Published online Sep 15, 2025. doi: 10.4239/wjd.v16.i9.104290
Revised: April 18, 2025
Accepted: August 11, 2025
Published online: September 15, 2025
Processing time: 265 Days and 6.7 Hours
Insulin is the preferred clinical treatment for hospitalized patients with type 2 diabetes mellitus (T2DM) to control blood glucose effectively. Hypoglycemia is one of the most common adverse events. Accurate prediction of the risk of hy
To develop and validate a hypoglycemia risk prediction tool for hospitalized patients with T2DM treated with insulin.
This retrospective study included 802 hospitalized patients with T2DM in the Department of Endocrinology, the Third Affiliated Hospital of Sun Yat-sen University, between January 2021 and December 2021. The hypoglycemia risk prediction model was developed using logistic regression and nomogram models. The model was validated and calibrated using receiver operating characteristic curves and the Hosmer-Lemeshow goodness of fit test.
The incidence of hypoglycemia among the enrolled patients was 44.9%. The hypoglycemic risk prediction model included six predictors: Body mass index, duration of diabetes, history of hypoglycemia within 1 year, glomerular filtration rate, blood triglyceride levels, and duration of treatment. The hypoglycemia risk prediction model displayed high discrimination ability (area under the curve = 0.67) and good calibration power (goodness of fit, χ2 =12.25, P = 0.14).
The hypoglycemia risk prediction model for hospitalized patients with T2DM on insulin therapy displayed high reliability and discrimination ability. The model is a promising tool for clinicians to screen hospitalized patients with T2DM and an elevated risk of hypoglycemia and guide personalized interventions to prevent and treat hypoglycemia.
Core Tip: The hypoglycemia risk-prediction model was developed using the logistic regression and nomogram models. The model was validated and calibrated using the receiver operating characteristic curves and the Hosmer-Lemeshow goodness of fit test. The incidence of hypoglycemia was 44.9%. The model included eight independent hypoglycemia risk factors. The hypoglycemia risk prediction model for hospitalized T2 diabetes mellitus patients treated with insulin showed high reliability and discrimination ability.
- Citation: Zhang Y, Hu XL, Xu WR, Chen YM, Guo XD, Liu SH, Gao LL. Development and validation of a hypoglycemia risk prediction tool for hospitalized patients with type 2 diabetes mellitus treated with insulin. World J Diabetes 2025; 16(9): 104290
- URL: https://www.wjgnet.com/1948-9358/full/v16/i9/104290.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i9.104290
Diabetes mellitus (DM) is a metabolic disorder associated with alterations in pathways involved in carbohydrate, fat, and protein metabolism[1]. Type 2 DM (T2DM) accounts for more than 90% of all DM cases, and it is the primary contributor to the global disease burden[2,3]. Insulin is the recommended clinical treatment of hyperglycaemia for hospitalized patients with T2DM[1,4]. Hypoglycemia is a key barrier to achieving euglycemic control in hospitalized patients with T2DM[5]. Hypoglycemia refers to a blood glucose level ≤ 3.9 mmol/L for hospitalized patients with T2DM[6]. Inpatient hypoglycaemia has been linked to adverse clinical outcomes, including mortality, longer stay in hospital, and cost to the healthcare system[5]. Furthermore, long-term severe hypoglycemia can cause multiple-organ function damage, including cardio
Accurate prediction of the risk of hypoglycemia is critical for reducing hypoglycemic events and related adverse events in hospitalized patients with DM. Moreover, an objective and straightforward tool for predicting the risk of hy
The Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (Guangzhou, China) approved this study. All participants provided written informed consent before participating in this study.
This retrospective study enrolled 802 hospitalized patients with T2DM who received insulin treatment between January 2021 and December 2021 at the Department of Endocrinology, Third Affiliated Hospital of Sun Yat-sen University.
Inclusion criteria: The inclusion criteria were as follows: Diagnosis of T2DM as defined by the World Health Organization in 1999; age ≥ 18 years; eligible for insulin intensive therapy (including newly diagnosed patients with glycated hemoglobin A1c (HbA1c) > 9.0%, fasting plasma glucose (FPG) at admission > 11.1 mmol/L, or obvious symptoms of hyperglycemia; patients with significant blood glucose elevation (HbA1c > 9.0%) after combined treatment with two or more oral hypoglycemic agents; or patients with HbA1c > 7.0% after adequate dose-adjusted initial insulin therapy); and ongoing intensive insulin therapy, including basal pre-prandial insulin injection regimen, continuous subcutaneous insulin infusion, and premixed insulin analog injection regimen three times daily.
Exclusion criteria: The exclusion criteria were as follows: Severe acute complications of DM; serious disease of the heart, brain, lungs, and/or kidneys; pregnancy; and surgical treatment.
Outcome measures: The diagnostic criteria for hypoglycemia in this study were venous plasma glucose or capillary blood glucose (peripheral blood glucose) levels of ≤ 3.9 mmol/L combined with other clinical symptoms including sense of hunger, palpitation, and trembling hands.
Baseline predictor variables: Based on the review of previous studies, the following clinical data were collected from the medical records of all hospitalized patients: General factors including sex, age, education, body mass index (BMI), blood pressure, smoking history, alcohol consumption, occupation, education level, and marital status; disease-related information, including duration of diabetes, diabetic nephropathy, diabetic peripheral nerve disease, diabetic peripheral vascular disease, hypertension, hyperlipidemia, fatty liver, HbA1c, fasting blood glucose (FBG), random blood glucose at admission, standard deviation of blood glucose (SDBG), fasting insulin, fasting C peptide, homeostasis model assessment for insulin resistance (HOMA-IR), homeostasis model assessment of β-cell function, aspartate aminotransferase, alanine aminotransferase (ALT), creatinine, glomerular filtration rate (GFR), total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, and C-reactive protein; treatment-related information, including treatment before admission, insulin injection regimen, insulin dose, type of combined oral drugs (antidiabetic drugs, antihypertensive drugs), and total treatment time; and hypoglycemic event-related information, including frequency of hypoglycemia, specific blood glucose levels, hypoglycemia classification (clinically significant hypoglycemia, blood glucose alert value, pseudo-hypoglycemia), time of hypoglycemia occurrence (before and after meals, before sleep, or night/early morning), hypoglycemia symptoms (asymptomatic, hunger, sweating, vertigo, palpitation, fatigue, hand shaking, and others), hypoglycemia inducement (unreasonable diet, unreasonable exercise, and other reasons), treatment methods [eating food, oral intake of 50% glucose solution (GS), intravenous injection of 50% GS, or oral 50% GS + food], duration of hypoglycemia, and FBG before the onset of hypoglycemia.
SPSS 25.0 software (IBM, Armonk, NY, United States) was used for statistical analysis. P < 0.05 (two-sided) was considered statistically significant. Continuous variables were expressed as the mean ± SD. Categorical variables were expressed as numbers, percentages, or frequencies. Intergroup differences were analyzed using the independent-samples t-test, χ2 test, or Mann-Whitney U test. A backward stepwise logistic regression model was used to screen the independent hypoglycemia risk factors and construct a hypoglycemia risk prediction model. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were used to verify the prediction performance of the risk model. The Hosmer-Lemeshow test was used to verify and calibrate the model. Ten-fold cross-validation and AUCs were used to validate the hypoglycemia risk prediction model.
The incidence of hypoglycemia in this study cohort was 44.9%, and 716 hypoglycemic events were recorded in 360 of 802 patients included in this study (Table 1). Among the patients with hypoglycemia, 53.9% experienced two or more episodes. The median (interquartile range) time for the occurrence of hypoglycemia during insulin treatment was 6.5 (4-9) days. Therefore, hypoglycemia generally occurred on days 4-9 of insulin treatment. Hypoglycemia occurred most frequently before lunch in 187 patients (26.1%), after breakfast in 117 patients (16.3%), and before breakfast in 106 patients (14.8%). Asymptomatic hypoglycemia was observed in 455 patients (63.5%), whereas symptomatic hypoglycemia occurred in 261 patients (36.5%). The cause of 88% of hypoglycemic events was unknown. The leading causes of hypoglycemia included reduced food intake (58 cases), excessive exercise (11 cases), examination-related hypoglycemia (15 cases), and excessive insulin dosage (one case).
| n (%) or mean ± SD | | n (%)/M (P25, P75) | |
| Frequency of hypoglycemic events | FPG before hypoglycemia | 5.80 (4.80, 7.30) | |
| 0 | 442 (55.1) | Days of insulin treatment when hypoglycemia occurred | 6.50 (4.00, 9.00) |
| 1 | 166 (20.7) | Occurrence time | |
| 2 | 108 (13.5) | Before breakfast | 106 (14.8) |
| 3 and more | 86 (10.7) | After breakfast | 117 (16.3) |
| Hypoglycemia (mmol/L) | 3.53 ± 0.40 | Before lunch | 187 (26.1) |
| Symptoms of hypoglycemia | After lunch | 86 (12.0) | |
| No symptom | 455 (63.5) | Before dinner | 69 (9.6) |
| Sweating | 67 (9.4) | After dinner | 48 (6.7) |
| Fatigue | 37 (5.2) | Before sleep | 76 (10.6) |
| Palpitation | 56 (7.8) | Midnight/before dawn | 27 (3.8) |
| Sense of hunger | 54 (7.5) | Hypoglycemic trigger | |
| Hands tremble | 19 (2.7) | None | 630 (88.0) |
| Dizzy | 69 (9.6) | Reduced food intake | 58 (8.1) |
| Blurred vision | 6 (0.8) | Excessive exercise | 11 (1.5) |
| Types of hypoglycemia | Examination related | 15 (2.1) | |
| Clinically significant | 81 (11.3) | Excessive insulin dosage | 2 (0.3) |
| Blood glucose alert value | 617 (86.2) | Methods for alleviating hypoglycemia | |
| Relative hypoglycemia | 18 (2.5) | Eating food | 475 (66.3) |
| Duration of hypoglycemia | Oral GS | 172 (24.0) | |
| 15 minutes | 617 (86.2) | Intravenous injection of GS | 10 (1.4) |
| 30 minutes | 87 (12.1) | Eating food + Oral GS | 59 (8.2) |
| > 30 minutes | 12 (1.7) | ||
Based on the occurrence of hypoglycemia, the patients in this study were categorized into the non-hypoglycemic group
| Non-hypoglycemic group (n = 442) | Hypoglycemic group (n = 360) | t/χ2 | P value | |
| Age (years) | 54.04 ± 13.84 | 56.06 ± 12.94 | -2.11 | 0.04a |
| Age (years) | 6.36 | 0.10 | ||
| 18-44 | 116 (63.0) | 68 (37.0) | ||
| 45-59 | 173 (52.6) | 156 (47.4) | ||
| 60-74 | 127 (52.3) | 116 (47.7) | ||
| ≥ 75 | 26 (56.5) | 20 (43.5) | ||
| Gender | 3.59 | 0.06 | ||
| Male | 147 (50.7) | 143 (49.3) | ||
| Female | 295 (57.6) | 217 (42.4) | ||
| BMI (kg/m2) | 24.79 ± 3.38 | 23.36 ± 3.34 | 5.98 | < 0.001a |
| BMI (kg/m2) | 26.56 | < 0.001a | ||
| < 18.5 | 12 (37.5) | 20 (62.5) | ||
| 18.5-23.9 | 174 (47.0) | 196 (53.0) | ||
| ≥ 24.0 | 256 (64.0) | 144 (36.0) | ||
| Systolic blood pressure (mmHg) | 130.24 ± 20.27 | 130.88 ± 20.67 | -0.44 | 0.66 |
| Diastolic blood pressure (mmHg) | 82.23 ± 11.53 | 82.40 ± 11.37 | 1.02 | 0.31 |
| Smoking | 0.23 | 0.63 | ||
| No | 345 (54.7) | 286 (45.3) | ||
| Yes | 97 (56.7) | 74 (43.3) | ||
| Alcohol consumption | 1.87 | 0.17 | ||
| No | 384 (54.2) | 324 (45.8) | ||
| Yes | 58 (61.7) | 36 (38.3) | ||
| Education level | 4.77 | 0.10 | ||
| Primary school or lower | 99 (52.1) | 91 (47.9) | ||
| Middle school | 191 (52.8) | 171 (47.2) | ||
| College or higher | 152 (60.8) | 98 (39.2) | ||
| Occupational status | 12.29 | 0.002a | ||
| Employed | 278 (60.3) | 183 (39.7) | ||
| Unemployed | 49 (45.4) | 59 (54.6) | ||
| Retired | 115 (49.4) | 118 (50.6) | ||
| Marital status | 1.82 | 0.40 | ||
| Married | 412 (55.2) | 335 (44.8) | ||
| Unmarried | 22 (61.1) | 14 (38.9) | ||
| Divorced/widowed | 8 (42.1) | 11 (57.9) | ||
| Treatment before admission | 0.55 | 0.97 | ||
| None | 133 (55.4) | 107 (44.6) | ||
| Oral antidiabetic drugs1 | 183 (54.5) | 153 (45.5) | ||
| Insulin | 45 (52.9) | 40 (47.1) | ||
| Oral antidiabetic drugs2 + insulin | 76 (57.6) | 56 (42.4) | ||
| Others | 5 (55.6) | 4 (44.4) | ||
| Treatment time (days) | 9.20 ± 3.13 | 10.34 ± 3.56 | −4.81 | < 0.001a |
| Insulin injection therapy | 0.42 | 0.52 | ||
| Basal-before meal | 4 (44.4) | 5 (55.6) | ||
| CSII | 438 (55.2) | 355 (44.8) | ||
| Total insulin dose (IU/d) | 34.63 ± 9.14 | 34.07 ± 9.79 | 0.84 | 0.40 |
| Combined oral hypoglycemic drugs3 | 6.18 | 0.01a | ||
| No | 341 (53.0) | 303 (47.0) | ||
| Yes | 101 (63.9) | 57 (36.1) | ||
| Combined antihypertensive drugs | 1.25 | 0.26 | ||
| No | 307 (53.9) | 263 (46.1) | ||
| Yes | 135 (58.2) | 97 (41.8) | ||
Univariate regression analysis revealed that the duration of diabetes, hyperlipidemia, 1-year history of hypoglycemia, HOMA-IR, and FPG, fasting insulin, fasting C-peptide, ALT, GFR, TG, and HDL-C levels significantly differed between the two groups (all P < 0.05; Table 3). Compared with the results in the non-hypoglycemic group, the hypoglycemic group featured a longer course of diabetes; lower FPG, fasting insulin, fasting C-peptide, and ALT levels; lower HOMA-IR; a lower incidence of hyperlipidemia; a higher rate of low GFR; a higher probability of TG and HDL-C within the target range of T2DM control; and a larger proportion of subjects with a history of hypoglycemia within 1 year before hospital admission (Table 3).
| | Non-hypoglycemic group (n = 442) | Hypoglycemic group (n = 360) | Z/χ2 | P value |
| n (%)/M (P25, P75) | n (%) /M (P25, P75) | |||
| Duration of diabetes | 5.00 (0.96, 10.00) | 8.00 (1.00, 12.00) | -2.49 | 0.01 |
| Duration of diabetes | 12.26 | < 0.001a | ||
| < 10 years | 308 (59.7) | 208 (40.3) | ||
| ≥ 10 years | 134 (46.9) | 152 (53.1) | ||
| Diabetic nephropathy | 0.10 | 0.76 | ||
| No | 326 (54.8) | 269 (45.2) | ||
| Yes | 116 (56.0) | 91 (44.0) | ||
| Diabetic neuropathy | 0.02 | 0.89 | ||
| No | 284 (54.9) | 233 (45.1) | ||
| Yes | 158 (55.4) | 127 (44.6) | ||
| Diabetic vascular disease | 0.05 | 0.83 | ||
| No | 182 (54.7) | 151 (45.3) | ||
| Yes | 260 (55.4) | 209 (44.6) | ||
| High blood pressure | 0.01 | 0.94 | ||
| No | 270 (55.2) | 219 (44.8) | ||
| Yes | 172 (55.0) | 141 (45.0) | ||
| Cardiovascular diseases | 1.04 | 0.31 | ||
| No | 381 (55.9) | 301 (44.1) | ||
| Yes | 61 (50.8) | 59 (49.2) | ||
| Hyperlipidemia | 7.59 | 0.01a | ||
| No | 276 (51.7) | 258 (48.3) | ||
| Yes | 166 (61.9) | 102 (38.1) | ||
| Fatty liver | 3.46 | 0.06 | ||
| No | 235 (52.2) | 215 (47.8) | ||
| Yes | 207 (58.8) | 145 (41.2) | ||
| History of hypoglycemia within a year | 6.73 | 0.01 | ||
| No | 435 (55.9) | 343 (44.1) | ||
| Yes | 7 (29.2) | 17 (70.8) | ||
| Blood glucose status | ||||
| Random blood glucose (mmol/L) | 13.1 (10.3, 17.3) | 13.9 (10.53, 18.18) | -1.42 | 0.16 |
| FPG (mmol/L) | 7.6 (6.4, 9.2) | 6.7 (5.2, 9.1) | -4.31 | < 0.001a |
| SDBG (mmol/L) | 3.75 (2.72, 4.97) | 3.92 (2.86, 5.47) | -1.64 | 0.10 |
| HbA1c (%) | 9.5 (8.1, 11.2) | 9.85 (8.1, 11.6) | -0.96 | 0.34 |
| Pancreas islet function | ||||
| HOMA-β (%) | 40.27 (26.45, 75.93) | 39.08 (22.91, 95.24) | -0.48 | 0.63 |
| HOMA-IR | 3.21 (1.91, 5.26) | 2.03 (1.29, 3.86) | -6.50 | < 0.001a |
| Fasting insulin (mu/L) | 8.96 (5.77, 14.22) | 6.82 (4.31, 11.17) | -5.01 | < 0.001a |
| Fasting C-peptide (nmol/L) | 0.36 (0.22, 0.55) | 0.24 (0.15, 0.42) | -6.75 | < 0.001a |
| Liver and renal functions | ||||
| AST | 19 (15, 26) | 19 (15, 24) | -1.49 | 0.14 |
| AST | 1.21 | 0.27 | ||
| ≤ 40 U/L | 395 (54.5) | 330 (45.5) | ||
| > 40 U/L | 47 (61.0) | 30 (39.0) | ||
| ALT | 23 (15, 34) | 19 (14, 29) | -3.39 | 0.001a |
| ALT | 2.87 | 0.09 | ||
| ≤ 35 U/L | 342 (53.6) | 296 (46.4) | ||
| > 35 U/L | 100 (61.0) | 64 (39.0) | ||
| Cr | 64 (52, 77) | 66 (54, 80) | -1.37 | 0.17 |
| Cr | 0.07 | 0.80 | ||
| ≤ 133 μmol/L | 431 (55.2) | 350 (44.8) | ||
| > 133 μmol/L | 11 (52.4) | 10 (47.6) | ||
| GFR | 102.77 (89.30, 113.93) | 98.675 (81.31, 109.32) | -3.31 | 0.001a |
| GFR | 7.56 | 0.006a | ||
| < 90 | 114 (47.7) | 125 (52.3) | ||
| ≥ 90 | 328 (58.3) | 235 (41.7) | ||
| Blood lipid level | ||||
| TC | 4.87 (3.89, 5.61) | 4.73 (3.78, 5.41) | -1.36 | 0.18 |
| TC | 0.81 | 0.37 | ||
| < 4.5 mmol/L | 174 (53.2) | 153 (46.8) | ||
| ≥ 4.5 mmol/L | 268 (56.4) | 207 (43.6) | ||
| TG | 1.52 (1.05, 2.42) | 1.25 (0.89, 1.81) | -5.55 | < 0.001a |
| TG | 19.31 | < 0.001a | ||
| < 1.7 mmol/L | 249 (49.2) | 257 (50.8) | ||
| ≥ 1.7 mmol/L | 193 (65.2) | 103 (34.8) | ||
| HDL-C | 0.92 (0.79, 1.08) | 0.99 (0.82, 1.18) | -3.04 | 0.002a |
| HDL-C | 6.86 | 0.01a | ||
| ≤ 1 mmol/L | 281 (58.9) | 196 (41.1) | ||
| > 1 mmol/L | 161 (49.5) | 164 (50.5) | ||
| LDL-C | 3.09 (2.16, 3.74) | 3.06 (2.20, 3.80) | -0.40 | 0.69 |
| LDL-C | 0.30 | 0.58 | ||
| < 2.6 mmol/L | 163 (56.4) | 126 (43.6) | ||
| ≥ 2.6 mmol/L | 279 (54.4) | 234 (45.6) | ||
| CRP | 1.8 (1.00, 3.73) | 1.8 (0.80, 3.50) | -0.94 | 0.35 |
| CRP | 0.17 | 0.68 | ||
| ≤ 6 mg/L | 327 (55.9) | 258 (44.1) | ||
| > 6mg/L | 115 (53.0) | 102 (47.0) | ||
As HOMA-IR is not routinely measured in clinical practice and is often unavailable in medical records, we selected seven variables to construct the hypoglycemia prediction model based on the results of single-factor logistic regression analysis (P < 0.2) after excluding factors with multicollinearity and including factors with clinical practical significance. The occurrence of hypoglycemia was the dependent variable in the model. In contrast, six variables, including BMI, duration of diabetes, history of hypoglycemia within 1 year, days of treatment, GFR, and TG, were the independent variables (Table 4).
| Variables | β | S.E | Z | OR (95%CI) | P value |
| BMI | -0.10 | 0.02 | -4.30 | 0.90 (0.86-0.95) | < 0.001 |
| Duration of diabetes | |||||
| < 10 years | Reference | ||||
| ≥ 10 years | 0.26 | 0.16 | 1.63 | 1.30 (0.95-1.78) | 0.10 |
| History of hypoglycemia within 1 year | |||||
| No | Reference | ||||
| Yes | 0.89 | 0.48 | 1.86 | 2.42 (0.99-6.56) | 0.06 |
| DII | 0.09 | 0.02 | 3.68 | 1.09 (1.04-1.14) | < 0.001 |
| GFR | |||||
| < 90 | Reference | ||||
| ≥ 90 | -0.34 | 0.17 | -2.02 | 0.71 (0.51-0.99) | 0.04 |
| TG | |||||
| < 1.7 mmol/L | Reference | ||||
| ≥ 1.7 mmol/L | -0.48 | 0.16 | -3.01 | 0.62 (0.45-0.85) | 0.003 |
In patients with T2DM undergoing intensive insulin treatment, the risk of hypoglycemia significantly differed for every
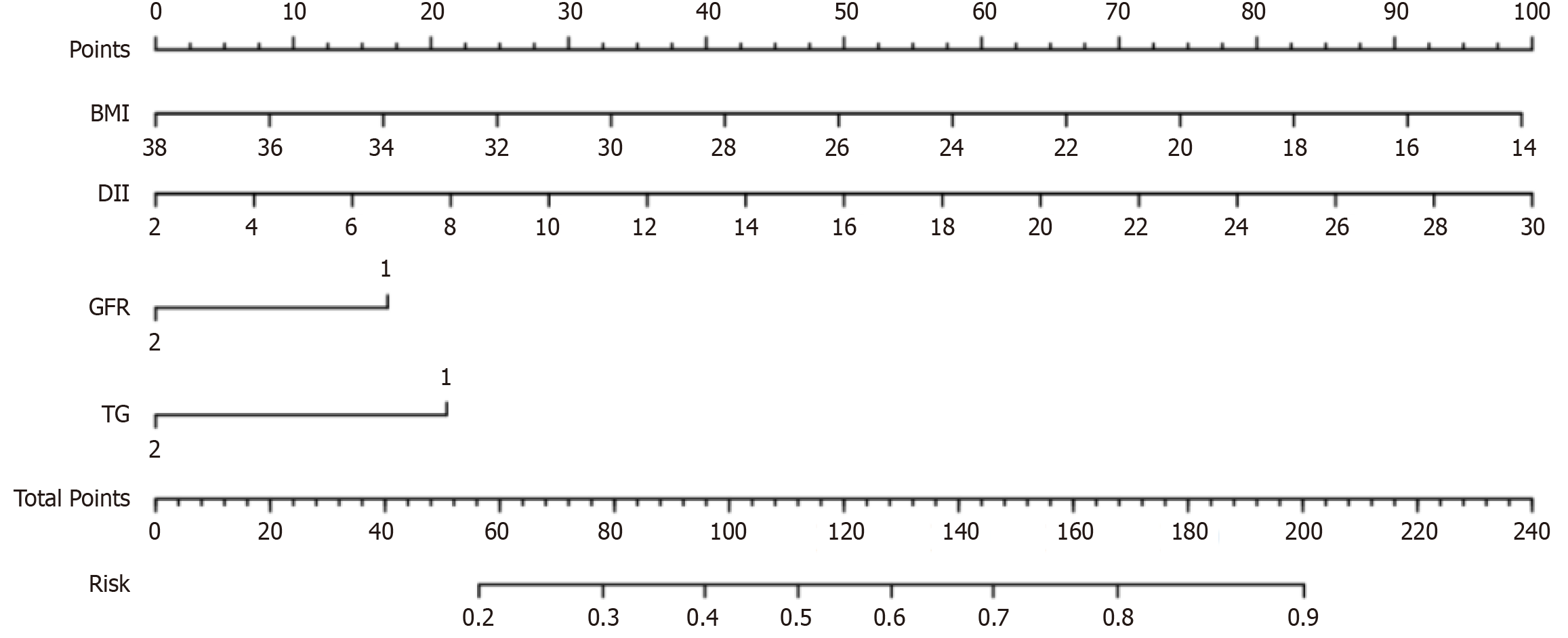
The multivariate logistic regression equation was as follows: Logit (P)= 1.89 - 0.10 × BMI + 0.09×DII - 0.42 × GFR -0.53 × TG. The nomogram model is presented in Figure 1.
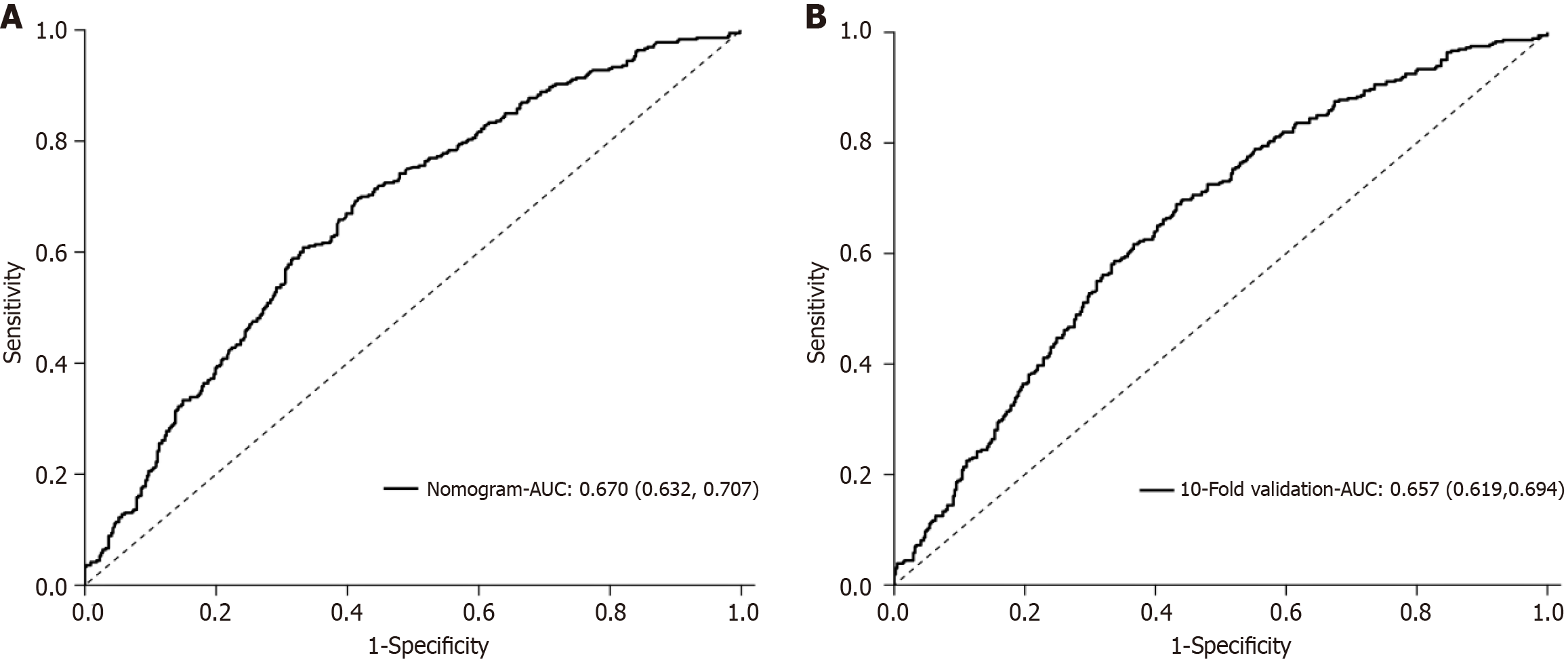
The Hosmer-Lemeshow test was used to calibrate the logistic regression model. The hypoglycemia risk prediction model displayed a reasonable degree of fit (χ2 = 12.54, P = 0.14). Based on ROC curve analysis, the model exhibited good discriminative ability with an AUC of 0.67 (95%CI: 0.63-0.71; P < 0.001; Figure 2). The risk prediction model based on six independent variables was evaluated by 10-fold cross-validation. The AUC for the cross-verified model was 0.66, thereby confirming its good discriminative ability (Figure 2).
The present study found that the incidence of hypoglycemia in hospitalized patients with T2DM treated with insulin was very high. The present study also developed and validated a hypoglycemic risk prediction model, which has six predictors including BMI, duration of diabetes, history of hypoglycemia within 1 year, glomerular filtration rate, blood triglyceride levels, and duration of treatment. The model displayed high reliability and discrimination ability and is a promising tool for clinicians to screen hospitalized patients with T2DM with an elevated risk of hypoglycemia and guide personalized interventions to prevent and treat hypoglycemia. We have compared our results with other studies. In our study, the incidence of hypoglycemia in hospitalized patients with T2DM treated with insulin was 44.9%, which significantly exceeded the rates of 10.3%-25% reported by Shah et al[16]. However, our data were comparable with the hypoglycemia incidence rate of 46.5% reported in the global HAT study[11]. The differences in incidence of hypoglycemia may be related to the different definitions of hypoglycemia used in each study and the level of glycemic control[15].
In this study, the median (interquartile range) duration of treatment for patients with hypoglycemia was 6.5 (4-9) days. This likely corresponded to a gradual recovery of islet function after approximately 1 week of intensive insulin treatment[17]. The frequency of hypoglycemia was highest before lunch (26.1%), followed by after breakfast (16.3%) and before breakfast (14.8%). Pazos-Couselo et al[18] also reported that hypoglycemia occurred more commonly among patients with diabetes before lunch (32.0%). This might be related to the high carbohydrate content of the Chinese breakfast. The present study also found that hypoglycemia was recorded once in 46.1% of the patients and two or more times in 53.9% of the patients in the hypoglycemic group. This demonstrated that the probability of recurrent hypoglycemia was high, which could further aggravate patients’ medical conditions and reduce the likelihood of recovery. The findings of the present study suggest that it is essential to assess the dynamic risk of hypoglycemia.
The present study found that the risk of hypoglycemia increased as the treatment duration of insulin therapy increased. Moreover, the treatment duration was an independent risk factor for hypoglycemia in hospitalized patients with insulin-treated T2DM. Jones et al[19] reported that hypoglycemia increased with the length of hospitalization in patients with T2DM. We postulated that patients with T2DM achieved partial remission of islet function in the middle and later stages of intensive insulin therapy. Still, the risk of hypoglycemia increased if the treatment plan was not adjusted over time. This suggested that the hypoglycemia evaluation results at admission should not be used to distinguish patients into groups with high or low hypoglycemia risks during the overall hospitalization period. The present study also found that BMI, biomarkers including GFR and TG, were predictors of hypoglycemia in hospitalized patients with T2DM treated with insulin. The risk of hypoglycemia significantly increased when the patients had a smaller BMI, with GFR < 90, and TG < 1.7 mmol/L. The findings of the present study suggest that healthcare providers should pay more attention to the blood glucose in these T2DM patients with an increased risk for hypoglycemia and modify the dosage of insulin timely.
In the present study, we used logistic regression analyses to build a hypoglycemia risk prediction model for hospitalized patients with T2DM treated with insulin, which has six independent hypoglycemia risk factors, including BMI, disease duration, history of hypoglycemia within 1 year, duration of treatment, GFR, and TG. The prediction accuracy of the hypoglycemia risk prediction model was good, as indicated by the Hosmer-Lemeshow goodness-of-fit test results. Moreover, the AUC for the model and the AUC for the 10-fold cross-validation model suggested that the prediction performance of the model was good and stable. Using 0.43 as the optimal cutoff for ROC curve analyses, the sensitivity, specificity, and Youden index were 70%, 58%, and 0.28, respectively.
We have compared our model with others. Shah et al[16] also developed a hypoglycemia prediction tool for hospitalized patients with diabetes by including age, emergency department visit because of hypoglycemia in the previous 6 months, use of insulin, use of oral drugs that do not cause hypoglycemia, and severe chronic kidney disease. They reported an AUC of 0.64, sensitivity of 65%, and specificity of 69%. Ena et al[20] validated a predictive model that included estimated GFR, daily insulin dose, length of hospitalization, and hypoglycemia episodes in the previous 3 months and reported an AUC of 0.72, sensitivity of 40.2%, and specificity of 87.2%. Stuart et al[21] developed a hypoglycemia prediction tool for hospitalized patients with diabetes by including factors such as age > 75 years, insulin and sulfonylurea treatment, Black and Asian ethnicity, emergency admission, low estimated GFR, high C-reactive protein levels, hyponatremia, and hypoalbuminemia and reported an AUC of 0.73, sensitivity of 59.3%, and specificity of 73.7%. The differences in the models may be related to the different subjects and variables included, as well as the different statistical models used.
There are some limitations in this study. First, the study cohort came from a single hospital, and all data were extracted retrospectively from the electronic medical record system. Second, because the present model has not yet undergone external validation, its clinical utility remains uncertain. Future studies could use a multi-center prospective design to modify this predictive model, confirm its generalizability, and inform safe clinical implementation.
Although the present study has some limitations, we used a logistic model to transform the regression equation into a simple visual graph to simplify the results of the prediction model[22], making it convenient for clinicians to obtain quantitative scores and predict the risk of hypoglycemia in patients. The nomogram model can be used for individualized risk prediction. The hypoglycemia risk can be estimated in each patient with T2DM using the nomogram model score to identify those at higher risk of hypoglycemia.
We developed a hypoglycemia risk prediction model for hospitalized patients with T2DM treated with insulin using independent risk factors, namely BMI, duration of diabetes, history of hypoglycemia within 1 year, GFR, TG, and duration of treatment. The model exhibited good reliability and clinical performance. Our data illustrated that this model is a promising tool for clinicians to screen hospitalized patients with T2DM with an elevated risk of hypoglycemia and guide prevention and treatment.
| 1. | American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S20-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 1018] [Article Influence: 509.0] [Reference Citation Analysis (2)] |
| 2. | Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G; 2010 China Noncommunicable Disease Surveillance Group. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1961] [Cited by in RCA: 2196] [Article Influence: 168.9] [Reference Citation Analysis (0)] |
| 3. | GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5495] [Cited by in RCA: 5345] [Article Influence: 485.9] [Reference Citation Analysis (0)] |
| 4. | Korytkowski MT, Muniyappa R, Antinori-Lent K, Donihi AC, Drincic AT, Hirsch IB, Luger A, McDonnell ME, Murad MH, Nielsen C, Pegg C, Rushakoff RJ, Santesso N, Umpierrez GE. Management of Hyperglycemia in Hospitalized Adult Patients in Non-Critical Care Settings: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2022;107:2101-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 5. | Ruan Y, Tan GD, Lumb A, Rea RD. Importance of inpatient hypoglycaemia: impact, prediction and prevention. Diabet Med. 2019;36:434-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/l (54 mg/dl) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2017;60:3-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 2015;38:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 8. | McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35:1897-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 417] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 9. | Karter AJ, Warton EM, Lipska KJ, Ralston JD, Moffet HH, Jackson GG, Huang ES, Miller DR. Development and Validation of a Tool to Identify Patients With Type 2 Diabetes at High Risk of Hypoglycemia-Related Emergency Department or Hospital Use. JAMA Intern Med. 2017;177:1461-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Stahn A, Pistrosch F, Ganz X, Teige M, Koehler C, Bornstein S, Hanefeld M. Relationship between hypoglycemic episodes and ventricular arrhythmias in patients with type 2 diabetes and cardiovascular diseases: silent hypoglycemias and silent arrhythmias. Diabetes Care. 2014;37:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Khunti K, Alsifri S, Aronson R, Cigrovski Berković M, Enters-Weijnen C, Forsén T, Galstyan G, Geelhoed-Duijvestijn P, Goldfracht M, Gydesen H, Kapur R, Lalic N, Ludvik B, Moberg E, Pedersen-Bjergaard U, Ramachandran A; HAT Investigator Group. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18:907-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | Ulmer BJ, Kara A, Mariash CN. Temporal occurrences and recurrence patterns of hypoglycemia during hospitalization. Endocr Pract. 2015;21:501-507. [PubMed] [DOI] [Full Text] |
| 13. | Lin L, Zhan LC, Zhang ST, Li YJ, Zhang XJ, Wang LY, Lai WH. [Active monitoring and analysis of hypoglycemic adverse events in hospitalized diabetic patients]. Chongqing Yixue. 2025;1-15. |
| 14. | Yu XH, Zhang XQ, Yang SJ, Wang ZW, Ding YN. [The risk prediction models for the occurrence of hypoglycemia in patients with diabetes mellitus: a systematic review and critical appraisal]. Zhonghua Huli Zazhi. 2022;57:1830-1839. |
| 15. | Cruz P. Inpatient Hypoglycemia: The Challenge Remains. J Diabetes Sci Technol. 2020;14:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Shah BR, Walji S, Kiss A, James JE, Lowe JM. Derivation and Validation of a Risk-Prediction Tool for Hypoglycemia in Hospitalized Adults With Diabetes: The Hypoglycemia During Hospitalization (HyDHo) Score. Can J Diabetes. 2019;43:278-282.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, Hu Y, Zhou Z, Yan X, Tian H, Ran X, Luo Z, Xian J, Yan L, Li F, Zeng L, Chen Y, Yang L, Yan S, Liu J, Li M, Fu Z, Cheng H. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 594] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 18. | Pazos-Couselo M, García-López JM, González-Rodríguez M, Gude F, Mayán-Santos JM, Rodríguez-Segade S, Rodríguez-García J, Casanueva F. High incidence of hypoglycemia in stable insulin-treated type 2 diabetes mellitus: continuous glucose monitoring vs. self-monitored blood glucose. Observational prospective study. Can J Diabetes. 2015;39:428-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Jones GC, Timmons JG, Cunningham SG, Cleland SJ, Sainsbury CAR. Hypoglycemia and Clinical Outcomes in Hospitalized Patients With Diabetes: Does Association With Adverse Outcomes Remain When Number of Glucose Tests Performed Is Accounted For? J Diabetes Sci Technol. 2017;11:720-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Ena J, Gaviria AZ, Romero-Sánchez M, Carretero-Gómez J, Carrasco-Sánchez FJ, Segura-Heras JV, Porto-Perez AB, Vázquez-Rodriguez P, González-Becerra C, Gómez-Huelgas R; Diabetes and Obesity Working Group of the Spanish Society of Internal Medicine. Derivation and validation model for hospital hypoglycemia. Eur J Intern Med. 2018;47:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Stuart K, Adderley NJ, Marshall T, Rayman G, Sitch A, Manley S, Ghosh S, Toulis KA, Nirantharakumar K. Predicting inpatient hypoglycaemia in hospitalized patients with diabetes: a retrospective analysis of 9584 admissions with diabetes. Diabet Med. 2017;34:1385-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Deng X, Hou H, Wang X, Li Q, Li X, Yang Z, Wu H. Development and validation of a nomogram to better predict hypertension based on a 10-year retrospective cohort study in China. Elife. 2021;10:e66419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |