diabetes. World J Diabetes 2013; 4(3): 76-81
- URL: https://www.wjgnet.com/1948-9358/full/v4/i3/76.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i3.76
The global prevalence of diabetes mellitus continues to escalate with the force of an approaching tsunami that affects about 350 million individuals worldwide[1]. Increasing prevalence of overweight and obesity in both developed and developing countries are the main factors for the alarming rise in the diabetic epidemic. Alterations in the glucose homeostasis among obese/overweight children and adolescents lead to the peculiar phenomenon of emergence of prediabetes and type 2 diabetes in young adults in the recent years[2]. Consequently the prevalence of diabetes among women of child-bearing age is also expected to rise.
Diabetes mellitus is a major cause of perinatal morbidity and mortality, as well as maternal morbidity that complicates about 3%-14% of all pregnancies[3-7]. About 85% of diabetes among pregnant women is due to gestational diabetes mellitus (GDM) and the remainder are due to type 2 or type 1 diabetes. It is well recognised that women with GDM have a higher risk of development of impaired glycaemicstate or type 2 diabetes over time, with studies suggesting a 17% to 63% risk of developing type 2 diabetes within 5 to 16 years of the index pregnancy[8]. The recurrence rate of GDM in a second consecutive pregnancy was found to be about 41% in a recent major study[9]. Despite majority of women with GDM becoming normoglycaemic immediately after delivery of the baby, the prevalence of persistent abnormal glucose metabolism (diabetes and impaired glycaemia) in the early postpartum period has been reported to be as high as 46%[10]. Identifying these high risk women with persistently impaired glucose regulation and frank diabetes would help in instigating early intervention strategies for better health outcomes. Thus, early postpartum diabetes screening is recommended by professional organizations, for all women with the diagnosis of GDM during pregnancy[11-15].
The American Diabetes Association (ADA) recommends diabetes screening with a 75 g oral glucose tolerance test (OGTT) at 6-12 wk after delivery in women with GDM[11] and the World Health Organization (WHO) recommends similar screening at least 6 wk after delivery[12]. A fasting glucose test, instead of the traditional OGTT, at the 6th week postpartum visit is recommended by the United Kingdom’s National Institute for Health and Clinical Excellence (NICE) for GDM patients as a screening tool[13]. Though estimation of glycated hemoglobin A1c (HbA1c) level is accepted as a screening tool for diagnosis of diabetes by WHO and other professional bodies, there is no official recommendation for its use for diabetes screening in the postpartum period.
Because of the ease of estimation of HbA1c in comparison to the cumbersome OGTT, it is being utilized increasingly for screening and diagnosis of diabetes and prediabetes states in the recent years. In this background we conducted a study to explore the utility of HbA1c in the early post-partum screening of women with gestational diabetes in a large university hospital in the United Kingdom.
All women who were diagnosed with GDM, managed by diet/lifestyle modifications and/or medical treatment, in the combined antenatal diabetes clinic (with obstetrician, diabetologist, dietician and diabetic specialist nurse) between January 2010 and August 2012, were offered post-partum screening in the 6th week postpartum visit. Along with the OGTT, HbA1c estimation was undertaken as a part of the post-partum screening test. All such women were identified and included, and there were no exclusion criteria. These women were given counselling by the diabetic team, during their antenatal follow up, regarding the implications of GDM diagnosis and the need for screening in the post-partum period.
OGTT was performed after a minimum of 8 h of overnight fast, by measuring fasting blood glucose (FBG) and 2-h postprandial blood glucose (PPBG). After taking blood sample for FBG check each participant was given a glucose drink (75 g of D-dextrose powder dissolved in 200 mL of water). Samples for FBG and 2-h PPBG were obtained by taking 2 mL of venous blood in tubes containing sodium fluoride. 3 mL each of venous blood samples were collected in tubes containing EDTA for HbA1c estimation while taking sample for fasting blood glucose. HbA1c was measured using high performance liquid chromatography on a Tosoh G7 analyser (Tosoh Bioscience Ltd., Worcestershire, United Kingdom). The performance scores in the United Kingdom National External Quality Assurance Scheme were: A scores < 100 and B scores < 2%. The between batch coefficient of variation was 1.8% and 1.4% for an HbA1c of 5.7% and 9.5% respectively.
The International Federation of Clinical Chemistry (IFCC) units for HbA1c levels were introduced in the United Kingdom since 1st June 2009. Locally, the IFCC reference system was adopted and the dual reporting of HbA1c with IFCC units and the corresponding calculated Diabetes Control and Complications Trial (DCCT) value was available during the period and utilised for the analysis of data among the participants. The equation describing the relationship between the IFCC and the DCCT units used was: IFCC-HbA1c (mmol/mol) = [DCCT - HbA1c (%) - 2.15] × 10.929[16].
Data of the test results from participants were collected and they were grouped into categories according to the values as normal, impaired glycaemiaor diabetes. FBG values less than 6.1 mmol/L was taken as normal; FBG values between 6.1 mmol/L and 6.9 mmol/L as impaired fasting glucose (IFG); and FBG ≥ 7.0 mmol/L as diabetes. The OGTT results were classified by the WHO criteria: normal glucose tolerance (FBG < 6.0 mmo/L and/or 2-h PPBG < 7.8 mmol/L); impaired glucose tolerance (FBG ≥ 6.1 mmol/L and < 7.0 mmol/L, and/or 2-h PPBG between 7.8 and 11.0 mmol/L); and diabetes (FBG ≥ 7.0 mmol/L and/or 2-h PPBG ≥ 11.1 mmol/L). Glycaemic categorization was also undertaken according to the HbA1c criteria recommended by the WHO in diagnosis of diabetes in the general population: Diabetes (HbA1c ≥ 48 mmol/mol or ≥ 6.5% DCCT) and non-diabetic (HbA1c < 48 mmol/mol or < 6.5%). Those with HbA1c values between 42 mmol/mol and 47 mmol/mol (6.0% and 6.4%) were considered as having an impaired glycaemicstate (high risk)[17].
Data was analysed using computer software SPSS Version 19.0 for Windows (SPSS Inc, Chicago, IL, United States). The continuous variables are presented as mean (± 2SD). Differences in the classifications between normal, impaired glycaemia and diabetes using FBG, OGTT and HbA1c were assessed using a non-parametric sign test. Receiver operating characteristics (ROC) curve analysis was used to determine the sensitivity and specificity of HbA1c in comparison with OGTT (as the gold standard) for postpartum screening of diabetes or high risk pre-diabetes state. P < 0.05 was considered statistically significant.
A total of 408 women with gestational diabetes were identified during the study period. However, only 203 women (49.8%) had simultaneous OGTT and HbA1c estimation as a part of the postpartum screening at the 6th postpartum week, the cohort which we used in the analysis for this study. Demographic characteristics were as follows: mean age: 29 ± 4.6 years; ethnic origin: 142 were Caucasians (70%) and 61 belonged to other racial groups (Asian: 60, Afro-Caribbean: 2, others: 9); body mass index: 30 ± 6.4 kg/m2 (Caucasians: 32 ± 5.1 kg/m2 and Asians 26 ± 4.2 kg/m2).
Mean FBG was 4.9 ± 0.7 mmol/L; mean 2-h PPBG 5.6 ± 2.0 mmol/L and HbA1c 38 ± 5 mmol/mol (5.6 ± 0.5%). The correlation for HbA1c vs FBG was r = 0.42 (P < 0.001) and for HbA1c vs 2-h PPBG was r = 0.42 (P < 0.001).
Categorization of the cohort into normal (= 1), impaired glycaemia (= 2) or diabetes (= 3) according to blood glucose and HbA1c criteria (WHO criteria) are shown in Table 1. Using the ADA criteria the prevalence of IGT in OGTT increased from 12.3% to 16.7% without altering the total number diagnosed diabetes[18]. The diabetes prevalence using FBG alone and OGTT were 3.0% and 3.5%. Further, OGTT diagnosed a higher proportion with impaired glycaemia compared to FBG (5.4% vs 12.3%). Of the 186 women with normal FBG, 15 (8.1%) had IGT on OGTT and none had diabetes based on the 2-h PPBG value on the OGTT. Amongst the 11 women with IFG, 1 had the 2-h PPBG in the diabetes range. Differences in the classifications using FBG and OGTT were assessed using a non-parametric sign test. The misclassifications identified 0 positive, 16 negative differences and 187 ties (P < 0.001), suggesting that OGTT is inclined to diagnose a significantly higher proportion of patients with impaired glycaemia and full diabetes compared to FBG.
| Category | FBG | OGTT | HbA1c |
| Diabetes | 6 (3.0) | 7 (3.5) | 11 (5.4) |
| Impaired | 11 (5.4) | 25 (12.3) | 23 (11.3) |
| Normal | 186 (91.6) | 171 (84.2) | 169 (83.3) |
The categorisation of glycaemia into normal (= 1), impaired glycaemia (= 2) and diabetes (= 3) using HbA1c identified different individuals compared to those identified using the OGTT and FBG (Table 2). HbA1c was in the diabetes range in more women when compared to the OGTT criteria (5.4% vs 3.5%, P > 0.05). Differences in the classifications using OGTT and HbA1c were also assessed using a non-parametric sign test. The misclassifications identified 22 positive, 23 negative differences and 160 ties (P > 0.05), suggesting that HbA1c classified different individuals with normal, impaired glycaemia and diabetes compared to those identified using the OGTT.
| Glycaemic status | HbA1c | |||
| Normal | Impaired glycaemia | Diabetes | ||
| OGTT | Normal | 149 (73.4) | 17 (8.4) | 5 (2.5) |
| Impaired glycaemia | 20 (9.9) | 4 (2.0) | 1 (0.5) | |
| Diabetes | 0 (0) | 2 (1.0) | 5 (2.5) | |
| FBG | Normal | 162 (79.8) | 19 (9.4) | 5 (2.5) |
| Impaired glycaemia | 7 (3.4) | 3 (1.5) | 1 (0.5) | |
| Diabetes | 0 (0) | 1 (0.5) | 5 (2.5) | |
For the diagnosis of diabetes, in the ROC curve (Figure 1), HbA1c values ≥ 48 mmol/mol (≥ 6.5%) showed a diagnostic sensitivity 71.4% and specificity 98.5% in comparison to the gold standard OGTT. When the HbA1c cut-off was lowered to 44 mmol/mol the sensitivity 100% and specificity 92.3% [area under the curve 0.98 (95%CI: 0.96-1.00)]. The sensitivity and specificity for detecting high risk impaired glycaemia using cut-off HbA1c 42 mmol/mol (6.0%) were 28% and 80% respectively.
In this study during early post-partum screening at 6 wk for women with GDM all women diagnosed to have diabetes by the OGTT had an abnormality in their fasting blood glucose. Thus, this supports a strategy to undertake FBG as the initial screening test, and a follow-on OGTT only in those with IFG, that would be sufficient to identify all women with diabetes. This is also in line with the current NICE recommendations for the use of FBG as the initial screening test in these women with GDM during early postpartum period. This would be a very cost-effective strategy avoiding OGTT in majority of women with GDM, but is likely to miss a significantly high proportion of women with IGT (8.1% in our study) who potentially may have a higher risk of developing diabetes earlier, than those with normalised postpartum glycaemia, in whom more aggressive interventions have been recommended by some authorities. However, others would argue that gestational diabetes itself is a significant risk for future diabetes, and that having IGT or with normalisation of glycaemia in postpartum period, there should be no practical difference in the degree of intervention and future screening for diabetes.
This study explored the utility of HbA1c in the early postpartum screening of women with gestational diabetes mellitus. Applying the recent WHO HbA1c criteria, the yield of diabetes was significantly higher compared to the blood glucose (OGTT) criteria (5.4% vs 3.5%) and the two tests diagnosed different women with diabetes with concordance only in 2.5%. Previous studies tested the utility of HbA1c in postpartum screening of glucose abnormalities at variable time periods from 6 wk to 36 mo[10,19-21]. Our data is unique in that estimation of all the 3 parameters for dysglycaemia (abnormal FBG, OGTT and HbA1c) were done in a single setting during the first postpartum visit at 6 wk. Many patients would have done postpartum screening at other health centres and there remained a variation in the test undertaken in a proportion of women making the response rate low (49.8%).
Use of HbA1c has been recommended as a test to diagnose diabetes by various international organisations[22-25]. Advantage of blood sampling in the non-fasting state without the need for OGTT makes HbA1c check as a promising tool for diabetes screening. However, the reliability of HbA1c as a true reflection of glycaemic status at 6 wk post-partum could be affected by several factors including glucose control in the later stages of pregnancy and/or the use or discontinuation of hypoglycaemic therapies peri-partum. HbA1c represents average glucose levels over the last 3 mo, not 6 wk, and therefore HbA1c at 6 wk postpartum partly reflects glucose levels during the pregnancy and hence the concern to use it at 6 wk post-partum as a screening test. The performance of HbA1c is likely better at about 12 wk or 3 mo postpartum. In addition, altered red cell turnover, anaemia of pregnancy and potential factors altering glycation - deglycation rates in the erythrocyte might affect the HbA1c value independent of prevailing glucose control that affect the categorisation of glycaemic status[26]. In our study higher proportion of patients were found to have HbA1c in the diabetes range compared to the previous reports, which may be due to the impact of prevailing glucose control just prior to delivery on the HbA1c estimation at 6 wk post-partum[19,20].
Though HbA1c did not appear to help in the diagnosis of diabetes in this study, it was noted that HbA1c had a high negative predictive value/sensitivity, with HbA1c cut-off < 44 mmol/mol practically excluding all those with diabetes (but not IGT). Thus HbA1c may be potentially used with FBG estimation to avoid OGTT in those women with an HbA1c < 44 mmol/mol. Further studies with larger cohort may be required to confirm this and prior to recommending HbA1c on its own as the test for early post-partum screening for women with gestational diabetes mellitus.
Hemoglobin A1c (HbA1c) has been accepted as a screening tool for diabetes mellitus by various professional bodies in the recent years. However, there is no data available on the efficacy of HbA1c in comparison to oral glucose tolerance test (OGTT) for screening diabetes and impaired glycaemia at the 6th postpartum week in patients with gestational diabetes mellitus (GDM).
OGTT, the gold standard test for postpartum screening of GDM cases, is cumbersome and the patients need to fast overnight, whereas HbA1c estimation needs only random blood testing. Limited data on postpartum screening with HbA1c at different timescales after childbirth showed variable results in terms of the sensitivity and specificity. It remains unclear if HbA1c estimation can be used as a screening tool for diabetes and impaired glycaemia at the 6th week postpartum clinic visit.
This study demonstrated that HbA1c has reasonable sensitivity and high specificity in comparison to OGTT for postpartum screening of diabetes in patients with GDM.
Results of the study shows that GDM patients with HbA1c values less than 44 mmol/mol and normal fasting blood glucose levels may not need the cumbersome OGTT to exclude diabetes mellitus.
HbA1c levels reflect the average glycaemic burden over 3-mo period and estimation of HbA1c at the 6th postpartum week may overestimate prevalence of diabetes in those with high glucose levels towards term of pregnancy and therefore may limit the diagnostic utility in screening patients with GDM. However, the study demonstrated reasonable sensitivity, high specificity of HbA1c as a postpartum screening tool.
| 1. | World Health Organization. Diabetes. Geneva: World Health Organization, 2012. (Assessed on 7th February 2013). Available from: http: //www.who.int/mediacentre/factsheets/fs312/en/index.html. |
| 2. | Caprio S. Development of type 2 diabetes mellitus in the obese adolescent: a growing challenge. Endocr Pract. 2012;18:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Gabbe SG, Graves CR. Management of diabetes mellitus complicating pregnancy. Obstet Gynecol. 2003;102:857-868. [PubMed] |
| 4. | Keshavarz M, Cheung NW, Babaee GR, Moghadam HK, Ajami ME, Shariati M. Gestational diabetes in Iran: incidence, risk factors and pregnancy outcomes. Diabetes Res Clin Pract. 2005;69:279-286. [PubMed] |
| 5. | Mamabolo RL, Alberts M, Levitt NS, Delemarre-van de Waal HA, Steyn NP. Prevalence of gestational diabetes mellitus and the effect of weight on measures of insulin secretion and insulin resistance in third-trimester pregnant rural women residing in the Central Region of Limpopo Province, South Africa. Diabet Med. 2007;24:233-239. [PubMed] |
| 6. | Flack JR, Ross GP, Ho S, McElduff A. Recommended changes to diagnostic criteria for gestational diabetes: impact on workload. Aust N Z J Obstet Gynaecol. 2010;50:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004;27 Suppl 1:S88-S90. [PubMed] |
| 8. | Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862-1868. [PubMed] |
| 9. | Khambalia AZ, Ford JB, Nassar N, Shand AW, McElduff A, Roberts CL. Occurrence and recurrence of diabetes in pregnancy. Diabet Med. 2013;30:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Picón MJ, Murri M, Muñoz A, Fernández-García JC, Gomez-Huelgas R, Tinahones FJ. Hemoglobin A1c versus oral glucose tolerance test in postpartum diabetes screening. Diabetes Care. 2012;35:1648-1653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29 Suppl 1:S43-S48. [PubMed] |
| 12. | World Health Organization. Definition, diagnosis, and classification of diabetes mellitus and its complications: report of WHO Consultation. Geneva: World Health Organization, 1999. (Assessed on 23rd March 2013). Available from: http: //whqlibdoc.who.int/hq/1999/who_ncd_ncs_99.2.pdf. |
| 13. | Guideline Development Group. Management of diabetes from preconception to the postnatal period: summary of NICE guidance. BMJ. 2008;336:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinman B, Hanley AJ. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metab. 2010;95:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Committee on Obstetric Practice. ACOG Committee Opinion No. 435: postpartum screening for abnormal glucose tolerance in women who had gestational diabetes mellitus. Obstet Gynecol. 2009;113:1419-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Sacks DB. Measurement of hemoglobin A(1c): a new twist on the path to harmony. Diabetes Care. 2012;35:2674-2680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care. 2010;33 Suppl 1:S11-S61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2231] [Cited by in RCA: 2322] [Article Influence: 145.1] [Reference Citation Analysis (1)] |
| 18. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27 Suppl 1:S5-S10. [PubMed] |
| 19. | Ogonowski J, Miazgowski T. The prevalence of 6 weeks postpartum abnormal glucose tolerance in Caucasian women with gestational diabetes. Diabetes Res Clin Pract. 2009;84:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Megia A, Näf S, Herranz L, Serrat N, Yañez RE, Simón I, Vendrell J. The usefulness of HbA1c in postpartum reclassification of gestational diabetes. BJOG. 2012;119:891-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Kim C, Herman WH, Cheung NW, Gunderson EP, Richardson C. Comparison of hemoglobin A1c with fasting plasma glucose and 2-h postchallenge glucose for risk stratification among women with recent gestational diabetes mellitus. Diabetes Care. 2011;34:1949-1951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2247] [Cited by in RCA: 2257] [Article Influence: 132.8] [Reference Citation Analysis (1)] |
| 23. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1:S62-S69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3654] [Cited by in RCA: 4360] [Article Influence: 272.5] [Reference Citation Analysis (0)] |
| 24. | World Health Organizatio. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. Geneva: World Health Organization, 2011. (Assessed on 23rd March 2013). Available from: http: //www.who.int/diabetes/publications/diagnosis_diabetes2011/en/index.html. |
| 25. | Inzucchi SE. Clinical practice. Diagnosis of diabetes. N Engl J Med. 2012;367:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 26. | Nayak AU, Holland MR, Macdonald DR, Nevill A, Singh BM. Evidence for consistency of the glycation gap in diabetes. Diabetes Care. 2011;34:1712-1716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
P- Reviewers Krcma M, Luo ZC, Wahabi HA, Wang CC S- Editor Wen LL L- Editor A E- Editor Li JY