Published online Sep 15, 2025. doi: 10.4239/wjd.v16.i9.106002
Revised: March 31, 2025
Accepted: August 6, 2025
Published online: September 15, 2025
Processing time: 209 Days and 0.5 Hours
Diabetes and its associated microvascular complications, such as nephropathy and retinopathy, significantly impact global health. These complications often begin in the prediabetic stage, emphasizing the importance of early detection and intervention. Inflammatory pathways are key contributors to these conditions, and recent research has identified members of the tumor necrosis factor (TNF) receptor superfamily as potential biomarkers. However, their association with renal and retinal dysfunction in individuals with intermediate hyperglycemia (IH) remains underexplored. The Early Prevention of Diabetes Complications (ePREDICE) trial provides a valuable cohort to investigate these associations and improve risk assessment strategies.
To identify inflammatory biomarkers associated with early renal and retinal dysfunction in individuals with IH. Specifically, we evaluate the diagnostic and prognostic potential of TNF receptor superfamily members [TNF receptor 1 (TNF-R1), TNF receptor 2 (TNF-R2)], T-cell immunoglobulin and mucin domain 3 (TIM-3)/HAVCR2, galectin-3, and interleukin-6 (IL-6) in detecting kidney dysfunction and retinopathy in this high-risk population. By understanding their roles, we seek to enhance early screening methods and inform personalized intervention strategies.
A cross-sectional analysis of 967 individuals with IH from the ePREDICE trial was conducted. Participants underwent comprehensive anthropometric and biochemical assessments. Key inflammatory biomarkers, including TNF-R1, TNF-R2, TIM-3/HAVCR2, galectin-3, and IL-6, were quantified using immunoassays. Renal function was assessed using estimated glomerular filtration rate (eGFR) and albuminuria, while retinopathy was evaluated through fundoscopic examination. Statistical analyses included adjusted mean comparisons, correlation studies, and receiver operating characteristic curve analysis to assess biomarker diagnostic accuracy.
TNF-R1, TNF-R2, and TIM-3/HAVCR2 were significantly associated with reduced filtration function (eGFR < 60 mL/minute/1.73 m2) and albuminuria, with area under the curve (AUC) values between 0.815 and 0.845. TIM-3/HAVCR2 emerged as the strongest predictor of retinopathy (AUC = 0.737). Strong correlations (r > 0.75) were observed among TNF-R1, TNF-R2, and TIM-3/HAVCR2, suggesting a coordinated role in inflammatory pathways.
Our findings highlight the potential of TNF receptor superfamily members as biomarkers for early-stage renal and retinal complications in individuals with IH. Their integration into clinical screening protocols could facilitate earlier detection, improving patient stratification and personalized management strategies. Further longitudinal studies are necessary to validate their predictive value and potential for guiding therapeutic interventions in IH and early diabetes management.
Core Tip: This study identifies tumor necrosis factor (TNF) receptor superfamily members as potential early biomarkers of renal and retinal dysfunction in individuals with intermediate hyperglycemia (IH). TNF receptor 1, TNF receptor 2, and T-cell immunoglobulin and mucin domain 3 (TIM-3) are significantly associated with early-stage renal impairment, while TIM-3 shows strong predictive value for retinopathy. These findings highlight the role of inflammation in microvascular complications and suggest that integrating these biomarkers into clinical screening may improve early detection and personalized intervention strategies in IH.
- Citation: Mas-Fontao S, Civantos E, Boukichou-Abdelkader N, Moreno JA, Gomez-Guerrero C, Gálvez MIL, Tuomilehto J, Lind M, Gabriel R, Egido J. Increased tumor necrosis factor-receptor superfamily plasma levels are associated with early renal or retinal involvement in intermediate hyperglycemia. World J Diabetes 2025; 16(9): 106002
- URL: https://www.wjgnet.com/1948-9358/full/v16/i9/106002.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i9.106002
Diabetes mellitus represents a significant global health challenge, characterized by its increasing prevalence and the severity of its long-term complications[1,2]. While macrovascular complications are a major cause of mortality in people with diabetes. Microvascular complications specifically nephropathy, retinopathy, and neuropathy are the primary contributors to morbidity in diabetes[3,4]. These complications often emerge during the prediabetic phase, underscoring the critical need for early diagnostic and preventive measures[5].
Intermediate hyperglycemia (IH), defined by blood glucose levels above normal but below diabetic thresholds, is increasingly prevalent worldwide[6]. Approximately 38% of United States adults have IH, though only 15% are aware of it[7]. Globally, 541 million adults were estimated to have IH in 2021, projected to rise to 730 million by 2045[2]. This trend is driven by sedentary lifestyles, poor diets, and rising obesity rates[8]. IH significantly increases the risk of diabetes, cardiovascular events, and mortality. Screening and management, particularly intensive lifestyle changes, have proven more effective than metformin in reducing progression to diabetes[5].
The elevated risk of complications in IH is associated with insulin resistance, impaired beta-cell function, chronic inflammation, and oxidative stress, underscoring the importance of understanding these mechanisms for effective prevention[9,10]. While the causal relationship between IH and chronic kidney disease (CKD) remains in some way undefined[11-13], the Early Prevention of Diabetes Complications (ePREDICE) trial, a large-scale European study, has provided valuable insights into the prevalence of renal involvement in individuals with IH and associated risk factors[14] Additionally, recent research has demonstrated the potential of early interventions, such as metformin and linagliptin, in reducing the risk of peripheral neuropathy and slowing kidney function decline in individuals with IH[15].
The standard clinical approach for diagnosing diabetic kidney disease (DKD) involves detecting albuminuria and reduced estimated glomerular filtration rate (eGFR)[16]. Although albuminuria is a prognostically significant marker, its early-stage relationship with kidney function and structural damage remains ambiguous. Thus, the development and implementation of new biomarkers are crucial for early detection of renal impairment in patients at high risk[17]. Changes in eGFR are key indicators in the monitoring of DKD, with formulas such as CKD epidemiology collaboration[18] and modification of diet in renal disease (MDRD)[19] both commonly used.
This study aimed to identify and evaluate novel biomarkers for early detection of renal impairment in individuals with IH. We investigated the relationship between these biomarkers and various stages of renal dysfunction, including hyperfiltration, albuminuria, and reduced filtration. Additionally, we explored their association with other microvascular complications, particularly retinopathy.
The ePREDICE trial is a double-blind, placebo-controlled clinical trial conducted independently across various centers in Europe, adhering to a randomization protocol that assigns participants to one of four trial arms metformin, linagliptin, a fixed-dose combination of linagliptin and metformin, or placebo with equal probability[14]. All participants received nutritional and exercise guidance as part of a lifestyle intervention that included a structured dietary plan to reduce caloric intake and promote balanced nutrition, alongside a supervised exercise program to enhance cardiovascular fitness and support weight management[14]. The study includes an active two-year intervention phase followed by a one-year observational phase.
This cross-sectional analysis focuses on baseline samples from individuals with IH at risk for nephropathy and retinopathy or neuropathy. The primary goal was to identify biomarkers indicative of early renal and retinal in
This study complies with the Declaration of Helsinki and was performed according to ethics committee approval of all national Medicine Agencies and local ethic committees of participating centers: Hospital Universitario La Paz, Madrid, Spain. Approval No. 3850 (February 01, 2013); Hellenic Republic Ministry of Health National Ethics Committee, Greece. Approval No. 74/00-01/14 (December 15, 2014); The Dean office, Istanbul Medical Faculty Ethical Board for Clinical Trials, Turkey. Approval No. 2014/495 (April 10, 2014); Etickog Odbora. Beograd, Republike Srbije, Serbia. Approval No. 61/1 (April 06, 2013); Ethics Committee of the Faculty of Medicine, University of Belgrade, Serbia. Approval No. 29/III-10 (March 07, 2013); Komisji Bioetycznej Uniwersytetu Jagiellonskiego, Poland. Approval No. BET/185/L/2014 (June 26, 2014); Executive Officer of Ethics Review Committee (RPAH) and Human Research Ethics Committee (HREC), Australia. Approval No. X13-0046 and No. HREC/13/RPAH/65 (March 12, 2014); комисия по етика “Александровска” ЕАД София, България, Bulgaria. Approval No. KИ-213/18.03.15 (March 18, 2015); Die Ethikkommission für das Bundesland Salzburg. “Landeskrankenhaus Salzburg-Universitätsklinikum der PMU, Universitätsklinik für Innere Medizin I”, Austria. Approval No. 415-E/1649/10-2014 (July 08, 2014). All study participants gave their written informed consent prior to the participation in the study.
As part of the ePREDICE study, anthropometric and biochemical data were collected, and lifestyle questionnaires were administered to participants for subsequent analysis[14]. Sex was defined based on biological characteristics reported by participants. Gender was not assessed in this study. Key biochemical variables included serum triglycerides, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein (LDL) cholesterol, as well as liver function tests: Alanine aminotransferase (ALT), aspartate aminotransferase, and gamma-glutamyl transferase. Additionally, the fatty liver index was used to estimate liver fat accumulation, and several indices were used to assess insulin sensitivity and glucose metabolism: The Matsuda index (MI), a measure of insulin sensitivity based on glucose and insulin levels during an oral glucose tolerance test (OGTT)[20,21]; The quantitative insulin sensitivity check index (QUICKI), a formula-based measure of insulin sensitivity; The oral glucose insulin sensitivity, which estimates whole-body insulin sensitivity; The disposition index (DI), which evaluates beta-cell function relative to insulin sensitivity; The insulinogenic index, a measure of the initial insulin response after glucose intake. Together, these metrics provide a comprehensive assessment of metabolic health and glucose regulation.
To determine the eGFR using the MDRD-4 formula, which includes four variables, serum creatinine was measured at each participating clinical center using standardized methods and calibrated to isotope dilution mass spectrometry standards[22]. The MDRD-4 formula is based on age, sex, ethnicity, and serum creatinine and was utilized due to its historical validation in similar populations with prediabetes and its consistency with previous studies[23], with a high degree of correlation when compared to newer equations[24]. Participants were categorized according to their eGFR as follows: G1 (> 90 mL/minute/1.73 m2), G2 (60-89 mL/minute/1.73 m2), and G3a (45-59 mL/minute/1.73 m2); None had an eGFR below 45 mL/minute/1.73 m2 (G4 and G5). Albumin and creatinine levels were measured in morning spot urine samples at the Fundación Jiménez Díaz Diabetes Laboratory in Madrid, Spain. Following Kidney Disease: Improving Global Outcomes (KDIGO) guidelines[25], participants were further stratified based on combined GFR and albuminuria levels into stages that range from G1 to G5 according to GFR and by albuminuria into categories A1 (< 30 mg/g), A2 (30-300 mg/g), and A3 (> 300 mg/g) to comprehensively assess renal involvement and the risk of progression (see Table 1).
| Variables | P value | FDR |
| IL-6 (pg/mL) | 8.81 × 10-18 | 6.35 × 10-16 |
| TIM-3 (pg/mL) | 2.10 × 10-5 | 0.00066 |
| TNF-R2 (pg/mL) | 2.37 × 10-5 | 0.0006 |
| Gal3 (pg/mL) | 0.0001 | 0.0029 |
| TNF-R1 (pg/mL) | 0.0002 | 0.0035 |
| ALT (UI/L) | 0.0130 | |
| Disposition index | 0.0253 | |
| Triglycerides (mmol/L) | 0.0484 |
Plasma biomarker determinations were conducted at specialized laboratories, including the Renal, Vascular, and Diabetes Research Laboratory and the Clinical Analysis Laboratory of the Jiménez Díaz Foundation. To minimize bias, researchers were blinded to clinical data. Key biomarkers, such as tumor necrosis factor (TNF) receptor 1 (TNF-R1), TNF receptor 2 (TNF-R2), T-cell immunoglobulin and mucin domain-containing protein 3 (TIM-3/HAVCR2), galectin-3 (Gal3), and interleukin-6 (IL-6), were quantified using an enzyme-linked immunosorbent assay with an automated immunoassay system (Ella, ProteinSimple custom assay SPCK-PS-005928, Bio-techne, Minneapolis, MN, United States). High-sensitivity C-reactive protein was measured by latex-enhanced immunoturbidimetry (ADVIA 2400 Chemistry System, Siemens, Munich, Germany).
Serum lipids, plasma glucose, and serum creatinine levels were determined using standard methods (ADVIA 2400 Clinical Chemistry System, Siemens, Germany). The eGFR was calculated using the MDRD-4 formula, a method to assess kidney function (Levey et al[19]). Insulin and C-peptide levels, which help evaluate insulin production and secretion, were analyzed using a second-generation automated chemiluminescence method (Elecsys 2010 platform, Roche Diagnostics, Mannheim, Germany). Phosphate levels were determined using an enzymatic method (Integra 400 analyzer, Roche Diagnostics, Mannheim, Germany).
Diabetic retinopathy was graded according to the final ETDRS scale by using digital fundus photographs (ETDRS Report Number 12. Early Treatment Diabetic Retinopathy Study Research Group 1991). The photograph’s protocol was the one validated by the Joslin Vision Network (three pictures covering posterior pole) performed under mydriasis with tropicamide and carried out by certified technicians. Retinal images were sent to the reading center at the IOBA (Eye Institute) of the University of Valladolid, Spain, and graded by trained ophthalmologists who were blinded to the participant’s treatment group assigned.
Data management was conducted using OpenClinica (https://www.openclinica.com/). Descriptive statistics were presented as percentages for qualitative factors and as median with interquartile ranges for quantitative variables. Non-parametric data were examined using the Mann-Whitney-Wilcoxon test for two-group comparisons and the Kruskal-Wallis test for multiple group comparisons. To account for multiple comparisons and control the false positive rate, we applied the false discovery rate (FDR) correction method[26,27].
Linear dependencies between quantitative variables were assessed using Pearson correlation coefficients and visualized in correlation matrices. For qualitative data, Pearson’s χ² test was employed. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the discriminatory power of biomarkers for different renal dysfunction stages and retinopathy. The area under the curve (AUC) was calculated for each biomarker, with higher AUC values indicating better discriminatory ability.
Multivariate logistic and linear regression models were employed to evaluate associations between inflammatory biomarkers and stages of renal dysfunction and retinopathy. Confounders such as age, sex, body mass index, and other relevant clinical variables were adjusted in all analyses. Interaction terms were incorporated into regression models to assess potential interactions, and likelihood ratio tests were used to determine statistical significance. Subgroup analyses were conducted based on renal function categories (hyperfiltration, albuminuria, and reduced filtration) and the presence or absence of retinopathy.
Statistical significance was established at P < 0.05 for initial analyses, with FDR-adjusted P values reported for multiple comparisons. All statistical analyses were conducted using R Studio (v1.4) with R software (v4.0.2). We utilized various R packages including ggplot2 for data visualization, jmv for descriptive statistics, finalfit for regression analyses, and gtsummary for creating summary tables (https://cran.r-project.org)[28].
The classification of renal involvement was guided by the KDIGO guidelines (Stevens, Levin, and Kidney Disease: Improving Global Outcomes CKD Guideline Development Work Group Members 2013). Our analysis included a total of 967 subjects. Among them, 20 individuals had an eGFR exceeding 130 mL/minute/1.73 m2, suggesting hyperfiltration; while 29 individuals had reduced kidney function, with an eGFR below 60 mL/minute/1.73 m2. Albumin excretion analysis revealed that 44 subjects had albuminuria levels ranging from 30 to 300 mg per g of creatinine (stage A2 according to KDIGO guidelines). Remarkably, one individual exhibited an albuminuria level of 510 mg/g (stage A3) probably indicating more severe renal involvement.
To comprehensively investigate all metabolic and inflammatory biomarkers associated with renal involvement, we analyzed data from 74 variables encompassing a diverse array of parameters, including anthropometric measurements, glycemic indices, lipid profiles, liver function indicators and various inflammatory biomarkers. Since the three different stages of renal dysfunction (hyperfiltration, albuminuria and reduced kidney function) could be differently influenced by the variety of anthropometric, metabolic status and inflammatory biomarkers, we first analyzed one by one the three renal conditions.
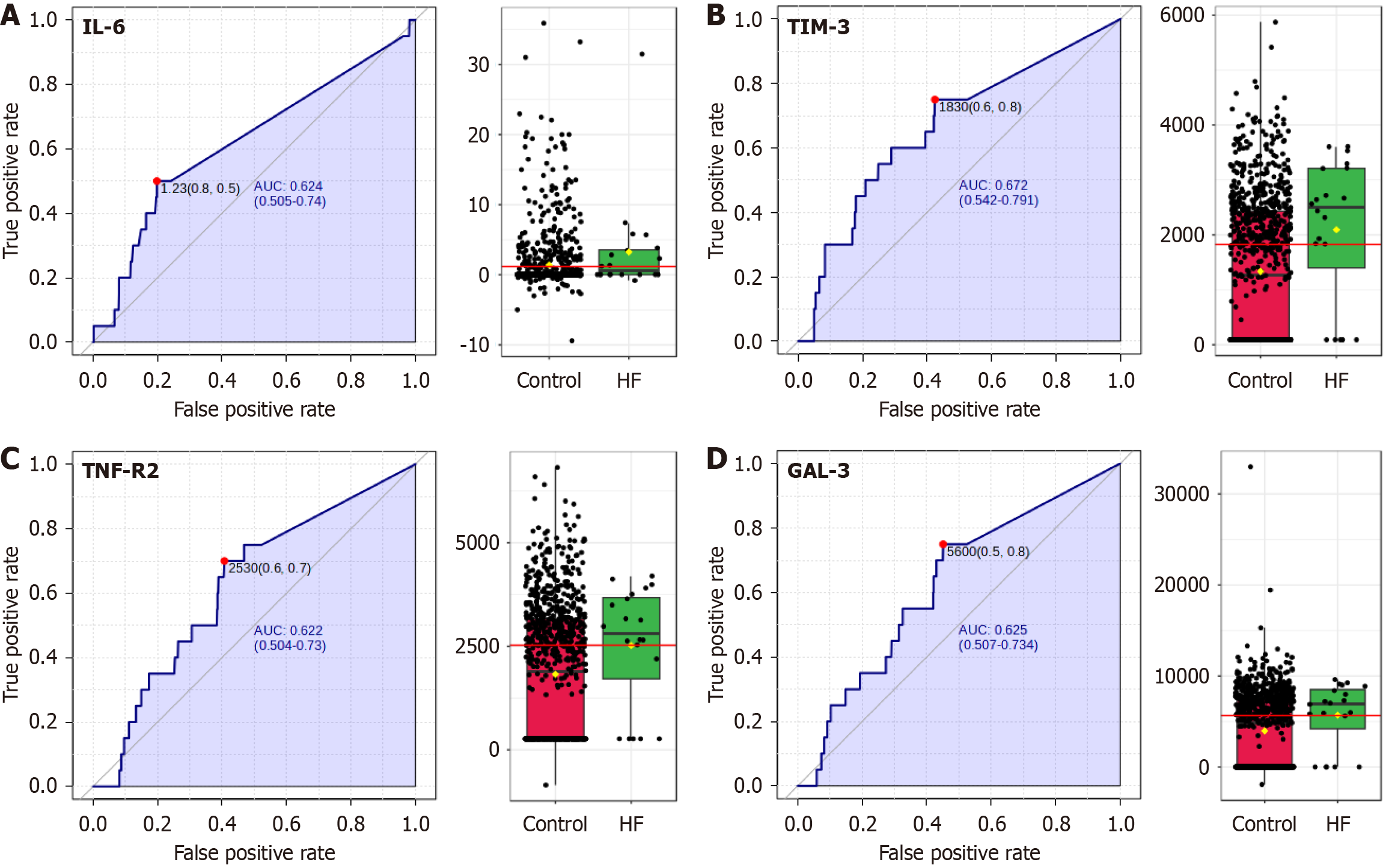
In our detailed analysis of biomarkers among individuals with IH and renal hyperfiltration, defined as an eGFR higher than 130 mL/minute/1.73 m2, several significant variables have been identified, underscoring the potential patho
The data indicated profound alterations in inflammatory markers among individuals with IH with hyperfiltration, with IL-6 showing an exceptionally strong association (P = 8.81 × 10-18, FDR = 6.35 × 10-6), reflecting possible systemic inflammatory responses linked to renal stress.
Similarly, biomarkers like TIM-3, TNF-R2, Gal3, and TNF-R1 also maintained significant associations after adjustment for multiple comparisons, highlighting their importance as indicators of altered immune response or tissue damage.
On the contrary, while the liver enzyme ALT, DI, and triglycerides showed initial statistical significance, they did not maintain significance after adjusting for false discovery, indicating that their relationships with hyperfiltration may be more complex or influenced by external factors. All 4 subsets (Figure 1) show good values as biomarkers individually, although none of them are discriminant for hyperfiltration.
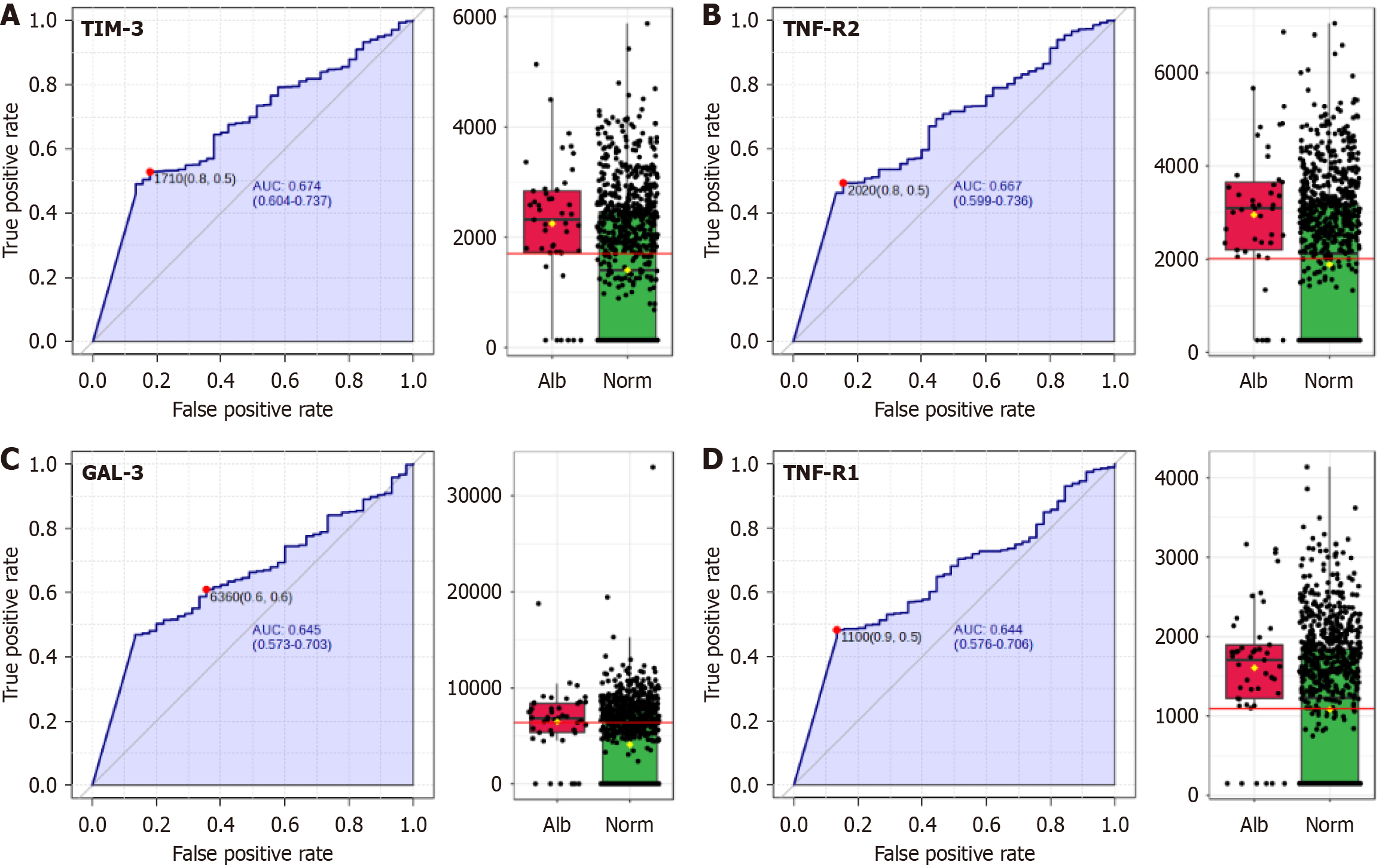
Our analysis revealed significant relationships between albuminuria and biomarkers related to inflammation, as detailed in Table 2. Inflammation-related biomarkers such as TIM-3, TNF-R2, TNF-R1, IL-6, and Gal3, displayed robust statistical significance after adjusting for multiple comparisons using the FDR.
| Variables | P value | FDR |
| TIM3 (pg/mL) | 4.10 × 10-5 | 0.0016 |
| TNF-R2 (pg/mL) | 7.75 × 10-5 | 0.0019 |
| IL-6 (pg/mL) | 0.0001 | 0.0019 |
| Gal3 (pg/mL) | 0.0006 | 0.0081 |
| TNF-R1 (pg/mL) | 0.0007 | 0.0081 |
| Disposition index | 0.0118 | |
| QUICKI | 0.0355 | |
| Matsuda index | 0.0429 |
Additionally, while certain glycemic measures, including the QUICKI, MI, and DI showed initial significance, they did not maintain their statistical relevance upon FDR adjustment. This observation suggests that although these measures are individually significant, their influence may be less robust in the context of albuminuria development. The 4 biomarkers with the higher AUC for the presence of albuminuria are depicted in Figure 2.
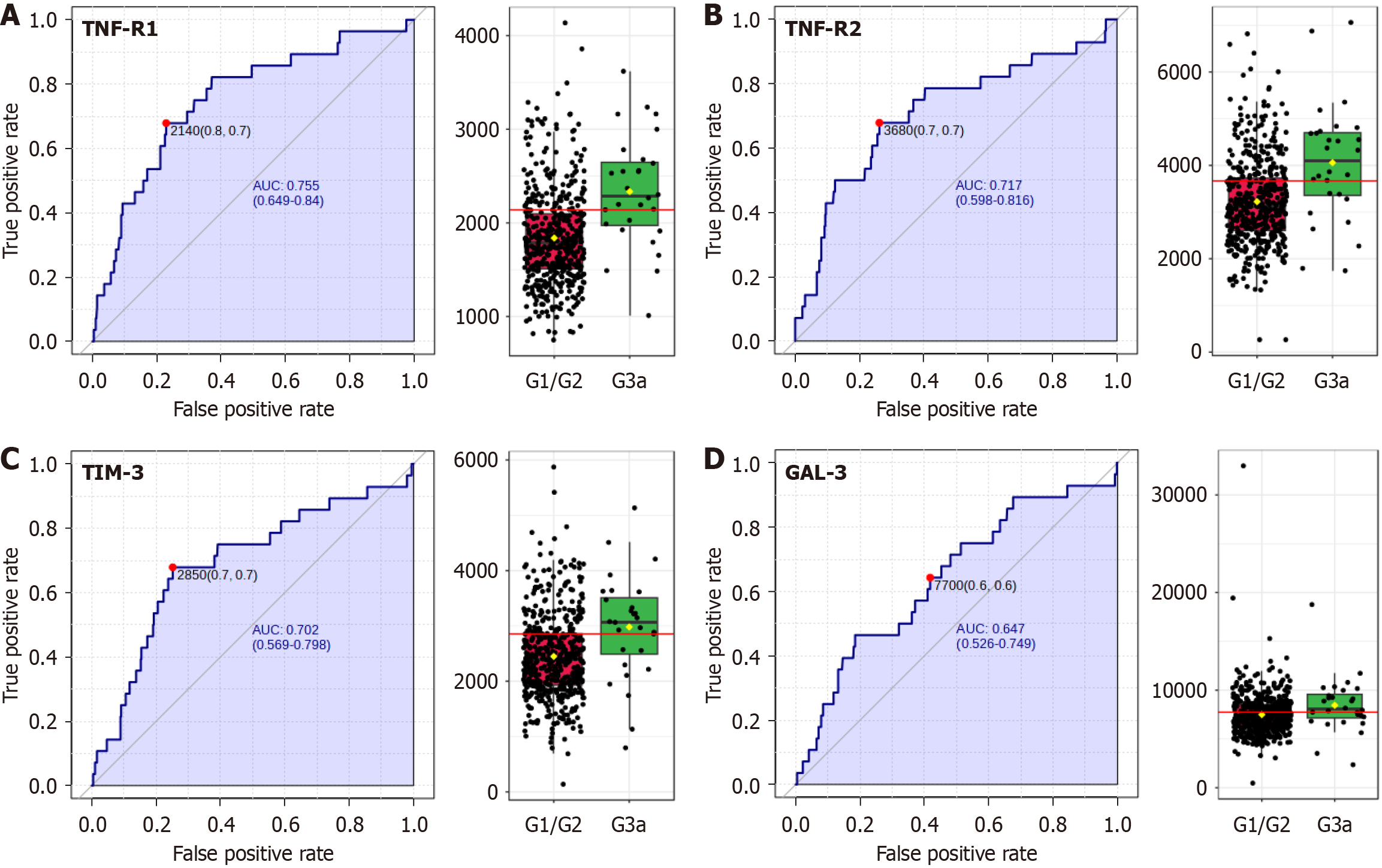
The final component of renal involvement examined was the reduction in glomerular filtration, defined as an eGFR lower than 60 mL/minute/1.73 m2. Among the 74 variables examined, 7 showed significant alterations when comparing individuals with IH and hypofiltration (stage G3a) vs those in G1 or G2 stages (Table 3).
| Variables | P value | FDR |
| TNF-R1 | 6.65 × 10-6 | 0.00044 |
| TNF-R2 | 0.00015 | 0.0046 |
| TIM-3 | 0.0006 | 0.012 |
| Gal3 | 0.01 | |
| Age | 0.02 | |
| IL-6 | 0.04 | |
| LDL | 0.045 |
Notably, three proteins within the TNF superfamily, TNF-R1, TNF-R2, and TIM-3, exhibited highly significant differences, with P values of 6.65 × 10-6, 0.00015, and 0.0006, respectively. Additional variables such as IL-6, LDL, Gal3, and age also showed initial significance.
To account for the risk of false positives due to multiple comparisons, the FDR correction was applied. After FDR adjustment, only TNF-R1, TNF-R2, and TIM-3 retained their statistical significance, with adjusted P values of 0.00044, 0.0046, and 0.012, respectively.
Further analysis using ROC curves revealed that the biomarkers most significantly altered in individuals with reduced glomerular filtration rates were TNF-R1, TNF-R2, and the TIM-3/HAVCR2 protein. These proteins demonstrated robust AUC values higher than 0.7, indicative of their strong discriminatory power in distinguishing between the two groups (Figure 3). Gal3 also emerged as a significant biomarker between the groups, although it displayed a slightly lower AUC value of 0.647.
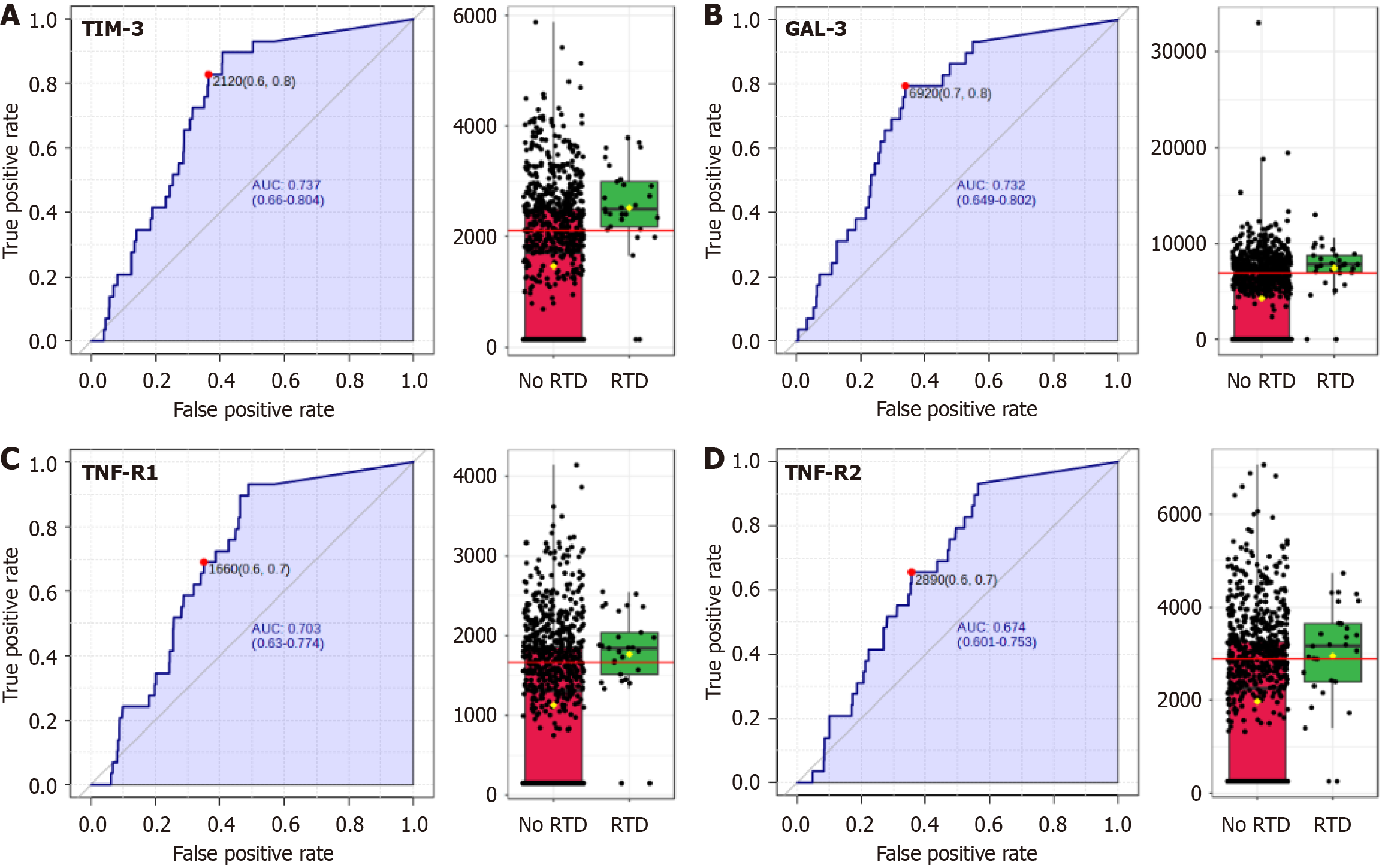
Our research also examined the association between various biomarkers and the development of retinopathy. In our cohort, fundoscopic examination was performed on all study participants, and 29 individuals were identified as pre
| Variables | P value | FDR adjusted |
| TIM-3 (pg/mL) | 7.10 × 10-6 | 0.0001023 |
| Gal3 (pg/mL) | 1.02 × 10-5 | 0.0001229 |
| TNF-R1 (pg/mL) | 0.00012715 | 0.0013078 |
| TNF-R2 (pg/mL) | 0.00093481 | 0.0084133 |
| Glucose > 155mg/dL at 1 hour | 0.057532 | |
| C-reactive protein | 0.058425 |
These results highlight the significant associations between these biomarkers, mainly TIM-3/HAVCR2 and Gal3, and retinopathy in individuals with IH.
On the other hand, 1-hour plasma glucose in the OGTT and C-reactive protein initially indicated potential association, but they did not maintain statistical significance after adjustment for multiple comparisons.
We also evaluated the predictive capacity of these biomarkers for the presence of retinopathy; TIM-3/HAVCR2 was the best biomarker with an AUC of 0.737 and an optimal cut-off of 2120 ng/dL, followed by Gal3 (AUC = 0.732) (Figure 4).
Our investigation also aimed to elucidate the interrelationships among the biomarkers within the TNF superfamily. We revealed strong positive correlations between TNF-R1, TNF-R2, and TIM-3, with Pearson correlation coefficients (r) exceeding 0.75 for each pairwise comparison. This high degree of interdependence suggests a coordinated role for these proteins in inflammatory pathways associated with IH (Supplementary Figure 1).
In contrast, correlation analyses with Gal3 (data not shown) yielded substantially weaker associations, with correlation coefficients below 0.35. Despite this weaker correlation, Gal3 exhibited similar trends to the TNF superfamily members across various analyses conducted. This discrepancy suggests that Gal3 may operate through distinct molecular mechanisms or signaling pathways in the context of renal dysfunction, warranting further investigation.
This study provides a detailed examination of various metabolic and inflammatory biomarkers associated with renal and retinal dysfunction in 967 individuals with IH from different regions across Europe. Our analysis revealed significant alterations in inflammatory markers across different stages of renal dysfunction. IL-6 showed the strongest association with hyperfiltration, confirming previous studies where IL-6 was associated to the pathogenesis of diabetic nephropathy[29-31]. We also found robust correlations and significant alterations of TNF super family members (TNF-R1, TNF-R2, and TIM-3) in people with IH with a reduced filtration, illustrating their potential in clinical stratification of renal dysfunction. While previous studies have emphasized the critical roles of these TNF superfamily members in the progression of DKD to end-stage renal failure in type 1 and type 2 diabetes[32-34], our findings highlight their in
The discriminatory capability of these biomarkers in identifying different stages of renal dysfunction, as demonstrated by our ROC curve analysis, bolsters their potential clinical utility. This finding is particularly significant given the limitations of the current diagnostic methods, which often fail to detect individual at early-stages of kidney dysfunction[16].
We also explored the associations of these biomarkers with retinopathy. We found significant correlations that underscore the systemic nature of diabetic complications. This interrelation stresses the utility of these biomarkers not only in diagnosing and managing kidney dysfunction but also in other microvascular complications, such as retinopathy[35]. The strong associations observed between TIM-3/HAVCR2, Gal3, TNF-R1 and TNF-R2 with retinopathy suggest their potential as early indicators of microvascular damage beyond the kidney. Moreover, the superior predictive value of TIM-3 for retinopathy may be related to its role in modulating immune responses and its effects on vascular endothelial cells, which are critical in the pathogenesis of retinal microvascular damage. Clinically, the integration of TIM-3, TNF-R1, and TNF-R2 measurements into existing screening algorithms could enhance early detection of microvascular complications. We propose the development of a composite risk score that incorporates these biomarkers, which should be validated in future prospective studies.
Our findings suggests that inflammatory processes mediated by TNF-R1, TNF-R2, and TIM-3/HAVCR2 may be initiated in IH, potentially contributing to early microvascular damage. The correlations among these markers indicate a coordinated inflammatory response that may precede clinically detectable renal dysfunction and diabetic retinopathy. Chronic inflammation is known to play a critical role in type 2 diabetes and DKD pathophysiology[33,36]. In fact, studies in populations with diabetes indicate a continuum of inflammatory processes from IH to diabetes[37], which could explain the increased risk of microvascular complications seen in some individuals with IH.
The weaker correlation of Gal3 with other inflammatory markers suggests a distinct regulatory mechanism in renal dysfunction associated with prediabetes. Given its established roles in fibrosis, inflammation, and immune cell activation, Gal3 may contribute to microvascular complications through pathways independent of acute inflammatory responses[38], studies have shown that elevated Gal3 Levels correlate with declining renal function and increased proteinuria, with higher circulating levels predicting a three-fold increased risk of renal function decline and a two-fold increased risk of massive proteinuria[39]. Additionally, Gal3 concentrations are negatively correlated with eGFR, even after adjusting for confounding factors such as urinary albumin-to-creatinine ratio[40], reinforcing its role as a biomarker for diabetic nephropathy. In diabetic retinopathy, Gal3 has been implicated in neuroinflammation and vascular remodeling, highlighting its involvement in retinal damage[41]. These findings suggest that Gal3 primarily reflects chronic tissue remodeling rather than acute inflammation, warranting further investigation into its mechanistic contributions and potential as a therapeutic target in diabetic microvascular complications.
Metabolic alterations in our population, including OGTT data, highlight the complex interplay between metabolic factors and inflammatory processes in the development of microvascular complications[42]. While markers of glucose metabolism such as DI, QUICKI, and Matsuda Index showed initial significance with albuminuria, they did not retain significance after FDR correction, suggesting a more complex relationship between insulin sensitivity, β-cell function, and early renal damage in IH[43]. Also, the observed alterations in lipid profiles, like LDL levels, in relation to reduced filtration rates emphasize the metabolic disturbances in IH.
While comparisons with existing literature are challenging due to methodological differences, available data provides a context for interpreting our data. Jiang et al[24] reported that combining serum creatinine with a metabolite profile yielded AUCs between 0.81 and 0.91 for predicting DKD. In another study microalbuminuria was used for CKD diagnostic with a AUC of 0.75[44,45]. Though these studies do not focus exclusively on prediabetes, they highlight the potential of combining biomarkers with established measures to improve risk assessment.
Our findings expand on this by analyzing specific DKD phenotypes, showing moderate but significant AUCs for hyperfiltration (0.622-0.672), albuminuria (0.644-0.674), and hypofiltration (0.647-0.755, with TNF-R1, TNF-R2, and TIM-3/HAVCR2 achieving AUC > 0.7). Given the limitations of eGFR and albuminuria in detecting early DKD or predicting progression in normoalbuminuric individuals, our biomarkers-particularly TNF-R1 and TNF-R2-may serve as prognostic indicators, as previously demonstrated[32].
Beyond diagnostics, these biomarkers offer insights into pathophysiology. TNF-R1/2, TIM-3, and Gal3 reflect inflammatory, immune-regulatory, and fibrotic pathways not captured by eGFR or albuminuria alone. Their established links to DKD progression[32-34] suggest potential utility in identifying high-risk patients and guiding personalized therapeutic strategies targeting inflammation and fibrosis. For retinopathy, TIM-3/HAVCR2 (AUC = 0.737) and Gal3 (AUC = 0.732) demonstrated moderate predictive value. While fundoscopy remains the gold standard, its requirement for specialized personnel and equipment limits accessibility for routine screening. Serum biomarkers could serve as initial screening tools, prioritizing high-risk individuals for ophthalmologic evaluation.
Our findings support a paradigm shift in approaching IH, viewing it as a state of active pathophysiological processes contributing to microvascular complications, beyond than merely a risk factor for diabetes[46]. These data also add to the evidence linking IH to an increased risk of DKD. The identification of significant biomarker alterations in our cohort with IH supports the notion that renal complications may begin to manifest before the onset of overt diabetes. This underscores the importance of early screening and intervention strategies in populations with IH[13].
Future efforts should focus on integrating these biomarkers into existing clinical frameworks, considering their cost-effectiveness and accessibility. The development of a composite risk score incorporating multiple biomarkers could provide more accurate prediction of microvascular complication, and DKD progression in particular, enabling targeted preventive strategies.
The cross-sectional nature of the study restricts our ability to establish causal relationships. Prospective, longitudinal studies are needed to delineate the temporal dynamics of these biomarkers in the progression of DKD. Additionally, the homogeneity of our study population may limit the generalisation of our findings. The biomarkers were evaluated at the baseline (trial entrance) and therefore it is unknown what their predictive value may be future research should include more diverse cohorts to enhance the external validity of the results.
Despite these limitations, the clinical relevance of our study remains significant. The identification of robust biomarkers, particularly those within the TNF superfamily, shows their potential as diagnostic and prognostic tools for early kidney dysfunction in individuals with IH. The ability of these biomarkers to discriminate between different stages of renal impairment suggests their utility in clinical stratification and may help in developing personalized treatment approaches.
Our study advances the understanding of biochemical factors linked to renal dysfunction in IH, laying a foundation for clinical application. Our final goal was to contribute to the development of more sensitive diagnostic tools for early detection of DKD, potentially enabling earlier interventions and improved patient outcomes in the population with IH. Addressing study limitations and validating results across diverse populations will be essential to fully realize the clinical potential of these biomarkers in managing diabetic renal complications. Integrating these biomarkers into clinical practice, with attention to cost-effectiveness, may enhance diagnostic precision and improve treatment strategies for early diabetic nephropathy.
Members of the e-PREDICE consortium (See annex). Annex 1: E-PREDICE Consortium investigators: Steering committee: Jaakko Tuomilehto, Rafael Gabriel, Jaana Lindström, Jesús Egido, Andrea Natali, José C Pastor, Michael Brainin, Marcus Lind, Luis Silva, Peter Schwartz, Aleksandra Gilis-Januszewska. Safety committee: Carmen Suárez Fernández: Hospital University La Princesa. Madrid, Spain. Beverly Balkau, INSERM Veifville. France. Matti Uusitupa, Institute of Public Health and Clinical Nutrition. University of Eastern Finland, Kuopio, Finland. Internal Ethic Committee: Julio Romero. Hospital University La Princesa, Madrid, Spain. Lars Ryden. Karolinska Institute, Stockholm, Sweden. External Scientific Advisory Board: Ralph DeFronzo, University of Texas. San Antonio, Texas, United States. Manuel Serrano Ríos. Universidad Complutense, Madrid, Spain. Michael Roden, German Center for Diabetes Research (DZD) Heinrich Heine University Düsseldorf, Düsseldorf, Germany. John Nolan, European Diabetes Forum, European Association for the Study of Diabetes, Trinity College Dublin, Ireland. Workpackage (WP). WP1: Coordinador. Evidem Consultores Rafael Gabriel, Jaakko Tuomilehto, Nisa Boukichou, Tania Acosta, Ruy López-Ridaura, Luis Silva, Eliana del Águila, Ana Rosón. WP2: Clinical Centres: Spain. Primary Care centres of SERMAS (Servicio Madrileño de Salud), Madrid. Tomás Gómez-Gascón, Juan Carlos Abánades Herranz, María Esther Sánchez Carranza, Alicia Rodríguez Blanco, Fernando Villasante Claudios, Consuelo Ugarte Pérez, Belén Peláez Raposo, Beatriz Cáceres Sánchez, Sergio González Gasca; Margarita Herrero Delgado, Isabel García Del Río, Mª Lorena Rodríguez Pérez, Mª Carmen Reyes Madridejos, Mª Carmen Castillo López, Mª Jesús Paloma Huerga González, Ana Alayeto Sánchez, Carmen Pascual Díez, Esperanza Villar Coloma, Tirso Galiano Arroyo, Mª Olga Peña Peña, Mª Elena Pejenaute Labari, Mª Mercedes Rojo Tardón, Mª Teresa Recio García, María Campos López-Carrión, Sara Criado Jorge, Virginia García Campo, Almudena Pazos González, Aranzazu Pérez Medina, Ricardo Benito Fernández, Mercedes Ricote Belinchón, Mª Teresa Sánchez-Villares Rodríguez, Noelia Polo Fernández, Antonio Cabrera Majada, Eva María Revuelta Marinez , Itziar Vázquez Carrión, Mª Nuria Fernández De Cano Martín, Manuel Gutiérrez Cabanas, Soledad Fernández Saavedra, Yolanda Jiménez Aguilar, Mª Ángeles Rodríguez Loarce, Emilia Pedroche Morales, Teresa Nieto Monreal, María Begoña Hernández Olivares, Carmen Domínguez Encinas, Ana Martínez-Cabrera Peláez, Mª Inés Casas Jiménez, Pilar Pérez Egea, Concepción Espariz Campano, Ángeles Brieva García, Mª Azucena SaezBerlana, Carlos Casanova García, Mª Carmen Belinchón Moya, Mª Dolores Parejo Pablos, Elisa Varona Lahuerta, Esther Labrador Arranz, Mª Ángeles Conde Llorente, Mª Teresa Gómez Martínez, Milagros Velázquez García, Mª Patrocinio Verde González, Mª Rosario Campo Martínez, Mª Rosario Del Álamo Gutiérrez, Mª Victoria Cantera Urcía, Alejandra González Esteban, Laura Rodríguez Cortizo, Sabrina Sosa Alés, Eva Torres Cantero, Idoia Baíllo Peña, Tamara García López, Cristina Calle Domínguez, Inmaculada Peña Sainz, Mª Antonia Minguito Lobos, Consuelo Viamonte Andrés, Francisco Manuel García García, José María Lobos Bejarano, Raquel Juez Pimienta, Emiliana Villares Motino, Elvira Pérez Peñas, Silvia Jiménez, Laura Manuyama Pacaya, Carmen Morales Guevara, Carmen Melero González, Blanca Novella Arribas, Marta Cuevas, Belén Sierra García, Marta Ruiz, Amelia González Gamarra, Rosa Mª Sánchez Alcalde, Belén Peláez Raposo, Ángela Gallego Arenas, Mª Soledad Mayayo Vicente, Javier López González, Manuel Jovino Arango Victoria, Ana María Santos Caballero, Isabel Jimeno, Juana Iribertegui, Ramón María Salgado, María Olga Ortega de Santos, María Gema García, María José LLorénsBalducel, Juan Carlos de la Fuente, Claudia Fernández Illen, Beatriz Manrique Olmedo, Tati Arévalo Gallego, Juan Machuca Gómez, Esther San José Blázquez, Teresa Castellanos Ruiz, Macarena Espejo Saucedo, María Esther Fernández Yedra, Carmen Torres Martínez, Ángel Lindo Torres, Víctor Raúl Montes Pina , khosrowDadbinDadbin, Rosa ArdáMaillo, Inmaculada Parra Álvarez, Justino Flores Ramos, Mª Dolores Vicente de Forondo, Antonio Calvo Cebrian, Yolanda Gines Díaz, Paloma Henares García, Luís La Puente Montoso, Aurora López Gil, Mª Concepción Marcello,Silvia Membrado Gómez, Nieves Puente García, Nuria Rodríguez Pata, Antonio Sánchez Calso, Mª Sánchez Casado, Mª Pilar Saladana Calzo, Carmen Velayos Rodríguez, Consuelo Velaz López, Miguel Ángel Venga Mendía, C. Susana Abad Guijarro, Gema Calderero Castellanos, Esperanza Corral Agüero, Ángeles Fernández Ortega, Carmen García Regidor, Edurne Hernández Sanzano, Pablo Martín Cano, Mª Del Carmen Martínez Coello, Sandrine Miguel Miguel, Mª Jesús Ramos Martín de Argenta, Beatriz Ruescas Aurrecoechea, Esteban González López, Elena Ramos Quirós, David Pérez Manchón, Leticia Pontón, Jon Koldo SagarduiVillamor, Mireia Rey Pérez de Pipaon, Mª Luisa Idarreta Zubiria, Juan Carlos Sánchez Ruiz, Ángela Rodríguez de Cossio, Milagros Merino Pella, Nuria Ruiz Hombrebueno, Rafael Llanes, Yolanda Vicente Prior, Mercedes Picó, Francisco Pérez Durán, Isabel Pérez Botella, Ángeles Cuevas, Francisco Martínez García, Raquel Cobeñas Mateo, María Teresa Rodríguez De Fonseca, Naldi Luz Cerdeña Ocola, Irene Alma Polanco García, María Esther Amez De Castro, Susana Barrios Espinoza, Antonio Guijarro Jiménez, Ana María De La Uz Pardos, Francisco Javier Cabrera Pérez, José Ignacio Torres Jiménez, Francisco Martínez García, María Isabel Vidal De La Riva, María Teresa Rodríguez De Fonseca, Gabriel Barderas Cuevas, Gonzalo Carrillo De Albornoz Martínez Pantoja, Mª Isabel García Romero. Castilla y León. Primary care centers of Ávila. Primary Care centre of Arévalo: Saturio Vega Quiroga; Roberto Aldrich García, Carlos Cañas Ruesgas, Carmen Vian Baron, Josefina Fernández Fernández, Mª Antonia Jiménez Carabias, Laureano López Gay, Mª Pilar Marqués Macías, Almudena Cantalejo Martín, Ana Benito Pérez, Modesta Mulero San José, Vanesa Martín Hernández, Laura Sánchez Domínguez, Rosa Mª García Martín, Victor Manuel Álvarez Zurdo. Primary Care centre of Sotillo de la Adrada: David Álvarez Suárez, Carmen Lázaro del Nogal, Lourdes González López, Mª del Mar Varas Reviejo, Juan Luis Martín Clavo, Mª Isabel Blázquez Blanco,Mª Luisa Ramos González, Guadalupe Rinaldi Català, Montserrat López Ramírez, Vanesa Hernández Blázquez, Vanessa Gutiérrez León, Raquel Pérez Cruz, Josefina Fernández Fernández, Almudena Fernández García, Raquel Alonso Moralejo. Primary care centers of Segovia. Primary Care centre of Carbonero El Mayor: María Soledad Fragua Gil, Virginia Silva Guisasola, Concepción Manrique de la Fuente, Ángeles Lazcoz Fontán, Héctor Aceves Gamarra, Alba Marina Hernández López, Mª Jesús Blanco Ledesma, Alfonso Santos López, Cristina de la Cruz Maeso, Mª del Espíritu Santo Otero Herrero, Cristina Olmos Marinero, Patricia Redondo Arranz, Mónica Álvaro García. Primary Care centre of Segovia III: Luis Gonzálvez López, María Ángeles Raquejo Grado, José Rodríguez Sanz, Juan Manuel de Andrés Rubio, Nuria González Acebes, Joaquina Galán Sánchez, Teresa López Fernández-Quesada, Almudena Sanz Prieto, Carmen Montero Morales, María Dolores Alba Jiménez, Beatriz Ayala Miranda. Castilla-La Mancha. Primary Care centres of CUENCA. Hospital General: Jaime Santiago Aranda Regules, Alba Caterina del Hoyo Herráiz, María Victoria Cantero Ayllón, María José Guillén Izquierdo, María Sandra Ruiz Mora, Ana Peña Cabia, Rosa Sánchez Amo, Mº Josefa Moya López. Cuenca I: Fructuoso Muelas Herráiz, Mº Ángeles Molina Morate, Fernando Salcedo Aguilar. Cuenca II: Nieves Valero Caracena, Beatriz Ortega Noheda, Mº Carmen García González. Cuenca III: Cristina Martínez Martín, Miryam Pardo Villalvilla, Mª Eugenia García Castellanos, María Elena de las Heras Martínez. Primary Care centre of Tarancón: Filomena del Saz Castellanos, Encarnación Palomares Cañada, María Concepción Fraile Jiménez, Pilar Palomar Moreno, Bárbara Martínez Garrido, María Pilar Orgaz Gallego, María José Tricio Armero, Cristina García del Pino Cañadas, Isabel Tierno Aparicio. Málaga. Fundación FIMABIS. Servicio Andaluz de Salud. Regional University Hospital of Málaga, Biomedical Research Institute of Málaga, University of Málaga, Málaga, Spain. Medicina Interna: Ricardo Gómez-Huelgas, María Dolores López-Carmona, Luis M Pérez-Belmonte, María Rosa Bernal-López, María Teresa Moyano Paris, Paula Moya Rodríguez, Antonio Vargas Candela, Alberto Vilches Pérez, María Isabel Ruiz Moreno, Maite Muñoz Melero, Pilar Gómez Martin. Oftalmología: Jacinto Villalvilla, Álvaro Santos, Antonio Archilla, Carlos Rocha, Silvia Lozano Ruiz. Primary care centers of Málaga. Alameda-Perchel: Francisco Javier Orellana Lozano, Manuel Guarino Nuño, Alhaurín de la Torre: Daniel Martin Castillo, María José Guerra Maldonado, José Rogelio Sánchez Ortiz. Alozaina-Yunquera: David Fernández Bonilla, J A Cortes, Juan Antonio Cordero Cabrera. Antequera: José Antonio Godínez, José Jesús Moreno Jiménez, David Paniagua Urbano. Archidona: Antonio Cansino Osuna, María Del Carmen Rojo Camacho, Celinda Lara Moreno, Ignacio Hinojosa Núñez, Almudena Puga González, Capuchinos: Yolanda Rey, Yolanda Rodríguez Gallego. Carranque: Carmen Aylón Moliner. Cartama Estacion-Cartama pueblo-Pizarra: Francisco Jose Guirado Hidalgo, María Eva Ruiz Coronado, Susana Barea Diañez, Beatriz Navarro Aranda. Casarabonela: M Carmen Arroyo Martínez. Ciudad Jardin: Antonio Baca Osorio, José Mancera, Salvador Ruiz Vera, Idelfonso Martos Cerezuela. Delicias: Fernando López Verde, Mª Carmen Barba Cañete, Cristóbal Gómez Acevedo, Margarita Sánchez Pavón. La Luz: Antonio Oropez Mesa, Antonio Rojas Barrilado. La Roca: Esther Martin Aurioles, Rocío Ramos, Francisco Javier Camino, María Eugenia Valdes, Dolores Bravo Fernández. Limonar: Silvia Hazañas, Amparo Vargas Machuca Benítez, Eva María Taboada Ríos. Puerta Blanca: Antonio Hormigo Pozo, Idelfonsa Martínez Zaragoza. Rincón de la Victoria: Milagrosa Espinar Toledo, María Del Rosario Rosillo Rein, Gloria Inmaculada Mestre Reoyo, María Auxiliadora Naranjo Sánchez, José Ángel Sánchez Ortiz, Mª José González Vega, José Carlos Pérez Sánchez, Antonia Cabra Navarro, Antonio Vivas Molina. San Andrés-El torcal: Antonio Ramírez Ceballos, Francisco Ruiz Solares. Tiro Pichón: Juan José Bedoya Belmonte, Germán Ortega Núñez, María Encarnación Bueno Caro. Trinidad-Jesús cautivo: Santos Agreda. Victoria: María José Bujalance Zafra, Montse Román Cereto, Rafael Ángel Maqueda. Poland. UniwersytetJagiellonski, Collegium Medicum, Poland: Aleksandra Gilis-Januszewska, Alicja Hubalewska-Dydejczyk, Beata Piwońska-Solska, JustynaBiegańska, Katarzyna Cybulska, Bernadeta Marcykiewicz, Magdalena Duraczyńska, Anna Cybulska, Joanna Stankiewicz-Góra, Alina Mruk, Grzegorz Młyński, Lucyna Rozpondek, Michał Sroka, Maciej Gilis-Januszewski, Edyta Sacha, Adela Justyńska, Magdalena Szopa, Bartłomiej Matejko. Greece. National and Kapodistrian University of Athens. Greece: Konstantinos Makrilakis, Stavros Liatis, Evangelia Siami, Chryssoula Stathi, Katerina Barmpagianni, Maria Nikoloudi, Meropi Kontogianni, Ioanna Kechrimpari, Aphroditi Tsiakou, Melina Karaolia. Alexandra Hospital. University of Athens. Greece: Asimina Mitrakou, Georgios Panagopoulos, Paraskevi Kontou, Petros Thomakos, Georgios Giagkou, Evangelia Avgeraki, Eirini Mamalaki. Bulgaria. University Multi-Profile Hospital for Active Treatment Alex and rovska EAD. Sofia, Bulgaria: Zdravko Asenov Kamenov, Antoaneta Trifonova Gateva, Yavor Sashov Assyov, Tsvetan Vladimirov Gatev, Vera Nacheva Karamfilova, Iveta Slavyanova Nedeva. Austria. Gemeinnutzige Salzburger Landeskliniken Betriebsgesellschaft. Salzburg, Austria: Bernhard Paulweber, Ludmilla Kedenko, Andrea Undeutsch. Turkey. Istanbul University Istanbul. Turkey: Ilhan Satman, B. Fulya Turker, Ayse K. Uzum, Sakin Tekin, Ramazan Çakmak, Elif T Bagdemir, Selda G Celik, Cemile C Idiz, Halime C Sackoparan, Zafer Cebeci, Nur Kir, Dilara Karsidag, Yildiz Tutuncu, Aslihan Demirbas, Busra Yildiz. Serbia. Medical System Beograd-MSB. Belgrade, Serbia. Predrag Djordjevic, Margarita Dodevska, Nevenka Raketic, Aleksandar Stamenkovic, Marko Jovic, Fadil Canovic, Ljiljana Milivojevic, Kristina Savic, Ljiljana Savic, Mirjana Sarkic. Faculty of Medicine, University of Belgrade. Serbia. Nebojsa Lalic, Katerina Lalic, Aleksandra Jotic, Jelena Stanarcic, Ljiljana Lukic, Tanja Milicic, Natasa Rajkovic, Marija Macesic, Dijana Risimic, Mladen Bila. Australia. The University of Sydney. Australia: Stephen Colagiuri, Anthony Keech, Kristine Maddock, Andrzej S. Januszewski, Liping Lee, Tegan Picone, Emma Sainsbury, Alison Coenen, Chelsea Hendy, Namson Lau, Tania Markovic, Erica Bessell, Nick Fuller. Kuwait. Dasman Diabetes Research Institute: Jaakko Tuomilehto, Abdullah Alkandari, Abdullah Bennakhi, Monira Alarouj, Mohammad Jalali, Medinella Fernandez, Makka Ali Osman, Jincy Raj, Ala’a Al-Obaid, Hyatt Alsayegh, Najeeba Almatrouk. WP3: Lifestyle Intervention. Terveyden ja Hyvinvoinnin Laitos. Finland: Jaana Lindström, Päivi Valve, Katri Hemiö, Katja Wikström, Esko Levälahti, Pirjo Saastamoinen. WP4. Central laboratories and Biobank. Instituto de Investigación Sanitaria de la Fundación Jiménez Díaz. Madrid, Spain (Central Laboratory, Biobank and Inflammatorybiomarkers): Jesús Egido, Sebastián Mas, Sandra Zazo, Esther Civantos, Rosario de Nicolás, Federico Rojo. Consiglio Nazionaledelle Ricerche. Pisa, Italy. (nonalcoholic fatty liver disease laboratory): Amalia Gastaldelli, Fabrizia Carli, Emma Buzzigoli, Melania Gaggini. Fundació Hospital Universitari Vall d’ Hebron, Institut de Recerca. Barcelona, Spain (Retinal biomarkers): Rafael Simó, Cristina Hernández, Marta García-Ramirez. Queen Mary University of London. United Kingdom (Genetic laboratory): Graham A Hitman. WP5: Microvascular assessment coordination. Universita di Pisa, Italy. (Endotelial assessment): Andrea Natali, Lucrecia Motta. WP6: Retinal Assessment. Instituto de Oftalmobiología Aplicada (IOBA) Universidad de Valladolid, Spain. (Central Retinal Reading Centre): Maribel López, José Carlos Pastor, Lucía Manzanas, Ignacio Alonso, Verónica Velasco, Laura Mena. e-DIAGNOSTIC Oftalmología. Madrid, Spain. (Retinal e-platform): Diana Bravo, Víctor González Rumayor, Marica D´Angelo, Álex Manau. WP7: Neuropsychological assessment. Department for Clinical Neurosciences and Preventive Medicine, Danube University Krems. Krems, Austria: Michael Brainin, Yvonne Teuschl, Alexandra Dachenhausen, Karl Matz. Instituto de Investigación y Asistencia Psiquiátrica. Madrid, Spain: Laura Ferrando Bundío. Oivauni Oy. Kuopio,Finland: Henri Tuomilehto, Seppo Silvennoinen. WP8: Statistical analysis. Vastra Gotalands Lans Landsting. Gotenburg, Sweden (statistical analysis coordinator): Marcus Lind, Aldina Pivodic, Hans Wedel. Institute of Neuroscience, National Research Council (Consiglio Nazionale delle Ricerche). Pavoda, Italy. (Insulin and C-Peptide modeling): Andrea Mari, Andrea Tura. University of Helsinki. Department of Public Health: Pekka Jousilahti. WP9: Technology Assessment. IMPETO Medical. Paris, France (Sudoscan assessment): Jean-Henri Calvet, GaelleLerise, Alice Vilier. Mezen Bouzaien. AARDEX Group SA. Geneva. Switzerland (MEMs drug´s adherence monitoring): Bernard Vrijens, Rodrigo Paiva, Eric Tousset. WP10: Dissemination and communication: Federation Internationale du Diabete Region Europe Lala Rabemananjara.
| 1. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 6370] [Article Influence: 910.0] [Reference Citation Analysis (12)] |
| 2. | International Diabetes Federation. IDF Diabetes Atlas 9th edition 2019. [cited April 4, 2021]. Available from: https://www.diabetesatlas.org/en/. |
| 3. | Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322-1335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 666] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 4. | Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1867] [Article Influence: 143.6] [Reference Citation Analysis (1)] |
| 5. | Echouffo-Tcheugui JB, Perreault L, Ji L, Dagogo-Jack S. Diagnosis and Management of Prediabetes: A Review. JAMA. 2023;329:1206-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 274] [Article Influence: 91.3] [Reference Citation Analysis (1)] |
| 6. | Bansal N. Prediabetes diagnosis and treatment: A review. World J Diabetes. 2015;6:296-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 276] [Cited by in RCA: 342] [Article Influence: 31.1] [Reference Citation Analysis (13)] |
| 7. | CDC. National Diabetes Statistics Report. Diabetes 2023. [cited July 3, 2024]. Available from: https://www.cdc.gov/diabetes/php/data-research/index.html. |
| 8. | Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2049] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 9. | Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 179] [Reference Citation Analysis (0)] |
| 10. | Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Häring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12:721-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 339] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 11. | Mutie PM, Pomares-Millan H, Atabaki-Pasdar N, Jordan N, Adams R, Daly NL, Tajes JF, Giordano GN, Franks PW. An investigation of causal relationships between prediabetes and vascular complications. Nat Commun. 2020;11:4592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Kim GS, Oh HH, Kim SH, Kim BO, Byun YS. Association between prediabetes (defined by HbA1(C), fasting plasma glucose, and impaired glucose tolerance) and the development of chronic kidney disease: a 9-year prospective cohort study. BMC Nephrol. 2019;20:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Plantinga LC, Crews DC, Coresh J, Miller ER 3rd, Saran R, Yee J, Hedgeman E, Pavkov M, Eberhardt MS, Williams DE, Powe NR; CDC CKD Surveillance Team. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5:673-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 14. | Gabriel R, Boukichou Abdelkader N, Acosta T, Gilis-Januszewska A, Gómez-Huelgas R, Makrilakis K, Kamenov Z, Paulweber B, Satman I, Djordjevic P, Alkandari A, Mitrakou A, Lalic N, Colagiuri S, Lindström J, Egido J, Natali A, Pastor JC, Teuschl Y, Lind M, Silva L, López-Ridaura R, Tuomilehto J; e-PREDICE Consortium. Early prevention of diabetes microvascular complications in people with hyperglycaemia in Europe. ePREDICE randomized trial. Study protocol, recruitment and selected baseline data. PLoS One. 2020;15:e0231196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Gabriel R, Boukichou-Abdelkader N, Gilis-Januszewska A, Makrilakis K, Gómez-Huelgas R, Kamenov Z, Paulweber B, Satman I, Djordjevic P, Alkandari A, Mitrakou A, Lalic N, Egido J, Más-Fontao S, Calvet JH, Pastor JC, Lindström J, Lind M, Acosta T, Silva L, Tuomilehto J; On Behalf Of The E-Predice Consortium. Reduction in the Risk of Peripheral Neuropathy and Lower Decrease in Kidney Function with Metformin, Linagliptin or Their Fixed-Dose Combination Compared to Placebo in Prediabetes: A Randomized Controlled Trial. J Clin Med. 2023;12:2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12:2032-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1956] [Article Influence: 217.3] [Reference Citation Analysis (0)] |
| 17. | Lousa I, Reis F, Beirão I, Alves R, Belo L, Santos-Silva A. New Potential Biomarkers for Chronic Kidney Disease Management-A Review of the Literature. Int J Mol Sci. 2020;22:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 21014] [Article Influence: 1236.1] [Reference Citation Analysis (0)] |
| 19. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11183] [Cited by in RCA: 11934] [Article Influence: 442.0] [Reference Citation Analysis (0)] |
| 20. | Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3971] [Cited by in RCA: 4467] [Article Influence: 165.4] [Reference Citation Analysis (6)] |
| 21. | Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A Model-Based Method for Assessing Insulin Sensitivity From the Oral Glucose Tolerance Test. Diabetes Care. 2001;24:539-548. [RCA] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 621] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 22. | Mari A, Tura A, Gastaldelli A, Ferrannini E. Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes. 2002;51 Suppl 1: S221-S226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Mendivil CO, Gnecco-González S, Herrera-Parra LJ, Hernández Vargas JA, Ramírez-García N, Acuña-Merchán L. MDRD is the eGFR equation most strongly associated with 4-year mortality among patients with diabetes in Colombia. BMJ Open Diabetes Res Care. 2023;11:e003495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Jiang JJ, Sham TT, Gu XF, Chan CO, Dong NP, Lim WH, Song GF, Li SM, Mok DK, Ge N. Insights into serum metabolic biomarkers for early detection of incident diabetic kidney disease in Chinese patients with type 2 diabetes by random forest. Aging (Albany NY). 2024;16:3420-3530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Bailey RA, Wang Y, Zhu V, Rupnow MF. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) staging. BMC Res Notes. 2014;7:415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 26. | Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:823-833. [PubMed] |
| 27. | Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B: Stat Methodol. 1995;57:289-300. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17030] [Cited by in RCA: 26080] [Article Influence: 3260.0] [Reference Citation Analysis (1)] |
| 28. | R Core Team. R: A language and environment for statistical computing. R Found Stat Comput. 2022;. |
| 29. | Gorostidi M, Santamaría R, Alcázar R, Fernández-Fresnedo G, Galcerán JM, Goicoechea M, Oliveras A, Portolés J, Rubio E, Segura J, Aranda P, de Francisco AL, Del Pino MD, Fernández-Vega F, Górriz JL, Luño J, Marín R, Martínez I, Martínez-Castelao A, Orte LM, Quereda C, Rodríguez-Pérez JC, Rodríguez M, Ruilope LM. Spanish Society of Nephrology document on KDIGO guidelines for the assessment and treatment of chronic kidney disease. Nefrologia. 2014;34:302-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2942] [Cited by in RCA: 2636] [Article Influence: 202.8] [Reference Citation Analysis (0)] |
| 31. | Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 847] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 32. | Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas TN, Warram JH, Krolewski AS. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 388] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 33. | Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, Park J, Nair V, Schlafly A, Saulnier PJ, Satake E, Simeone CA, Shah H, Qiu C, Looker HC, Fiorina P, Ware CF, Sun JK, Doria A, Kretzler M, Susztak K, Duffin KL, Nelson RG, Krolewski AS. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. 2019;25:805-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 330] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 34. | Lee JE, Gohda T, Walker WH, Skupien J, Smiles AM, Holak RR, Jeong J, McDonnell KP, Krolewski AS, Niewczas MA. Risk of ESRD and all cause mortality in type 2 diabetes according to circulating levels of FGF-23 and TNFR1. PLoS One. 2013;8:e58007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Xie Z, Xiao X. Novel biomarkers and therapeutic approaches for diabetic retinopathy and nephropathy: Recent progress and future perspectives. Front Endocrinol (Lausanne). 2022;13:1065856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Donate-Correa J, Martín-Núñez E, Muros-de-Fuentes M, Mora-Fernández C, Navarro-González JF. Inflammatory cytokines in diabetic nephropathy. J Diabetes Res. 2015;2015:948417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 191] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 37. | Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11:1185-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 512] [Cited by in RCA: 696] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 38. | Pugliese G, Iacobini C, Pesce CM, Menini S. Galectin-3: an emerging all-out player in metabolic disorders and their complications. Glycobiology. 2015;25:136-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Li Y, Li T, Zhou Z, Xiao Y. Emerging roles of Galectin-3 in diabetes and diabetes complications: A snapshot. Rev Endocr Metab Disord. 2022;23:569-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 40. | Chung JO, Park SY, Lee SB, Kang NR, Cho DH, Chung DJ, Chung MY. Plasma galectin-3 concentration and estimated glomerular filtration rate in patients with type 2 diabetes with and without albuminuria. Sci Rep. 2022;12:16328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Mendonca HR, Carpi-Santos R, da Costa Calaza K, Blanco Martinez AM. Neuroinflammation and oxidative stress act in concert to promote neurodegeneration in the diabetic retina and optic nerve: galectin-3 participation. Neural Regen Res. 2020;15:625-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 42. | Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, Yang Y, Hu Y, Huang Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370:m2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 461] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 43. | Bergman M, Chetrit A, Roth J, Jagannathan R, Sevick M, Dankner R. One-hour post-load plasma glucose level during the OGTT predicts dysglycemia: Observations from the 24year follow-up of the Israel Study of Glucose Intolerance, Obesity and Hypertension. Diabetes Res Clin Pract. 2016;120:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Hayashi Y. Detection of Lower Albuminuria Levels and Early Development of Diabetic Kidney Disease Using an Artificial Intelligence-Based Rule Extraction Approach. Diagnostics (Basel). 2019;9:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 663] [Article Influence: 66.3] [Reference Citation Analysis (1)] |
| 46. | Echouffo-Tcheugui JB, Narayan KM, Weisman D, Golden SH, Jaar BG. Association between prediabetes and risk of chronic kidney disease: a systematic review and meta-analysis. Diabet Med. 2016;33:1615-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |