Published online Jun 15, 2013. doi: 10.4239/wjd.v4.i3.70
Revised: March 28, 2013
Accepted: April 13, 2013
Published online: June 15, 2013
Processing time: 70 Days and 11.2 Hours
AIM: To investigate the cytotoxic mechanism of caribbean maitotoxin (MTX-C) in mammalian cells.
METHODS: We used whole-cell patch-clamp techniques and fluorescence calcium imaging to determine the cellular toxic mechanisms of MTX-C in insulin secreting HIT-T15 cells, which is a system where the effects of MTX have been observed. HIT-T15 cells stably express L-type calcium current, making it a suitable model for this study. Using the fluorescence calcium indicator Indo-1 AM, we found that there is a profound increase in HIT-T15 intracellular free calcium 3 min after application of 200 nmol/L MTX-C.
RESULTS: About 3 min after perfusion of MTX-C, a gradual increase in free calcium concentration was observed. This elevation was sustained throughout the entire recording period. Application of MTX-C did not elicit the L-type calcium current, but large cationic currents appeared after applying MTX-C to the extracellular solution. The current-voltage relationship of the cation current is approximately linear within the voltage range from -60 to 50 mV, but flattened at voltages at -80 and -100 mV. These results indicate that MTX-C induces a non-voltage activated, inward current under normal physiological conditions, which by itself or through a secondary mechanism results in a large amount of cationic influx. The biophysical mechanism of MTX-C is different to its isoform, pacific maitotoxin (MTX-P), when the extracellular calcium is removed.
CONCLUSION: We conclude that MTX-C causes the opening of non-selective, non-voltage-activated ion channels, which elevates level of intracellular calcium concentration and leads to cellular toxicities.
Core tip: The toxicity of maitotoxin is estimated to affect over 50000 people annually. Baracuda, snapper, grouper, jacks, and moray eel are particularly notorious for their potential to carry high toxin load. The symptoms of the toxicity include numbness of the perioral area and extremities, reversal of temperature sensation, muscle and joint aches, headache, itching tachycardia, hypertension, blurred vision, and paralysis. Our study aims to elucidate the cellular toxic mechanism of caribbean maitotoxin in mammalian cells. We conclude that it causes opening of non-selective, non-voltage activated ion channels, which elevates level of intracellular calcium concentration and leads to cellular toxicities.
- Citation: Lu XZ, Deckey R, Jiao GL, Ren HF, Li M. Caribbean maitotoxin elevates [Ca2+]i and activates non-selective cation channels in HIT-T15 cells. World J Diabetes 2013; 4(3): 70-75
- URL: https://www.wjgnet.com/1948-9358/full/v4/i3/70.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i3.70
Ciguatera fish poisoning is caused by ladder-like polyether toxins[1]. Ciguatera occurs circumglobally in tropical coral reef regions, and results from the consumption of fish that have accumulated toxin through the food web[2-4]. It is estimated to affect over 50000 people annually, and is no longer a disease limited to the tropics, due both to travel to the tropics and to shipping of tropical fish species to markets elsewhere in the world[5]. Large carnivorous fishes associated with coral reefs are the most frequent source of ciguatera[6]. Baracuda, snapper, grouper, jacks, and moray eel are particularly notorious for their potential to carry high toxin load[7]. The symptoms of ciguatera comprise early onset (2-6 h) gastrointestinal disturbance, including nausea, vomiting, and diarrhea, and may be followed by a variety of later onset (18 h) neurological sequelae, including numbness of the perioral area and extremities, reversal of temperature sensation, muscle and joint aches, headache, itching tachycardia, hypertension, blurred vision, and paralysis[7-9]. Ciguatera symptoms in the Caribbean differ somewhat from those in the Pacific in that gastrointestinal symptoms are dominant in Caribbean cases, while in Pacific cases neurological symptoms tend to dominate[10].
Maitotoxin (MTX) is one of toxins implicated in ciguatera. This toxin is the most potent marine toxin known today, with a lethal dose of 0.17 ug/kg[11]. MTX elicits calcium influx in all cells and tissues tested[12,13]. This calcium influx however, is not mediated by the MTX itself but by activating existing non-selective cation ion channels[14-16]. MTX induced calcium influx is observed in pancreatic β-cells[17-19]. In β-cells, MTX-induced nonselective cation current is indistinguishable from the insulin stimulating hormone glucagon-like peptide-1 and PACAP-activated current[20]. It was reported that the MTX-induced calcium current is dependent on extracellular calcium[21].
We have isolated a novel Caribbean isoform of MTX (MTX-C) from tropical fish. In the present study, we used whole-cell patch-clamp techniques and fluorescence calcium imaging to determine the cytotoxic mechanisms of MTX-C in a hamster pancreatic islet cell line, HIT-T15, which stably expresses L-type calcium currents and thus is a suitable model[22]. We also used a pacific maitotoxin (MTX-P) to perform parallel comparisons for all experiments.
The Caribbean and Pacific maitotoxins were gifts from the Food and Drug Administration of the United States, Mobile, AL.
Transformed hamster pancreatic islet cells HIT-T15 (American Type Culture Collection CRL-1777) were routinely cultured in Dulbecco’s modified Eagle’s medium (25 mmol/L glucose) supplemented with 10% fetal bovine serum (Gibco). For patch clamp recording in the whole-cell and cell-attached configurations, the cells were grown to 30%-80% confluence in 35-mm dishes (Corning). All culture media contained penicillin-G (25 U/mL) and streptomycin (25 mg/mL). Cultures were maintained at 37 °C in 5% CO2 atmosphere incubator.
Whole-cell patch clamp recording followed standard techniques. Calcium currents were recorded with an EPC-9 patch clamp amplifier (HEKA Electronics, Lambrecht, Germany). All data were analyzed with PULSE/PULSFIT acquisition and analysis software (HEKA Electronics). The filter frequency was 2.8 kHz and sample frequency was 5 kHz. Patch pipettes were pulled by a two-stage puller (PC-10; Narishige, Greenvale, NY), and heat-polished with a microforge (MF-200; World Precision Instruments, Sarasota, FL) before use. For high voltage gated calcium current measurement, the extracellular solution contained (in mmol/L) 90 TEA-Cl, 40 BaCl2, 2 CaCl2, 2 MgCl2, 10 HEPES and 40 sucrose, with pH adjusted to 7.4. The pipette solution contained (in mmol/L) 130 TEA-Cl, 20 EGTA, with pH adjusted to 7.3. For non-selective cation current recording, the extracellular solution contained (in mmol/L): 120 NaCl, 20 TEA-Cl, 5.6 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, with pH adjusted to 7.4; the pipette solution contained (in mmol/L): 130 CsCl, 10 NaCl, 5 Cs-EGTA, 2 MgCl2, 5 HEPES, with pH adjusted to 7.4. For cation substitution experiments, bath solutions were the same as the pipette solution except CsCl (120 mmol/L) was replaced by the same amount of KCl or NaCl, accordingly.
Intracellular free calcium concentration was measured in HIT-15 cells using Indo-1 fluorescence imaging. Cells were loaded with 2.5 mmol/L Indo-1 for 30 min. The cells were incubated at 37 °C for 45 min to allow for de-esterification. Fluorescent measurement was conducted by using an ACAS 570 confocal laser scanning microscope. The measurement solution contained (in mmol/L) 135 NaCl, 2 CaCl2, 5 KCl, 10 HEPES, with pH adjusted to 7.3. The loading solution consisted of measurement solution augmented with indo-1 AM (2.5 μmol/L), DMSO (0.4%) and pluronic F-127 (0.1%). The de-esterification washing solution contained (in mmol/L): 82 Na2SO4, 30 K2SO4, 5 MgCl2, 10 HEPES, 10 glucose, and 1 mg/mL BSA. Cells were illuminated with monochromatic light (350 nm), and emitted light was detected at wavelengths of 405 and 485 nm using photomultiplier tubes. The signals from the photomultipliers and force transducer were digitized and stored on a dedicated computer. Data were acquired and analyzed with a separate data analysis work station.
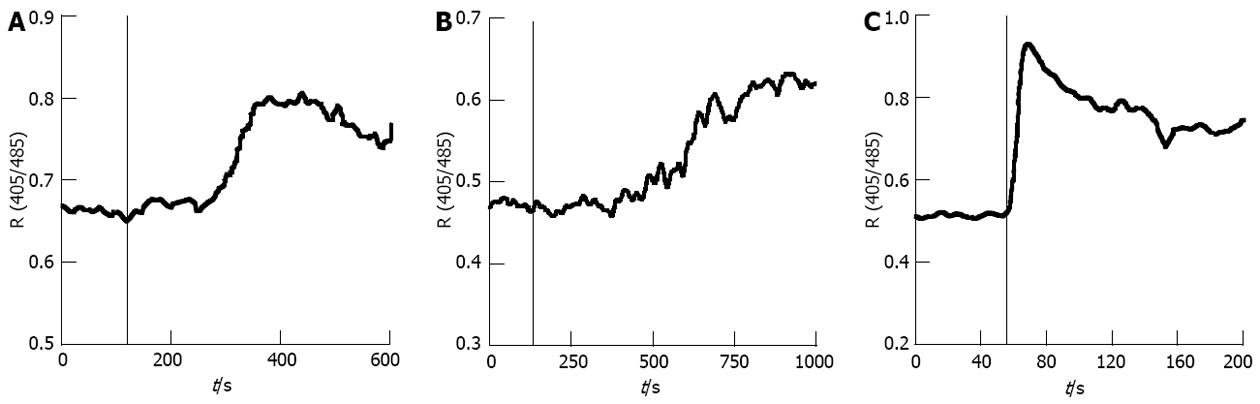
It is known that MTX-P’s cytotoxicity is related to disturbing of intracellular calcium homeostasis. In order to determine the effect of MTX-C on intracellular calcium regulation, experiments using the fluorescent dye indo-1 to directly measure the intracellular free calcium concentration were performed in insulin secreting HIT-T15 cells. Both MTX-C and MTX-P induce a sustained elevation in intracellular free calcium concentration. Figure 1A shows the intracellular free calcium concentration before and after the application of 200 nmol/L MTX-C. About 3 min after perfusion of MTX-C, a gradual increase in free calcium concentration was observed. This elevation was sustained throughout the entire recording period. Similar results were obtained from a MTX-P (10 μmol/L) perfusion experiment, as shown in Figure 1B. The increase of free intracellular calcium started after a delay, and then increased to a plateau over 4-5 min. The pattern of maitotoxin-induced intracellular calcium elevation is noticeably different than the calcium elevation caused by opening of voltage-gated calcium channels where a sharp peak of calcium is followed by a quick decay, as was observed in the same type of cells (Figure 1C).
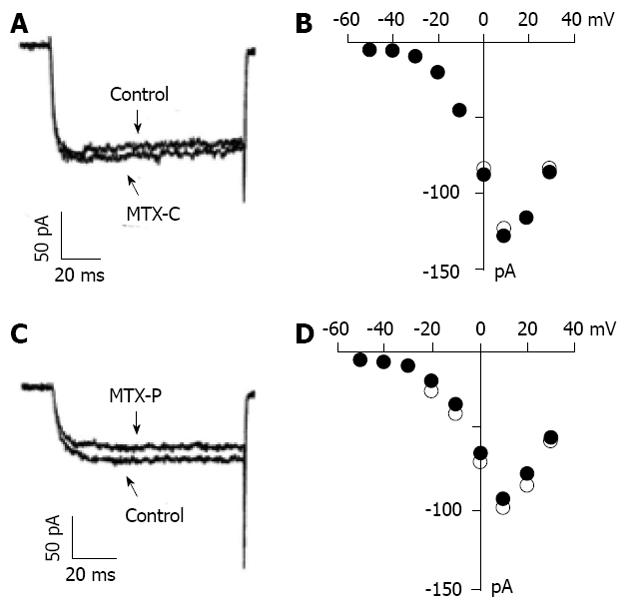
Although the calcium fluorescence experiments suggest that MTX-C might not activate voltage gated calcium channels directly, it is important to determine whether MTX-C alters the kinetics of voltage gated calcium channels. To address this question, whole cell patch clamp experiments were employed to record voltage calcium channel currents and analyze the I-V relationship under conditions with or without the presence of MTX-C or MTX-P. Figure 2A and C show barium current traces measured at 10 mV when held at -70 mV. Figure 2B and D show the I-V plots of the channels before and after adding MTX-C and MTX-P, respectively. These results show that the currents measured in these experiments are generated from the activation of high voltage gated calcium channels. Neither MTX-C nor MTX-P has significant effect on the current amplitude or the voltage dependence of these channels.
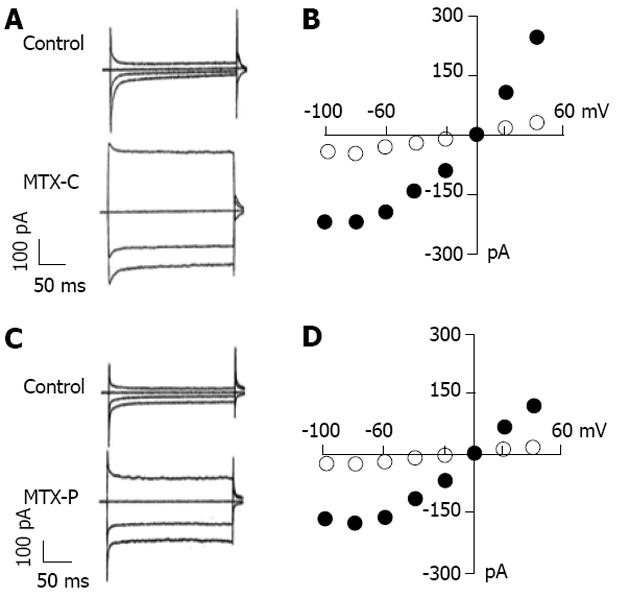
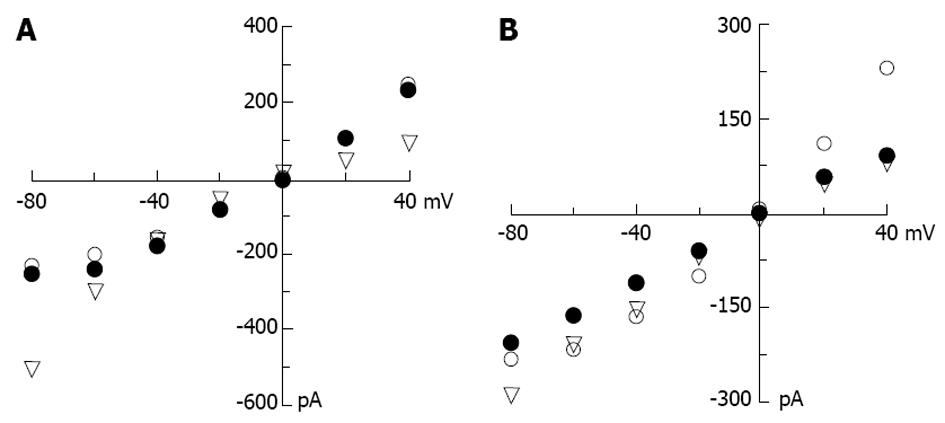
Since voltage gated calcium channels are not the primary targets of MTX-C, could its effect of intracellular calcium elevation result from the activation of non-voltage activated cation channels? Experiments were performed to delineate the effect of MTX-C on non-voltage activated cation currents, which were measured using extracellular sodium and intracellular cesium solutions. In these whole cell patch clamp experiments, the membrane potential was held at 0 mV, and the test pulses were from -100 to 50 mV in increments of 10 mV. As shown in Figure 3, both MTX-C and MTX-P caused profound cation currents, which are approximately linear within the voltage range from -60 to 50 mV, but reduced or flattened at voltages at -80 and -100 mV (Figure 3B and D). Since the electrical driving force for calcium ions are higher at these voltages, this rectification may due to a partial closure of non-voltage gated calcium channels.
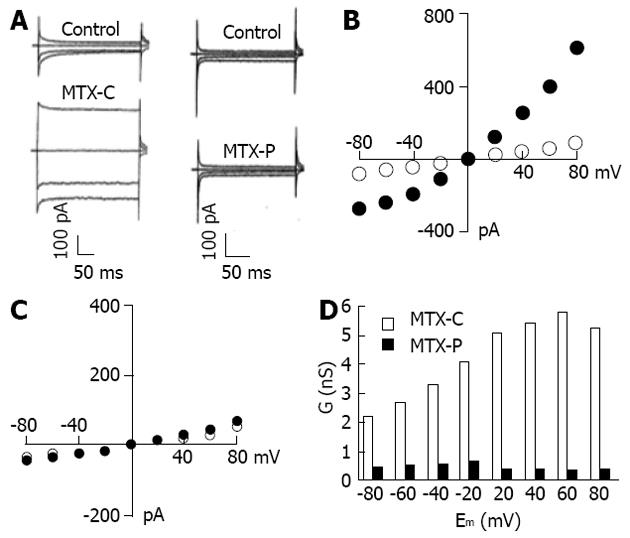
Further experiments revealed that these profound non-voltage activated currents induced by MTX-C and MTX-P had a different characterization in extracellular calcium dependency. Under the condition in which EGTA was used to chelate the free extracellular calcium, MTX-P failed to elicit non-voltage activated cation current, as shown in Figure 4A and C. In contrast, this current was continually detected in MTX-C treated cells bathed in the calcium free extracellular solution (Figure 4A and B). However, the inward current conductance was non-linear at voltages lower than 0 mV, as shown in Figure 4D. The discrepancy in calcium-dependent activation of non-voltage activated current between MTX-C and MTX-P indicates that the toxicological mechanisms in activating non-voltage activated cation currents by the two isoforms of maitotoxin have small but distinct differences.
The selectivity of MTX-C and MTX-P sensitive channels for other cations were also examined. The I-V relationship analyses were performed under conditions of symmetrical CsCl in the pipette and bath solutions, or the bath cesium was replaced by potassium or sodium ions. Since the reversal potentials across at 0 mV for both toxin-elicited currents in all cation solutions, the selectivity is the same for Na+, Cs+ and K+. These results suggest that maitotoxins elicit a non-selective, non-voltage activated current. However, for MTX-C, while the I-V relationships in extracellular CsCl were similar to that in NaCl as shown above (Figure 3B), it was very different when the extracellular solution contained KCl, where a clear inward rectification was observed (Figure 5A). In MTX-P sensitive channel experiments, unlike in the NaCl extracellular solution, this channel showed non-linear reduction at very negative test potential (Figure 3D). The I-V relationships were linear in all voltages between 100 to 50 mV when the bath solutions contain CsCl or KCl (Figure 5B). These results suggest that MTX-C and MTX-P may act on different channel proteins or act on different parts of the same channel protein.
In conclusion, MTX-C causes an opening of non-selective, non-voltage activated ion channels, which permits or elicits further abnormal calcium influx. The elevated level of intracellular calcium concentration resulting from this calcium influx may lead to cellular toxicities.
The toxic potency of MTX-P exceeds that of ciguatera toxins (LD50 0.05/kg ip in mice). Its biological activity is strictly calcium dependent and causes both membrane depolarization and calcium influx in many different cell types. It was originally believed to be an activator of voltage dependent calcium channels[23]. However, voltage dependent calcium channel antagonists can block MTX-P-stimulated calcium influx, but not MTX-P-induced membrane depolarization[24]. The results in the present study show that the opening of non-selective cation channels will result in a net positive ion influx due to the higher electrochemical driving force for sodium ions than for potassium ions. Such a net sodium ion influx can depolarize cell membrane potential and caused activation of voltage gated calcium channels in HIT-T15 cells. This depolarization is a gradual process, which could explain why there was a time delay in the intracellular calcium experiments (Figure 1A and B). MTX-C-induced membrane depolarization also likely causes anomalies of cellular functions and possibly reduces chances of cell survival.
Although the structure of MTX-P has been described previously[25,26], the mechanism of calcium dependence in MTX-P-induced current activation is unclear. It is possible that extracellular calcium creates a significant surface potential that provides a necessary influent to help MTX-P open non-selective cation channels, or it may affect an enzymatic mechanism on the cell surface to achieve the same purpose. The fact that MTX-C could effectively activate these channels regardless of the presence of extracellular calcium indicates that it may be a more potent isoform than MTX-P. However, this likely has little physiological significance since living cells are exposed to an extracellular solution containing calcium ions.
The authors thank Dr. Jonathan Pottle for his assistance in the preparation of this manuscript.
Ciguatera occurs circumglobally in tropical coral reef regions, and results from the consumption of fish that have accumulated toxin through the food web. Maitotoxin (MTX) is one of the toxins implicated in ciguatera. MTX is the most potent marin toxin which causes severe illness in gastrointesting system. MTX presents primarily as an acute neurologic disease manifested by a constellation of gastrointestinal, neurologic and cardiovascular signs and symptoms. Acute fatality, usually due to respiratory failure, circulatory collapse or arrhythmias, ranges from 0.1% to 12% of reported cases. Although the mechansim of pacific isoform of MTX induced cell damage has been studied extensively, the mechanism of caribbean isoform of MTX toxicity is largely unknown.
MTX activates Ca2+ permeable, non-selective cation channels, leading to an increase in levels of cytosolic Ca2+ ions, which triggers a cell death cascade, resulting in membrane blebbing and eventually cell lysis. MTX is known to activate cytosolic calcium-activated proteases calpain-1 and calpain-2, contributing to necrosis. The molecular characters of MTX activated ion channels, however, remains unknown. This study charactered the electrophysiogical differences between currents induced by two isoforms of MTX, provided more insights to the physiological properties of the channel.
The study shows that pacific and caribben isoforms of MTX share similar potency in eliciting calcium influx, carried by similar non-selective, non-voltage activated outward-rectified calcium currents. However, the currents induced by two isoforms of MTX had a difference in extracellular calcium dependency: function of pacific MTX is extracellular calcium dependent whereas the caribbean MTX is not. This difference indicates that the toxicological mechanisms is different between the two isoforms of MTX.
The conclusion of this study can be applied to further characterize the molecular nature of the MTX activated ion channels and to eventually elucidate the molecular mechanism of MTX toxicity.
Rectified current-voltage relationship: The changes in the size of current is non-linear with the changes of voltage values.
This succinct paper characterizes the effects of caribbean maitotoxin (MTX-C) on Ca2+ influx in insulin-secreting HIT-T15 cells by means of cytosolic free Ca2+ concentration ([Ca2+]i) measurement and whole-cell patch-clamp analysis. [Ca2+]i measurement reveals that this toxin induces a marked increase in [Ca2+]i. Whole-cell patch-clamp analysis verifies that MTX-C had no influence on voltage-gated Ca2+ currents, but evidently activated non-selective cation channels. The data are interesting. They indicate that MTX-C may exert their cellular toxicity by promoting Ca2+ influx through non-selective cation channels in insulin-secreting HIT-T15 cells.
| 1. | Takahashi M, Ohizumi Y, Yasumoto T. Maitotoxin, a Ca2+ channel activator candidate. J Biol Chem. 1982;257:7287-7289. [PubMed] |
| 2. | Yokoyama A, Murata M, Oshima Y, Iwashita T, Yasumoto T. Some chemical properties of maitotoxin, a putative calcium channel agonist isolated from a marine dinoflagellate. J Biochem. 1988;104:184-187. [PubMed] |
| 3. | Trevino S. Fish and shellfish poisoning. Clin Lab Sci. 1998;11:309-314. [PubMed] |
| 4. | Holmes MJ, Lewis RJ, Gillespie NC. Toxicity of Australian and French polynesian strains of Gambierdiscus toxicus (Dinophyceae) grown in culture: characterization of a new type of maitotoxin. Toxicon. 1990;28:1159-1172. [PubMed] |
| 5. | Ahmed FE. Seafood Safety. Washington, DC: National Academy Press 1991; . |
| 6. | Swift AE, Swift TR. Ciguatera. J Toxicol Clin Toxicol. 1993;31:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Ciguatera poisoning: an increasing occurrence in New Zealand. N Z Med J. 2010;123:100-102. [PubMed] |
| 8. | Isbister GK, Kiernan MC. Neurotoxic marine poisoning. Lancet Neurol. 2005;4:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Clark RF, Williams SR, Nordt SP, Manoguerra AS. A review of selected seafood poisonings. Undersea Hyperb Med. 1999;26:175-184. [PubMed] |
| 10. | Vernoux JP, Lewis RJ. Isolation and characterisation of Caribbean ciguatoxins from the horse-eye jack (Caranx latus). Toxicon. 1997;35:889-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Yasumoto T, Nakajima I, Oshima Y, Bagnis R. A new toxic dinoflagellate found in assocation with ciguatera. Toxic Dinoflagellate Blooms. North Holland: Elsevier 1979; 65. |
| 12. | Escobar LI, Salvador C, Martínez M, Vaca L. Maitotoxin, a cationic channel activator. Neurobiology (Bp). 1998;6:59-74. [PubMed] |
| 13. | Estacion M, Schilling WP. Maitotoxin-induced membrane blebbing and cell death in bovine aortic endothelial cells. BMC Physiology. 2001;1:2. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Dietl P, Völkl H. Maitotoxin activates a nonselective cation channel and stimulates Ca2+ entry in MDCK renal epithelial cells. Mol Pharmacol. 1994;45:300-305. [PubMed] |
| 15. | Musgrave IF, Seifert R, Schultz G. Maitotoxin activates cation channels distinct from the receptor-activated non-selective cation channels of HL-60 cells. Biochem J. 1994;301:437-441. [PubMed] |
| 16. | Schilling WP, Sinkins WG, Estacion M. Maitotoxin activates a nonselective cation channel and a P2Z/P2X(7)-like cytolytic pore in human skin fibroblasts. Am J Physiol. 1999;277:C755-C765. [PubMed] |
| 17. | Leech CA, Habener JF. A role for Ca2+-sensitive nonselective cation channels in regulating the membrane potential of pancreatic beta-cells. Diabetes. 1998;47:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Worley JF, McIntyre MS, Spencer B, Dukes ID. Depletion of intracellular Ca2+ stores activates a maitotoxin-sensitive nonselective cationic current in beta-cells. J Biol Chem. 1994;269:32055-32058. [PubMed] |
| 19. | Roe MW, Worley JF, Qian F, Tamarina N, Mittal AA, Dralyuk F, Blair NT, Mertz RJ, Philipson LH, Dukes ID. Characterization of a Ca2+ release-activated nonselective cation current regulating membrane potential and [Ca2+]i oscillations in transgenically derived beta-cells. J Biol Chem. 1998;273:10402-10410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Leech CA, Habener JF. Insulinotropic glucagon-like peptide-1-mediated activation of non-selective cation currents in insulinoma cells is mimicked by maitotoxin. J Biol Chem. 1997;272:17987-17993. [PubMed] |
| 21. | Morales-Tlalpan V, Vaca L. Modulation of the maitotoxin response by intracellular and extracellular cations. Toxicon. 2002;40:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Yang SN, Berggren PO. The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr Rev. 2006;27:621-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Gusovsky F, Daly JW. Maitotoxin: a unique pharmacological tool for research on calcium-dependent mechanisms. Biochem Pharmacol. 1990;39:1633-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 106] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Xi D, Van Dolah FM, Ramsdell JS. Maitotoxin induces a calcium-dependent membrane depolarization in GH4C1 pituitary cells via activation of type L voltage-dependent calcium channels. J Biol Chem. 1992;267:25025-25031. [PubMed] |
| 25. | Murata M, Naoki H, Iwashita T, Mutsunaga S, Sasaki M, Yokoyama A, Yasumoto T. Structure of maitotoxin. J Am Chem Soc. 1993;115:2060-2062. [RCA] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Zheng W, DeMattei JA, Wu J-P, Duan JJ, Cook LR, Oinuma H, Kishi Y. Complete relative sterochemistry of maitotoxin. J Am Chem Soc. 1996;118:7946-7968. [DOI] [Full Text] |
P- Reviewer Yang SN S- Editor Song XX L- Editor A E- Editor Li JY