Published online Sep 15, 2025. doi: 10.4239/wjd.v16.i9.109130
Revised: June 18, 2025
Accepted: August 20, 2025
Published online: September 15, 2025
Processing time: 133 Days and 21.6 Hours
Exogenous insulin may trigger immune-mediated complications, particularly among East Asian populations. Double diabetes, characterized by overlapping features of type 1 diabetes (T1D) and type 2 diabetes (T2D), may arise from insulin-induced autoimmunity. This study aimed to explore the association bet
To investigate clinical and immunogenic features of patients who develop double diabetes following exogenous insulin therapy.
We retrospectively analyzed five cases from Peking Union Medical College Hospital and 18 cases identified from published literature. Patients were ca
A total of 23 patients were included in the analysis. Of these, 10 progressed from theT2D→T1D with autoimmune features, while 13 remained in the stable T2D→T2D group. There was no statistically significant difference in age at diagnosis between the two groups (57.10 ± 16.11 years vs 60.31 ± 17.41 years). In the T2D→T1D group, 70% of patients carried the HLA-DRB1 04: 05 allele and 40% carried DRB1 09: 01, both of which are commonly associated with a high risk of T1D. In contrast, the T2D→T2D group showed greater genetic heterogeneity, with a broader distribution of HLA-DRB1 alleles, including DRB1 03: 02 (n = 4), DRB1 09: 01 (n = 4), and several lower frequency alleles such as DRB1*04: 05, *08: 03, *03: 01, *04: 06, *14: 01, *04: 01, *12: 02,*15: 02 and *02: 01.
These findings suggest that patients in the T2D→T1D group exhibit a stronger autoimmune genetic predisposition, characterized by an enrichment of high-risk HLA class II alleles. In contrast, individuals with stable T2D demonstrate greater HLA diversity and lack definitive autoimmune-associated markers.
Core Tip: We report a rare and under-recognized subtype of double diabetes, in which insulin autoantibodies induced by exogenous insulin therapy accelerate β-cell failure in patients with type 2 diabetes. This immune-mediated phenotype-predominantly observed in East Asian populations and associated with high-risk human leukocyte antigen class II alleles highlights a novel immunogenic mechanism underlying diabetes progression.
- Citation: Li XG, Qi MY, Li X, Ping F. Exogenous insulin-associated autoimmunity and the emergence of double diabetes in type 2 diabetes. World J Diabetes 2025; 16(9): 109130
- URL: https://www.wjgnet.com/1948-9358/full/v16/i9/109130.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i9.109130
Advancements in synthesis of recombinant human insulin and its analogues have significantly improved glycemic control in individuals with diabetes. However, several serious immune-mediated adverse responses to insulin therapy have been reported, including insulin allergy, localized lipoatrophy at injection sites, diabetic ketoacidosis (DKA), and acute hypoglycemia[1]. Notably, such immune-related events occur more frequently in Asian populations, particularly among individuals of East Asian descent[2,3]. Both experimental and clinical studies have shown that exogenous insulin can stimulate immune activation, including monocyte/macrophage infiltration into pancreatic islets, reflecting an autoimmune process reminiscent of type 1 diabetes (T1D) pathogenesis[4].
In recent years, the phenomenon of "double diabetes" has received growing clinical attention. Double diabetes is characterized by the coexistence of features from both type 1 and type 2 diabetes (T2D), arising either from autoimmune
As of 2024, approximately 589 million people are living with diabetes worldwide, including more than 9.5 million individuals affected by T1D (International Diabetes Federation, IDF Diabetes Atlas, 2025). The potential role of exogenous insulin in precipitating autoimmune activation among genetically susceptible individuals adds a novel dimension to the concept of double diabetes[8]. In some genetically predisposed patients-especially those carrying high-risk human leukocyte antigen (HLA) class II alleles-exogenous insulin administration may act as a trigger for autoimmunity. This mechanism may contribute to a unique form of double diabetes that resembles Exogenous Insulin Autoimmune Syndrome (EIAS), where the immune system produces pathogenic IAAs following insulin exposure[9]. The resulting autoimmune attack on pancreatic β-cells may accelerate disease progression and complicate glycemic control, nece
Our study aim to explore the potential association between high-risk HLA genotypes and susceptibility to a unique form of exogenous insulin-triggered double diabetes, with the goal of identifying individuals at elevated risk for autoimmune activation during insulin therapy.
Subjects included in this study were from two sources: (1) Retrospective clinical cases from the Department of En
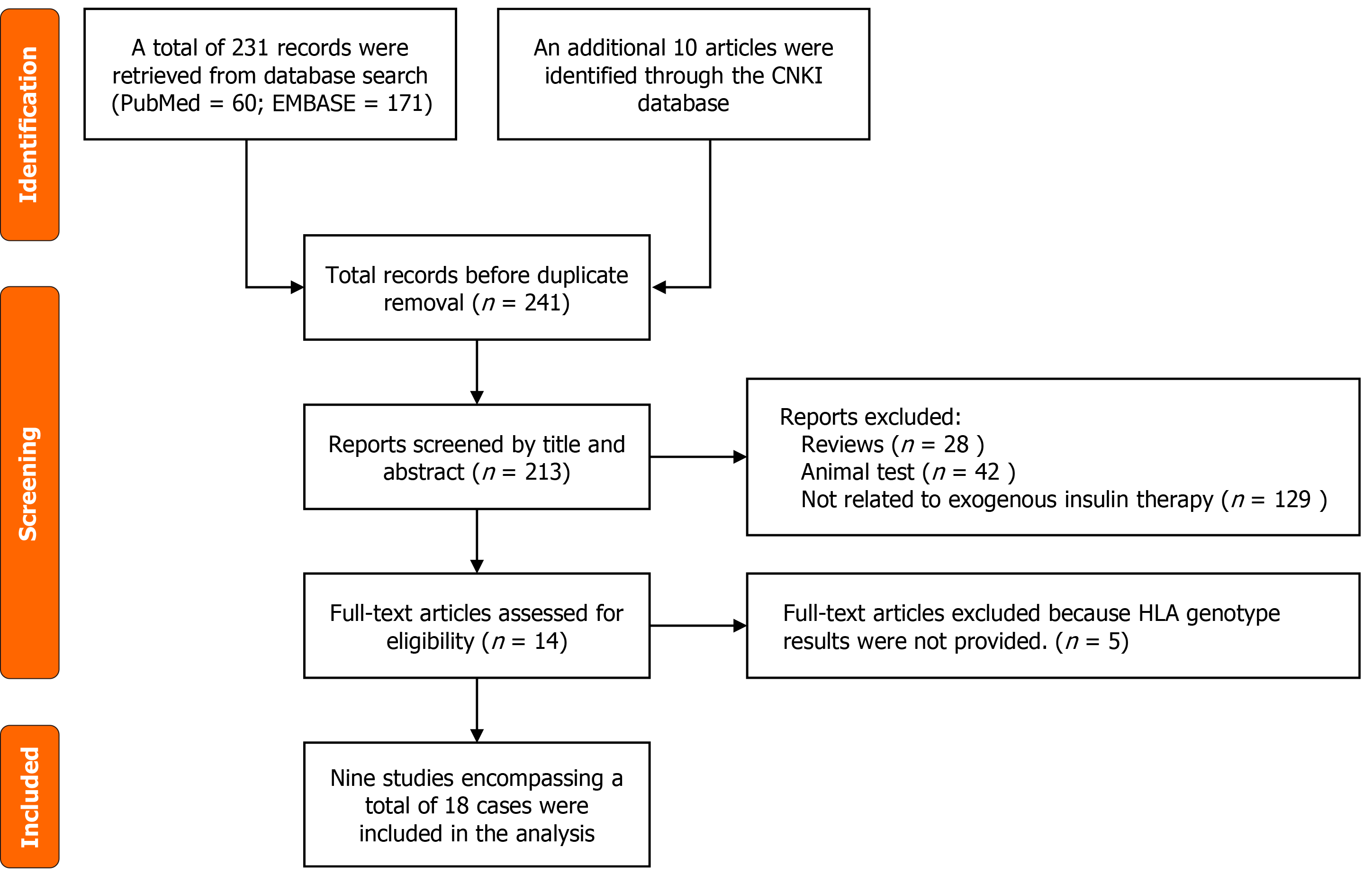
A retrospective review was performed on PUMCH cases up to December 1, 2024. In addition, a comprehensive literature search was conducted using PubMed, Embase, and CNKI databases (up to March 1, 2025), covering both Chinese and English publications. Search terms included: “Insulin autoantibody”, “insulin autoimmune syndrome”, “insulin allergy”, “DKA”, and “β-cell destruction”. Of the 241 records screened, 9 studies met eligibility criteria, yielding 18 Literature-derived cases (Figure 1).
Cases from both sources were included if they met all of the following criteria: (1) Initial diagnosis of T2D; (2) Development of IAAs following subcutaneous insulin therapy; and (3) Availability of HLA genotyping data.
Subjects were classified into the T2D→T1D group if they developed undetectable C-peptide levels or DKA within two years of initiating insulin therapy, indicating progression to a type 1-like autoimmune phenotype. Those who retained typical T2D features despite IAA positivity were assigned to the T2D→T2D group.
Among the five PUMCH cases, one (Case 1) demonstrated clear autoimmune progression (T2D→T1D), characterized by IAA seroconversion, undetectable C-peptide levels, the onset of DKA, and clinical signs of insulin allergy. Notably, this patient initially responded to insulin therapy with transient glycemic improvement, but experienced rapid deterioration within one month, ultimately developing life-threatening metabolic decompensation. She also exhibited marked glycemic variability and subcutaneous lipoatrophy at injection sites, reinforcing the presence of an immune-mediated process. Given the abrupt β-cell failure shortly after insulin initiation, Case 1 serves as a representative example of exogenous insulin-triggered double diabetes. The remaining four cases (Cases 11-14) tested positive for IAAs following insulin therapy but remained clinically with features consistent with T2D.
In total, 23 cases were included and categorized into two subgroups: T2D→T1D group (n = 10): Autoimmune progression with insulin dependence; (Cases 1-10) T2D→T2D group (n = 13): IAA-positive but stable T2D phenotype (Cases 11-23).
Extracted data included demographic information (age, sex, ethnicity), insulin regimen details (type, duration), duration of diabetes, autoantibody profiles-including GAD-Ab, islet cell antibodies (ICA) and insulinoma-associated antigen 2 antibodies (IA-2Ab), and particularly IAA-insulin-related adverse events (e.g., allergy, lipoatrophy, hypoglycemia, DKA), fasting C-peptide levels before and after insulin therapy, and HLA genotypes.
Due to the limited sample size and heterogeneity of data sources, all analyses were descriptive. Categorical variables were summarized as counts and percentages, while continuous variables were reported as raw values. HLA class II allele distributions were compared qualitatively between subgroups. Clinical characteristics and allele frequencies were visualized using summary tables and figures.
Normally distributed quantitative variables were expressed as the mean ± SD, whereas categorical variables were shown as percentages. Parameters that were not normally distributed were transformed or presented as the median (25-75th percentile). Comparison of variables between groups was performed using Student t-test or non-parametric Mann-Whitney U-test or χ2-test. SPSS (version 22.0) and Excel were used to perform all statistical analyses. P < 0.05 (two-sided) indicated statistical significance.
Table 1[8,10-17] summarizes the demographics, clinical characteristics, and HLA class II genotyping (DRB1 and DQB1) of the 23 included individuals. All participants were of East Asian descent, including both Chinese and Japanese patients. Based on initial diagnostic classification, 10 patients were assigned to the T2D→T1D group, and 13 patients were classified into the T2D→T2D group. The mean age at diagnosis was 57.10 ± 16.11 years in the T2D→T1D group and 60.31 ± 17.41 years in the T2D→T2D group. Sex distribution was approximately balanced in the T2D→T1D group (male-to-female ratio of 5:5), whereas the T2D→T2D group included more female (male-to-female ratio 5: 8). There were no statistically significant differences in age or sex distribution between the two groups (P > 0.05).
| Ethnicity | Sex/age (year) | Insulin preparation | Duration of insulin use (month) | Duration since initial diagnosis (year) | ICA | IA-2A | GADA | IAA-pre insulin | IAA titer | IAA detection method | Insulin allergy | Lipoatrophy | Hypoglycemia | DKA | C-P (pre) (pmol/L) | C-P (post) (pmol/L) | Treatment | DRB1 | DQB1 | |
| Case 1 | Chinese | F/49 | NR, L | 1 | NA | - | - | - | - | NA | CL | + | + | + | + | 329.7 | < 30 | NA | 04:05 15:01 | 04:01 04:01 |
| Case 2 | Japanese | M/70 | N | 5 | 30 | - | - | + | NA | 13 | RI | + | NA | + | + | 560 | < 16.65 | NR | 04:05 09:01 | 04:01 03:03 |
| Case 3 | Japanese | M/50 | A, M30 | 2 | 5 | + | + | + | NA | 12 | RI | + | NA | - | - | 370 | 33.3 | NU, H | 09:01 08:03 | 03:03 03:01 |
| Case 4 | Japanese | M/69 | H70/30 | 2 | 20 | - | - | - | NA | 14 | RI | - | NA | - | - | 900 | 33.3 | H, HN | 04:05 04:05 | 04:01 04:01 |
| Case 5 | Japanese | M/63 | M30 | 17 | 29 | - | - | - | NA | 2 | RI | - | NA | NA | - | NA | < 30 | NA | 09:01 01:01 | 03:03 05:01 |
| Case 6 | Japanese | F/67 | R, H70/30 | 7 | 15 | - | - | - | NA | 9 | RI | + | NA | NA | - | NA | < 30 | NA | 04:05 14:07 | 04:01 05:02 |
| Case 7 | Japanese | F/38 | N, NR | 13 | 2 | + | NA | + | NA | 7 | RI | - | NA | NA | - | NA | NA | NA | 09:01 09:01 | 03:03 03:03 |
| Case 8 | Chinese | F/25 | NR, D | 0.5 | 1 | - | - | - | NA | 75 | RI | + | - | - | + | NA | 8 | NR, DG | 04:05 | 04:01 |
| Case 9 | Japanese | M/75 | R, N | 12 | 20 | + | + | + | NA | 9 | RI | - | + | + | - | NA | < 30 | Pred | 04:05 13:02 | 04:01 06:04 |
| Case 10 | Chinese | F/65 | A | 6 | 10 | - | - | - | NA | 125# | RI | - | NA | - | + | NA | 50 | Pred | 04:03 | NA |
| Case 11 | Chinese | M/53 | GI | 30 | 8.4 | - | - | - | NA | 256 | CL | NA | + | NA | NA | NA | NA | NA | 02:01 03:02 | 04:03 07:01 |
| Case 12 | Chinese | F/24 | GI | 2 | 1.4 | NA | NA | NA | NA | 7.82 | CL | NA | NA | NA | NA | NA | NA | NA | 03:01 03:02 | 04:06 11:01 |
| Case 13 | Chinese | M/55 | H3/7 | 18 | 2.2 | - | - | - | NA | 390 | CL | NA | NA | NA | NA | NA | NA | NA | 03:01 03:01 | 12:01 12:02 |
| Case 14 | Chinese | F/50 | GI | 0.5 | 11.1 | - | - | - | NA | 691.5 | CL | + | NA | NA | NA | NA | NA | NA | 03:02 03:02 | 04:06 04:06 |
| Case 15 | Japanese | M/66 | H3/7 | 12 | 27 | NA | - | - | NA | 20 | RI | + | NA | + | NA | NA | 0.47 | Liraglutide, voglibose, mitiglinide | 04:01 04:06 | 03:02 05:01 |
| Case 16 | Japanese | M/46 | NR | 12 | NA | NA | NA | NA | NA | 13.6 | RI | NA | NA | + | NA | NA | 0.40 | Nateglinide | 09:01 15:02 | 03:03 06:01 |
| Case 17 | Chinese | M/49 | N30R | 12 | 10 | NA | NA | NA | NA | NA | RI | NA | NA | + | NA | NA | 1.43 | Acarbose | 04:05 | NA |
| Case 18 | Japanese | M/86 | NA | 20 | NA | NA | NA | NA | NA | 20.9 | RI | NA | NA | + | NA | NA | NA | Cyclophosphamidepulse and Preds | 04:06 | NA |
| Case 19 | Japanese | M/83 | NA | 15 | NA | NA | NA | NA | NA | 22.4 | RI | NA | NA | + | NA | NA | NA | DFPP and Preds | 08:03 09:01 | NA |
| Case 20 | Japanese | F/60 | R | 21 | NA | NA | NA | + | NA | 10 | RI | NA | NA | + | NA | NA | NA | NA | 04:05 09:01 | NA |
| Case 21 | Japanese | F/69 | M30 | 20 | NA | NA | NA | - | NA | 13 | RI | NA | NA | + | NA | NA | NA | NA | 08:03 09:01 | NA |
| Case 22 | Japanese | F/60 | M30 | 2 | NA | NA | NA | - | NA | 14 | RI | NA | NA | + | NA | NA | NA | NA | 04:05 14:01 | NA |
| Case 23 | Chinese | M/83 | N, GI | 20 | NA | - | - | NA | NA | 14 | NA | NA | NA | + | NA | NA | 1.19 | Linagliptin | 08:03 12:02 | NA |
Prior to insulin initiation, most patients in both groups tested negative for classical T1D–associated autoantibodies, including GAD-Ab, ICA and IA-2Ab. However, within the T2D→T1D group, two patients tested positive for all three autoantibodies, one was positive for both GAD-Ab and ICA, and another tested positive for GAD-Ab alone. In contrast, only one patient in the T2D→T2D group was positive for GAD-Ab. These findings suggest a higher baseline autoimmune burden in a subset of patients who later progressed to insulin-dependent diabetes, despite an initial diagnosis of T2D. In the T2D→T1D group, C-peptide levels were initially detectable but declined significantly following insulin therapy. The median duration from the initiation of to either undetectable C-peptide or the onset of DKA was 6 months (ranging: 0.5 to 17 months) in these originally T2D-diagnosed patients.
The duration of insulin exposure differed significantly between groups. In the T2D→T1D group (n = 10), the median duration was 5.5 months, compared to 15.0 months in the T2D→T2D group (n = 13). This difference was statistically significant (Mann–Whitney U test, P = 0.046), suggesting that longer insulin exposure was associated with a lower likelihood of autoimmune progression. The median duration of diabetes at the time of insulin initiation was 12.5 years in the T2D→T1D group (n = 10) and 8.4 years in the T2D→T2D group (n = 5). However, due to substantial missing data in the T2D→T2D group, statistical comparison was not performed. Insulin allergy was reported in 5 of 10 patients (50.0%) in the T2D→T1D group, while only two patients in the T2D→T2D group experienced local allergic reactions. Lipoatrophy occurred in 2 of 3 patients (66.7%) in the T2D→T1D group and in the only evaluable case in the T2D→T2D group. Hypoglycemia was observed in 3 of 7 evaluable cases (42.9%) in the T2D→T1D group and in all 9 patients (100%) with available data in the T2D→T2D group. DKA occurred in 4 of 10 patients (40.0%) in the T2D→T1D group, whereas no DKA events were reported in the T2D→T2D group.
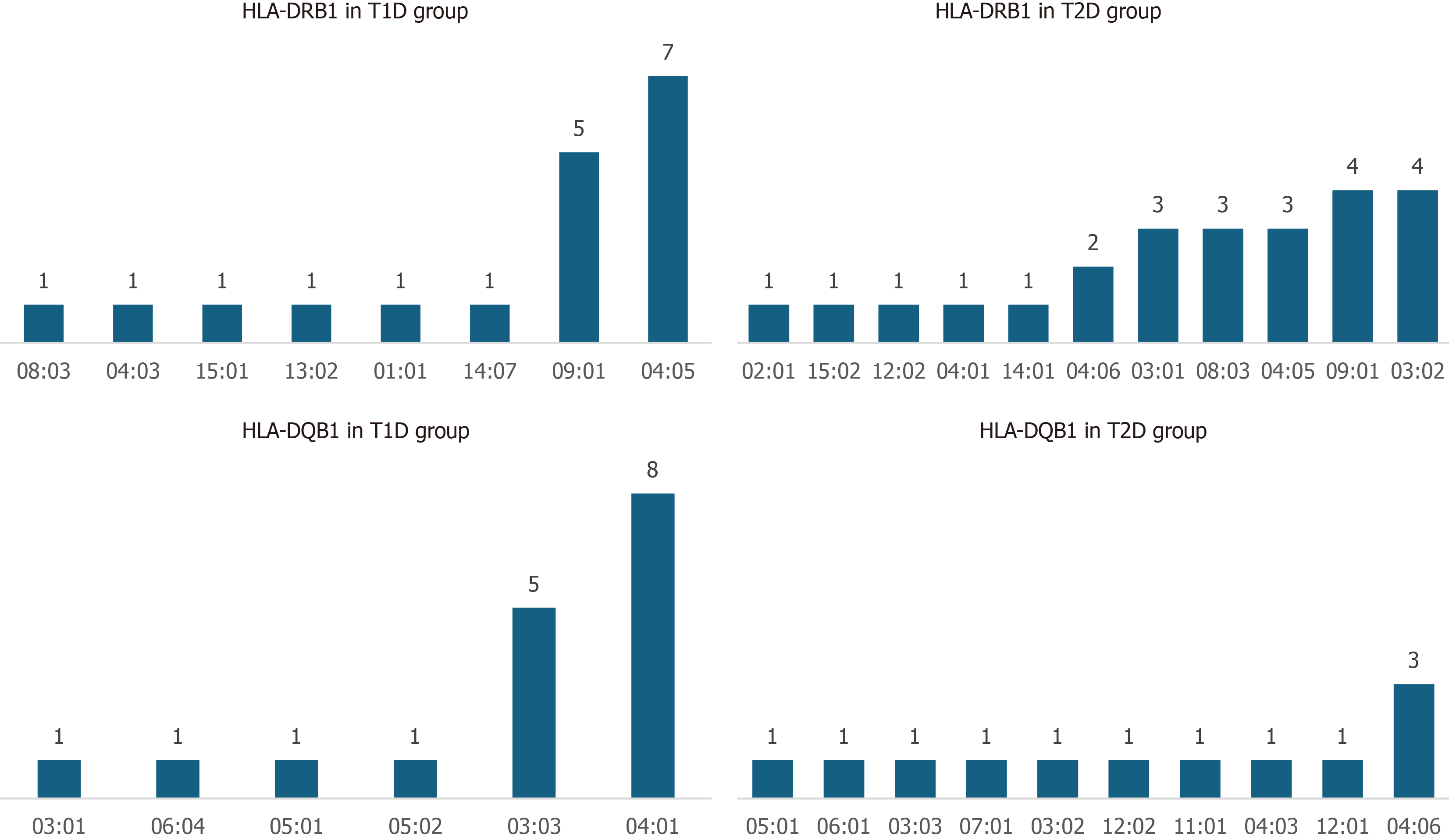
Our study revealed a distinct distribution of HLA class II alleles between the T2D→T1D group and the T2D→T2D groups. In the T2D→T1D group, DRB1*04:05 was the most frequently observed allele, present in 7 individuals, followed by DRB1*09: 01 in 5 individuals. Less common alleles included DRB1*08:03, DRB1*13:02, DRB1*14:07, DRB1*15:01, and DRB1*01:01, each detected in one patient. Consistent with these DRB1 findings, the corresponding DQB1 alleles-DQB1*04:01 (typically paired with DRB1*04:05) and DQB1*03:03 (associated with DRB1*09:01)-were the most common in this group, with frequencies of 8 and 5, respectively (Figure 2).
In contrast, the T2D→T2D group exhibited a more heterogeneous HLA profile. DRB1 alleles such as DRB1*03:02 (in 4 individuals), DRB1*09:01 (in 4 individuals), and a variety of others including DRB1*02:01, 15:02, 12:02, 04:01, 14:01 and 04:06 appeared less frequently and did not form dominant cluster as observed in the T2D→T1D group. The DQB1 alleles in this group were similarly diverse, including DQB1*04:06 (3 individuals), and several other lower-frequency variants such as DQB1*05:01, 06:01, 03:03, 07:01, 03:02, 12:02, 11:01, 04:03 and 12:01 (Figure 2). Importantly, the T2D→T2D group lacked the enrichment of classical T1D high-risk haplotypes seen in the T2D→T1D group, supporting the hypothesis that these patients have a more metabolically driven disease course with limited autoimmune involvement.
These findings support the hypothesis that individuals who progress from T2D→T1D may carry a genetic predisposition associated with classical T1D-linked HLA class II haplotypes, particularly DRB104:05-DQB104:01 and DRB109:01–DQB103:03.
The emergence of IAAs in all cases following insulin therapy—contrasted with the predominantly negative status of other T1D-associated autoantibodies prior to insulin initiation-suggests that exogenous insulin may act as a trigger for autoimmune β-cell destruction in genetically susceptible individuals[18]. This concern is particularly relevant for East Asian populations, where unique immunogenetic factors may exacerbate the risk of such immune-mediated responses. The high incidence of insulin allergy followed by subsequent β-cell failure highlights the importance of monitoring autoimmune markers in patients receiving insulin therapy, especially those who develop local allergic reactions to injection sites[19]. Recent evidence suggests that insulin-derived fibrils formed at subcutaneous injection sites may contribute to these complications by inducing macrophage cytotoxicity, promoting inflammation, and leading to localized tissue damage and poor glycemic control[20].
The immune response to exogenous insulin may arise through several mechanisms that disrupt immune tolerance. As an antigen, insulin can be presented by antigen-presenting cells (APCs) and activate both CD4+ and CD8+ T cells[21], triggering immune cascades that promote the development of IAAs[22]. Exogenous insulin-particularly formulations that differ in purity or molecular structure from endogenous insulin-may breach immune tolerance, even in insulin-naïve individuals[23].
At the injection site, dendritic cells and other APCs process the insulin and present it to T-helper cells, initiating a response that stimulates B cells to produce insulin-specific IgG antibodies[24]. Several factors influence susceptibility to this response, including the site and route of insulin administration, insulin formulation, and host genetic predisposition-especially HLA class II genotypes[18]. Variations in these factors, along with structural modifications of insulin analogs, can also increase immunogenicity and the likelihood of breaking immune tolerance. The resulting antibodies may form immune complexes that contribute to β-cell damage, reduced insulin secretion, and clinical manifestations such as insulin allergy and hypersensitivity reactions.
In our study, the term “double diabetes” refers primarily to individuals initially diagnosed with T2D who later transitioned to an insulin-dependent state[25]. In this context, this transformation is largely driven by immune responses to exogenous insulin, which appears to play a pivotal role in the progression toward insulin dependence[23]. Compared to patients with EIAS who do not develop features of T1D, this emerging subtype is more frequently associated with high-risk HLA class I alleles. These findings suggest a genetic predisposition that may contribute to a more complex clinical course and present additional challenges for therapeutic decision-making.
Our findings reveal a clear divergence in HLA class Il allele distribution between patients with stable T2D and those who progressed to double diabetes following insulin therapy. The overrepresentation of DQB1*04: 01, DQB1*03: 03, DRB1*04: 05, and DRB1*09: 01 in the T2D-T1D group is consistent with alleles known to be associated with T1D and autoimmune β-cell destruction. These genotypes are believed to enhance antigen presentation and T-cell activation. thereby facilitating autoimmune responses in genetically predisposed individuals[26].
The predominant haplotypes-DRB1 04: 05-DQB1 04: 01 and DRB1 09: 01- DQB1 03: 03-are well-established high-risk markers for T1D. Their presence in the T2D→T1D group supports the notion that these individuals harbor classical T1D-susceptibility HLA haplotypes. This aligns with previously reported genetic patterns in autoimmune diabetes. Notably, among East Asian populations, these haplotypes represent major susceptibility markers and differ significantly from those observed in Caucasian populations[27,28]. These findings underscore a population-specific genetic predisposition influencing progression to T1D in East Asians.
These observations support the hypothesis that exogenous insulin may act as an environmental trigger in individuals carrying high-risk HLA genotypes, promoting the development of IAAs, progressive β-cell dysfunction, and eventual clinical transition from T2D to a double diabetes phenotype[29]. In contrast, the broader and more heterogeneous HLA profile observed in the T2D→T2D group may indicate a lower genetic predisposition to autoimmunity and a patho
From a clinical perspective, these findings underscore the potential utility of HLA class II genotyping as a predictive biomarker for insulin-induced autoimmune progression in patients with T2D. Early identification of genetically sus
This study has several limitations that should be acknowledged to support the accurate interpretation and credibility of our findings. First, the retrospective design and small sample size limit the generalizability of the results and preclude definitive conclusions regarding causality. Second, although we focused on immunogenetic characteristics, particularly HLA class II alleles, other potential causes of pancreatic β-cell destruction were not comprehensively assessed due to data limitations. Detailed clinical information on factors such as alcohol consumption, prior pancreatitis, or recent viral infections-known contributors to β-cell dysfunction-was unavailable for many literature-derived cases and partially in the institutional records. Third, concomitant autoimmune conditions, such as Hashimoto’s thyroiditis or polyglandular autoimmune syndromes, were not systematically evaluated, which may have influenced disease progression and immune activation. Lastly, as an observational study, it is not possible to establish exogenous insulin as a definitive environmental trigger for autoimmune diabetes in genetically susceptible individuals. Despite these limitations, our findings offer a plausible hypothesis that warrants further investigation in larger, prospective studies incorporating genetic screening and longitudinal monitoring of autoimmune markers.
In summary, our findings support the existence of a distinct subset of patients with T2D who, following exposure to exogenous insulin, develop autoimmune features and progress to a “double diabetes” phenotype. This transition is closely associated with the development of IAAs and a high frequency of HLA class II alleles known to confer susceptibility to T1D. These results suggest that genetic predisposition—particularly specific HLA-DR and DQ variants-may play a pivotal role in disrupting immune tolerance to insulin and accelerating β-cell failure.
Given the potential for immune-mediated complications, HLA class II genotyping may serve as a valuable tool for identifying individuals at increased risk prior to initiating insulin therapy. Early identification could enable personalized monitoring strategies and timely immunomodulatory interventions aimed at preserving endogenous insulin secretion and improving long-term metabolic outcomes.
| 1. | Huynh T. Clinical and Laboratory Aspects of Insulin Autoantibody-Mediated Glycaemic Dysregulation and Hyperinsulinaemic Hypoglycaemia: Insulin Autoimmune Syndrome and Exogenous Insulin Antibody Syndrome. Clin Biochem Rev. 2020;41:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Wang M, Jjiang G, Meng X, Wang L. A Case of Exogenous Insulin Autoimmune Syndrome: A Case Report. Cureus. 2024;16:e72067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Jerkins T, Bell DSH. Development of Exogenous Insulin Antibody Syndrome in a Patient with Newly Diagnosed Type 1 Diabetes Successfully Treated with Oral Immunosuppressive Monotherapy. Diabetes Ther. 2021;12:2795-2799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Teoli J, Chikh K, Jouini-Bouhamri R, Charriere S, Fabien N, Raverot V. When discordant insulin and C-peptide levels lead to a medical diagnosis in a patient with transient hypoglycemia: Varying degrees of interference of insulin-antibody complexes on three insulin immunoassays. Heliyon. 2024;10:e34009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Bielka W, Przezak A, Molęda P, Pius-Sadowska E, Machaliński B. Double diabetes-when type 1 diabetes meets type 2 diabetes: definition, pathogenesis and recognition. Cardiovasc Diabetol. 2024;23:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 6. | Popovic DS, Papanas N. Double Diabetes: A Growing Problem Requiring Solutions. Exp Clin Endocrinol Diabetes. 2022;130:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Hu X, Chen F. Exogenous insulin antibody syndrome (EIAS): a clinical syndrome associated with insulin antibodies induced by exogenous insulin in diabetic patients. Endocr Connect. 2018;7:R47-R55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Zhou J, Cai Y, Luo S, Su H. [Exogenous insulininduced type 1 diabetes mellitus: a case report]. Zhongguo Tangniaobing Zazhi. 2023;15:1139-1141. [DOI] [Full Text] |
| 9. | Li Z, Yi D, Zheng L, Li S, Fang W, Wang C. Analysis of the clinical characteristics of insulin autoimmune syndrome induced by exogenous insulin in diabetic patients. Diabetol Metab Syndr. 2021;13:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Nakamura M, Nishida W, Yamada Y, Chujo D, Watanabe Y, Imagawa A, Hanafusa T, Kawasaki E, Onuma H, Osawa H, Makino H. Insulin administration may trigger pancreatic beta-cell destruction in patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;79:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Nishida W, Nagata M, Imagawa A, Hanafusa T, Ohashi J, Takahashi K, Suehiro T, Yamada Y, Chujo D, Kawasaki E, Kawamura R, Onuma H, Osawa H, Makino H. Insulin administration may trigger type 1 diabetes in Japanese type 2 diabetes patients with type 1 diabetes high-risk HLA class II and the insulin gene VNTR genotype. J Clin Endocrinol Metab. 2014;99:E1793-E1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Matsuyoshi A, Shimoda S, Tsuruzoe K, Taketa K, Chirioka T, Sakamoto F, Sakakida M, Miyamura N, Araki E. A case of slowly progressive type 1 diabetes with unstable glycemic control caused by unusual insulin antibody and successfully treated with steroid therapy. Diabetes Res Clin Pract. 2006;72:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Han CY, Ye XM, Lu JP, Jin HY, Xu WW, Wang P, Zhang M. Exogenous Insulin Antibody Syndrome in Patients with Type 2 Diabetes. Diabetes Metab Syndr Obes. 2023;16:1895-1902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Kaneko K, Satake C, Izumi T, Tanaka M, Yamamoto J, Asai Y, Sawada S, Imai J, Yamada T, Katagiri H. Enhancement of postprandial endogenous insulin secretion rather than exogenous insulin injection ameliorated insulin antibody-induced unstable diabetes: a case report. BMC Endocr Disord. 2019;19:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Yoshida M, Murakami M, Ogawa K, Asai M, Miyata M, Maeda H, Oiso Y. Repeated hypoglycemia caused by the overproduction of anti-insulin antibodies and isolated ACTH deficiency in a type 2 diabetic patient receiving insulin therapy. Diabetes Care. 2013;36:e22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Ishizuka T, Ogawa S, Mori T, Nako K, Nakamichi T, Oka Y, Ito S. Characteristics of the antibodies of two patients who developed daytime hyperglycemia and morning hypoglycemia because of insulin antibodies. Diabetes Res Clin Pract. 2009;84:e21-e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Kure M, Katsura Y, Kosano H, Noritake M, Watanabe T, Iwaki Y, Nishigori H, Matsuoka T. A trial to assess the amount of insulin antibodies in diabetic patients by surface plasmon resonance. Intern Med. 2005;44:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Obata Y, Takayama K, Nishikubo H, Tobimatsu A, Matsuda I, Uehara Y, Maruo Y, Sho H, Kosugi M, Yasuda T. Exogenous insulin antibody syndrome in a patient with diabetes secondary to total pancreatectomy. Diabetol Int. 2023;14:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Liu Y, Ping F, Yu J, Lv L, Zhao Y, Qi M, Li W, Xu L, Yu M, Li M, Zhang H, Li Y. Hypoglycemia Caused by Exogenous Insulin Antibody Syndrome: A Large Single-Center Case Series From China. J Clin Endocrinol Metab. 2023;108:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Teo CB, Tan PY, Lee SX, Khoo J, Tan JG, Ang SF, Tan SH, Tay TL, Tan E, Lim SC, Boehm BO, Loh WJ. Insulin Allergy to Detemir Followed by Rapid Onset of Diabetic Ketoacidosis: A Case Report and Literature Review. Front Endocrinol (Lausanne). 2022;13:844040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Wenzlau JM, Peterson OJ, Vomund AN, DiLisio JE, Hohenstein A, Haskins K, Wan X. Mapping of a hybrid insulin peptide in the inflamed islet β-cells from NOD mice. Front Immunol. 2024;15:1348131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Kawamura R, Miyao S, Onuma H, Uchigata Y, Kawasaki E, Ohashi J, Shiraishi S, Nishida W, Yokomoto-Umakoshi M, Takata Y, Osawa H, Makino H. Recurrent Hypoglycemia Due to a High Titer of Insulin Antibody in Response to Exogenous Insulin Administration in Two Cases of Type 1 Diabetes. Intern Med. 2022;61:687-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Jerkins T, Stockham K, Bell DSH. Exogenous Insulin Antibody Syndrome (EIAS) Presenting in an Elderly, Long-Term Patient with Type 1 Diabetes Mellitus that Resolved with Low-Cost Outpatient Therapy with Mycophenolate Mofetil and Regular Insulin by Pump. Diabetes Ther. 2024;15:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Burg AR, Tse HM. Redox-Sensitive Innate Immune Pathways During Macrophage Activation in Type 1 Diabetes. Antioxid Redox Signal. 2018;29:1373-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Pozzilli P, Buzzetti R. A new expression of diabetes: double diabetes. Trends Endocrinol Metab. 2007;18:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (2)] |
| 26. | Xu LL, Chen JX, Cheng JP, Luo N. Exogenous insulin autoimmune syndrome: A case report and review of literature. World J Clin Cases. 2024;12:1691-1697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Kawabata Y, Ikegami H, Kawaguchi Y, Fujisawa T, Shintani M, Ono M, Nishino M, Uchigata Y, Lee I, Ogihara T. Asian-specific HLA haplotypes reveal heterogeneity of the contribution of HLA-DR and -DQ haplotypes to susceptibility to type 1 diabetes. Diabetes. 2002;51:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, Lavant E, Louey A, Moonsamy P; Type 1 Diabetes Genetics Consortium. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 612] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 29. | Luo S, Lin J, Xie Z, Xiang Y, Zheng P, Huang G, Li X, Liao Y, Hagopian WA, Wang CY, Zhou Z. HLA Genetic Discrepancy Between Latent Autoimmune Diabetes in Adults and Type 1 Diabetes: LADA China Study No. 6. J Clin Endocrinol Metab. 2016;101:1693-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Ma ZJ, Sun P, Guo G, Zhang R, Chen LM. Association of the HLA-DQA1 and HLA-DQB1 Alleles in Type 2 Diabetes Mellitus and Diabetic Nephropathy in the Han Ethnicity of China. J Diabetes Res. 2013;2013:452537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/