Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.106720
Revised: March 22, 2025
Accepted: April 16, 2025
Published online: June 15, 2025
Processing time: 100 Days and 6.3 Hours
Diabetic osteoporosis (DOP) is a common complication in diabetes, driven by hyperglycemia-induced metabolic disturbances, chronic inflammation, and oxi
Core Tip: Diabetic osteoporosis (DOP) is becoming increasingly prevalent, driven by global aging and diabetes-related metabolic disturbances. Elevated glucose levels impair osteoblast function and activate osteoclasts via oxidative stress and chronic inflammation, accelerating bone loss. Dysregulated iron metabolism, particularly iron overload, triggers ferroptosis - a novel form of cell death marked by lipid peroxidation and reactive oxygen species production-further exacerbating DOP. Therapeutic strategies targeting iron metabolism and ferroptosis, including antioxidants, iron chelators, and personalized interventions, hold significant potential for improving bone health. Future research should prioritize unraveling underlying mechanisms and refining targeted treatment approaches.
- Citation: Wang YB, Li ZP, Wang P, Wang RB, Ruan YH, Shi Z, Li HY, Sun JK, Mi Y, Li CJ, Zheng PY, Zhang CJ. Iron dysregulation, ferroptosis, and oxidative stress in diabetic osteoporosis: Mechanisms, bone metabolism disruption, and therapeutic strategies. World J Diabetes 2025; 16(6): 106720
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/106720.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.106720
The relationship between diabetes and osteoporosis has received increasing attention, particularly in the context of global aging, as the incidence of diabetes-related osteoporosis (DOP) has risen significantly. Studies have shown that the bone mineral density (BMD) of diabetic patients is generally lower than that of non-diabetic individuals, and as diabetes progresses, the risks of osteoporosis and fractures significantly increase[1-3]. Fracture risk is increased sixfold in patients with type 1 diabetes and twofold in those with type 2 diabetes, which is closely associated with diabetes-induced metabolic changes[2,4].
With the aging global population, the coexistence of diabetes and osteoporosis has become an increasingly prominent issue[5]. Data indicate that approximately 50% to 66% of diabetic patients exhibit decreased BMD, and approximately 33% are diagnosed with DOP[1]. This phenomenon not only affects patients’ quality of life but also imposes a substantial burden on public health systems[1,5,6]. The development of DOP is associated with multiple factors, including chronic inflammation, oxidative stress, and iron metabolism dysregulation[7,8].
The market demand for anti-inflammatory iron preparations continues to significantly expand as healthcare providers increasingly recognize the complex relationship between chronic inflammation and iron metabolism dysregulation. Traditional iron supplements are often ineffective for patients with inflammatory conditions due to inflammation-induced hepcidin upregulation, which leads to functional iron deficiency. This creates a significant unmet need for specialized formulations[9,10]. Recent advancements in intravenous iron treatments, such as ferric carboxymaltose, as well as new oral formulations like malto-ferric iron, are addressing these challenges by offering improved efficacy, safety, and tolerability for patients with inflammatory conditions[11-13]. The projected growth of the global iron preparation market, particularly in regions with high prevalence of inflammatory diseases, underscores the significant commercial opportunity and therapeutic need in this space[11,14]. As research continues to elucidate the intricate connections between inflammation, iron metabolism, and various disease states, we can anticipate further innovations in anti-inflammatory iron preparations that will enhance treatment outcomes and quality of life for patients worldwide[13].
This review aims to explore how diabetes contributes to osteoporosis through mechanisms involving bone metabolism, inflammatory responses, and oxidative stress, with a particular focus on the role of iron metabolism dysregulation in DOP. Research suggests that under hyperglycemic conditions, abnormal iron metabolism may lead to cell death (such as iron-dependent cell death, or ferroptosis), further exacerbating osteoporosis progression[1,5,15]. By analyzing these mechanisms in depth, we hope to provide new insights and strategies for clinical treatment to improve bone health in diabetic patients.
Bone metabolism represents the dynamic balance between bone formation and resorption, primarily governed by the coordinated activities of osteoblasts, osteoclasts, and osteocytes. Under normal physiological conditions, this balance ensures skeletal health and stability[16,17]. However, in pathological conditions such as diabetes, bone resorption frequently outpaces bone formation, leading to bone loss and the development of osteoporosis. Table 1 provides an overview of the effects of diabetes on bone metabolism.
| Cell type | Mechanism of action | Influencing factors | Specific manifestations | Ferroptosis-related mechanisms | Ref. |
| Osteoblasts | High-glucose environments inhibit osteoblast proliferation and differentiation | High glucose, AGEs, oxidative stress | Decreased ALP activity, reduced mineralization capacity | High glucose induces ferroptosis via lipid peroxidation and GPX4 inhibition; AGEs promote ferroptosis, disrupting osteoblast function and mineralization | Wu et al[4], Hygum et al[18] |
| Osteoclasts | Osteoclast formation and function are suppressed in high-glucose conditions | High glucose, inflammatory factors | Reduced number of osteoclasts, diminished bone resorption function | Iron overload in diabetic conditions enhances osteoclast activity through ferroptosis-associated pathways, increasing bone resorption in some contexts | Bao et al[5], Kim et al[21] |
| Osteocytes | High glucose and inflammatory environments impair osteocyte function | High glucose, inflammatory factors | Decreased osteocyte activity, reduced bone matrix quality | Ferroptosis induced by high glucose and lipid peroxidation leads to osteocyte death; upregulation of HO-1 and intracellular iron overload exacerbate bone matrix deterioration | Bao et al[5], Saadi et al[25], Yang et al[68] |
Osteoblasts are responsible for synthesizing and mineralizing the bone matrix and play a pivotal role in bone formation. They secrete collagen and non-collagenous proteins while regulating mineralization. Research indicates that hyperglycemia impairs osteoblast proliferation and function, resulting in diminished new bone formation[4,18]. Moreover, osteoblast function relies on critical signaling molecules such as insulin and insulin-like growth factor-1 (IGF-1), and deficiencies in these factors exacerbate the risk of osteoporosis in individuals with diabetes[19,20].
Conversely, osteoclasts are specialized cells that degrade bone tissue by secreting acidic substances and enzymes, facilitating the remodeling process. In diabetic patients, osteoclast activity is often heightened, driven by the upregulation of osteoclast-promoting factors such as receptor activator of nuclear factor-κB ligand (RANKL) and tumor necrosis factor-α (TNF-α). Elevated levels of these factors enhance osteoclast function, accelerating bone resorption[20-22].
Osteocytes are mature osteoblasts embedded within the mineralized bone matrix, where they act as mechanosensors to detect changes in mechanical stress and coordinate osteoblast and osteoclast activities. They release signaling molecules that influence nearby cells to maintain the delicate balance of bone metabolism[23,24]. However, in diabetic patients, oxidative stress and chronic inflammation can impair osteocyte function, further disrupting overall bone metabolic equilibrium[8,25].
In addition to cellular-level disruptions, hyperglycemia also affects the periosteum, a highly vascularized membrane that envelops bones and contains progenitor cells essential for bone growth and repair. In diabetic conditions, the vascular network and cellular activity within the periosteum are significantly impaired, leading to reduced osteogenic capacity and delayed fracture healing. Hyperglycemia-induced microangiopathy and inflammation in the periosteum compromise nutrient delivery and stem cell function, thereby exacerbating bone loss and reducing regenerative potential in diabetic patients[23-25].
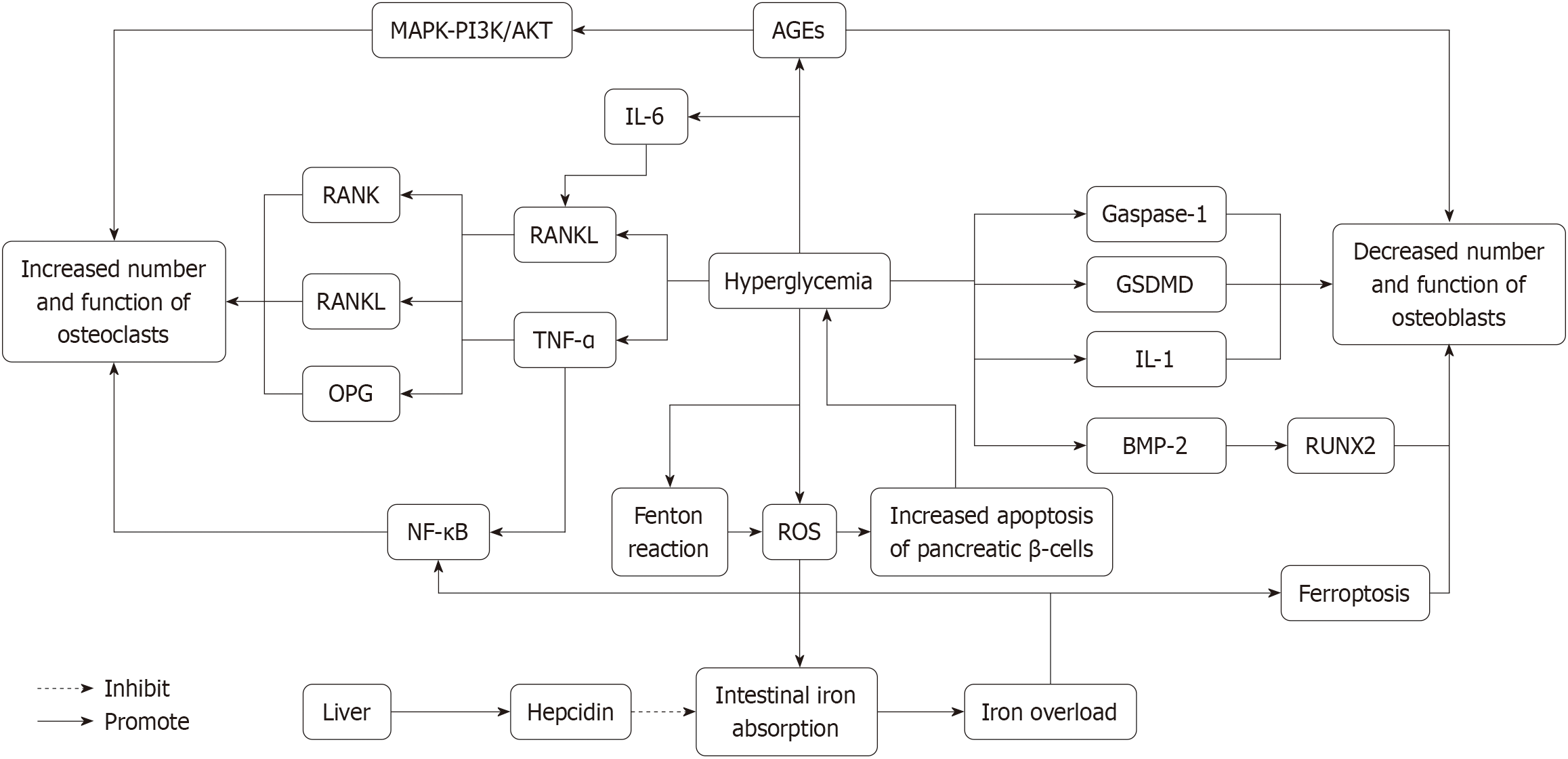
The mechanisms through which hyperglycemia disrupts bone metabolism are depicted in Figure 1, highlighting the multifactorial impacts of diabetes on skeletal health.
A hyperglycemic environment significantly impairs osteoblast proliferation and differentiation. Studies have revealed that under high-glucose conditions, osteoblasts exhibit diminished proliferative capacity, reduced matrix synthesis, and delayed mineralization. This suppression is partially attributed to elevated levels of advanced glycation end products (AGEs) in diabetic patients. AGEs not only directly damage osteoblasts but also activate inflammatory pathways, ultimately leading to cell apoptosis[26,27].
Hyperglycemia specifically promotes inflammatory cytokine release, such as interleukin-1β (IL-1β), through activation of the caspase-1/Gasdermin D (member of Gasdermin family D)/IL-1 signaling pathway. These inflammatory mediators further inhibit osteoblast proliferation and functionality. Research indicates that high glucose concentrations (e.g., 25 mmol/L) suppress osteoblast proliferation and mineralization while reducing the expression of key osteogenic markers, including runt-related transcription factor 2 (RUNX2), alkaline phosphatase, and osteocalcin[28-30]. Furthermore, AGE accumulation is a critical contributor to osteoblast dysfunction, as it induces oxidative stress and exacerbates inflammatory responses, thereby compounding osteoblast damage[31,32].
Insulin plays an essential role in promoting osteoblast proliferation and differentiation. Its absence or insufficiency can result in osteoblast dysfunction. Insulin stimulates the expression of key genes such as RUNX2, facilitating osteoblast differentiation and mineralization[31]. In diabetic models, insulin significantly restores osteoblast function, enhancing their proliferation and differentiation capabilities. These findings underscore insulin's crucial physiological role in regulating bone metabolism[33].
Moreover, hyperglycemia may disrupt osteoblast function by interfering with other signaling pathways. For instance, studies have shown that a high-glucose environment suppresses the bone morphogenetic protein-2 (BMP-2) signaling pathway, which is pivotal for osteoblast differentiation. Under hyperglycemic conditions, BMP-2 levels are significantly diminished, leading to reduced expression of downstream target genes, such as RUNX2, ultimately impairing osteoblast mineralization[26,27].
Diabetes significantly increases osteoclast activity and accelerates bone resorption. Under hyperglycemic conditions, the expression levels of osteoclast-promoting factors, such as RANKL and TNF-α, are markedly elevated. These factors enhance osteoclast differentiation and activity through the RANK/RANKL/Osteoprotegerin signaling pathway[34,35]. RANKL is secreted by osteoblasts and binds to the receptor RANK on osteoclast precursors, driving their maturation into active osteoclasts[21].
In diabetic models, elevated RANKL and TNF-α levels have been directly linked to increased osteoclast activity[36]. For instance, studies show that diabetic rats exhibit significantly higher TNF-α levels than controls. TNF-α further promotes RANKL expression by activating the nuclear factor-κB (NF-κB) signaling pathway, enhancing osteoclastogenesis and activity. This mechanism not only accelerates bone resorption but also exacerbates chronic inflammation, thereby hastening DOP progression[37,38].
Additionally, a hyperglycemic environment exacerbates osteoclast function by elevating oxidative stress, leading to more pronounced bone loss. Oxidative stress impacts bone metabolism through various pathways, including increasing osteoclast activity and apoptosis. Research indicates that AGE accumulation in diabetic patients stimulates osteoclast activity by activating specific signaling pathways, such as MAPK and PI3K/Akt[26,27].
Moreover, persistently elevated levels of inflammatory cytokines in diabetic patients, such as IL-6, IL-1β, and TNF-α, directly enhance osteoclastogenesis. These inflammatory mediators also impair osteoblast function, indirectly exacerbating the imbalance between bone resorption and formation[19]. The resulting disruption leads to reduced BMD and osteoporosis progression.
Chronic low-grade inflammation associated with diabetes is a key mechanism in the development of DOP[39]. Diabetic patients often exhibit a persistent inflammatory state driven by obesity, insulin resistance, and metabolic dysfunction. This state is characterized by elevated levels of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α[40,41]. These cytokines directly affect bone metabolism but also disrupt the balance between bone formation and resorption by impairing osteoblast and osteoclast functions[42] (Table 2).
| Mechanism type | Key factor | Pathway | Impact on bone metabolism | Ref. |
| Inflammatory response | TNF-α | Activates NF-κB pathway, promotes osteoclast differentiation and activity | Increases bone resorption, leading to osteoporosis | Qi et al[20] |
| IL-6 | Stimulates RANKL expression, enhances osteoclast formation | Increases bone resorption, decreases bone density | Wu et al[4] | |
| Oxidative stress | ROS | Damages osteoblasts, inhibits differentiation and function | Reduces bone formation, promotes osteoporosis | Iantomasi et al[53] |
| AGEs | Binds to receptors, induces oxidative stress and inflammatory responses | Disrupts bone matrix, reduces bone strength | Wang et al[26], Zhang et al[27] | |
| High-throughput sequencing | miRNAs | Identifies differentially expressed miRNAs (e.g., miR-140-5p, miR-486-3p) involved in bone metabolism pathways like Wnt and TGF-β signaling | Predicts osteoporosis progression; regulates osteoblast and osteoclast activities through gene silencing or activation | Huang et al[110] |
| RNA-seq | Transcript variants | Detects oxidative stress-induced transcriptomic changes, including activation of TNF and NRF2 signaling pathways | Highlights mitochondrial dysfunction and inflammation contributing to bone loss | Nyunt et al[111], Chen et al[112] |
Elevated IL-6 levels are a hallmark of diabetes and play a pivotal role in DOP. IL-6 promotes osteoclastogenesis while inhibiting osteoblast differentiation and function. Specifically, IL-6 enhances osteoclast activity by upregulating RANKL expression, thereby accelerating bone resorption[4,43]. Similarly, TNF-α is a significant driver of osteoclastogenesis, further stimulating osteoclast activity through activation of the NF-κB signaling pathway. In a chronic inflammatory state, sustained elevation of these cytokines contributes to progressive bone loss and worsening osteoporosis[44,45].
In experimental models of diabetes, there is a strong correlation between inflammatory cytokines and bone metabolism markers[46]. For instance, elevated TNF-α levels are inversely correlated with BMD, underscoring the critical role of inflammation in DOP[47,48]. Furthermore, persistent low-grade inflammation may increase osteoblast apoptosis, leading to reduced bone formation and exacerbating DOP progression[4,27].
Hyperglycemia significantly increases reactive oxygen species (ROS) production, which represents another critical mechanism in DOP pathogenesis[49,50]. Research indicates that hyperglycemia induces ROS generation through multiple pathways, including the accumulation of AGEs, mitochondrial dysfunction, and activation of the polyol pathway[51]. Excessive ROS production causes oxidative damage to cells and disrupts the function of both osteoblasts and osteoclasts[49,52].
In osteoblasts, ROS inhibits proliferation and differentiation while inducing apoptosis. For example, under high-glucose conditions, MC3T3-E1 osteoblasts undergo increased rates of apoptosis and decreased mineralization capacity[27]. This effect is partially attributed to ROS-induced mitochondrial dysfunction, which reduces ATP synthesis and leads to cellular energy deficiency[31]. Additionally, ROS activates signaling pathways such as MAPK and NF-κB, further promoting osteoblast apoptosis and inhibiting differentiation[53].
In osteoclasts, ROS enhances activity and function. Studies have shown that hyperglycemia-induced ROS amplifies osteoclast responsiveness to RANKL, promoting their differentiation and activity. This process accelerates bone resorption, resulting in more severe bone loss[54,55].
Thus, hyperglycemia-induced oxidative stress in diabetic patients exerts a dual impact: Impairing osteoblast function and enhancing osteoclast activity. This imbalance exacerbates DOP progression, highlighting oxidative stress as a key therapeutic target in managing diabetes-related bone disorders[56].
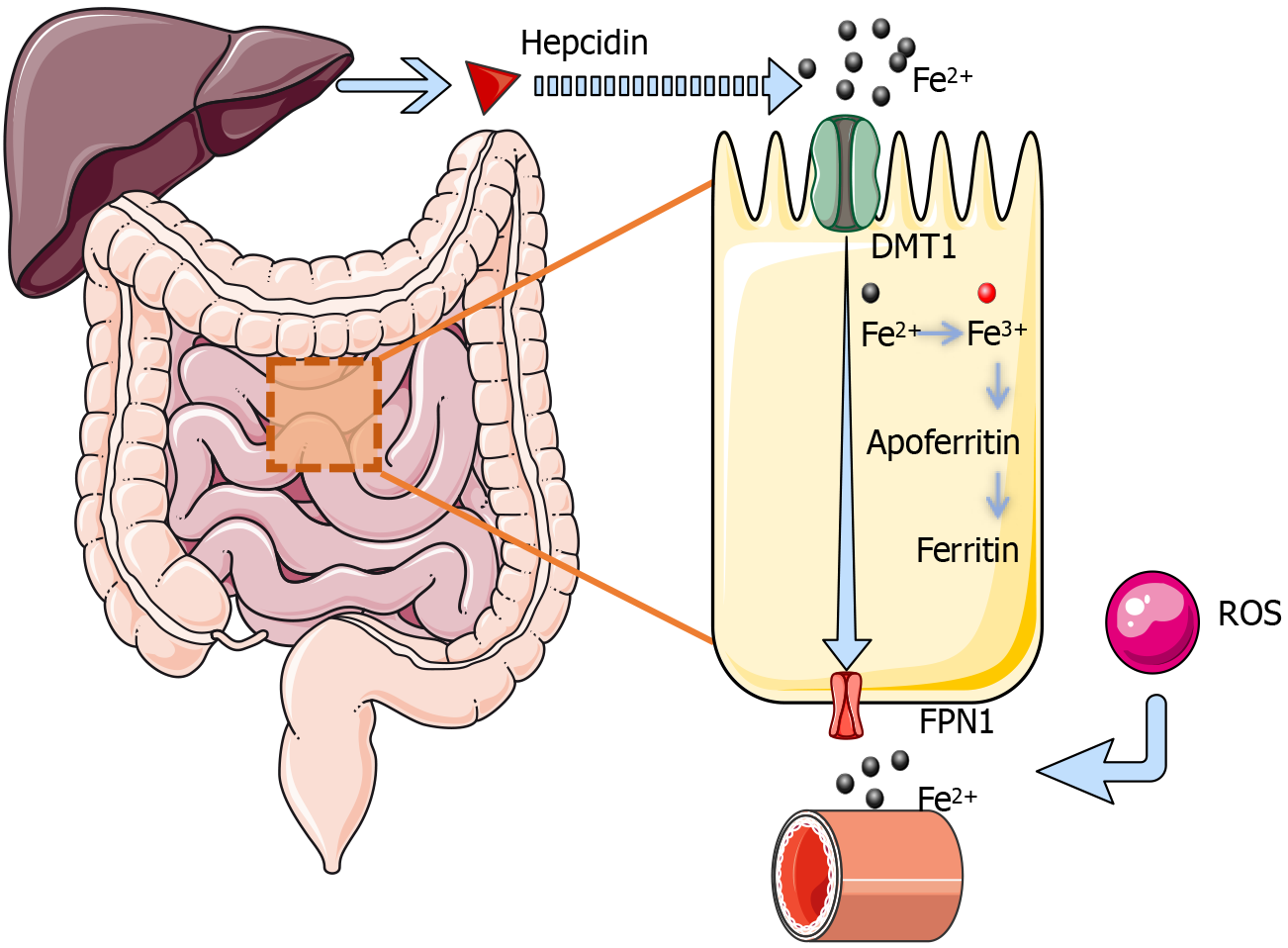
Iron is an essential element in the body, playing a pivotal role in various physiological processes such as oxygen transport, DNA synthesis, and energy production. Iron metabolism involves several key stages: absorption, transport, utilization, recycling, regulation, and storage. Iron is primarily absorbed in the small intestine, where it enters cells in its ferrous form (Fe2+) and is transported throughout the body via transferrin, a carrier protein in the bloodstream[57]. Within cells, iron can be stored as ferritin or utilized in the synthesis of hemoglobin and other iron-dependent enzymes[58].
Iron homeostasis is tightly regulated by hepcidin, a hormone produced by the liver. Under normal conditions, increased hepcidin levels inhibit intestinal iron absorption and reduce iron release from storage sites, thereby preventing iron overload[59]. Conversely, during hypoxic conditions, hepcidin expression decreases, facilitating iron release and utilization. This dynamic regulatory mechanism ensures that the body’s iron demands are met under varying physiological conditions while protecting against the toxicity associated with excessive iron levels[59,60].
The interplay between diabetes and iron metabolism is complex and multifaceted. Studies have revealed that diabetic patients frequently exhibit abnormalities in iron metabolism, manifesting as either iron overload or iron deficiency. These conditions are often associated with inflammation, insulin resistance, and metabolic dysregulation[61-63]. Specifically, iron metabolism disturbances in diabetes can be categorized into two main categories: Iron overload and iron deficiency. Table 3 provides a summary of the relationship between diabetes and iron metabolism.
| Mechanism type | Key factors | Pathway | Impact on bone metabolism | Ref. |
| Iron overload | Iron ions (Fe2+/Fe3+) | Excess iron generates ROS via the Fenton reaction, causing oxidative stress and lipid peroxidation | Damages osteoblasts, inhibits their differentiation and function, reduces bone formation; promotes osteoclast differentiation, increasing bone resorption | Zang et al[59], Liu et al[62], Harrison et al[65] |
| Ferroptosis (iron-dependent cell death) | GPX4, SLC7A11, ROS | Iron-dependent cell death involving lipid peroxidation and antioxidant system imbalance | Induces bone cell death, disrupts bone tissue structure, and promotes the progression of osteoporosis | Yang et al[68] |
| Hepcidin dysregulation | Hepcidin, FPN1 | Overexpression of hepcidin inhibits iron export protein FPN1, leading to intracellular iron accumulation | Increases iron content in bone marrow mesenchymal stem cells, inhibits their differentiation into osteoblasts, reduces bone formation | Zang et al[59] |
| Hyperglycemia-induced dysregulation | Hyperglycemia, AGEs, ROS | High-glucose environment promotes AGEs formation; AGEs bind to their receptors, inducing ROS production and iron metabolism dysregulation | Causes osteoblast dysfunction, enhances osteoclast activity, and exacerbates osteoporosis | Xie et al[71], Dludla et al[72], Zhao et al[73] |
Iron overload and diabetes: Chronic low-grade inflammation in diabetic patients may elevate hepcidin levels, a key hormone responsible for regulating iron homeostasis. Increased hepcidin inhibits intestinal iron absorption and reduces the release of stored iron, potentially leading to inadequate iron availability. Furthermore, oxidative stress, which is increased in diabetes, exacerbates iron metabolism dysregulation[5]. Elevated ROS levels promote iron release and enhance its bioavailability, contributing to iron overload. The detailed process of iron absorption is illustrated in Figure 2.
Iron overload intensifies oxidative stress, aggravating insulin resistance and impairing β-cell function, thereby complicating diabetes management. Excessive iron facilitates ROS production through the Fenton reaction, which is toxic to β-cells, resulting in their dysfunction and apoptosis[1,64]. Additionally, studies indicate that iron overload disrupts insulin signaling pathways in the liver and adipose tissue, further exacerbating insulin resistance[65].
Iron deficiency and diabetes: Conversely, diabetic patients may also experience iron deficiency. Chronic inflammation and elevated hepcidin levels are contributors to functional iron deficiency in diabetes. This condition impairs ery
In diabetic models, iron deficiency has been shown to diminish the glucose-stimulated response of β-cells, thereby reducing insulin secretion. These findings highlight the critical importance of maintaining optimal iron levels in diabetes management to preserve β-cell functionality and ensure metabolic stability[69,70].
Recent research highlights a strong relationship between abnormal iron metabolism and bone metabolism, particularly in the context of DOP. Iron-dependent cell death, or ferroptosis, has emerged as a critical mechanism underlying DOP. Ferroptosis is a novel form of programmed cell death characterized by the accumulation of lipid peroxides and ROS generation[68]. Under hyperglycemic conditions, ferroptosis is significantly elevated in diabetic patients, closely linked to bone cell death and the development of osteoporosis[71-73].
Definition of ferroptosis and its association with DOP: Ferroptosis is an iron-dependent form of cell death that differs from traditional mechanisms such as apoptosis, necrosis, and autophagy. It is defined by lipid peroxidation triggered by intracellular iron overload, which ultimately causes membrane rupture and cell death. In diabetic patients, hyper
For instance, one study demonstrated that osteoblasts exposed to high glucose levels exhibit substantial lipid peroxidation and apoptosis, effects that can be mitigated by inhibiting ferroptosis[74]. Furthermore, iron overload amplifies this process, as excessive iron enhances ROS generation, further promoting ferroptosis[76].
Effects of abnormal iron metabolism on osteoblast and osteoclast function: In diabetic models, both osteoblasts and osteoclasts are vulnerable to ferroptosis. Elevated glucose levels and iron overload increase ROS levels in osteoblasts, inducing ferroptosis. For example, studies have shown that MC3T3-E1 osteoblasts exposed to high glucose concentrations accumulate lipid peroxides, experience mitochondrial dysfunction, and undergo apoptosis[77]. These changes not only impair osteoblast proliferation and differentiation but also reduce bone matrix synthesis, exacerbating osteoporosis[78,79].
Similarly, in osteoclasts, iron overload induces ferroptosis and enhances osteoclast activity. Under high iron conditions, osteoclasts demonstrate increased responsiveness to RANKL, which promotes their differentiation and activity. This accelerates bone resorption and exacerbates bone loss. Studies in rat models have shown that in diabetes, osteoclast activity is significantly elevated and strongly correlated with increased iron levels[78].
The clinical management of DOP primarily involves pharmacological interventions and lifestyle modifications. Current pharmacological strategies focus on the combined use of antidiabetic and anti-osteoporotic drugs, aiming to control blood glucose levels while simultaneously enhancing bone metabolism.
Regarding pharmacotherapy, antidiabetic agents such as metformin, GLP-1 receptor agonists, and SGLT-2 inhibitors are considered relatively safe for bone health[80]. Research has demonstrated that metformin not only effectively reduces blood glucose levels but may also offer protective effects on bone density[80-82]. Furthermore, GLP-1 receptor agonists promote bone quality by stimulating osteoblast proliferation and differentiation, whereas SGLT-2 inhibitors exhibit bone-protective effects and also lower blood glucose levels[81].
Anti-osteoporotic treatments typically include bisphosphonates and RANKL inhibitors, such as denosumab. Bisphosphonates work by inhibiting osteoclast activity, thereby reducing bone resorption, while RANKL inhibitors prevent the binding of RANKL to its receptor RANK, further suppressing osteoclastogenesis[83,84]. Calcium and vitamin D supplementation also constitute a core treatment component, collectively supporting bone health in patients[80,85].
Lifestyle interventions are crucial for enhancing both overall and bone health. These include a balanced diet, regular exercise, and avoiding smoking. A nutritious diet provides the necessary nutrients to maintain bone strength, while moderate physical activity, especially weight-bearing exercises, helps increase bone density and decrease osteoporosis risk[82,86]. Additionally, healthy lifestyle habits contribute to better blood glucose control. A study on postmenopausal women demonstrated that rigorous dietary and exercise management significantly improved bone density in patients with both type 2 diabetes and osteoporosis[87].
Future research should prioritize the areas discussed below to improve the management and treatment outcomes of DOP (summarized in Table 4).
| Treatment strategy/research direction | Mechanism of action | Clinical effect | Challenges and prospects |
| Bisphosphonates | Inhibit osteoclast activity, reduce bone resorption | Increase bone density, reduce fracture risk | Long-term use may lead to side effects such as osteonecrosis of the jaw; risks need to be balanced |
| Calcitonin | Inhibit osteoclasts, promote osteoblast activity | Relieve bone pain, increase bone density | Limited efficacy; long-term use may lead to drug resistance |
| Selective estrogen receptor modulators | Mimic estrogen effects, reduce bone resorption | Increase bone density, lower risk of spinal fractures | May increase risk of thrombosis; use with caution |
| Choice of anti-diabetic drugs | Different drugs have varying impacts on bone metabolism | Metformin may benefit bone health; thiazolidinediones may increase fracture risk | Need to select appropriate medications based on the patient's specific condition |
| Vitamin D and calcium supplementation | Provide raw materials for bone mineralization, promote calcium absorption | Improve bone density, prevent osteoporosis | Excessive supplementation may lead to hypercalcemia; dosage needs monitoring |
| New anti-osteoporosis drugs | Agents like denosumab inhibit RANKL, reducing osteoclast formation | Significantly increase bone density, reduce fracture risk | Long-term safety requires further research |
| Personalized treatment strategies | Develop comprehensive plans based on the patient's specific situation | Improve treatment effectiveness, reduce side effects | Requires multidisciplinary collaboration to formulate individualized plans |
| Traditional Chinese medicine therapy | Improve bone metabolism through multi-target regulation | Some herbal medicines show potential to enhance bone density | Lack of large-scale clinical research data; further validation needed |
| Gene therapy | Target specific genes to regulate bone metabolism pathways | Potentially curative treatment method | Technology is not yet mature; ethical and safety issues need to be addressed |
| Stem cell therapy | Use stem cells to differentiate into osteoblasts and repair bone tissue | Animal studies show some efficacy | Clinical application is still in early stages; more research is necessary |
The role of ferroptosis in DOP: Ferroptosis is a novel form of programmed cell death and is characterized by the accumulation of lipid peroxides and ROS generation[88]. Recent findings suggest that ferroptosis is a critical mechanism underlying DOP onset and progression. While preliminary studies have highlighted the influence of ferroptosis on bone cells in the diabetic microenvironment, its precise molecular mechanisms remain incompletely understood[68,89]. Future research should explore the following areas:
(1) Regulating ferroptosis to protect bone cells: Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key transcription factor that orchestrates cellular antioxidant defenses. Under oxidative stress, Nrf2 dissociates from its inhibitor Keap1 and translocates to the nucleus, where it induces the expression of antioxidant genes such as heme oxygenase-1 (HO-1) and glutathione peroxidase 4 (GPX4)[90,91]. These genes mitigate iron-dependent lipid peroxidation, thereby inhibiting ferroptosis.
Nrf2 plays a protective role in osteoblasts and osteoclasts by modulating intracellular free iron levels and lipid metabolism, effectively decelerating DOP progression[92,93]. Therapeutic approaches targeting pathways like the Nrf2/HO-1 signaling axis to suppress ferroptosis could offer innovative strategies for bone cell protection and DOP mitigation. For example, previous studies have demonstrated that Nrf2 activation decreases iron-dependent lipid peroxidation, significantly reducing ferroptosis[94].
(2) Application of antioxidants and iron chelators; the role of antioxidants Glutathione (GSH) and melatonin: GSH is a pivotal intracellular antioxidant that protects cells from oxidative damage by scavenging ROS. Studies indicate that GSH deficiency can trigger ferroptosis, as GSH is an essential substrate for GPX4, an enzyme responsible for detoxifying lipid peroxides[95,96].
Melatonin is also an effective ferroptosis inhibitor[95]. In addition to its potent antioxidant properties, melatonin enhances cellular antioxidant defenses by activating the Nrf2/HO-1 signaling pathway. Research has demonstrated that melatonin increases GSH and superoxide dismutase levels and reduces the accumulation of lipid peroxides and malondialdehyde, thereby alleviating ferroptosis[97,98].
Role of iron chelators: Deferoxamine (DFO) applications. DFO is a highly effective iron chelator that mitigates ferroptosis by binding free iron and reducing intracellular iron levels[76]. DFO has been extensively studied in various disease models to counteract iron-induced cell death. For example, in osteoarthritis models, DFO inhibits chondrocyte ferroptosis and activates the Nrf2 antioxidant system, thereby protecting cartilage cells[99,100].
DFO shows promise in the treatment of osteoporosis-related conditions. Research indicates that DFO prevents bone loss caused by excessive iron accumulation and improves osteoblast function by inhibiting ferroptosis. These findings suggest new therapeutic opportunities for managing DOP[100].
Combined applications: The simultaneous use of antioxidants and iron chelators may provide synergistic effects, offering enhanced protection against ferroptosis-induced cellular damage. For instance, co-administration of melatonin and DFO could amplify bone cell protection by concurrently reducing ROS levels and alleviating iron overload, thereby improving bone cell functionality[93,101,102].
Developing novel therapies targeting iron metabolism and oxidative stress: With growing insights into the intricate relationship between iron metabolism and oxidative stress, developing targeted therapies has become a critical research priority. Potential approaches include:
(1) Interventions in iron metabolism: Investigating the use of iron chelators in diabetic patients and their potential benefits for bone health. Studies have shown that DFO effectively reduces iron overload and may protect bone cells by mitigating ROS production[93,99,100];
(2) Antioxidant therapies: Antioxidants targeting oxidative stress, such as GSH precursors or small-molecule drugs, represent promising therapeutic options for DOP. These therapies aim to alleviate cellular damage by neutralizing excess ROS, thereby enhancing bone metabolism[103].
Numerous studies have highlighted the antioxidant effects of small-molecule drugs and natural compounds in animal models, demonstrating significant improvements in diabetes-induced bone metabolic abnormalities. For example, the natural polyphenolic compound resveratrol reduces blood glucose levels while promoting osteoblast differentiation and suppressing adipogenesis through activation of the Sirtuin1 signaling pathway, thereby supporting bone health[104,105]. Furthermore, innovative nanomaterials with specific structural properties have shown exceptional antioxidant capabilities, enabling the sustained local release of antioxidant agents, thereby enhancing the repair of diabetic bone defects[106,107].
Personalized treatment strategies: Given the substantial variability among diabetic patients, future efforts should focus on personalized assessments to create more precise treatment plans. This includes selecting the most suitable anti-diabetic and anti-osteoporosis medications based on individual patient conditions, complemented by lifestyle modifications to maximize therapeutic outcomes[68]. For instance, tailoring interventions to address specific iron metabolism imbalances could significantly enhance treatment efficacy.
Clinical trials and long-term observations: Large-scale, long-term clinical trials are essential to evaluate the efficacy of various treatment strategies on bone health and overall quality of life in DOP patients[68]. These trials will provide strong evidence to inform clinical practice. Moreover, studies focusing on emerging therapies, such as drugs targeting ferroptosis and iron metabolism, will deliver crucial data to drive the development of innovative treatment strategies.
Application of high-throughput sequencing technology in ferroptosis and DOP research: High-throughput sequencing technology, especially RNA sequencing (RNA-seq), serves as a powerful tool for exploring the molecular mechanisms of ferroptosis in DOP. This technique allows for the simultaneous detection of millions of DNA molecules, enabling the identification of specific genes or genomic regions, thereby offering profound insights into the role of ferroptosis in DOP[30] (Table 2).
RNA-seq technology offers significant advantages in the study of DOP. It enables a comprehensive analysis of transcriptomic changes in bone tissue, facilitating the identification of differentially expressed genes associated with ferroptosis. For instance, RNA-seq analysis of diabetic bone tissue has led to the identification of key ferroptosis-related genes, including GPX4, Ftl1, Tp53, and GPLD1. These findings suggest that along with the GPI-anchored biosynthesis signaling pathway, ferroptosis plays a crucial role in high-phosphate-induced vascular smooth muscle cell calcification[68,108,109]. These insights provide valuable understanding of the mechanisms underlying ferroptosis in bone cells within the diabetic microenvironment.
The application of single-cell RNA-seq (scRNA-seq) technology has further advanced the precision of DOP research[110-112]. This technology can distinguish gene expression patterns across different cell types, making it particularly important for studying the ferroptosis status of distinct cell types in the bone marrow microenvironment under diabetic conditions, such as osteoblasts, osteoclasts, and bone marrow mesenchymal stem cells. Studies by Bao et al[5] and Zhang et al[113] have shown that iron metabolism dysregulation in the bone marrow microenvironment is a key factor in developing osteoporosis under diabetic conditions, and scRNA-seq can accurately capture these changes.
In addition, the integration of multi-omics data analysis methods has provided new perspectives for DOP research. Combining RNA-seq data with proteomics and metabolomics data can help construct a comprehensive regulatory network linking ferroptosis and DOP[68,114]. For example, Yang et al[68] performed a joint analysis of RNA-seq and proteomics and found that inhibiting ferroptosis could alleviate glucolipotoxicity in bone cells and improve diabetic osteopathy. This study confirmed the crucial roles of key genes such as MAPK3, CDKN1A, MAP1 LC3A, TNF, RELA, and TGF-β1 in this process[68,115].
Future research should focus on the following key areas: high-throughput sequencing technologies to identify specific biomarkers of ferroptosis, which could facilitate the early diagnosis of DOP. Second, conducting functional genomics studies to validate the roles of key genes in DOP, thereby supporting the discovery of drug targets and the development of personalized treatment strategies. Additionally, the creation of a large-scale database integrating transcriptomic and clinical data will provide a more comprehensive understanding of ferroptosis in DOP pathogenesis, laying a solid foundation for the development of novel ferroptosis-targeted therapies.
This study provides a comprehensive analysis of DOP pathogenesis, with a particular emphasis on the roles of iron metabolism dysregulation and ferroptosis, an iron-dependent form of programmed cell death. The findings demonstrate that diabetes adversely affects bone metabolism through multiple interconnected pathways, including impaired osteoblast function, enhanced osteoclast activity, chronic inflammation, persistent oxidative stress, and abnormalities in iron metabolism.
The results further confirm the inhibitory effects of hyperglycemia on bone cell metabolism. Under high-glucose conditions, osteoblasts exhibit significantly reduced proliferation and differentiation, which is closely associated with oxidative stress-induced apoptosis and functional impairment. Simultaneously, pro-inflammatory factors such as RANKL and TNF-α markedly enhance osteoclast activity, leading to accelerated bone resorption and further bone loss. These findings suggest that targeting the dysregulated functions of osteoblasts and osteoclasts may provide novel therapeutic avenues for DOP.
Moreover, the study underscores the pivotal role of iron metabolism dysregulation in DOP, revealing its dual impact on bone metabolism through iron overload and deficiency. In diabetic patients, abnormal iron metabolism not only impairs bone cell function but also exacerbates osteoporosis progression by inducing ferroptosis. Particularly under iron overload conditions, elevated ROS levels intensify lipid peroxidation and lead to bone cell death. This mechanism highlights the importance of addressing iron metabolism abnormalities as part of DOP management.
Ferroptosis has been identified as a key contributor to the detrimental effects of diabetes on bone metabolism, as it accelerates the death of both osteoblasts and osteoclasts while aggravating the overall metabolic imbalance through oxidative stress and lipid peroxidation. These findings suggest that inhibiting ferroptosis could represent a promising therapeutic strategy for the treatment of DOP.
While this study provides valuable insights into DOP pathogenesis, particularly the impact of iron metabolism and ferroptosis on bone metabolism, it has certain limitations. For example, the differences in mechanisms between in vivo and in vitro models may restrict the generalizability of the findings. Additionally, the study does not comprehensively address how various diabetes treatments influence the regulation of iron metabolism and ferroptosis, leaving this as an important area for future research.
Future studies should prioritize a detailed exploration of the specific mechanisms underlying iron metabolism dysregulation and ferroptosis in DOP, particularly their interactions with oxidative stress and pro-inflammatory factors. The development of novel therapeutic agents targeting these abnormalities, such as iron chelators and antioxidants, and their validation through large-scale clinical trials will be essential. Furthermore, establishing representative animal models that integrate genetic, metabolic, and environmental factors could facilitate the creation of personalized treatment strategies. Such advancements would support the transition of DOP management toward individualized and precision medicine.
| 1. | Chen Y, Zhao W, Hu A, Lin S, Chen P, Yang B, Fan Z, Qi J, Zhang W, Gao H, Yu X, Chen H, Chen L, Wang H. Type 2 diabetic mellitus related osteoporosis: focusing on ferroptosis. J Transl Med. 2024;22:409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 2. | Ghodsi M, Larijani B, Keshtkar AA, Nasli-Esfahani E, Alatab S, Mohajeri-Tehrani MR. Mechanisms involved in altered bone metabolism in diabetes: a narrative review. J Diabetes Metab Disord. 2016;15:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Luo W, Li X, Zhou Y, Xu D, Qiao Y. Correlation between bone mineral density and type 2 diabetes mellitus in elderly men and postmenopausal women. Sci Rep. 2024;14:15078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Wu B, Fu Z, Wang X, Zhou P, Yang Q, Jiang Y, Zhu D. A narrative review of diabetic bone disease: Characteristics, pathogenesis, and treatment. Front Endocrinol (Lausanne). 2022;13:1052592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 5. | Bao J, Yan Y, Zuo D, Zhuo Z, Sun T, Lin H, Han Z, Zhao Z, Yu H. Iron metabolism and ferroptosis in diabetic bone loss: from mechanism to therapy. Front Nutr. 2023;10:1178573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 6. | Li W, Xie S, Zhong S, Lan L. The synergistic effect of diabetes mellitus and osteoporosis on the all-cause mortality: a cohort study of an American population. Front Endocrinol (Lausanne). 2023;14:1308574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Wen Y, Li H, Zhang X, Liu P, Ma J, Zhang L, Zhang K, Song L. Correlation of Osteoporosis in Patients With Newly Diagnosed Type 2 Diabetes: A Retrospective Study in Chinese Population. Front Endocrinol (Lausanne). 2021;12:531904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Wongdee K, Charoenphandhu N. Osteoporosis in diabetes mellitus: Possible cellular and molecular mechanisms. World J Diabetes. 2011;2:41-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 134] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 9. | Cherayil BJ. Pathophysiology of Iron Homeostasis during Inflammatory States. J Pediatr. 2015;167:S15-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Loveikyte R, Duijvestein M, Mujagic Z, Goetgebuer RL, Dijkstra G, van der Meulen-de Jong AE. Predicting response to iron supplementation in patients with active inflammatory bowel disease (PRIme): a randomised trial protocol. BMJ Open. 2024;14:e077511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Kumar A, Sharma E, Marley A, Samaan MA, Brookes MJ. Iron deficiency anaemia: pathophysiology, assessment, practical management. BMJ Open Gastroenterol. 2022;9:e000759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 12. | Gordon M, Sinopoulou V, Iheozor-Ejiofor Z, Iqbal T, Allen P, Hoque S, Engineer J, Akobeng AK. Interventions for treating iron deficiency anaemia in inflammatory bowel disease. Cochrane Database Syst Rev. 2021;1:CD013529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 13. | Gupte S, Mukhopadhyay A, Puri M, Gopinath PM, Wani R, Sharma JB, Swami OC. A meta-analysis of ferric carboxymaltose versus other intravenous iron preparations for the management of iron deficiency anemia during pregnancy. Rev Bras Ginecol Obstet. 2024;46:e-rbgo21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Malesza IJ, Bartkowiak-Wieczorek J, Winkler-Galicki J, Nowicka A, Dzięciołowska D, Błaszczyk M, Gajniak P, Słowińska K, Niepolski L, Walkowiak J, Mądry E. The Dark Side of Iron: The Relationship between Iron, Inflammation and Gut Microbiota in Selected Diseases Associated with Iron Deficiency Anaemia-A Narrative Review. Nutrients. 2022;14:3478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 69] [Reference Citation Analysis (0)] |
| 15. | Ren J, Meng C, Li R, Xu Y, Li C. Targeting oxidative stress, iron overload and ferroptosis in bone-degenerative conditions. Turk J Biochem. 2025;50:1-16. [DOI] [Full Text] |
| 16. | Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells. 2020;9:2073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 795] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 17. | Delgado-Calle J, Bellido T. The osteocyte as a signaling cell. Physiol Rev. 2022;102:379-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 18. | Hygum K, Starup-Linde J, Harsløf T, Vestergaard P, Langdahl BL. Mechanisms in Endocrinology: Diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis. Eur J Endocrinol. 2017;176:R137-R157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (1)] |
| 19. | Kalaitzoglou E, Popescu I, Bunn RC, Fowlkes JL, Thrailkill KM. Effects of Type 1 Diabetes on Osteoblasts, Osteocytes, and Osteoclasts. Curr Osteoporos Rep. 2016;14:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Qi J, Hu KS, Yang HL. Roles of TNF-α, GSK-3β and RANKL in the occurrence and development of diabetic osteoporosis. Int J Clin Exp Pathol. 2015;8:11995-12004. [PubMed] |
| 21. | Kim JH, Kim AR, Choi YH, Jang S, Woo GH, Cha JH, Bak EJ, Yoo YJ. Tumor necrosis factor-α antagonist diminishes osteocytic RANKL and sclerostin expression in diabetes rats with periodontitis. PLoS One. 2017;12:e0189702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Xu J, Yue F, Wang J, Chen L, Qi W. High glucose inhibits receptor activator of nuclear factorκB ligand-induced osteoclast differentiation via downregulation of vATPase V0 subunit d2 and dendritic cellspecific transmembrane protein. Mol Med Rep. 2015;11:865-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Choi JUA, Kijas AW, Lauko J, Rowan AE. The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Front Cell Dev Biol. 2021;9:770143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 24. | Qin L, Liu W, Cao H, Xiao G. Molecular mechanosensors in osteocytes. Bone Res. 2020;8:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 300] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 25. | Saadi MSS, Das R, Mullath Ullas A, Powell DE, Wilson E, Myrtziou I, Rakieh C, Kanakis I. Impact of Different Anti-Hyperglycaemic Treatments on Bone Turnover Markers and Bone Mineral Density in Type 2 Diabetes Mellitus Patients: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2024;25:7988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Wang J, Wang B, Li Y, Wang D, Lingling E, Bai Y, Liu H. High glucose inhibits osteogenic differentiation through the BMP signaling pathway in bone mesenchymal stem cells in mice. EXCLI J. 2013;12:584-597. [PubMed] |
| 27. | Zhang P, Liao J, Wang X, Feng Z. High glucose promotes apoptosis and autophagy of MC3T3-E1 osteoblasts. Arch Med Sci. 2023;19:138-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Yang L, Liu J, Shan Q, Geng G, Shao P. High glucose inhibits proliferation and differentiation of osteoblast in alveolar bone by inducing pyroptosis. Biochem Biophys Res Commun. 2020;522:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Takeno A, Kanazawa I, Tanaka KI, Notsu M, Kanasaki K, Oono T, Ogawa Y, Sugimoto T. High glucose promotes mineralization via bone morphogenetic protein 4-Smad signals in early stage of osteoblast differentiation. Diabetol Int. 2021;12:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Zhang Y, Li M, Lou P, Zhang M, Shou D, Tong P. miRNA-seq analysis of high glucose induced osteoblasts provides insight into the mechanism underlying diabetic osteoporosis. Sci Rep. 2024;14:13441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 31. | Donat A, Knapstein PR, Jiang S, Baranowsky A, Ballhause TM, Frosch KH, Keller J. Glucose Metabolism in Osteoblasts in Healthy and Pathophysiological Conditions. Int J Mol Sci. 2021;22:4120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | He HQ, Qu YQ, Kwan Law BY, Qiu CL, Han Y, Ricardo de Seabra Rodrigues Dias I, Liu Y, Zhang J, Wu AG, Wu CW, Fai Mok SW, Cheng X, He YZ, Wai Wong VK. AGEs-Induced Calcification and Apoptosis in Human Vascular Smooth Muscle Cells Is Reversed by Inhibition of Autophagy. Front Pharmacol. 2021;12:692431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | E L, Shan Y, Luo Y, Feng L, Dai Y, Gao M, Lv Y, Zhang C, Liu H, Wen N, Zhang R. Insulin promotes the bone formation capability of human dental pulp stem cells through attenuating the IIS/PI3K/AKT/mTOR pathway axis. Stem Cell Res Ther. 2024;15:227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 34. | De Leon-Oliva D, Barrena-Blázquez S, Jiménez-Álvarez L, Fraile-Martinez O, García-Montero C, López-González L, Torres-Carranza D, García-Puente LM, Carranza ST, Álvarez-Mon MÁ, Álvarez-Mon M, Diaz R, Ortega MA. The RANK-RANKL-OPG System: A Multifaceted Regulator of Homeostasis, Immunity, and Cancer. Medicina (Kaunas). 2023;59:1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 35. | Udagawa N, Koide M, Nakamura M, Nakamichi Y, Yamashita T, Uehara S, Kobayashi Y, Furuya Y, Yasuda H, Fukuda C, Tsuda E. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. 2021;39:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 469] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 36. | Xing B, Yu J, Zhang H, Li Y. RANKL inhibition: a new target of treating diabetes mellitus? Ther Adv Endocrinol Metab. 2023;14:20420188231170754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 37. | Marahleh A, Kitaura H, Ohori F, Kishikawa A, Ogawa S, Shen WR, Qi J, Noguchi T, Nara Y, Mizoguchi I. TNF-α Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Front Immunol. 2019;10:2925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 38. | Reni C, Mangialardi G, Meloni M, Madeddu P. Diabetes Stimulates Osteoclastogenesis by Acidosis-Induced Activation of Transient Receptor Potential Cation Channels. Sci Rep. 2016;6:30639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Isidro ML, Ruano B. Bone disease in diabetes. Curr Diabetes Rev. 2010;6:144-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Zhao K, Wu X, Han G, Sun L, Zheng C, Hou H, Xu BB, El-Bahy ZM, Qian C, Kallel M, Algadi H, Guo Z, Shi Z. Phyllostachys nigra (Lodd. ex Lindl.) derived polysaccharide with enhanced glycolipid metabolism regulation and mice gut microbiome. Int J Biol Macromol. 2024;257:128588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 41. | Wen X, Qi LM, Zhao K. Influence of gut bacteria on type 2 diabetes: Mechanisms and therapeutic strategy. World J Diabetes. 2025;16:100376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (4)] |
| 42. | Molina-Ayala M, Cruz-Soto RC, Vargas-Ortega G, González-Virla B, Ferreira-Hermosillo A, Mac Gregor-Gooch J, Mendoza-Zubieta V. [Association of inflammatory cytokines with bone turnover markers in type 1 diabetes]. Rev Med Inst Mex Seguro Soc. 2016;54 Suppl 2:S191-S195. [PubMed] |
| 43. | Leanza G, Faraj M, Cannata F, Viola V, Pellegrini N, Tramontana F, Pedone C, Vadalà G, Piccoli A, Strollo R, Zalfa F, Civitelli R, Maccarrone M, Papalia R, Napoli N. Increased bone inflammation in type 2 diabetes and obesity correlates with Wnt signaling downregulation and reduced bone strength. 2024 Preprint. Available from: eLife 13:RP102146. [DOI] [Full Text] |
| 44. | Huang D, He Q, Pan J, Zhai Z, Sun J, Wang Q, Chu W, Huang J, Yu J, Qiu X, Lu W. Systemic immune-inflammatory index predicts fragility fracture risk in postmenopausal anemic females with type 2 diabetes mellitus: evidence from a longitudinal cohort study. BMC Endocr Disord. 2024;24:256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 45. | Mitama Y, Fujiwara S, Yoneda M, Kira S, Kohno N. Association of type 2 diabetes and an inflammatory marker with incident bone fracture among a Japanese cohort. J Diabetes Investig. 2017;8:709-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Phimphilai M, Pothacharoen P, Chattipakorn N, Kongtawelert P. The trajectory of osteoblast progenitor cells in patients with type 2 diabetes and the predictive model for their osteogenic differentiation ability. Sci Rep. 2023;13:2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (2)] |
| 47. | Jiao H, Xiao E, Graves DT. Diabetes and Its Effect on Bone and Fracture Healing. Curr Osteoporos Rep. 2015;13:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 377] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 48. | Wu YY, Xiao E, Graves DT. Diabetes mellitus related bone metabolism and periodontal disease. Int J Oral Sci. 2015;7:63-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 49. | Hu H, Zhang W, Zhou Y, Zhao K, Kuang J, Liu X, Li G, Xi Y. Engineered mitochondrial ROS scavenger nanocomplex to enhance lung biodistribution and reduce inflammation for the treatment of ARDS. Adv Compos Hybrid Mater. 2024;7:194. [DOI] [Full Text] |
| 50. | González P, Lozano P, Ros G, Solano F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int J Mol Sci. 2023;24:9352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 342] [Reference Citation Analysis (0)] |
| 51. | Chen Y, Meng Z, Li Y, Liu S, Hu P, Luo E. Advanced glycation end products and reactive oxygen species: uncovering the potential role of ferroptosis in diabetic complications. Mol Med. 2024;30:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 52. | Jia X, Zhang G, Yu D. Application of extracellular vesicles in diabetic osteoporosis. Front Endocrinol (Lausanne). 2024;15:1466775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 53. | Iantomasi T, Romagnoli C, Palmini G, Donati S, Falsetti I, Miglietta F, Aurilia C, Marini F, Giusti F, Brandi ML. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int J Mol Sci. 2023;24:3772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 233] [Reference Citation Analysis (0)] |
| 54. | Wittrant Y, Gorin Y, Woodruff K, Horn D, Abboud HE, Mohan S, Abboud-Werner SL. High d(+)glucose concentration inhibits RANKL-induced osteoclastogenesis. Bone. 2008;42:1122-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 55. | Wang X, Wang H, Zhang T, Cai L, Kong C, He J. Current Knowledge Regarding the Interaction Between Oral Bone Metabolic Disorders and Diabetes Mellitus. Front Endocrinol (Lausanne). 2020;11:536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Ren X, Liu H, Wu X, Weng W, Wang X, Su J. Reactive Oxygen Species (ROS)-Responsive Biomaterials for the Treatment of Bone-Related Diseases. Front Bioeng Biotechnol. 2021;9:820468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 57. | Mackenzie B, Garrick MD. Iron Imports. II. Iron uptake at the apical membrane in the intestine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G981-G986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 58. | Vogt AS, Arsiwala T, Mohsen M, Vogel M, Manolova V, Bachmann MF. On Iron Metabolism and Its Regulation. Int J Mol Sci. 2021;22:4591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 59. | Zang ZS, Xu YM, Lau ATY. Molecular and pathophysiological aspects of metal ion uptake by the zinc transporter ZIP8 (SLC39A8). Toxicol Res (Camb). 2016;5:987-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Gao G, Li J, Zhang Y, Chang YZ. Cellular Iron Metabolism and Regulation. Adv Exp Med Biol. 2019;1173:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 61. | Ren J, Liu R, Qin J. Research Progress on Relationship between Iron Metabolism and Type 2 Diabetes and Intervention Effect of GLP-1. J Neurol Res Rev Rep. 2023;5:1-4. [DOI] [Full Text] |
| 62. | Liu J, Li Q, Yang Y, Ma L. Iron metabolism and type 2 diabetes mellitus: A meta-analysis and systematic review. J Diabetes Investig. 2020;11:946-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 63. | Parham M, Tavasoli GR, Arsang-Jang S, Habibi MA, Dameshgi DO, Pashaei MR, Ahmadpour S, Vafaeimanesh J. Effect of Iron Deficiency Anemia on Blood Glucose and Insulin Resistance in Women with Type II Diabetes: A Single-group, Clinical Interventional Study. Rev Recent Clin Trials. 2024;19:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Rochette L, Dogon G, Rigal E, Zeller M, Cottin Y, Vergely C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int J Mol Sci. 2022;24:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 597] [Reference Citation Analysis (0)] |
| 65. | Harrison AV, Lorenzo FR, McClain DA. Iron and the Pathophysiology of Diabetes. Annu Rev Physiol. 2023;85:339-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 66. | Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 480] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 67. | Ndevahoma F, Mukesi M, Dludla PV, Nkambule BB, Nepolo EP, Nyambuya TM. Body weight and its influence on hepcidin levels in patients with type 2 diabetes: A systematic review and meta-analysis of clinical studies. Heliyon. 2021;7:e06429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Yang Y, Lin Y, Wang M, Yuan K, Wang Q, Mu P, Du J, Yu Z, Yang S, Huang K, Wang Y, Li H, Tang T. Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res. 2022;10:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 69. | Miao R, Fang X, Zhang Y, Wei J, Zhang Y, Tian J. Iron metabolism and ferroptosis in type 2 diabetes mellitus and complications: mechanisms and therapeutic opportunities. Cell Death Dis. 2023;14:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 125] [Reference Citation Analysis (0)] |
| 70. | Fernández-Real JM, McClain D, Manco M. Mechanisms Linking Glucose Homeostasis and Iron Metabolism Toward the Onset and Progression of Type 2 Diabetes. Diabetes Care. 2015;38:2169-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 71. | Xie T, Yao L, Li X. Advance in Iron Metabolism, Oxidative Stress and Cellular Dysfunction in Experimental and Human Kidney Diseases. Antioxidants (Basel). 2024;13:659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 72. | Dludla PV, Mabhida SE, Ziqubu K, Nkambule BB, Mazibuko-Mbeje SE, Hanser S, Basson AK, Pheiffer C, Kengne AP. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J Diabetes. 2023;14:130-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 159] [Article Influence: 53.0] [Reference Citation Analysis (12)] |
| 73. | Zhao X, Ma Y, Shi M, Huang M, Xin J, Ci S, Chen M, Jiang T, Hu Z, He L, Pan F, Guo Z. Excessive iron inhibits insulin secretion via perturbing transcriptional regulation of SYT7 by OGG1. Cell Mol Life Sci. 2023;80:159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Jin C, Tan K, Yao Z, Lin BH, Zhang DP, Chen WK, Mao SM, Zhang W, Chen L, Lin Z, Weng SJ, Bai BL, Zheng WH, Zheng G, Wu ZY, Yang L. A Novel Anti-Osteoporosis Mechanism of VK2: Interfering with Ferroptosis via AMPK/SIRT1 Pathway in Type 2 Diabetic Osteoporosis. J Agric Food Chem. 2023;71:2745-2761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 75. | Du YX, Zhao YT, Sun YX, Xu AH. Acid sphingomyelinase mediates ferroptosis induced by high glucose via autophagic degradation of GPX4 in type 2 diabetic osteoporosis. Mol Med. 2023;29:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 76. | Jiang Z, Wang H, Qi G, Jiang C, Chen K, Yan Z. Iron overload-induced ferroptosis of osteoblasts inhibits osteogenesis and promotes osteoporosis: An in vitro and in vivo study. IUBMB Life. 2022;74:1052-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 77. | Deng L, Mo MQ, Zhong J, Li Z, Li G, Liang Y. Iron overload induces islet β cell ferroptosis by activating ASK1/P-P38/CHOP signaling pathway. PeerJ. 2023;11:e15206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 78. | Jia P, Xu YJ, Zhang ZL, Li K, Li B, Zhang W, Yang H. Ferric ion could facilitate osteoclast differentiation and bone resorption through the production of reactive oxygen species. J Orthop Res. 2012;30:1843-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 79. | Li Y, Bai B, Zhang Y. Bone abnormalities in young male rats with iron intervention and possible mechanisms. Chem Biol Interact. 2018;279:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Wikarek A, Grabarczyk M, Klimek K, Janoska-Gawrońska A, Suchodolska M, Holecki M. Effect of Drugs Used in Pharmacotherapy of Type 2 Diabetes on Bone Density and Risk of Bone Fractures. Medicina (Kaunas). 2024;60:393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (16)] |
| 81. | Al-Mashhadi ZK, Viggers R, Starup-Linde J, Vestergaard P, Gregersen S. SGLT2 inhibitor treatment is not associated with an increased risk of osteoporotic fractures when compared to GLP-1 receptor agonists: A nationwide cohort study. Front Endocrinol (Lausanne). 2022;13:861422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Al-Mashhadi ZK, Viggers R, Fuglsang-Nielsen R, Vestergaard P, Gregersen S, Starup-Linde J. The risk of major osteoporotic fractures with GLP-1 receptor agonists when compared to DPP-4 inhibitors: A Danish nationwide cohort study. Front Endocrinol (Lausanne). 2022;13:882998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Lu J, Hu D, Zhang Y, Ma C, Shen L, Shuai B. Current comprehensive understanding of denosumab (the RANKL neutralizing antibody) in the treatment of bone metastasis of malignant tumors, including pharmacological mechanism and clinical trials. Front Oncol. 2023;13:1133828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (1)] |
| 84. | Zhang N, Zhang ZK, Yu Y, Zhuo Z, Zhang G, Zhang BT. Pros and Cons of Denosumab Treatment for Osteoporosis and Implication for RANKL Aptamer Therapy. Front Cell Dev Biol. 2020;8:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 85. | Yang YJ, Chen XE, Zhou XC, Liang FX. Mesenchymal stem cell-derived extracellular vesicles: A promising therapeutic strategy in diabetic osteoporosis. World J Diabetes. 2024;15:2399-2403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 86. | Bigford GE, Mendez AJ, Betancourt L, Burns-Drecq P, Backus D, Nash MS. A lifestyle intervention program for successfully addressing major cardiometabolic risks in persons with SCI: a three-subject case series. Spinal Cord Ser Cases. 2017;3:17007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 87. | Song LY, Xuan YL, Yang J, Xuan M, Wang YL, Song LG, Wang WX, Zhang XZ. [The association among the management of diet and sport, glucose metabolism, bone metabolism, and bone mineral density in postmenopausal women with type II diabetes and osteoporosis:a clinical study]. Zhongguo Guzhi Shusong Zazhi. 2014;20:156-160. |
| 88. | Zhang L, Luo YL, Xiang Y, Bai XY, Qiang RR, Zhang X, Yang YL, Liu XL. Ferroptosis inhibitors: past, present and future. Front Pharmacol. 2024;15:1407335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 89. | Deng Q, Zhu Y, Zhang M, Fei A, Liang J, Zheng J, Zhang Q, Cheng T, Ge X. Ferroptosis as a potential new therapeutic target for diabetes and its complications. Endocr Connect. 2023;12:e220419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 90. | Chen H, Han Z, Wang Y, Su J, Lin Y, Cheng X, Liu W, He J, Fan Y, Chen L, Zuo H. Targeting Ferroptosis in Bone-Related Diseases: Facts and Perspectives. J Inflamm Res. 2023;16:4661-4677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 91. | Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1891] [Cited by in RCA: 2979] [Article Influence: 595.8] [Reference Citation Analysis (1)] |
| 92. | Song X, Long D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front Neurosci. 2020;14:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 447] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 93. | Ru Q, Li Y, Xie W, Ding Y, Chen L, Xu G, Wu Y, Wang F. Fighting age-related orthopedic diseases: focusing on ferroptosis. Bone Res. 2023;11:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 124] [Reference Citation Analysis (0)] |
| 94. | Xiang Y, Song X, Long D. Ferroptosis regulation through Nrf2 and implications for neurodegenerative diseases. Arch Toxicol. 2024;98:579-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 95. | Saha M, Das S, Manna K, Saha KD. Melatonin targets ferroptosis through bimodal alteration of redox environment and cellular pathways in NAFLD model. Biosci Rep. 2023;43:BSR20230128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 96. | Kajarabille N, Latunde-Dada GO. Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int J Mol Sci. 2019;20:4968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 500] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 97. | Yehia A, Abulseoud OA. Melatonin: a ferroptosis inhibitor with potential therapeutic efficacy for the post-COVID-19 trajectory of accelerated brain aging and neurodegeneration. Mol Neurodegener. 2024;19:36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 98. | Ma H, Wang X, Zhang W, Li H, Zhao W, Sun J, Yang M. Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis. Oxid Med Cell Longev. 2020;2020:9067610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 99. | Guo Z, Lin J, Sun K, Guo J, Yao X, Wang G, Hou L, Xu J, Guo J, Guo F. Deferoxamine Alleviates Osteoarthritis by Inhibiting Chondrocyte Ferroptosis and Activating the Nrf2 Pathway. Front Pharmacol. 2022;13:791376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 100. | Zhang H, Wang A, Li G, Zhai Q, Huang Z, Wang X, Cao Z, Liu L, Liu G, Chen B, Zhu K, Xu Y, Xu Y. Osteoporotic bone loss from excess iron accumulation is driven by NOX4-triggered ferroptosis in osteoblasts. Free Radic Biol Med. 2023;198:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 101. | Ramadoss T, Weimer DS, Mayrovitz HN. Topical Iron Chelator Therapy: Current Status and Future Prospects. Cureus. 2023;15:e47720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 102. | Tao Y, Zhao Q, Lu C, Yong W, Xu M, Wang Z, Leng X. Melatonin suppresses atherosclerosis by ferroptosis inhibition via activating NRF2 pathway. FASEB J. 2024;38:e23678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 103. | Wang R, Su S, Chen Z, Zhou F. Type 2 Diabetes Mellitus Mediated Oxidative Stress in Bone Tissues and Novel Challenges for Biomaterials. Adv Ther. 2024;7:2300231. [DOI] [Full Text] |
| 104. | Liu Z, Mao J, Li W, Xu C, Lao A, Shin A, Wu J, Gu A, Zhang Z, Mao L, Lin K, Liu J. Smart glucose-responsive hydrogel with ROS scavenging and homeostasis regulating properties for diabetic bone regeneration. Chem Eng J. 2024;497:154433. [DOI] [Full Text] |
| 105. | Gong Y, Gan Y, Wang P, Gong C, Han B, Li P, Liu E, Yu Z, Sheng L, Wang X. Injectable foam-like scaffolds release glucose oxidase-integrated metal–organic framework hybrids for diabetic bone defects. Appl Mater Today. 2024;38:102190. [DOI] [Full Text] |
| 106. | Zhang Q, Chen W, Li G, Ma Z, Zhu M, Gao Q, Xu K, Liu X, Lu W, Zhang W, Wu Y, Shi Z, Su J. A Factor-Free Hydrogel with ROS Scavenging and Responsive Degradation for Enhanced Diabetic Bone Healing. Small. 2024;20:e2306389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 56] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 107. | Wang Z, Zhang Y, Chen S, Qu Y, Tang M, Wang W, Li W, Gu L. Multifunctional CeO2 nanozymes for mitigating high-glucose induced senescence and enhancing bone regeneration in type 2 diabetes mellitus. Chem Eng J. 2024;485:149842. [DOI] [Full Text] |
| 108. | Dong Q, Han Z, Gao M, Tian L. FNDC5/irisin ameliorates bone loss of type 1 diabetes by suppressing endoplasmic reticulum stressmediated ferroptosis. J Orthop Surg Res. 2024;19:205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 109. | Ye Y, Chen A, Li L, Liang Q, Wang S, Dong Q, Fu M, Lan Z, Li Y, Liu X, Ou JS, Lu L, Yan J. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. 2022;102:1259-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 259] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 110. | Huang J, Yang H, Chai S, Lin Y, Zhang Z, Huang H, Wan L. Identification of miRNAs related to osteoporosis by high-throughput sequencing. Front Pharmacol. 2024;15:1451695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 111. | Nyunt T, Britton M, Wanichthanarak K, Budamagunta M, Voss JC, Wilson DW, Rutledge JC, Aung HH. Mitochondrial oxidative stress-induced transcript variants of ATF3 mediate lipotoxic brain microvascular injury. Free Radic Biol Med. 2019;143:25-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 112. | Chen LJ, Li JY, Nguyen P, He M, Chen ZB, Subramaniam S, Shyy JY, Chien S. Single-cell RNA sequencing unveils unique transcriptomic signatures of endothelial cells and role of ENO1 in response to disturbed flow. Proc Natl Acad Sci U S A. 2024;121:e2318904121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 113. | Zhang Z, Ji C, Wang YN, Liu S, Wang M, Xu X, Zhang D. Maresin1 Suppresses High-Glucose-Induced Ferroptosis in Osteoblasts via NRF2 Activation in Type 2 Diabetic Osteoporosis. Cells. 2022;11:2560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 114. | Wei L, Gao J, Wang L, Tao Q, Tu C. Multi-omics analysis reveals the potential pathogenesis and therapeutic targets of diabetic kidney disease. Hum Mol Genet. 2024;33:122-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Song Z, Wang J, Zhang L. Ferroptosis: A New Mechanism in Diabetic Cardiomyopathy. Int J Med Sci. 2024;21:612-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/