Published online Aug 15, 2025. doi: 10.4239/wjd.v16.i8.105532
Revised: April 9, 2025
Accepted: July 4, 2025
Published online: August 15, 2025
Processing time: 177 Days and 3 Hours
Diabetic foot ulcers (DFUs) in patients with type 2 diabetes (T2D) are associated with heightened risks of infection and amputation and thus require effective surgical interventions to enhance outcomes. Free anterolateral thigh (ALT) per
To identify key factors affecting intraoperative blood perfusion during free ALT perforator flap repair in patients with T2D and DFUs, thereby providing insights to improve surgical outcomes.
This retrospective case-control study included 100 patients with T2D who un
Old age, high body mass index, long diabetes duration, and presence of diabetic peripheral neuropathy were associated with impaired perfusion. Abnormal perfusion was correlated with poor ankle-brachial index and elevated glycated hemoglobin (HbA1c), creatinine, triglycerides, and partial pressure of carbon dioxide. Conversely, high hemoglobin, albumin, and prealbumin levels and partial pressure of oxygen (PaO2) were protective. Multivariate analysis identified diabetes duration, HbA1c, PaCO2, PaO2, and albumin as independent predictors of perfusion, underscoring the roles of metabolic control and vascular health.
Optimizing metabolic control, vascular health, and nutritional status was crucial to enhance intraoperative blood perfusion in diabetic patients undergoing ALT perforator flap repair for DFUs.
Core Tip: This study investigates the key factors influencing intraoperative blood perfusion in patients with type 2 diabetes undergoing free anterolateral thigh perforator flap repair for diabetic foot ulcers. We identified that prolonged diabetes duration, elevated glycated hemoglobin, and poor nutritional status significantly impair perfusion. Conversely, higher hemoglobin, albumin, and prealbumin levels were protective. These findings underscore the importance of integrated diabetes management strategies, including metabolic control and nutritional support, to optimize surgical outcomes. Prospective studies are needed to validate these results and refine perioperative care protocols for improved patient outcomes.
- Citation: Wu HL, Huang H, Chen BQ, Xia J, Jiang LB. Intraoperative blood perfusion factors in free anterolateral thigh flap repair for diabetic foot ulcers: A retrospective analysis. World J Diabetes 2025; 16(8): 105532
- URL: https://www.wjgnet.com/1948-9358/full/v16/i8/105532.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i8.105532
Diabetic foot ulcers (DFUs) represent a formidable challenge in the management of patients with type 2 diabetes (T2D) because of their prevalence and serious complications if not adequately treated. These ulcers significantly increase the risk of infection, lower extremity amputation, and, consequently, morbidity and mortality in individuals with diabetes[1,2]. The lifetime risk of a patient with diabetes developing a foot ulcer reaches as high as 19% to 34%[3]. Patients with T2D are particularly vulnerable due to associated peripheral neuropathy, peripheral arterial disease, and impaired wound healing[4].
The surgical management of DFUs aims to promote wound healing, reduce the risk of infection, and ultimately preserve limb function and quality of life. Free flap transfer is an integral part of reconstructive surgery that provides sufficient tissue coverage and enhanced blood supply, which are critical to healing in ischemic and neuropathic tissues[5,6]. The anterolateral thigh (ALT) perforator flap is increasingly recognized for its versatility and reliability in re
Despite the proven utility of the ALT flap, successful application in diabetic patients, particularly in the context of DFUs, relies heavily on ensuring optimal intraoperative blood perfusion. Diabetic vasculopathy, characterized by endothelial dysfunction, arterial stenosis, and microvascular disease, poses significant challenges in this regard. These vascular impairments can compromise flap perfusion, increase the risk of partial or total flap loss, and delay or complicate wound healing[9,10]. Consequently, understanding factors that influence intraoperative perfusion is crucial to improve surgical outcomes.
Several studies have highlighted potential factors affecting flap perfusion, including patient-specific factors[11,12], such as severity and duration of diabetes, tobacco use, and comorbidities like hypertension. Surgical factors, including the choice of recipient vessels, anastomosis technique, and intraoperative fluid management, also play critical roles. Additionally, the use of intraoperative monitoring techniques, such as laser Doppler flowmetry or indocyanine green angiography, has been investigated for their potential to provide real-time data on perfusion status and guide surgical decision-making[13,14].
A comprehensive understanding of these factors, especially within the specific context of ALT perforator flap repair in DFUs, remains underexplored. The interplay between microvascular diseases due to diabetes and the specific surgical approach, particularly the selection and handling of perforator vessels during flap harvest, requires careful analysis to optimizing outcomes. Filling this gap could have profound implications for clinical practice. By identifying key factors influencing intraoperative perfusion, surgeons can better tailor their approaches to individual patients, potentially reducing the risk of flap failure and improving overall surgical outcomes. Future studies should evaluate the relative impact of these factors to inform surgical practices and enhance patient outcomes.
In this study, we seek to fill this gap by systematically analyzing factors that influence intraoperative blood perfusion in free ALT perforator flap repair of DFUs in patients with T2D.
This retrospective case-control study included patients with T2D who underwent repair surgery using a free ALT perforator flap at our hospital between June 2016 and June 2024 to treat DFUs. A stratified sampling method was employed to select 50 patients with normal blood perfusion during surgery and another 50 with abnormal perfusion. To ensure the groups were appropriately stratified, we matched patients based on age, ensuring similar age distributions between the two groups to avoid confounding factors related to age; gender, maintaining consistent gender ratios to minimize gender-related physiological differences; duration of diabetes, ensuring similar diabetes durations to account for microvascular and macrovascular complications; patency of major lower extremity arteries, ensuring similar artery patency to minimize perfusion differences due to vascular occlusion; and Wagner grade, ensuring similar ulcer severity to avoid perfusion differences due to varying wound conditions. This retrospective study utilized de-identified patient data and has no potential risk of harm or impact on the medical care of the patients. As a result, informed consent was waived. This waiver and the study itself received approval from the institutional review board and the ethics committee of the First Hospital of Nanchang (No. IIT2024018), adhering to regulatory and ethical guidelines for retrospective studies.
Inclusion criteria: Patients aged between 18 and 75 years old; individuals with DFUs necessitating repair using a free ALT perforator flap under general anesthesia; preoperative lower extremity major artery computed tomography angiography findings demonstrating at least one patent artery among the anterior tibial, posterior tibial, and peroneal arteries; and Wagner grade III or IV DFUs.
Exclusion criteria: Those who experienced chest pain or tightness; routine preoperative echocardiogram indicating left ventricular outflow tract obstruction or severe organic heart disease; those with severe arrhythmia; those suffering from uremia; those with systolic blood pressure ≥ 160 mmHg (1 mmHg = 0.133 kPa) and/or diastolic blood pressure ≥ 100 mmHg; and those with an iodine allergy.
Sources of main reagents and instruments: Pentolinium hydrochloride was procured from Jinzhou Aohong Pharmaceutical Co., Ltd. Midazolam, sufentanil, and remifentanil were sourced from Yichang Renfu Pharmaceutical Co., Ltd. Cisatracurium besylate injection was obtained from Jiangsu Hengrui Medicine Co., Ltd. Etomidate and propofol were supplied by Jiangsu Enhua Pharmaceutical Co., Ltd. Dobutamine hydrochloride injection was acquired from Shandong Fangming Pharmaceutical Co., Ltd. Indocyanine green was sourced from Dandong Yichuang Pharmaceutical Co., Ltd.
The MI-4 infrared imaging instrument was purchased from Jinan Xianwei Intelligent Technology Co., Ltd. The anesthesia machine was acquired from Shenzhen Mindray Biomedical Electronics Co., Ltd. The BeneVision N15 electrocardiograph monitor was sourced from Shenzhen Mindray Bio-Medical Electronics Co., Ltd. The 3M Bair Hugger 775 warming unit was supplied by 3M, the United States. The DNP-9272A liquid warming cabinet was obtained from Shanghai Hongdu Electronic Technology Co., Ltd. The ABL90 flex blood gas analyzer was procured from Radiometer Medical APS (Shanghai) Co., Ltd. The micro-infusion pump was acquired from Shenzhen Mindray Biomedical Technology Co., Ltd.
Anesthesia management protocol: All patients received standardized endotracheal intubation general anesthesia administered by an experienced anesthesia team. Thirty minutes before anesthesia induction, patients were given pentolinium hydrochloride (0.01 mg/kg) intramuscularly to inhibit glandular secretion. Upon entering the operating room, electrocardiogram monitoring and pulse oximetry were initiated, and radial artery puncture was performed under local anesthesia to monitor mean arterial pressure (MAP). Baseline blood pressure was established using the MAP recorded prior to anesthesia induction.
Anesthesia induction involved intravenous administration of midazolam (0.1 mg/kg), sufentanil (0.3-0.4 μg/kg), cisatracurium besylate (0.15 mg/kg), and etomidate (0.15-0.30 mg/kg). Following mask oxygenation, endotracheal intubation was performed, and mechanical ventilation was initiated with a tidal volume of 6-8 mL/kg, inspiratory to expiratory ratio of 1.0:1.5 to 1.0:2.0, and positive end-expiratory pressure at 3-6 cmH2O. The inspired oxygen concentration was maintained between 50%-60%, and respiratory rate was adjusted to keep end-tidal CO2 pressure within 35-45 mmHg. Body temperature was monitored using a nasopharyngeal probe, and warming devices were used to maintain oropharyngeal temperature between 36.0-37.0 °C. Bispectral index (BIS) was maintained at 40-60.
During surgery, normal saline and hydroxyethyl starch 130/0.4 were used to maintain central venous pressure between 8-12 cmH2O. Electrolyte and acid-base balance adjustments were made in real-time based on blood gas analysis results. Blood glucose levels were strictly controlled between 5.5-10.0 mmol/L, and hemoglobin (Hb) levels were maintained above 70 g/L. No vasoactive drugs were used prior to vascular anastomosis.
ALT flap transplantation scheme and assessment of flap perfusion: Flap transplantation surgery was conducted by a consistent team of experienced and well-trained surgeons. The donor artery, which is the lateral circumflex femoral artery, was selected as the pedicle artery for the flap, while the recipient artery was either the anterior tibial artery or the posterior tibial artery, depending on the case. End-to-side anastomosis was utilized. The accompanying veins from both arteries were also connected using an end-to-end anastomosis to the corresponding recipient veins to ensure that the distal blood supply to the foot remained uncompromised.
Following vascular anastomosis completion, the flap’s blood circulation was observed for 10 minutes to rule out vasospasm and anastomotic occlusion. Indocyanine green dye (2.5 mg/mL, 3 mL) was then injected intravenously, and an infrared imaging device was immediately used to monitor flap perfusion. Data were analyzed using the device's integrated software. Different colorations within the flap areas indicated variations in perfusion, distinguishing high-perfusion (red) from low-perfusion (blue) areas. A transparent grid paper was used to cover the flap surface, marking and measuring the total flap area and the maximum high- and low-perfusion areas, to calculate their respective proportions.
At 30 minutes after the initial contrast injection, dobutamine was intravenously infused via a central venous pump, starting at a dose of 3 μg/kg/minute and increased by 1 μg/kg/minute every 30 seconds until the MAP reached the target level (6-10 mmHg above MAP0). The same dose of indocyanine green was re-injected to reassess flap perfusion. Dobutamine infusion rates were adjusted as necessary to maintain MAP near pre-anesthesia levels until surgery completion. The endotracheal tube was removed once the patient regained consciousness and normal spontaneous respiration. Heart rate and MAP were reassessed 10 minutes later.
Dobutamine infusion was immediately halted if any of the following conditions occurred: (1) Heart rate reached the maximum level [defined as 2 × (20 - age) × 85%]; (2) The dobutamine dose reached the maximum of 15 μg/kg/minute; (3) Systolic blood pressure dropped more than 20 mmHg below the baseline; (4) Electrocardiogram showed an ST segment elevation or depression exceeding 2 mm; and (5) Any form of arrhythmia occurred (≥ 5 times/minute). This protocol was designed to balance optimal flap perfusion with patient safety.
Grouping criteria: Patients were categorized into two groups based on intraoperative blood perfusion status[15]: The normal perfusion group (n = 50) and the abnormal perfusion group (n = 50). The normal perfusion group comprised patients whose high perfusion area (indicated by red) covered at least 70% of the total area, as monitored by an infrared imaging device following indocyanine green injection. A high perfusion zone covering ≥ 70% of the total area was used as the criterion for the normal perfusion group. This cutoff value was determined using advanced computational methods to optimize classification accuracy based on clinical outcomes. These patients also demonstrated good blood circulation immediately after vascular anastomosis, with no evidence of vasospasm or anastomotic occlusion. The abnormal perfusion group included patients whose high perfusion area was less than 70% of the total and had significant low perfusion areas (indicated by blue), as detected using the same method. The final grouping was determined by the optimized blood perfusion assessment rather than the initial evaluation. Patients who did not achieve the ≥ 70% high perfusion area after dobutamine intervention were classified into the abnormal perfusion group. This group might show mild to moderate vasospasm or signs of partial anastomotic occlusion after vascular anastomosis.
Before 8 am, the researchers collected 5 mL of fasting venous blood from each patient for hematological and metabolic assessments. For the hematological analysis, a DxH800 blood analyzer (Beckman Coulter, Inc., Brea, CA, United States) was employed to measure counts for red blood cells (RBC), white blood cells (WBC), neutrophils (NEUT), lymphocytes (LYM), eosinophils (EOS), basophils (BASO), Hb, platelets (PLT), and monocytes (MON).
Glycated Hb (HbA1c) levels were determined using high-performance liquid chromatography on a Variant II Turbo system (Bio-Rad Laboratories, Hercules, CA, United States) to assess metabolic control. Blood glucose levels were measured using hexokinase enzymatic method on a Synchron LX20 automated biochemical analyzer (Beckman Coulter, Inc., Brea, CA, United States).
After completing hematological and HbA1c tests, the blood samples were centrifuged at 3000 rpm for 5 minutes. The supernatant was then collected for further analysis. The Synchron LX20 analyzer was used to measure concentrations of total bilirubin, albumin, creatinine, prealbumin, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides by using dedicated reagent kits compatible with the equipment.
About 2 mL of arterial blood was taken from patients as needed to measure blood gas indicators, including pH, partial pressure of oxygen (PaO2), and the partial pressure of carbon dioxide (PaCO2), by using a ABL90 flex blood gas analyzer (Radiometer Medical APS, Shanghai, China).
Measurement data were presented as mean ± SD or median with interquartile range, depending on whether the data conform to a normal distribution. Categorical data were expressed as frequency and percentage. Unpaired t-tests were used to compare continuous variables between the two groups. Univariate and multivariate logistic regression analyses were conducted to calculate the odds ratio (OR) and 95%CI for each parameter considered as a continuous variable. Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS 19 software (SPSS Inc., Chicago, IL, United States) and the R software package version 3.0.2 (Free Software Foundation, Inc., Boston, MA, United States).
Patients with abnormal blood perfusion were notably older, with a mean age of 55.29 ± 8.12 years compared with 50.47 ± 8.64 years old in the normal perfusion group (t = 2.88, P < 0.01; Table 1). Body mass index (BMI) was higher in patients with abnormal perfusion, with an average of 25.96 ± 4.17 kg/m² vs 23.44 ± 3.82 kg/m² in the normal perfusion group (t = 3.15, P < 0.01). The duration of diabetes was another significant factor, as patients with abnormal perfusion had been living with diabetes for a longer period (12.91 ± 4.09 years) than those with normal perfusion (10.18 ± 3.88 years, t = 3.43, P < 0.01). Notably, diabetic peripheral neuropathy (DPN) was more prevalent in the abnormal blood perfusion group (50.00%) compared with the normal group (26.00%), presenting a significant difference (χ2 = 6.11, P = 0.01). Other factors such as gender, educational level, residential status, marital status, smoking and drinking history, hypertension, cardiovascular disease, diabetic retinopathy, diabetic nephropathy, walking impairment, the results of wound specimen bacterial culture, Wagner grade, and foot ulcer size did not show statistically significant differences between the two groups. These findings suggest that age, BMI, duration of diabetes, and presence of DPN were important influencing factors for intraoperative blood perfusion in the surgical treatment of DFUs in patients with T2D.
| Parameters | Normal blood perfusion (n = 50) | Abnormal blood perfusion (n = 50) | t/χ2 | P value |
| Age (years) | 50.47 ± 8.64 | 55.29 ± 8.12 | 2.88 | < 0.01 |
| BMI (kg/m²) | 23.44 ± 3.82 | 25.96 ± 4.17 | 3.15 | < 0.01 |
| Education level (years) | 9.39 ± 3.67 | 8.62 ± 3.84 | 1.02 | 0.31 |
| Gender | 0.05 | 0.83 | ||
| Male | 33 (66.00) | 34 (68.00) | ||
| Female | 17 (34.00) | 16 (32.00) | ||
| Employment, work for pay | 22 (44.00) | 20 (40.00) | 0.16 | 0.69 |
| Residential status | 0.18 | 0.67 | ||
| Rural | 15 (30.00) | 17 (34.00) | ||
| Urban | 35 (70.00) | 33 (66.00) | ||
| Marital status | 0.27 | 0.87 | ||
| Married | 41 (82.00) | 39 (78.00) | ||
| Single | 6 (12.00) | 7 (14.00) | ||
| Divorced | 3 (6.00) | 4 (8.00) | ||
| Smoking history | 16 (32.00) | 18 (36.00) | 0.18 | 0.67 |
| Drinking history | 13 (26.00) | 15 (30.00) | 0.20 | 0.66 |
| Hypertension | 24 (48.00) | 28 (56.00) | 0.64 | 0.42 |
| Cardiovascular disease | 9 (18.00) | 14 (28.00) | 1.41 | 0.24 |
| DR | 16 (32.00) | 20 (40.00) | 0.69 | 0.41 |
| DPN | 13 (26.00) | 25 (50.00) | 6.11 | 0.01 |
| DN | 11 (22.00) | 16 (32.00) | 1.27 | 0.26 |
| Affected toes | 1.07 | 0.30 | ||
| Yes | 16 (32.00) | 21 (42.00) | ||
| No | 34 (68.00) | 29 (58.00) | ||
| Walking impairment | 1.21 | 0.27 | ||
| Yes | 12 (24.00) | 17 (34.00) | ||
| No | 38 (76.00) | 33 (66.00) | ||
| Results of wound specimen bacterial culture | 0.93 | 0.33 | ||
| Positive | 9 (18.00) | 13 (26.00) | ||
| Negative | 41 (82.00) | 37 (74.00) | ||
| Wagner grade | 0.64 | 0.42 | ||
| 3 | 26 (52.00) | 22 (44.00) | ||
| 4 | 24 (48.00) | 28 (56.00) | ||
| Foot ulcer size (cm²) | 12.39 ± 4.67 | 13.48 ± 5.12 | 1.11 | 0.27 |
| Duration of diabetes (years) | 10.18 ± 3.88 | 12.91 ± 4.09 | 3.43 | < 0.01 |
The ankle-brachial index (ABI), a measure of vascular health, showed a statistically significant disparity. A higher percentage of patients in the abnormal perfusion group had poor ABI (32.00%) compared with those in the normal perfusion group (10.00%), indicating compromised vascular function among patients with abnormal perfusion (χ² = 7.29, P < 0.01; Table 2). HbA1c levels, which indicate long-term blood glucose control, were significantly higher in the abnormal perfusion group (8.44 ± 1.43) than in the normal perfusion group (7.39 ± 1.62, t = 3.44, P < 0.01), highlighting poor metabolic control. Although MAP was slightly lower in the abnormal group (86.79 ± 11.28 mmHg compared with 89.10 ± 10.43 mmHg) and blood glucose levels were higher (8.45 ± 2.13 mmol/L vs 7.83 ± 2.17 mmol/L), these differences were not statistically significant (P = 0.29 and P = 0.15, respectively). Therefore, poor vascular status as measured by ABI and inadequate metabolic control as indicated by elevated HbA1c levels were associated with abnormal intraoperative blood perfusion in this patient cohort.
| Parameters | Normal blood perfusion (n = 50) | Abnormal blood perfusion (n = 50) | t/χ2 | P value |
| Ankle-brachial index | 7.29 | < 0.01 | ||
| Poor | 5 (10.00) | 16 (32.00) | ||
| Good | 45 (90.00) | 34 (68.00) | ||
| MAP (mmHg) | 89.10 ± 10.43 | 86.79 ± 11.28 | 1.06 | 0.29 |
| Glycated hemoglobin HbA1c (%) | 7.39 ± 1.62 | 8.44 ± 1.43 | 3.44 | < 0.01 |
| Blood glucose (mmol/L) | 7.83 ± 2.17 | 8.45 ± 2.13 | 1.44 | 0.15 |
Patients in the normal perfusion group had a higher mean Hb level (135.83 ± 12.47 g/L) than those in the abnormal perfusion group (128.15 ± 13.12 g/L), and the difference was statistically significant (t = 3.00, P < 0.01; Table 3). Other hematological parameters such as RBC, WBC, NEUT, LYM, EOS, BASO, PLT, and MON did not exhibit statistically significant differences between the two groups. Specifically, RBC levels showed a marginally non-significant trend towards lower values in the abnormal perfusion group (4.76 ± 0.48 vs 4.57 ± 0.51, t = 1.87, P = 0.06). Other parameters such as WBC and PLT had P-values of 0.096 and 0.170, respectively. These findings suggest that lower Hb levels may be associated with abnormal intraoperative blood perfusion in patients with DFUs undergoing this surgical procedure.
| Parameters | Normal blood perfusion (n = 50) | Abnormal blood perfusion (n = 50) | t | P value |
| RBC (1012/L) | 4.76 ± 0.48 | 4.57 ± 0.51 | 1.89 | 0.06 |
| WBC (109/L) | 6.67 ± 1.83 | 7.31 ± 1.96 | 1.68 | 0.10 |
| NEUT (109/L) | 4.16 ± 1.34 | 4.25 ± 1.41 | 0.31 | 0.75 |
| LYM (109/L) | 1.99 ± 0.54 | 1.91 ± 0.57 | 0.70 | 0.49 |
| EOS (109/L) | 0.24 ± 0.11 | 0.23 ± 0.12 | 0.73 | 0.47 |
| BASO (109/L) | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.43 | 0.67 |
| Hb (g/L) | 135.83 ± 12.47 | 128.15 ± 13.12 | 3.00 | < 0.01 |
| PLT (109/L) | 241.68 ± 58.74 | 224.92 ± 62.37 | 1.38 | 0.17 |
| MON (109/L) | 0.53 ± 0.18 | 0.51 ± 0.19 | 0.58 | 0.56 |
To account for potential gender differences, we performed a subgroup analysis based on gender (Table 4). In male patients, lower Hb levels were significantly associated with abnormal intraoperative blood perfusion (P < 0.01). However, in female patients, no significant association was observed (P = 0.16). This suggests that gender may play a role in the relationship between Hb levels and intraoperative blood perfusion during free ALT perforator flap repairs in patients with DFUs.
| Parameters | Normal blood perfusion (n = 50) | Abnormal blood perfusion (n = 50) | t | P value |
| Male | 33 | 34 | ||
| Hemoglobin (g/L) | 143.11 ± 11.25 | 134.74 ± 12.01 | 2.94 | < 0.01 |
| Female | 17 | 16 | ||
| Hemoglobin (g/L) | 122.72 ± 9.87 | 117.67 ± 10.23 | 1.44 | 0.16 |
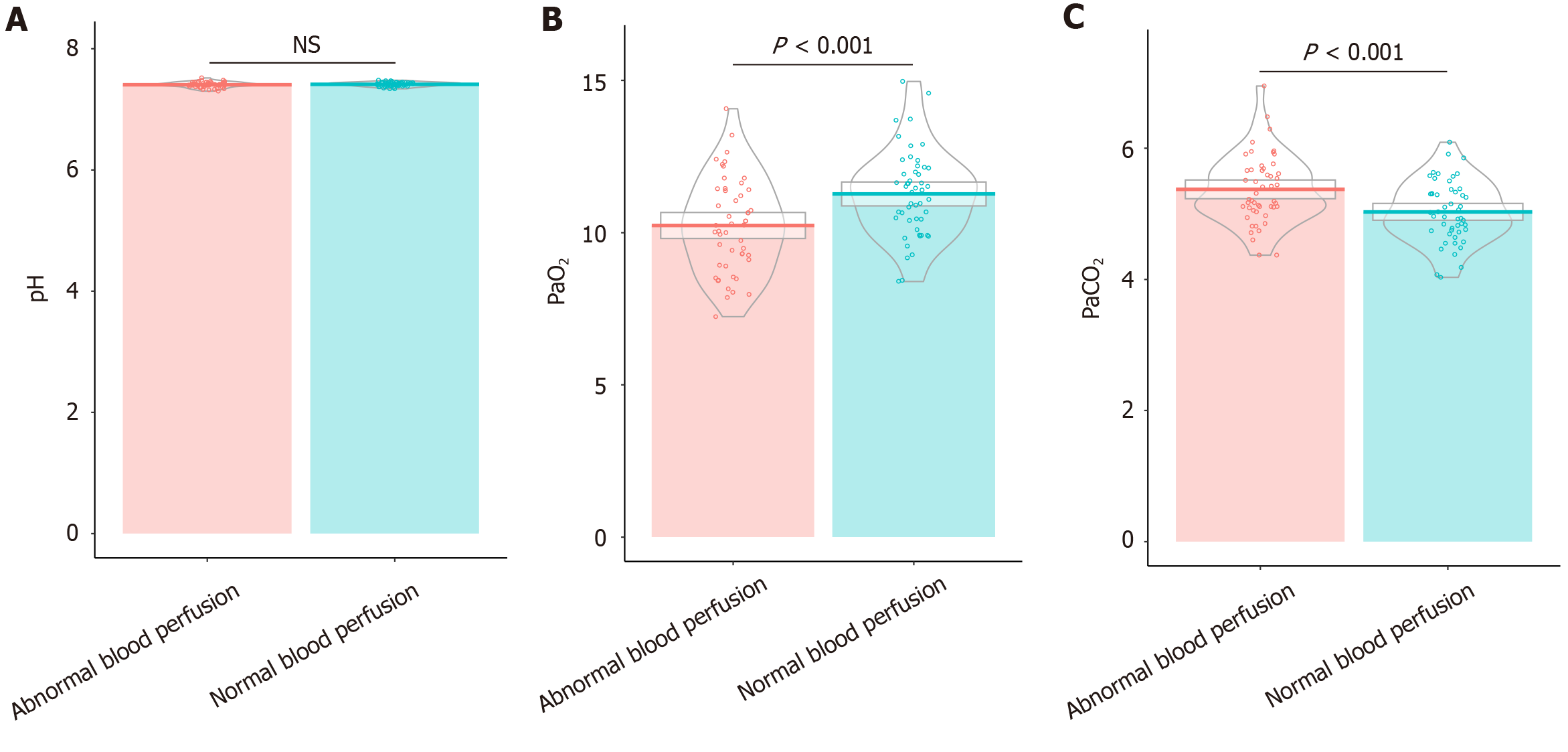
Patients with abnormal blood perfusion exhibited a significantly lower PaO2 (averaging 10.24 ± 1.53 kPa) compared patients in the normal perfusion group (11.27 ± 1.42 kPa; t = 3.50, P < 0.01), indicating reduced oxygenation (Figure 1). Additionally, PaCO2 was higher in the abnormal perfusion group, with an average of 5.37 ± 0.52 kPa vs 5.03 ± 0.47 kPa in the normal perfusion group (t = 3.48, P < 0.01), suggesting impaired carbon dioxide exchange. However, the pH levels, indicative of blood acidity or alkalinity, were similar across both groups (7.41 ± 0.03 in the normal group and 7.41 ± 0.04 in the abnormal group), with no statistically significant difference observed (t = 0.93, P = 0.35). These findings reveal that impaired oxygenation and elevated PaCO2 may be associated with abnormal intraoperative blood perfusion in patients with DFUs undergoing this surgical procedure.
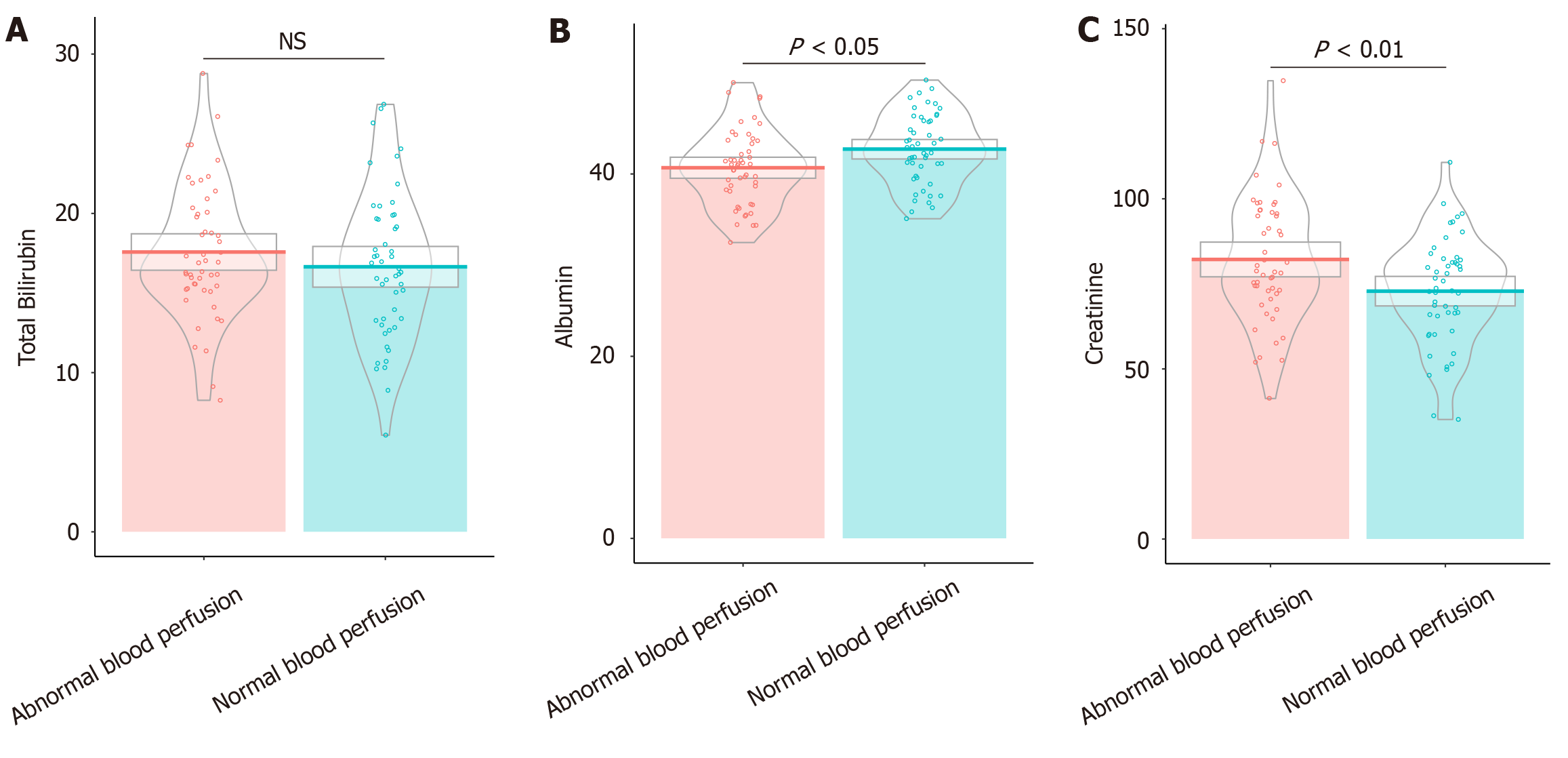
Patients with abnormal blood perfusion displayed lower albumin levels, averaging 40.69 ± 4.12 g/L, compared with 42.73 ± 3.87 g/L in the normal perfusion group (t = 2.55, P = 0.01). This finding suggests a potential compromise in protein synthesis or distribution (Figure 2). Additionally, creatinine levels were significantly elevated in the abnormal perfusion group (82.21 ± 18.42 μmol/L) vs the normal group (72.87 ± 15.64 μmol/L; t = 2.73, P < 0.01), indicating altered renal function that may impact postoperative outcomes. Total bilirubin levels, however, did not differ significantly between the groups (16.65 ± 4.62 μmol/L in the normal group vs 17.57 ± 4.11 μmol/L in the abnormal group; t = 1.06, P = 0.29). These findings highlight that low albumin level and high creatinine levels were associated with impaired blood perfusion in this patient cohort.
Patients with abnormal perfusion had lower prealbumin levels, averaging 227.88 ± 51.47 mg/L, compared to 253.03 ± 45.62 mg/L in the normal perfusion group (t = 2.59, P = 0.01), indicating a potential deficit in nutritional reserves or protein synthesis (Table 5). Triglyceride levels were also higher in the abnormal perfusion group (1.91 ± 0.61 mmol/L) than in the normal group (1.60 ± 0.53 mmol/L, t = 2.77, P < 0.01), suggesting differences in lipid metabolism. No sig
| Parameters | Normal blood perfusion (n = 50) | Abnormal blood perfusion (n = 50) | t | P value |
| Prealbumin (mg/L) | 253.03 ± 45.62 | 227.88 ± 51.47 | 2.59 | 0.01 |
| Total cholesterol (mmol/L) | 4.87 ± 0.89 | 4.66 ± 0.94 | 1.16 | 0.25 |
| LDL (mmol/L) | 2.91 ± 0.74 | 2.82 ± 0.79 | 0.62 | 0.54 |
| HDL (mmol/L) | 1.24 ± 0.32 | 1.21 ± 0.34 | 0.42 | 0.68 |
| Triglycerides (mmol/L) | 1.60 ± 0.53 | 1.91 ± 0.61 | 2.77 | < 0.01 |
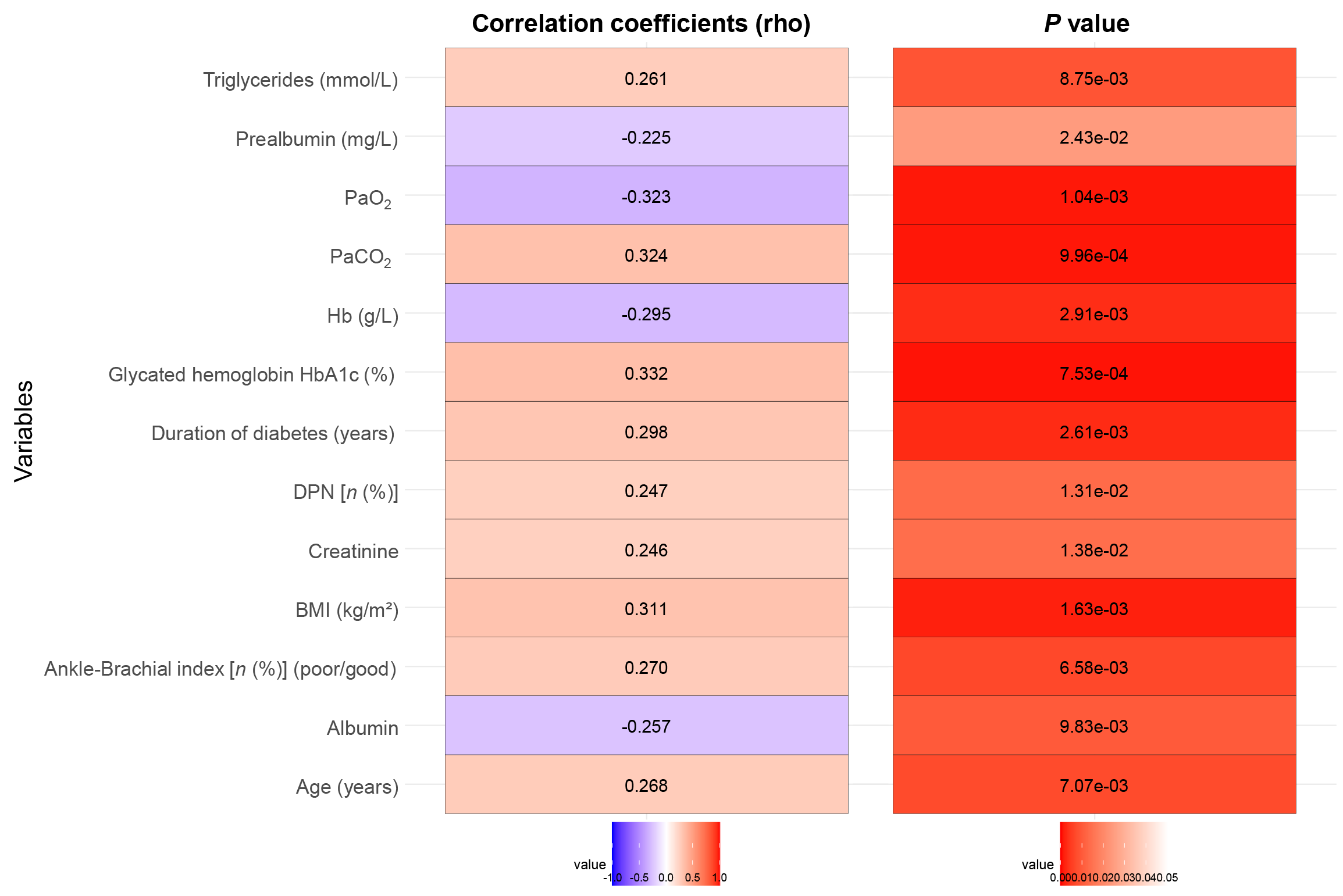
Correlation analysis was conducted to identify several factors significantly associated with intraoperative blood perfusion in patients undergoing free ALT perforator flap repair for DFUs (Figure 3). Age (rho = 0.27, P < 0.01), BMI (rho = 0.31, P < 0.01), DPN presence (rho = 0.25, P = 0.01), and duration of diabetes (rho = 0.30, P < 0.01) were positively correlated with abnormal perfusion. Hence, old age, high BMI, longer diabetes duration, and presence of DPN were associated with impaired perfusion. Poor ABI also showed a positive correlation (rho = 0.27, P < 0.01), indicating compromised vascular function. HbA1c levels (rho = 0.33, P < 0.01) and PaCO2 (rho = 0.32, P < 0.01) were higher in association with abnormal perfusion. Conversely, negative correlations were observed with Hb levels (rho = -0.30, P < 0.01), PaO2 (rho = -0.32, P < 0.01), albumin levels (rho = -0.26, P = 0.01), and prealbumin levels (rho = -0.23, P = 0.02), indicating that low values of these parameters were linked to worse perfusion outcomes. Serum creatinine (rho = 0.25, P = 0.01) and triglycerides (rho = 0.26, P < 0.01) were positively correlated, suggesting that higher levels were associated with poor perfusion. These results highlight critical factors affecting blood perfusion during this surgical procedure, with implications for preoperative assessment and management.
The univariate logistic regression analysis identified several significant factors affecting intraoperative blood perfusion during free ALT perforator flap repair in patients with DFUs (Table 6). Age was associated with increased risk (OR = 1.07; 95%CI: 1.02-1.13; P < 0.01), suggesting that older patients were more likely to experience abnormal perfusion. Higher BMI also elevated this risk (OR = 1.17; 95%CI: 1.06-1.31; P < 0.01). The presence of DPN was particularly influential, nearly doubling the likelihood of poor perfusion (OR = 2.85; 95%CI: 1.25-6.75; P = 0.02). Consistent with these findings, each additional year of diabetes management increased the odds of impaired perfusion (OR = 1.19; 95%CI: 1.07-1.34; P < 0.01), similar to poor ABI (OR = 4.24; 95%CI: 1.50-14.00; P = 0.01). Elevated HbA1c further exacerbated this risk (OR = 1.57; 95%CI: 1.20-2.11; P < 0.01).
| Parameters | Coefficient | Std Error | Wald | P value | OR (95%CI) |
| Age (years) | 0.07 | 0.03 | 2.69 | < 0.01 | 1.07 (1.02-1.13) |
| BMI (kg/m²) | 0.16 | 0.06 | 2.91 | < 0.01 | 1.17 (1.06-1.31) |
| DPN [n (%)] | 1.05 | 0.43 | 2.44 | 0.02 | 2.85 (1.25-6.75) |
| Duration of diabetes (years) | 0.18 | 0.06 | 3.10 | < 0.01 | 1.19 (1.07-1.34) |
| Ankle-brachial index (poor) | 1.44 | 0.56 | 2.58 | 0.01 | 4.24 (1.50-14.00) |
| Glycated hemoglobin HbA1c (%) | 0.45 | 0.14 | 3.15 | < 0.01 | 1.57 (1.20-2.11) |
| Hb (g/L) | -0.05 | 0.02 | -2.80 | < 0.01 | 0.95 (0.92-0.99) |
| PaO2 (kPa) | -0.48 | 0.15 | -3.16 | < 0.01 | 0.61 (0.45-0.82) |
| PaCO2 (kPa) | 1.47 | 0.47 | 3.13 | < 0.01 | 4.34 (1.82-11.59) |
| Albumin (g/L) | -0.13 | 0.05 | -2.43 | 0.02 | 0.88 (0.79-0.97) |
| Creatinine (μmol/L) | 0.03 | 0.01 | 2.56 | 0.01 | 1.03 (1.01-1.06) |
| Prealbumin (mg/L) | -0.01 | < 0.01 | -2.45 | 0.01 | 0.99 (0.98-1.00) |
| Triglycerides (mmol/L) | 0.98 | 0.38 | 2.61 | < 0.01 | 2.68 (1.31-5.82) |
By contrast, blood parameters such as Hb (OR = 0.95; 95%CI: 0.92-0.99; P < 0.01), PaO2 (OR = 0.62; 95%CI: 0.45-0.82; P < 0.01), albumin (OR = 0.88; 95%CI: 0.79-0.97; P = 0.02), and prealbumin (OR = 0.99; 95%CI: 0.98-1.00; P = 0.01) were protective factors against abnormal perfusion. Elevated PaCO2 significantly increased the risk of poor perfusion (OR = 4.34; 95%CI: 1.82-11.59; P < 0.01). Additionally, higher creatinine (OR = 1.03; 95%CI: 1.01-1.06; P = 0.01) and triglycerides (OR = 2.68; 95%CI: 1.31-5.82; P < 0.01) were linked with greater odds of impaired perfusion. The main goal was to identify factors affecting intraoperative blood perfusion. This method evaluated the relationship between multiple predictors and the binary outcome of perfusion status. By calculating ORs and 95%CI, we quantified each factor's impact on abnormal perfusion risk. These findings highlight the importance of metabolic, nutritional, and vascular factors in perfusion outcomes, providing strong evidence for clinical decision-making and improving patient outcomes.
The multivariate logistic regression analysis revealed independent risk factors that significantly influenced intraoperative blood perfusion during free ALT perforator flap repairs in patients with DFUs (Table 7). Higher BMI was significantly associated with decreased perfusion quality (OR = 1.31, 95%CI: 1.05-1.64, P = 0.02), indicating that poor weight control adversely affects perfusion. The duration of diabetes was a notable factor, with a longer duration significantly increasing the likelihood of impaired perfusion (OR = 1.31, 95%CI: 1.06-1.63, P = 0.01). Elevated HbA1c levels were also significantly associated with decreased perfusion quality (OR = 1.37; 95%CI: 1.12-1.67; P < 0.01), indicating that poor glycemic control adversely affects perfusion. Hb levels were a significant protective factor, with higher Hb levels reducing the odds of impaired perfusion (OR = 0.88; 95%CI: 0.81-0.96; P < 0.01). Additionally, a high PaCO2 substantially increased the risk (OR = 2.84; 95%CI: 1.56-5.17; P < 0.01), while lower PaO2 levels were protective against poor perfusion (OR = 0.33; 95%CI: 0.16-0.71; P < 0.01). Albumin levels demonstrated a protective effect, with higher levels associated with better perfusion (OR = 0.91; 95%CI: 0.85-0.98; P = 0.01). A high creatinine levels were significantly associated with increased odds of impaired perfusion (OR = 1.13; 95%CI: 1.04-1.22; P < 0.01). Prealbumin levels were also a significant protective factor, with higher prealbumin levels reducing the odds of impaired perfusion (OR = 0.97; 95%CI: 0.95-0.99; P < 0.01).
| Parameters | Coefficient | Std Error | Wald | P value | OR (95%CI) |
| Age (years) | 0.09 | 0.05 | 1.85 | 0.07 | 1.09 (1.00-1.20) |
| BMI (kg/m²) | 0.27 | 0.11 | 2.39 | 0.02 | 1.31 (1.05-1.64) |
| DPN [n (%)] | 1.80 | 0.98 | 1.84 | 0.07 | 6.03 (0.89-41.06) |
| Duration of diabetes (years) | 0.27 | 0.11 | 2.47 | 0.01 | 1.31 (1.06-1.63) |
| Ankle-brachial index (poor) | 0.36 | 1.20 | 0.30 | 0.77 | 1.43 (0.14-14.85) |
| Glycated hemoglobin HbA1c (%) | 0.31 | 0.10 | 3.07 | < 0.01 | 1.37 (1.12-1.67) |
| Hb (g/L) | -0.13 | 0.04 | -2.97 | < 0.01 | 0.88 (0.81-0.96) |
| PaO2 (kPa) | -1.10 | 0.38 | -2.87 | < 0.01 | 0.33 (0.16-0.71) |
| PaCO2 (kPa) | 1.04 | 0.31 | 3.41 | < 0.01 | 2.84 (1.56-5.17) |
| Albumin (g/L) | -0.10 | 0.04 | -2.59 | 0.01 | 0.91 (0.85-0.98) |
| Creatinine (μmol/L) | 0.12 | 0.04 | 3.08 | < 0.01 | 1.13 (1.04-1.22) |
| Prealbumin (mg/L) | -0.03 | 0.01 | -2.81 | < 0.01 | 0.97 (0.95-0.99) |
| Triglycerides (mmol/L) | 1.14 | 0.82 | 1.39 | 0.17 | 3.11 (0.62-15.53) |
These variables may have shown trends due to the sample size limitations, which could have reduced their statistical power. Other variables including ABI, and triglycerides, did not independently predict perfusion outcomes after controlling for other factors in the multivariate analysis. These findings emphasize the roles of metabolic control and respiratory gas exchange in influencing intraoperative blood perfusion during flap repair surgeries. However, it is important to note that some variables, such as age, and DPN, exhibited trends toward significance (P < 0.10) but did not achieve statistical significance. This may be due to the limited sample size, which could have affected the statistical power of these analyses. Future studies with larger sample sizes are needed to further explore these potential associations.
Our study identified key independent risk factors for intraoperative blood perfusion during free ALT flap repair in T2D patients with DFUs. These factors included high BMI, longer diabetes duration, elevated HbA1c, higher PaCO2, and lower levels of Hb, PaO2, albumin, and prealbumin. We confirmed known vascular health risk factors and highlighted the importance of metabolic control and respiratory gas exchange. Additionally, we found potential protective factors that could improve surgical outcomes by maintaining adequate blood perfusion.
One of the most significant findings was the correlation between long duration of diabetes and poor blood perfusion. Prolonged diabetes leads to microvascular and macrovascular complications through mechanisms, such as endothelial dysfunction and advanced glycation end-product accumulation. These pathophysiological changes result in vascular stiffening and reduced blood flow and impair perfusion during surgery[16-18]. Moreover, chronic hyperglycemia damages small blood vessels and reduces their ability to supply adequate blood to tissues, which could explain the observed perfusion deficits in patients with long diabetes durations[19,20].
Inadequate glycemic control, as indicated by elevated HbA1c levels, is a key factor in compromised perfusion. High HbA1c reflects poor long-term glycemic management, which contributes to vascular complications in diabetic patients. Persistent hyperglycemia leads to oxidative stress and inflammation, which further degrade vascular function. This study supports the hypothesis that glycemic control may enhance surgical outcomes by stabilizing blood vessel integrity and improving microvascular perfusion[21,22]. Based on current guidelines and our findings, aiming for an HbA1c level below 7% before surgery could be beneficial. Achieving this target can reduce the risk of postoperative complications and improve overall surgical outcomes. Preoperative optimization of glycemic control should involve close collaboration with endocrinologists or primary care providers to ensure patients are adequately managed.
The observed relationship between increased BMI and impaired perfusion may be attributed to obesity-related inflammatory processes and metabolic dysregulation. Adipose tissue serves not only as an energy reserve but also as an active endocrine organ that secretes pro-inflammatory cytokines such as tumor necrosis factor-alpha and interleukin-6. These cytokines activate signaling pathways like nuclear factor kappa-light-chain-enhancer of activated B cells, exacerbating endothelial dysfunction and insulin resistance. These inflammatory signals can disrupt vascular homeostasis and lead to poor perfusion. Furthermore, increased BMI is often associated with comorbid conditions such as hypertension, which can compound vascular stiffness and compromise blood flow during flap repair surgeries[23-25].
DPN emerged as another influencing factor because of its effects on autonomic regulation of blood vessel tone. Neuropathy can impair the nerves responsible for vasodilation and vasoconstriction, leading to an inadequate physiological response during surgical procedures that require precise blood flow adjustments[26-28]. The presence of DPN as a predictor of perfusion issues highlights the need for comprehensive preoperative assessment of neurological function, which could enable tailored interventions to stabilize perfusion intraoperatively.
Vascular status, assessed by the ABI, played a critical role. Patients with low ABI values, which indicate poor vascular health, had significant perfusion deficits. ABI below 0.9 indicates severe peripheral artery disease, requiring preoperative collaboration with vascular specialists to optimize treatment and reduce surgical risks. This finding further supports the principle that pre-existing peripheral artery disease, common in diabetic populations, directly impacts the success of surgical interventions reliant on microvascular perfusion. The use of ABI as a preoperative diagnostic tool might thus guide the stratification of patient risk and the customization of perioperative care plans to ensure improved surgical outcomes[29-31].
An intriguing finding is the association of lower Hb, prealbumin, and albumin levels with impaired perfusion. Hb, a key mediator of oxygen delivery capacity, when deficient, may lead to inadequate tissue oxygenation, stressing peripheral wound sites with already limited blood supply due to diabetic vasculopathy. The role of albumin and prealbumin levels might be explained by their involvement in maintaining oncotic pressure and facilitating nutrient delivery to tissues. Low levels of these proteins could exacerbate edema and the sequestration of necessary inputs for tissue repair, thereby hindering perfusion. Elevated PaCO2 might reflect inadequate ventilation or a post-operative respiratory complication potentially affecting tissue oxygenation indirectly. Meanwhile, lower PaO2 could mean insufficient oxygen delivery to already compromised flap tissues. These findings emphasize the importance of perioperative respiratory assessment and stabilization measures to support optimal surgical outcomes and postoperative recovery.
Although univariate logistic regression analysis identified several factors impacting perfusion, multivariate analysis helped determine factors that independently drove these outcomes. Intriguingly, while age showed significance in the univariate model, it did not independently predict perfusion quality in the multivariate assessment. As such, age-related factors might be mediated through indirect pathways, such as the duration of diabetes or comorbidities impacting vascular function or metabolic control.
A possible limitation of our study is the retrospective design, which introduces inherent biases such as selection and information bias. Future prospective studies could reinforce our findings and provide nuanced insights into the dynamic perioperative changes affecting perfusion. Additionally, while our study focused on metabolic and physiological parameters, psychosocial conditions affecting diabetes management and adherence to preoperative instructions could further modulate surgical outcomes. Factors such as patient education, socioeconomic status, psychological stress, and social support can significantly influence treatment plan adherence and overall health outcomes. These aspects should be considered in future research to better understand their impact on surgical success and patient recovery.
Our study identified key risk factors affecting intraoperative blood perfusion during free ALT flap repair in patients with DFUs. Key findings showed that optimizing HbA1c, anemia, and nutritional status significantly improved intraoperative blood perfusion during DFU free ALT flap repairs. These insights suggested that preoperative optimization of metabolic and vascular health could enhance surgical outcomes and overall patient care. By focusing on these specific risk factors, healthcare providers could develop more effective treatment protocols, ultimately improving the success rates of free ALT perforator flap reconstructions in diabetic foot ulcer patients.
| 1. | Rehman ZU, Khan J, Noordin S. Diabetic Foot Ulcers: Contemporary Assessment And Management. J Pak Med Assoc. 2023;73:1480-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 2. | Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic Foot Ulcers: A Review. JAMA. 2023;330:62-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 789] [Article Influence: 263.0] [Reference Citation Analysis (2)] |
| 3. | McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care. 2023;46:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 568] [Article Influence: 189.3] [Reference Citation Analysis (1)] |
| 4. | Jiang P, Li Q, Luo Y, Luo F, Che Q, Lu Z, Yang S, Yang Y, Chen X, Cai Y. Current status and progress in research on dressing management for diabetic foot ulcer. Front Endocrinol (Lausanne). 2023;14:1221705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 5. | Tran R, Haffner ZK, Slamin RP, Akbari CM, Evans KK. Arterialized Vein Bypass Graft Recipient Vessel in Free Tissue Transfer Covering Diabetic Foot Ulcers Complicated by Critical Limb Ischemia. Ann Plast Surg. 2023;90:S570-S573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Bhat S, Chia B, Barry IP, Panayi AC, Orgill DP. Free Tissue Transfer in Diabetic Foot Ulcers: A Systematic Review and Meta-Analysis. Eur J Vasc Endovasc Surg. 2023;66:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Li X, Liu H, Yang C, Xiong A, He X, Tian X, Li Y, Yang R, Yan H. [Application of free anterolateral thigh flap with fascia lata for diabetic foot ulcers with bone exposure]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022;36:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Chen W, Chang SS, Zhou J, Zhang F, Yang CL, Nie KY, Deng CL, Wei ZR. [Clinical effects of antibiotic bone cement combined with free anterolateral thigh flap in sequential treatment of diabetic foot ulcer]. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2023;39:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Kim HJ, Kim WJ, Lee HS, Koh YY, Shin YB, Yeo ED. Clinical utility of skin perfusion pressure measurement in diabetic foot wounds: An observational study. Medicine (Baltimore). 2022;101:e30454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Boonen PT, Buls N, Vandemeulebroucke J, Van Gompel G, Van Den Bergh F, Leiner T, Aerden D, de Mey J. Combined evaluation of blood flow and tissue perfusion in diabetic feet by intra-arterial dynamic 4DCT imaging. Eur Radiol Exp. 2023;7:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Park JY, Jin SJ, Suh HP, Lee SA, Cho C, Yu J, Hwang JH, Hong JP, Kim YK. The role of age in determining the effects of lipo-PGE1 infusion on immediate arterial maximal flow velocity in patients with diabetes undergoing free flap surgery for lower extremity reconstruction: A prospective observational study. J Plast Reconstr Aesthet Surg. 2020;73:885-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Lee ZH, Daar DA, Stranix JT, Anzai L, Levine JP, Saadeh PB, Thanik VD. Free-Flap Reconstruction for Diabetic Lower Extremity Limb Salvage. J Surg Res. 2020;248:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Hu XX, Xing XM, Zhang ZM, Zhang C, Chen L, Huang JZ, Wang X, Ma X, Geng X. Wearable laser Doppler flowmetry for non-invasive assessment of diabetic foot microcirculation: methodological considerations and clinical implications. J Biomed Opt. 2024;29:065001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Hajhosseini B, Chiou GJ, Virk SS, Chandra V, Moshrefi S, Meyer S, Kamperman KJ, Gurtner GC. Hyperbaric Oxygen Therapy in Management of Diabetic Foot Ulcers: Indocyanine Green Angiography May Be Used as a Biomarker to Analyze Perfusion and Predict Response to Treatment. Plast Reconstr Surg. 2021;147:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Alshomer F, Hong JP. Evaluation of Overall Circulation of the Lower Limb. In: Hong JP, Lee BT, Hayashi A, Visconti G, editors. Singapore: Springer, 2024. [DOI] [Full Text] |
| 16. | Parmar UM, Jalgaonkar MP, Kansara AJ, Oza MJ. Emerging links between FOXOs and diabetic complications. Eur J Pharmacol. 2023;960:176089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 17. | Cox ER, Brown WJ, Gajanand T, Bailey TG, Gomersall SR, Chachay VS, Burton NW, Fassett RG, Cox SV, Coombes JS, Keating SE. Effects of fitness and fatness on age-related arterial stiffening in people with type 2 diabetes. Clin Obes. 2022;12:e12519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Meir J, Huang L, Mahmood S, Whiteson H, Cohen S, Aronow WS. The vascular complications of diabetes: a review of their management, pathogenesis, and prevention. Expert Rev Endocrinol Metab. 2024;19:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Li LJ, Kramer M, Tapp RJ, Man RE, Lek N, Cai S, Yap F, Gluckman P, Tan KH, Chong YS, Koh JY, Saw SM, Cheung YB, Wong TY. Gestational diabetes mellitus and retinal microvasculature. BMC Ophthalmol. 2017;17:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Bui HDT, Jing X, Lu R, Chen J, Ngo V, Cui Z, Liu Y, Li C, Ma J. Prevalence of and factors related to microvascular complications in patients with type 2 diabetes mellitus in Tianjin, China: a cross-sectional study. Ann Transl Med. 2019;7:325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Mazarello Paes V, Barrett JK, Taylor-Robinson DC, Chesters H, Charalampopoulos D, Dunger DB, Viner RM, Stephenson TJ. Effect of early glycemic control on HbA1c tracking and development of vascular complications after 5 years of childhood onset type 1 diabetes: Systematic review and meta-analysis. Pediatr Diabetes. 2019;20:494-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Guo K, Zhao Q, Wang M, Lu Y, Wo M, Zhou X, Ying C. The Scope of HbA1c Variability and Risk of Vascular Complications Among Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Prospective Studies. Horm Metab Res. 2022;54:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 23. | Schinzari F, Tesauro M, Campia U, Cardillo C. Increased fractalkine and vascular dysfunction in obesity and in type 2 diabetes. Effects of oral antidiabetic treatment. Vascul Pharmacol. 2020;128-129:106676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Ryder JR, Northrop E, Rudser KD, Kelly AS, Gao Z, Khoury PR, Kimball TR, Dolan LM, Urbina EM. Accelerated Early Vascular Aging Among Adolescents With Obesity and/or Type 2 Diabetes Mellitus. J Am Heart Assoc. 2020;9:e014891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 25. | Meakin PJ, Coull BM, Tuharska Z, McCaffery C, Akoumianakis I, Antoniades C, Brown J, Griffin KJ, Platt F, Ozber CH, Yuldasheva NY, Makava N, Skromna A, Prescott A, McNeilly AD, Siddiqui M, Palmer CN, Khan F, Ashford ML. Elevated circulating amyloid concentrations in obesity and diabetes promote vascular dysfunction. J Clin Invest. 2020;130:4104-4117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Shillo P, Yiangou Y, Donatien P, Greig M, Selvarajah D, Wilkinson ID, Anand P, Tesfaye S. Nerve and Vascular Biomarkers in Skin Biopsies Differentiate Painful From Painless Peripheral Neuropathy in Type 2 Diabetes. Front Pain Res (Lausanne). 2021;2:731658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Ding R, Zhu S, Zhao X, Yue R. Vascular endothelial growth factor levels in diabetic peripheral neuropathy: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1169405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Ando A, Miyamoto M, Saito N, Kotani K, Kamiya H, Ishibashi S, Tavakoli M. Small Fibre Neuropathy Is Associated With Impaired Vascular Endothelial Function in Patients With Type 2 Diabetes. Front Endocrinol (Lausanne). 2021;12:653277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Wei Y, Liu C, Liu Y, Zhang Z, Feng Z, Yang X, Liu J, Lei H, Zhou H, Shen Q, Lu B, Gu P, Shao J. The association between time in the glucose target range and abnormal ankle-brachial index: a cross-sectional analysis. Cardiovasc Diabetol. 2022;21:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Ugwu E, Anyanwu A, Olamoyegun M. Ankle brachial index as a surrogate to vascular imaging in evaluation of peripheral artery disease in patients with type 2 diabetes. BMC Cardiovasc Disord. 2021;21:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Alshehri MM, Alqahtani AS, Alenazi AM, Aldhahi M, Alothman S, Gray C, Alqahtani B, Khunti K, Kluding P. Associations between ankle-brachial index, diabetes, and sleep apnea in the Hispanic community health study/study of Latinos (HCHS/SOL) database. BMC Cardiovasc Disord. 2020;20:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |