Published online Sep 15, 2025. doi: 10.4239/wjd.v16.i9.110639
Revised: July 23, 2025
Accepted: August 27, 2025
Published online: September 15, 2025
Processing time: 92 Days and 17.4 Hours
The global rise in overweight and obesity has reached alarming levels, substantially increasing the risk of metabolic disorders such as dyslipidemia. We outlined the evolving trends in baseline blood lipid levels among patients experiencing overweight or obesity, as observed in placebo-controlled randomized trials, to address the unmet clinical requirements.
To assess long-term trends in lipid profiles in overweight or obese populations and their association with clinical and treatment factors.
EMBASE, PubMed, Cochrane Library, and Web of Science databases were searched up to October 9, 2024. Randomized placebo-controlled trials of participants with overweight or obesity, with reports of baseline lipid levels, were included. The main outcome was a correlation between pooled baseline levels of triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) with study year. Subgroup analysis was conducted based on characteristics of the populations and intervention types.
A comprehensive meta-analysis encompassing 866 studies across nearly 60 countries and regions worldwide, involving 3300 participants, revealed significant temporal trends in baseline lipid profiles. The analysis revealed a significant decline in TG (Rs = -0.704, P < 0.001, I2 = 98.6%), TC (Rs = -0.884, P < 0.001, I2 = 99.6%), and LDL-C (Rs = -0.808, P < 0.001, I2 = 96.8%) levels. In contrast, HDL-C (Rs = 0.336, P = 0.041, I2 = 99.2%) levels exhibited a progressive increase over the study period. Subgroup analyses revealed that sex, body mass index, blood pressure, diabetes status, and type of intervention influenced the observed trends, especially with patients receiving pharmacological therapies demonstrating more pronounced improvements (TG: Rs = -0.449, Padj = 0.011; I2 = 98.9%; TC: Rs = -0.650, Padj = 0.001; I2 = 99.4%; HDL-C: Rs = 0.650, Padj = 0.002; I2 = 98.6%; LDL-C: Rs = -0.417, Padj = 0.031; I² = 98.0%).
Despite rising obesity rates, lipid control has improved over three decades among individuals with overweight or obesity, reflecting the positive impact of public health efforts and effective dyslipidemia treatment strategies.
Core Tip: This global meta-analysis of 866 randomized trials (n = 3300) reveals significant improvements in lipid profiles among overweight/obese individuals over three decades, with triglycerides, total cholesterol and low-density lipoprotein-cholesterol declining while high-density lipoprotein-cholesterol increased. Notably, pharmacological interventions showed the most pronounced benefits. These findings highlight that despite rising obesity rates, concerted public health efforts and therapeutic advances have successfully mitigated dyslipidemia risks in this high-risk population, offering crucial insights for clinical practice and health policy.
- Citation: Wang YQ, Xiao QZ, Zhang ZM, Yang Y. Trends in baseline blood lipid levels in randomized placebo-controlled trials of overweight or obesity from 1990 to 2024. World J Diabetes 2025; 16(9): 110639
- URL: https://www.wjgnet.com/1948-9358/full/v16/i9/110639.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i9.110639
The prevalence of overweight and obesity has risen dramatically over the past three decades, reaching levels considered pandemic worldwide. Projections suggest that if current trends continue, more than half of the global adult population could be affected by 2050[1]. This concerning trend is not merely a cosmetic concern but a significant public health issue that is intricately linked to a range of metabolic disorders, including dyslipidemia[2].
Dyslipidemia is a clinical condition characterized by abnormal blood lipid levels, specifically elevated triglycerides (TG) and total cholesterol (TC), reduced high-density lipoprotein cholesterol (HDL-C), and a predominance of small, dense low-density lipoprotein cholesterol (LDL-C) particles[3]. The relationship between adiposity and dyslipidemia is complex and multifactorial. Mechanistically, several interrelated pathways like insulin resistance[4], chronic inflammation[5], altered adipokine secretion[6], and hepatic lipid metabolism[7] play pivotal roles in exacerbating lipid dysre
As the number of placebo-controlled clinical trials focusing on populations with overweight and obesity continues to rise, it is now feasible to assess global patterns of obesity-related lipid disorders through the lens of randomized controlled trials (RCTs). To the best of our knowledge, extensive studies with standardized designs have not yet thoroughly examined the long-term trends in lipid control among individuals with overweight or obesity enrolled in RCTs. Additionally, the unique trajectories of lipid profiles (such as TC, LDL-C, HDL-C, and TG), stratified by factors including sex, age, body mass index (BMI), blood pressure, blood glucose, and types of intervention within this group, remain inadequately characterized.
Accordingly, this study aims to elucidate trends in baseline lipid profiles among populations struggling with obesity and overweight, utilizing data sourced from placebo-controlled randomized trials. This investigation has the potential to offer fresh perspectives for the design of future RCTs aimed at assessing anti-obesity interventions, and it may also encourage advancements in therapeutic strategies and research methods focused on weight management and enhancing metabolic health.
The meta-analysis protocol was prospectively registered on the PROSPERO platform (ID: CRD420250650197) prior to data extraction. Study implementation rigorously followed the PRISMA guidelines.
We selected EMBASE, PubMed, Cochrane Library, and Web of Science databases for publications available up to October 9, 2024. Our search utilized a combination of medical terms and free-text search terms, including obesity, overweight, randomized controlled trials, blood lipids, cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, exercise, lifestyle, bariatric surgery, orlistat, bupropion/naltrexone, phentermine/topiramate, and glucagon-like peptide-1 receptor agonists. To ensure the inclusion of relevant studies, we also reviewed the reference lists of pertinent reviews in this field.
The inclusion criteria for this study were as follows: (1) Availability of full text; (2) Inclusion of adult populations aged ≥ 18 years classified as overweight and/or obese; (3) Studies must provide data on baseline blood lipid levels, including TG, TC, HDL-C, and LDL-C; (4) The study design must be a randomized placebo-controlled trial; and (5) Studies must be published in English. The exclusion criteria were: (1) Inability to extract blood lipid data separately; (2) Inclusion of populations with comorbidities such as pregnancy, mental illness, cancer, secondary obesity, or the use of concurrent medications or interventions likely to influence lipid levels; and (3) Studies that excluded populations based on restricted blood lipid levels at baseline. Two investigators (Wang YQ and Xiao QZ) independently browsed the title, abstract, full text, and reference list for potentially eligible trials. Discrepancies were resolved through discussion with a third reviewer (Yang Y).
Two reviewers independently extracted data using predefined forms, including the study characteristics (first author, publication year, country, and sample size), participant characteristics (age, sex, baseline BMI, baseline blood pressure, and blood glucose control condition), and intervention methods (e.g., diet, exercise, pharmacotherapy, and surgery). The quality assessment of selected studies used the Cochrane risk of bias assessment tool.
This meta-analysis aimed to evaluate the pooled effect sizes of baseline blood lipid levels, including TG, TC, HDL-C, and LDL-C, derived from participants in studies that were published in the same year. We utilized a random-effects model for our analysis due to the significant heterogeneity noted among the studies. To facilitate comparisons among studies, we standardized the measurements of blood lipids and glucose levels, with all values expressed in milligrams per deciliter (mg/dL). For studies reporting cholesterol levels (TC, HDL-C, or LDL-C) in mmol/L, we applied a conversion factor of 38.67 to obtain mg/dL. Similarly, triglyceride values reported in mmol/L were converted to mg/dL using a factor of 88.55, and blood glucose levels were standardized to mg/dL by applying a factor of 18. This approach ensured consistent interpretation of estimates across different studies[9,10].
Subgroup analyses were conducted based on participant characteristics, including age group (< 65 or ≥ 65 years old), baseline BMI levels (< 30 kg/m2 or ≥ 30 kg/m2), sex (male predominant or female predominant), blood pressure (hypertension or non-hypertension) and blood glucose measures (fasting blood glucose level (> 110 or ≤ 110 mg/dL), glycated hemoglobin A1c (HbA1c) (≥ 6.5% or < 6.5%), diabetes mellitus (DM) complication (DM combined or DM uncombined)); and treatment characteristics, including treatment type (monotherapy or combination therapy), the use of diet, lifestyle, medicine, surgery, and exercise (user or non-users). Hypertension was defined as a baseline mean systolic blood pressure ≥ 140 mmHg and/or a mean diastolic blood pressure ≥ 90 mmHg among study participants. In this meta-analysis, monotherapy refers to the use of a single intervention, whereas combination therapy involves two or more concurrent treatment strategies. Given the focus on overweight and obese populations, subsequent subgroup analyses were stratified by intervention type, including dietary interventions, lifestyle modifications, pharmacologic treatments, bariatric surgery, and structured exercise programs. Changes in blood lipid levels (∆ values) were defined as the dif
To compare and analyze the baseline data of TG, TC, HDL-C, and LDL-C in predefined subgroups, the Wilcoxon test was used based on the non-normal distribution of the data. Linearity was assessed through visual inspection of residual-versus-fitted value plots. Subsequently, Spearman correlation and linear regression analyses were performed to evaluate associations. Statistical analyses were conducted in STATA 13.0 and SPSS 26.0. A P value of < 0.05 was considered statistically significant.
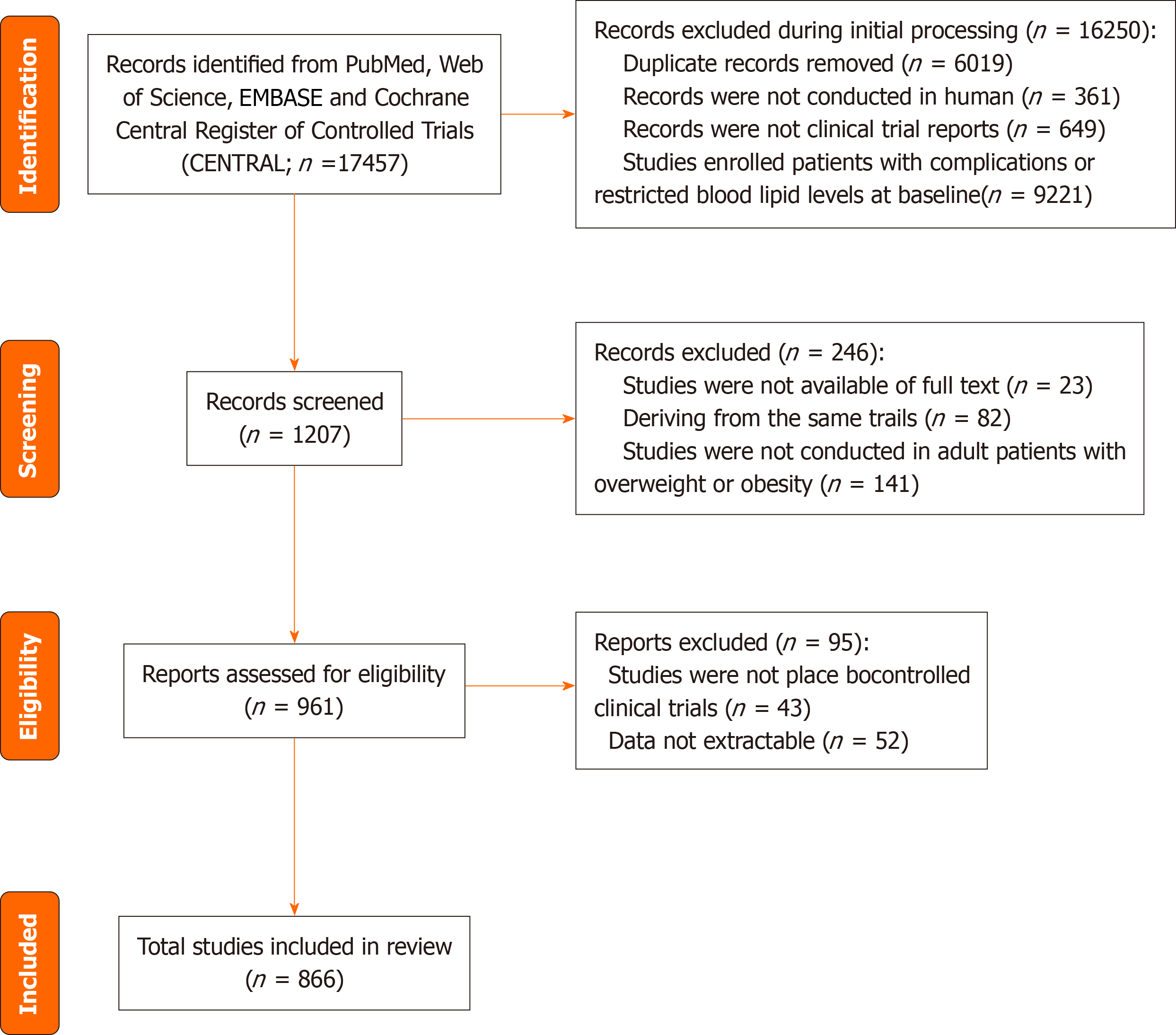
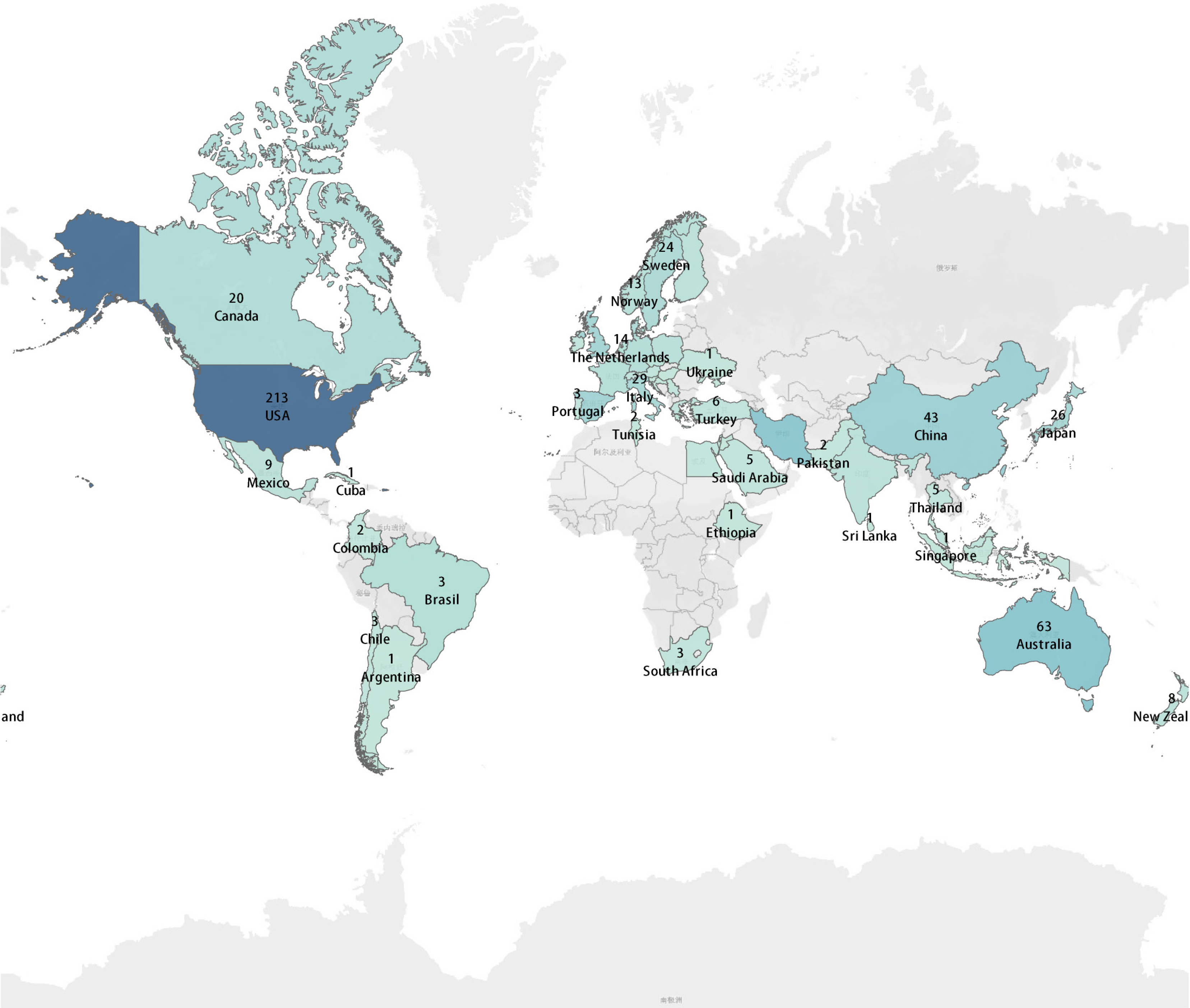
We identified a total of 17457 citations, of which 866 studies involving 3,300 participants were included in our subsequent analysis (Figure 1). The quality assessment conducted using the Cochrane risk-of-bias tool is presented in Supplementary Table 1. The baseline characteristics of the included studies are summarized in Supplementary Table 2. Egger’s funnel plots showed potential publication bias in the analyses of baseline blood lipid levels (Supplementary Figure 1). Our research encompasses trials from nearly 60 countries and regions worldwide, with 213 studies originating from the United States, which accounts for the highest proportion at 24.6% (Figure 2).
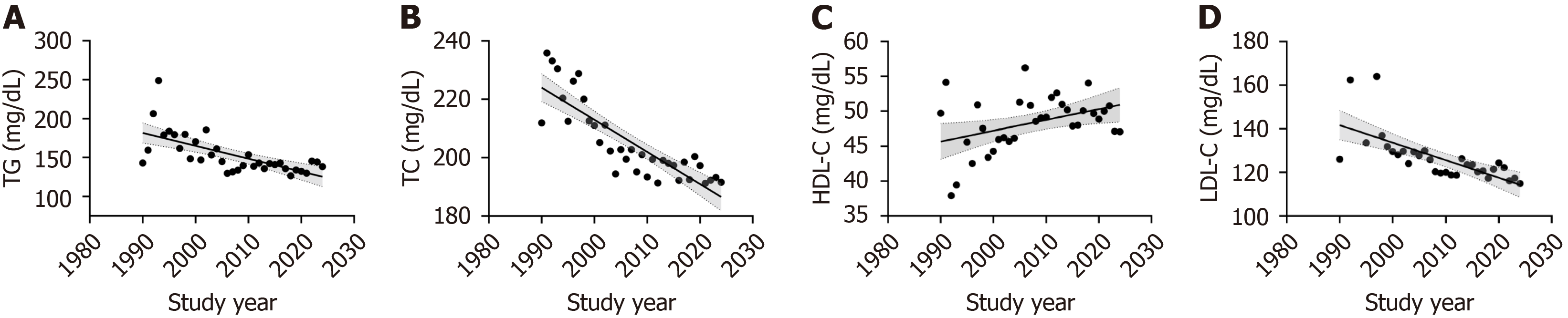
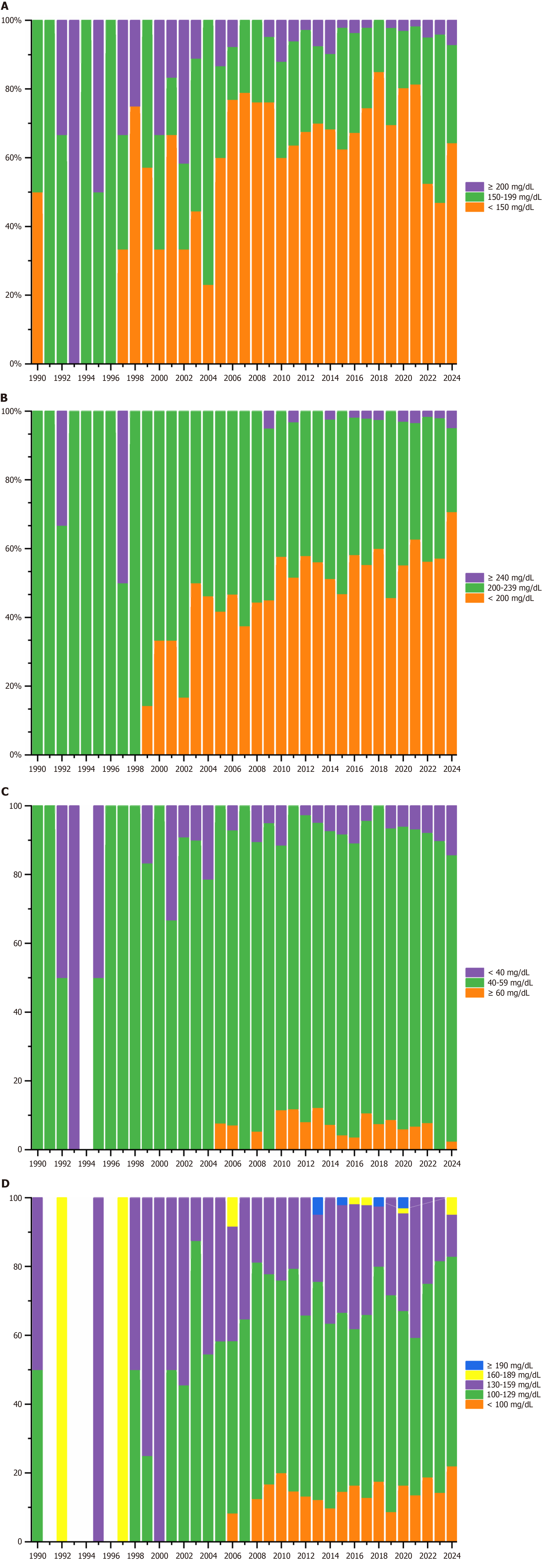
A significant decline in baseline TG levels was observed over time (Rs = -0.704, P < 0.001, I2 = 98.6%; Figure 3A). The mean baseline TG concentrations demonstrated a pronounced acceleration in the downward trend prior to 2010, followed by a slower decline thereafter, with a peak observed in 1993 and a nadir in 2018. As for the distribution pattern of TG, the proportion of trials in which participants in categories of TG ≥ 200 mg/dL dropped substantially from 66.7% in 1992 to 7.0% in 2024 (Figure 4A).
Concurrently, a marked reduction in baseline TC was demonstrated during 1990-2024 (Rs = -0.884, P < 0.001, I2 = 99.6%; Figure 3B). Mean TC values exhibited a steady decline from 235 mg/dL in the 1990s to 192 mg/dL by 2024. Regarding distribution patterns, the percentage of studies with participants classified in the low-risk TC category (< 200 mg/dL) increased from 0.0% in 1990 to 67.4% in 2024 (Figure 4B). The proportion of patients with baseline TC exceeding 240 mg/dL remained below 10%, except for 33.3% in 1992 and 50.0% in 1997 (Figure 4B).
In contrast to other lipid parameters, HDL-C levels showed a progressive increase over time (Rs = 0.336, P = 0.041, I2 = 99.2%; Figure 3C). Baseline HDL-C levels exhibited greater fluctuations during the 1990s, which can be attributed to the limited number of studies conducted during that period. In contrast, since 2005, HDL-C levels have stabilized at higher values, exceeding 45 mg/dL. Meanwhile, patients with HDL-C below 40 mg/dL remained less than 15.0% in most study years (Figure 4C).
Similarly, baseline LDL-C levels decreased substantially (Rs = -0.808, P < 0.001, I² = 96.8%), dropping by approximately 8.1 mg/dL per decade in patients with overweight or obesity worldwide (Figure 3D). Moreover, patients classified as LDL-C more than 130 mg/dL declined from 50% in 1998 to less than 20% in 2024 (Figure 4D). Supplementary Figure 2 presents the trial number range from 1990 to 2024 of different TG, TC, HDL-C, and LDL-C categories among patients with overweight or obesity in placebo-controlled RCTs.
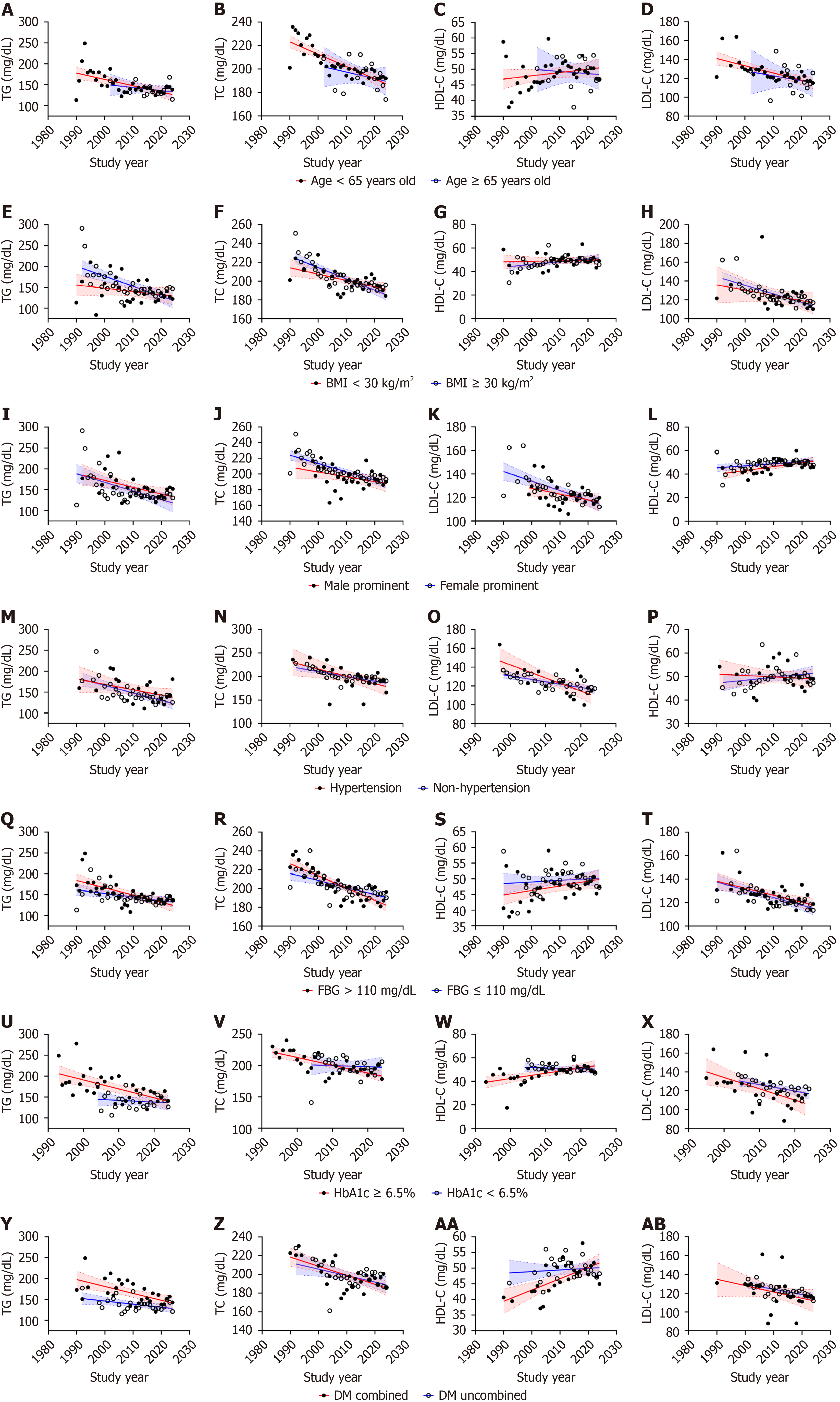
Age: Non-elderly patients (< 65 years) exhibited a marked inverse association between baseline TG levels and study year, whereas elderly patients showed no significant temporal trend (nonelderly individuals: Rs = -0.575, Padj = 0.002, I2 = 98.7%; elderly individuals: Rs = -0.121, Padj = 0.694, I2 = 96.0%, Figure 5A). Notably, inter-subgroup comparisons demonstrated no statistically significant difference in mean TG levels (Z = -0.130, P = 0.897, Supplementary Table 3).
This age-dependent divergence was also observed in TC. Non-elderly individuals displayed a robust negative correlation between TC and study year (nonelderly individuals: Rs = -0.856, Padj = 0.002, I2 = 99.6%; elderly individuals: Rs = -0.323, Padj = 0.260, I2 = 96.5%, Figure 5B). Similarly, mean baseline TC levels remained comparable across subgroups (Z = -0.411, P = 0.681, Supplementary Table 3).
In contrast, HDL-C levels showed no discernible temporal association in either age cohort (non-elderly individuals: Rs = 0.268, Padj = 0.250, I2 = 99.2%; elderly individuals: Rs = -0.022, Padj = 0.940, I2 = 98.2%, Figure 5C). Furthermore, there was no significant difference identified in the average HDL-C levels between the two subgroups (Z = -0.300, P = 0.764, Supplementary Table 3).
In non-elderly patients, a negative relationship was observed between the average baseline LDL-C levels and the year of the study (nonelderly individuals: Rs = -0.755, Padj = 0.002, I2 = 99.0%; elderly individuals: Rs = -0.231, Padj = 0.448, I2 = 98.2%, Figure 5D). Subgroup comparisons again revealed no significant LDL-C disparity (Z = -1.011, P = 0.312, Supplementary Table 3).
BMI: Significant longitudinal trends in baseline lipid profiles were observed among participants with BMI ≥ 30 kg/m² during the 1990-2024 study period. Specifically, TG, TC, and LDL-C levels exhibited statistically significant decreasing trends, while HDL-C did not demonstrate a significant association (TG: Rs = -0.711, Padj = 0.002, I2 = 98.7%; TC: Rs =
Sex: We observed a reduction in the trends of TG over the years in studies predominantly involving males and females (male predominant: Rs = -0.472, Padj = 0.011, I2 = 98.5%; female predominant: Rs = -0.500, Padj = 0.006, I2 = 98.2%, Figure 5I). There was no significant difference in the magnitude of TG level between males and females (Z = -1.942, P = 0.052, Supplementary Table 3).
In studies predominantly involving females, the overall trends of TC and LDL-C showed a decrease over time (TC: Rs = -0.874, Padj = 0.002, I2 = 98.4%; LDL-C: Rs = -0.696, Padj = 0.002, I2 = 99.1%). However, a sex-stratified analysis did not reveal a notable trend in TC and LDL-C levels in male-predominant studies (TC: Rs = -0.338, Padj = 0.078, I2 = 99.8%; LDL-C: Rs = -0.340, Padj = 0.089, I2 = 98.0%, Figure 5J and K). No significant differences were found in TC and LDL-C levels between these two groups (TC: Z = -1.812, Padj = 0.070; LDL-C: Z = -0.449, Padj = 0.653, Supplementary Table 3).
Regarding HDL-C, we observed positive correlations between HDL-C levels and time in male predominant studies (male predominant: Rs = 0.565, Padj = 0.004, I2 = 98.9%; female predominant: Rs = 0.344, Padj = 0.050, I2 = 99.2%, Figure 5L). Additionally, the average HDL-C level for females was markedly greater than that of males (Z = -2.736, P = 0.006, Supplementary Table 3).
Blood pressure: Among individuals with hypertension and non-hypertension, significant inverse correlations over time were observed for TG (hypertension: Rs = -0.345, Padj = 0.027, I2 = 99.1%; non-hypertension: Rs = -0.154, Padj = 0.002, I2 = 98.7%), TC (hypertension: Rs = -0.513, Padj = 0.002, I2 = 99.2%; non-hypertension: Rs = -0.162, Padj = 0.002, I2 = 99.7%), and LDL-C (hypertension: Rs = -0.401, Padj = 0.026, I2 = 98.9%; non-hypertension: Rs = -0.144, Padj = 0.030, I2 = 99.1%), whereas HDL-C trends remained statistically nonsignificant (hypertension: Rs = -0.142, Padj = 0.740, I2 = 97.3%; non-hypertension: Rs = -0.015, Padj = 0.747, I2 = 99.5%; Figure 5M-P). Between-strata comparisons demonstrated a significant difference in TG trajectory (Z = -2.232, P = 0.026, Supplementary Table 3).
Blood glucose: Fasting blood glucose level: In patients with elevated fasting blood glucose (> 110 mg/dL), a markedly negative temporal association was detected for TG levels (Rs = -2.02, Padj = 0.004, I2 = 98.7%), alongside significant declines in TC (Rs = -0.351, Padj = 0.002, I2 = 97.2%) and LDL-C (Rs = -0.137, Padj = 0.042, I2 = 99.5%), although the HDL-C trend remained statistically insignificant (Rs = 0.097, Padj = 0.272, I2 = 98.8%). Among subgroup of fasting blood glucose ≤ 110 mg/dL, lipid patterns were still showed statistically significant reductions in TG (Rs = -0.093, Padj = 0.022, I2 = 98.6%), TC (Rs = -0.133, Padj = 0.001, I2 = 99.7%), and LDL-C (Rs = -0.122, Padj = 0.006, I2 = 97.4%), with no significant HDL-C trend (Rs = 0.054, Padj = 0.186, I2 = 99.3%; Figure 5Q-T). Between-group comparisons revealed a more pronounced downward trajectory of TG levels in individuals with hyperglycemia (Z = –2.540, P = 0.011, Supplementary Table 3).
HbA1c: In individuals with HbA1c ≥ 6.5%, significantly decreasing trends were observed for TG (Rs = -0.359, Padj = 0.002, I2 = 99.3%), TC (Rs = -0.43, Padj = 0.002, I2 = 96.6%), and LDL-C (Rs = -0.319, Padj = 0.006, I2 = 99.7%), while HDL-C showed a moderate yet statistically significant upward association (Rs = 0.303, Padj = 0.008, I2 = 98.9%). In contrast, participants with HbA1c < 6.5% did not exhibit statistically significant temporal trends in any lipid parameter (TG: Rs =
Combined with DM: Notable between-group differences emerged in lipid trends when stratified by DM status. Patients with comorbid DM demonstrated statistically significant negative temporal trends for TG, TC, and LDL-C, accompanied by a robust positive HDL-C association (TG: Rs = -0.581, Padj = 0.002, I2 = 99.3%; TC: Rs = -0.563, Padj = 0.004, I2 = 98.3%; HDL-C: Rs = 0.608, Padj = 0.002, I2 = 98.6%; LDL-C: Rs = -0.588, Padj = 0.004, I2 = 99.7%). In diabetic-free participants, significant downward trends persisted for TC and LDL-C, while TG and HDL-C showed non-significant associations with temporal progression (TG: Rs = -0.315, Padj = 0.117, I2 = 98.4%; TC: Rs = -0.422, Padj = 0.032, I2 = 99.8%; HDL-C: Rs = 0.060, Padj = 0.781, I2 = 99.4%; LDL-C: Rs = -0.558, Padj = 0.006, I2 = 97.9%, Figure 5Y-AB). Significant between-strata differences were specifically observed for TG trajectories (Z = -5.907, P < 0.001), whereas TC (Z = -0.801, P = 0.423), HDL-C (Z = -1.425, P = 0.154), and LDL-C (Z = -0.738, P = 0.460) patterns remained statistically indistinguishable between groups (Supplementary Table 3).
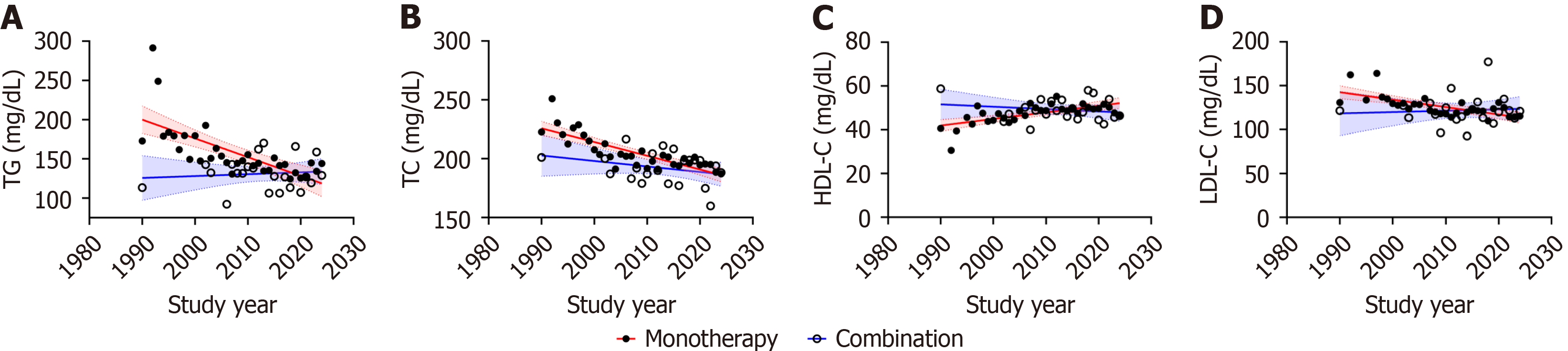
Monotherapy vs combination therapy: We observed a downward trend in baseline TG, TC, and LDL-C levels and upward trends in baseline HDL-C levels with study year in monotherapy (TG: Rs = -0.826, Padj = 0.002, I2 = 98.9%; TC: Rs = -0.833, Padj = 0.002, I2 = 99.7%; HDL-C: Rs = 0.626, Padj = 0.002, I2 = 99.3%; LDL-C: Rs = -0.688, Padj = 0.002, I2 = 99.3%). No correlation was observed between baseline blood lipid levels and time in combination therapy (TG: Rs = -0.102, Padj = 0.651, I2 = 98.5%; TC: Rs = -0.271, Padj = 0.222, I2 = 97.9%; HDL-C: Rs = -0.102, Padj = 0.651, I2 = 96.0%; LDL-C: Rs = -0.012, Padj = 0.960, I2 = 99.0%, Figure 6). Furthermore, no significant difference in baseline lipid levels was detected between the two groups (Supplementary Table 3).
Subgroup analysis of therapeutic methods: The mean baseline blood lipid levels (including TG, TC, and LDL-C) showed a significant correlation with study year in patients without diet intervention, while HDL-C did not show a significant correlation (TG: Rs = -0.604, Padj = 0.002, I2 = 98.6%; TC: Rs = -0.836, Padj = 0.002, I2 = 99.3%; HDL-C: Rs = 0.387, Padj = 0.052, I2 = 97.2%; LDL-C: Rs = -0.850, Padj = 0.002, I2 = 99.2%). Furthermore, a negative correlation between the mean baseline levels of TG, TC, and LDL-C and the study year was observed in patients who underwent dietary intervention (TG: Rs =
As for exercise, significant temporal trends in baseline lipid profiles were identified among non-exercising patients. Specifically, TG, TC, and LDL-C levels exhibited strong inverse correlations with study year (TG: Rs = -0.739, Padj = 0.002, I2 = 99.8%; TC: Rs = -0.864, Padj = 0.002, I2 = 99.2%; LDL-C: Rs = -0.833, Padj = 0.002, I2 = 98.3%), while HDL-C demonstrated a positive temporal association (Rs = 0.482, Padj = 0.008, I2 = 98.8%). Notably, patients receiving exercise intervention displayed attenuated temporal correlations for blood lipid and time, which were no longer statistically significant (TG: Rs = 0.331, Padj = 0.098; I2 = 99.2%; TC: Rs = -0.348, Padj = 0.088; I2 = 98.5%; HDL-C: Rs = -0.078, Padj = 0.709; I2 = 98.6%; LDL-C: Rs = -0.357, Padj = 0.086; I2 = 99.3%; Supplementary Figure 3E-H).
Similar trends of baseline mean blood lipid profiles with study year were observed in patients with lifestyle in
Significant temporal correlations were observed in baseline TG, TC, HDL-C, and LDL-C levels in medicine intervention patients (TG: Rs = -0.449, Padj = 0.011; I2 = 98.9%; TC: Rs = -0.650, Padj = 0.001; I2 = 99.4%; HDL-C: Rs = 0.650, Padj = 0.002; I2 = 98.6%; LDL-C: Rs = -0.417, Padj = 0.031; I² = 98.0%). In non-medicine intervention patients, baseline TG, TC, and LDL-C exhibited negative correlations with study year (TG: Rs = -0.480, Padj = 0.010, I2 = 97.6%; TC: Rs = -0.795, Padj = 0.001, I2 = 97.8%; LDL-C: Rs = -0.743, Padj = 0.002, I2 = 98.8%), while HDL-C demonstrated no temporal association in this group (Rs = 0.022, Padj = 0.904, I² = 98.5%; Supplementary Figure 3M-P).
Regarding surgery, a significant negative correlation was observed in baseline mean TG, TC, and LDL-C levels in participants without surgical intervention, except for HDL-C, which not indicated a significant correlation (TG: Rs =
The compared subgroup analysis of different therapeutic methods is detailed in Supplementary Table 3.
The change in value of blood lipid (including ∆TG, ∆TC, ∆HDL-C, and ∆LDL-C) did not present a significant correlation with time in different subgroups of the therapeutic regimen. Compared to the subgroup without surgery, the patients with surgery exhibited a greater reduction in ∆TG, ∆TC, and ∆LDL-C, as well as a more significant increase in ∆HDL-C (TG: Z = -6.301, P < 0.001; TC: Z = -5.322, P < 0.001; LDL-C: Z = -5.287, P < 0.001; HDL-C: Z = -4.804, P < 0.001). Moreover, ∆TG, ∆TC, and ∆HDL-C levels in patients with diet intervention were much lower than in patients without diet intervention (TG: Z = -3.143, P = 0.002; TC: Z = -2.756, P = 0.006; HDL-C: Z = -3.474, P = 0.001). Detailed results of subgroup analysis were reported in Supplementary Table 4.
In our analysis, among participants with overweight or obesity in placebo-controlled randomized trials, baseline blood lipid levels have improved over the past 34 years (1990–2024). Specifically, baseline mean TG, TC, and LDL-C levels have decreased, while HDL-C levels have increased. These findings indicate that blood lipid control has improved in patients with obesity despite the emergence and development of the obesity pandemic[11].
Individuals with obesity exhibit significantly elevated risks of cardiovascular disease[12], diabetes[13], cancer[14], as well as an increased risk of all-cause mortality compared to the general population[15]. Dysregulation of lipid metabolism is multifactorial, linked to genetic predisposition, hypertension, insulin resistance, chronic inflammatory states, dietary imbalance (e.g., high intake of saturated fatty acids), and sedentary lifestyle[16]. While the pathophysiological interactions remain complex, scientific consensus confirms that obesity can significantly elevate LDL-C levels through multiple pathophysiological pathways (including but not limited to the spillover effect of free fatty acids induced by visceral fat accumulation[17,18], increased synthesis of very low-density lipoprotein in the liver[19], and inhibition of lipoprotein lipase activity[20]), while simultaneously reducing HDL-C concentration and its reverse cholesterol transport function[21,22]. Current evidence offers limited insight into whether weight loss or LDL-C reduction is more effective in mitigating cardiovascular risk. However, combined intensive lipid-control and weight-control strategies in patients with obesity have been shown to lower cardiovascular risk substantially[23]. Based on the results of the Look AHEAD study, mo
According to data from the Non-communicable Disease Risk Factor Collaboration, the global age-standardized mean levels of TC and non-HDL-C exhibited little change between 1980 and 2018. Specifically, among the 200 countries and regions included in the study, the median age-standardized mean non-HDL-C levels in men increased slightly from 3.36 mmol/L in 1980 to 3.37 mmol/L in 2018, while in women, it decreased slightly from 3.44 mmol/L in 1980 to 3.34 mmol/L in 2018[26]. Notably, our analysis shows that nearly half of the data originates from the United States, with African randomized placebo-controlled trials contributing less than 1% of the total data. This suggests that our results are more representative of the health management standards in developed regions. To facilitate more accurate conclusions, systematic collection of additional data from underrepresented regions remains imperative.
Currently, there is a lack of globally unified epidemiological data regarding lipid levels and their distribution characteristics in overweight or obese populations. In addition to documenting global trends in the average lipid levels among overweight or obese patients, our findings also illustrate the unique distribution of all lipid categories within this group. Over the past 30 years, in placebo-controlled randomized trials, the proportion of overweight or obese patients with LDL-C levels exceeding 160 mg/dL has ranged from 20% to 40%, while the proportion with HDL-C levels below 40 mg/dL has consistently remained around 10%. This provides a foundation for future clinical research tailored to specific population characteristics.
Our analysis revealed no significant differences in baseline lipid trends between younger and older participants. Notably, the findings indicate that non-elderly patients exhibit a notable inverse relationship between baseline TG, TC, LDL-C, and study year. In contrast, elderly patients did not present significant temporal trends for TG, TC, HDL-C, and LDL-C, highlighting a possible age-related resistance to changes in lipid profiles over time[27].
According to existing research, obesity management has shifted from traditional, generalized approaches to more individualized treatment strategies[28]. However, current weight loss interventions for individuals with obesity remain relatively limited, with a predominant reliance on lifestyle modifications[29]. In the included studies, fewer than 10% employed pharmacological interventions. Nevertheless, these agents demonstrated significant improvements in lipid profiles[30]. Our analysis of randomized placebo-controlled studies involving pharmacological interventions de
Nevertheless, our research does have certain limitations. First, the individuals enrolled in the included RCTs were drawn from relatively specific populations under strict eligibility criteria, which limits the external validity of baseline findings. Although we excluded trials with lipid-level-based inclusion criteria, the baseline lipid profiles of RCT participants may not be generalizable to the broader real-world population. This distinction is critical, as trends in RCT-derived baseline values may reflect shifts in trial design philosophies or population characteristics rather than true epidemiological changes in the general population. Second, Egger’s test suggested a possible publication bias in the evaluation of baseline blood lipid. Therefore, the findings must be considered carefully. Third, there was significant variation in the publication years, study designs, treatment regimens, and research methodologies of the studies included, which resulted in considerable heterogeneity among them. While a random-effects model was employed for the analysis, and subgroup analyses were performed to investigate the origins of the pronounced heterogeneity, the substantial level of heterogeneity in this meta-analysis remains a limitation. This heterogeneity may compromise the precision and robustness of the pooled estimates, suggesting the results should be interpreted with certain settings. Fourth, there was a marked geographic imbalance in the distribution of included studies, with nearly half of the data derived from the United States and less than 1% from Africa. This uneven geographic representation may introduce region-specific biases and limit the generalizability of our findings to global populations, particularly to low- and middle-income countries. Consequently, the observed trends may better reflect patterns in developed nations or regions, and caution is warranted when extrapolating these results to populations in other regions. Future research efforts should aim to include more data from underrepresented regions to enhance global applicability. Fifth, only a limited number of the studies included reported clinical endpoint events, such as all-cause mortality or major adverse cardiovascular events. Due to the scarcity of such data, we were unable to perform a quantitative analysis on these outcomes. Future studies that report clinical endpoints will offer profiles that facilitate comprehensive evaluations.
Our findings indicate a significant decline in baseline TG, TC, and LDL-C levels over time, coupled with an increase in HDL-C levels. These trends reflect the positive impact of public health initiatives and effective treatment strategies aimed at managing dyslipidemia.
| 1. | GBD 2021 Adult BMI Collaborators. Global, regional, and national prevalence of adult overweight and obesity, 1990-2021, with forecasts to 2050: a forecasting study for the Global Burden of Disease Study 2021. Lancet. 2025;405:813-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 384] [Article Influence: 384.0] [Reference Citation Analysis (3)] |
| 2. | Pershina OV, Pakhomova AV, Widera D, Ermakova NN, Epanchintsev AA, Pan ES, Krupin VA, Vaizova OE, Putrova OD, Sandrikina LA, Kurochkina IV, Morozov SG, Kubatiev AA, Dygai AM, Skurikhin EG. Gender Differences in the Pharmacological Actions of Pegylated Glucagon-Like Peptide-1 on Endothelial Progenitor Cells and Angiogenic Precursor Cells in a Combination of Metabolic Disorders and Lung Emphysema. Int J Mol Sci. 2019;20:5414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421. [PubMed] |
| 4. | Kahn CR, Wang G, Lee KY. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest. 2019;129:3990-4000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 471] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 5. | Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 2173] [Article Influence: 135.8] [Reference Citation Analysis (1)] |
| 6. | Kirichenko TV, Markina YV, Bogatyreva AI, Tolstik TV, Varaeva YR, Starodubova AV. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int J Mol Sci. 2022;23:14982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 164] [Reference Citation Analysis (0)] |
| 7. | Samuel VT, Shulman GI. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2018;27:22-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 557] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 8. | Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 5985] [Article Influence: 997.5] [Reference Citation Analysis (0)] |
| 9. | Liu B, Zhu L, Wang M, Sun Q. Associations between Per- and Polyfluoroalkyl Substances Exposures and Blood Lipid Levels among Adults-A Meta-Analysis. Environ Health Perspect. 2023;131:56001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 10. | Ried K, Fakler P. Protective effect of lycopene on serum cholesterol and blood pressure: Meta-analyses of intervention trials. Maturitas. 2011;68:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Global Obesity Observatory. Obesity Atlas 2025. [cited 10 June 2025]. Available from: https://data.worldobesity.org/publications/?cat=23. |
| 12. | Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge MP; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2021;143:e984-e1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1982] [Article Influence: 396.4] [Reference Citation Analysis (0)] |
| 13. | Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah-Pollack R, Plodkowski R; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American association of clinical endocrinologists and american college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1165] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 14. | Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity's impact. Fertil Steril. 2017;107:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 554] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 15. | GBD 2015 Obesity Collaborators; Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5669] [Cited by in RCA: 5390] [Article Influence: 598.9] [Reference Citation Analysis (3)] |
| 16. | Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls SJ, Nordestgaard BG, Watts GF, Bruckert E, Fazio S, Ference BA, Graham I, Horton JD, Landmesser U, Laufs U, Masana L, Pasterkamp G, Raal FJ, Ray KK, Schunkert H, Taskinen MR, van de Sluis B, Wiklund O, Tokgozoglu L, Catapano AL, Ginsberg HN. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41:2313-2330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1209] [Cited by in RCA: 1037] [Article Influence: 172.8] [Reference Citation Analysis (0)] |
| 17. | Lee MS, Bensinger SJ. Reprogramming cholesterol metabolism in macrophages and its role in host defense against cholesterol-dependent cytolysins. Cell Mol Immunol. 2022;19:327-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 18. | Altuzar J, Notbohm J, Stein F, Haberkant P, Hempelmann P, Heybrock S, Worsch J, Saftig P, Höglinger D. Lysosome-targeted multifunctional lipid probes reveal the sterol transporter NPC1 as a sphingosine interactor. Proc Natl Acad Sci U S A. 2023;120:e2213886120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Staels B, Fonseca VA. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care. 2009;32 Suppl 2:S237-S245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Stadler JT, Marsche G. Obesity-Related Changes in High-Density Lipoprotein Metabolism and Function. Int J Mol Sci. 2020;21:8985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 21. | Bays HE, Kirkpatrick C, Maki KC, Toth PP, Morgan RT, Tondt J, Christensen SM, Dixon D, Jacobson TA. Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024. Obes Pillars. 2024;10:100108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 22. | Yang H, Fogo AB, Kon V. Kidneys: key modulators of high-density lipoprotein levels and function. Curr Opin Nephrol Hypertens. 2016;25:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Ramos Salas X, Saquimux Contreras MA, Breen C, Preiss Y, Hussey B, Forhan M, Wharton S, Campbell-Scherer D, Vallis M, Brown J, Pedersen SD, Sharma AM, Woodward E, Patton I, Pearce N. Review of an international pilot project to adapt the Canadian Adult Obesity Clinical Practice Guideline. Obes Pillars. 2023;8:100090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L; Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1082] [Cited by in RCA: 1310] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 25. | Domanski MJ, Tian X, Wu CO, Reis JP, Dey AK, Gu Y, Zhao L, Bae S, Liu K, Hasan AA, Zimrin D, Farkouh ME, Hong CC, Lloyd-Jones DM, Fuster V. Time Course of LDL Cholesterol Exposure and Cardiovascular Disease Event Risk. J Am Coll Cardiol. 2020;76:1507-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 253] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 26. | NCD Risk Factor Collaboration. [cited 10 June 2025]. Available from: https://www.ncdrisc.org. |
| 27. | Wang H, Wang C, Xuan X, Xie Z, Qiu Y, Qin H, Xiaoning Z. Association between triglyceride to high-density lipoprotein cholesterol ratio and type 2 diabetes risk in Japanese. Sci Rep. 2023;13:3719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 28. | Glanz K, Shaw PA, Kwong PL, Choi JR, Chung A, Zhu J, Huang QE, Hoffer K, Volpp KG. Effect of Financial Incentives and Environmental Strategies on Weight Loss in the Healthy Weigh Study: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2124132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Qian Y, Zhang Y, Zhong P, Peng K, Xu Z, Chen X, Lu K, Chen G, Li X, Liang G. Inhibition of inflammation and oxidative stress by an imidazopyridine derivative X22 prevents heart injury from obesity. J Cell Mol Med. 2016;20:1427-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Gorgojo-Martínez JJ, Mezquita-Raya P, Carretero-Gómez J, Castro A, Cebrián-Cuenca A, de Torres-Sánchez A, García-de-Lucas MD, Núñez J, Obaya JC, Soler MJ, Górriz JL, Rubio-Herrera MÁ. Clinical Recommendations to Manage Gastrointestinal Adverse Events in Patients Treated with Glp-1 Receptor Agonists: A Multidisciplinary Expert Consensus. J Clin Med. 2022;12:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 192] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/