Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.105447
Revised: March 30, 2025
Accepted: May 15, 2025
Published online: June 15, 2025
Processing time: 142 Days and 6.1 Hours
The coronavirus disease 2019 (COVID-19) pandemic has disproportionately impacted individuals with type 2 diabetes mellitus (T2DM), increasing their risk of severe illness and mortality. Vaccination has been a crucial intervention in mitigating these risks. However, the metabolic effects of COVID-19 vaccination, particularly the Johnson & Johnson (J&J) vaccine, in diabetic populations remain inadequately explored. This study investigated the longitudinal effects of the J&J vaccine on lipid and eicosanoid biomarkers to assess its metabolic safety and potential cardiovascular benefits.
To evaluate the long-term impact of the J&J COVID-19 vaccine on lipid and eicosanoid biomarkers in Ethiopian patients with T2DM.
This prospective cohort study was conducted at Adama Hospital Medical College (Oromia, Ethiopia) from May 2023 to June 2024. A total of 224 T2DM patients (57 vaccinated, 167 unvaccinated) were monitored for 1 year. Biomarkers including triglycerides (TGs), high-density lipoprotein (HDL), total cholesterol (TC), pro
TG and PG levels remained stable across all time points. HDL levels showed a temporary decline at 3 months (mean difference [MD] = -4.33; P < 0.001) and 6 months (MD = -2.62; P < 0.001) but recovered by 9 months (MD = 2.09; P = 0.001) and 1 year (MD = 2.38; P < 0.001). TC exhibited a significant decrease at 3 months (MD = -16.44, P = 0.001) before stabilizing. TX levels showed a consistent decline across all follow-ups (e.g., 1 year: MD = -0.08; P = 0.036), suggesting a reduced thrombotic risk. Correlation analysis indicated significant interrelations among biomarkers, emphasizing their roles in metabolic and inflammatory pathways.
The J&J COVID-19 vaccine exhibited metabolic safety in patients with T2DM, with transient HDL and TC reductions that later stabilized and a sustained TX decline, suggesting potential cardiovascular benefits. Further studies are needed to explore long-term immunometabolic effects on high-risk populations.
Core Tip: This cohort study assessed the long-term effects of the Johnson & Johnson coronavirus disease 2019 vaccine on metabolic markers in Ethiopian patients with type 2 diabetes mellitus. Key findings include temporary drops in high-density lipoprotein and total cholesterol levels post-vaccination, followed by recovery, whereas thromboxane levels showed lasting reductions, indicating potential thrombosis protection. The stability of triglycerides and prostaglandins underscores the vaccine's metabolic safety. These results contribute to understanding the vaccine's metabolic effects and highlight its safety and possible cardiovascular benefits for high-risk diabetic individuals.
- Citation: Edae CK, Bedada AT, Teklemariam MD, Girma T, Gebre SG. Longitudinal effects of Johnson & Johnson COVID-19 vaccination on metabolic biomarkers in type 2 diabetes mellitus in Ethiopia. World J Diabetes 2025; 16(6): 105447
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/105447.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.105447
Diabetes mellitus (DM) has been identified as a chronic metabolic condition affecting about 420 million individuals globally, with persistent hyperglycemia arising from deficiencies in insulin production, insulin resistance, or both[1,2]. Among the different kinds of diabetes, type 2 DM (T2DM) is the most common, defined by a combination of insulin resistance and beta-cell dysfunction affected by hereditary and environmental factors[3]. T2DM considerably increases the risk of consequences such as cardiovascular illnesses, renal failure, and mortality, mostly due to metabolic de
The coronavirus disease 2019 (COVID-19) pandemic, produced by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, disproportionately affects individuals with diabetes, leading to greater chances of severe disease, complications, and mortality[5]. Inadequate glycemic control exacerbates outcomes in diabetic individuals infected with COVID-19[6,7]. Vaccination has been emphasized as a vital intervention to minimize these hazards, particularly among susceptible groups such as individuals with T2DM[8].
The Johnson & Johnson (J&J) COVID-19 vaccine (Ad26. COV2. S), a single-dose adenovirus-vectored vaccine, has been widely deployed due to its proven efficacy and adaptability for usage in resource-limited situations[9]. While its protective effects against severe COVID-19 have been well established[10-12], its metabolic impacts on high-risk po
Researchers have identified that lipid and eicosanoid biomarkers serve as crucial indications of metabolic and inflammatory status[13]. Biomarkers such as triglycerides (TGs), high-density lipoprotein (HDL), and total cholesterol (TC) have been closely linked to immune responses and inflammation, with dysregulation contributing to poor outcomes in infections, including COVID-19[14,15]. Similarly, eicosanoids, particularly prostaglandins (PGs) and thromboxanes (TXs), play critical roles in inflammation and coagulation, pathways involved with catastrophic COVID-19 outcomes[16,17]. Longitudinal evaluations of these biomarkers have been recommended as a tool to investigate the systemic effects of vaccination on metabolic homeostasis[18].
Despite an increasing corpus of research on COVID-19 immunization, the evidence on its metabolic effects on diabetic populations from LMICs are sparse[19]. This study intends to fill this gap by analyzing long-term changes in lipid and eicosanoid biomarkers—including TG, HDL, TC, PG, and TX—in Ethiopian individuals with T2DM who received the J&J COVID-19 vaccination. To this end, the study explored the vaccine's immunometabolic effects and provided information regarding its safety and efficacy in managing high-risk diabetic groups.
This study utilized a prospective cohort design conducted at Adama Hospital Medical College (Oromia, Ethiopia) from May 2023 to June 2024. Participants were separated into two groups: A vaccinated group, who received the J&J COVID-19 vaccine, and an unvaccinated group. The vaccinated group received a single dose of the vaccine at baseline, followed by sample collection at 3 months, 6 months, 9 months, and 1 year. This strategy allowed for a longitudinal examination of the vaccine's influence on lipid and eicosanoid biomarkers.
The study comprised persons aged 18 years and older diagnosed with T2DM and receiving regular care at Adama Hospital Medical College. Exclusion criteria included a history of severe adverse reactions to immunizations, co
The sample size for the study was determined by considering the longitudinal (repeated measures from each subject over time) nature of the research. The sample size was calculated using the formula from Diggle et al[20] for comparing two means to detect a 0.5 mean difference (MD) in lipid and eicosanoid biomarkers between the vaccinated and unvaccinated groups, as obtained from a study conducted in 2023 by Irún et al[21]. Five measurements were planned in the study, which occurred at baseline, 3 months, 6 months, 9 months, and 1 year. The following parameters were used for sample size determination: Number of time points (t = 5), type I error rate (α = 0.05), power (90%), smallest meaningful difference (d = 0.5), and a ratio of 1:3 (λ = 3) between the vaccinated and unvaccinated groups. We assumed a standard deviation (σ) of 1 and a correlation (r = 0.02) of repeated measures. The unequal sample size between the groups was due to various reasons. The expected effect size (ES) was small (the 0.5 MD observed in our study). Having a larger control group (unvaccinated individuals) can enhance the precision of our estimates and increase statistical power. During the initial COVID-19 vaccination season, unvaccinated individuals were more readily available due to low vaccination coverage. Additionally, we anticipated that many of these unvaccinated individuals may choose to get vaccinated and drop out of the study during the follow-up period.
A minimum sample size of 13 vaccinated and 39 unvaccinated participants was required. To account for potential attrition, a 10% adjustment was added to the minimum required sample sizes; therefore, 15 vaccinated and 45 un
Particularly, given our continuous counseling efforts and ongoing national vaccination campaigns, we initially recruited 75 vaccinated and 225 unvaccinated participants who met the inclusion criteria. However, during the follow-up period, 18 participants in the vaccinated group and 58 in the unvaccinated group were lost due to vaccination uptake or other factors. Ultimately, 57 vaccinated and 167 unvaccinated participants completed the study, which remained above the minimum required sample size for the study.
Baseline data, including demographic characteristics, medical history, and lifestyle factors such as smoking, alcohol use, and dietary habits, were collected by structured interviews and medical record reviews. Blood samples were taken at baseline and during follow-up visits at 3 months, 6 months, 9 months, and 1-year post-vaccination. Standardized protocols were followed for blood collection and all samples were kept at -80 °C until analysis.
Blood samples were taken at each follow-up visit using standardized venipuncture techniques under rigorous aseptic circumstances[22]. Serum was separated by centrifugation and kept in sterile Eppendorf tubes at -80 °C until analysis. Fasting lipid profiles, including TC, HDL, and TG, were tested at the Adama Public Health Research and Referral Laboratory Center (Adama, Ethiopia) using known techniques[23]. Prostaglandin E2 (PGE2) and thromboxane B2 (TXB2) levels were determined using enzyme-linked immunosorbent assay kits from Biosource (Invitrogen Corporation, Carlsbad, California, United States) and Abcam (Cambridge, United Kingdom), respectively, according to the manufacturers’ instructions. All assays were performed in accordance with the manufacturer’s instructions, ensuring complete compliance with quality control and biosafety requirements.
Data were analyzed using SPSS version 26 (IBM SPSS Statistics, Armonk, NY, United States) and Stata version 18 (StataCorp, College Station, TX, United States). Descriptive statistics summarized baseline characteristics. Independent t-tests were employed to compare continuous data, whereas χ2 tests analyzed differences in categorical variables. Longitudinal variations in biochemical parameters were analyzed using Generalized Estimating Equations (GEE) to account for within-subject correlations between time points. Statistical significance was set at P < 0.05. Effect sizes, including Cohen's d, were assessed to determine the clinical relevance of observed changes.
The work was approved by the institutional review board of Addis Ababa University College of Health Sciences (Protocol No. 019/23/biochemistry) and the National Research Ethics Review Committee (Ref No: 17/152/235/24). Written informed consent was obtained from all participants, and the study adhered to the principles described in the Declaration of Helsinki[24].
The baseline characteristics of study participants, as given in Table 1, were evaluated using the χ2 test to assess their association with J&J COVID-19 vaccination status. Among the individuals, 25.5% were vaccinated, whereas 74.5% were unvaccinated. Age distribution showed no statistically significant difference between groups (P = 0.446), with participants aged ≤ 40 comprising 36.2% of the total, and those > 40 contributing 63.8%. Similarly, sex distribution was comparable across vaccinated and unvaccinated groups (P = 0.936), with females constituting 51.3% and males 48.7%. Alcohol use and chat usage were also not significantly linked with vaccination status (P = 0.211 and P = 0.348, respectively). Smoking prevalence was low (2.7%) and showed no significant variation across groups (P = 0.174). Regarding medication use, those using insulin, metformin, or both demonstrated no significant changes in vaccination status (P = 0.399). These findings suggest no significant relationships between J&J COVID-19 immunization status and age, sex, alcohol drinking, smoking and medication of subjects.
| Variables | Categories | COVID-19 vaccination status | P value1 | Total | |
| Vaccinated, n = 57 (25.5) | Unvaccinated, n = 167 (74.5) | ||||
| Age | ≤ 40 years | 23 (28.4) | 58 (71.6) | 0.446 | 81 (36.2) |
| > 40 years | 34 (23.8) | 109 (76.2) | 143 (63.8) | ||
| Sex | Female | 29 (25.2) | 86 (74.8) | 0.936 | 115 (51.3) |
| Male | 28 (25.7) | 81 (74.3) | 109 (48.7) | ||
| Alcohol | Yes | 15 (32.6) | 31 (67.4) | 0.211 | 46 (20.5) |
| No | 42 (23.6) | 136 (76.4) | 178 (79.5) | ||
| Chat | Yes | 8 (33.3) | 16 (66.7) | 0.348 | 24 (10.7) |
| No | 49 (24.5) | 151 (75.5) | 200 (89.3) | ||
| Smoking | Yes | 3 (50.0) | 3 (50.0) | 0.174 | 6 (2.7) |
| No | 54 (24.8) | 164 (75.2) | 218 (97.3) | ||
| Medications | Insulin | 13 (23.2) | 43 (76.8) | 0.399 | 56 (25.0) |
| Metformin | 24 (22.9) | 81 (77.1) | 105 (46.9) | ||
| Both | 20 (31.8) | 43 (68.3) | 63 (28.1) | ||
The results in Table 2 indicate the changes in TG, HDL, TC, PG, and TX over 1 year following vaccination. TG levels revealed no significant change across all time periods following vaccination, with P > 0.05 and ESs near zero, showing that immunization did not influence TG levels. HDL levels, however, showed a significant reduction at 3 months (mean difference [MD] = -4.33, ES = -0.60; P < 0.001,) and 6 months (MD = -2.62, ES = -0.40; P < 0.001), followed by a significant increase at 9 months (MD = 2.09, ES = 0.27; P = 0.001) and 1 year (MD = 2.38, ES = 0.33; P < 0.001), suggesting that vaccination may have initially lowered HDL levels, but these levels subsequently recovered and even increased over time. TC levels revealed a substantial drop at 3 months (MD = -16.44, ES = -0.70; P = 0.001), but remained stable thereafter, showing a transitory benefit of immunization in lowering cholesterol that did not continue in the long term. PG levels remained stable throughout the trial, demonstrating that immunization had no impact on PG synthesis. TX levels revealed statistically significant decreases at all time intervals post-immunization (e.g., 1 year: MD = -0.08, ES = -0.30; P = 0.036), showing a prolonged reduction in TX following vaccination. These findings indicate that whereas immunization did not alter TG or PG, it had transitory effects on HDL and TC, with a sustained drop in TX over the 1-year follow-up period.
| Outcomes | Baseline | 3 months | 6 months | 9 months | 1 year | |||||
| MD | ES | MD | ES | MD | ES | MD | ES | MD | ES | |
| TG | -0.57 (-7.46, 6.32) | -0.00 | 3.29 (-0.37, 6.96) | 0.14 | 1.33 (-2.31, 4.97) | 0.06 | -1.34 (-5.09, 2.41) | -0.10 | -2.34 (-6.09, 1.41) | -0.10 |
| P value | 0.869 | 0.078 | 0.471 | 0.482 | 0.220 | |||||
| HDL | 1.42 (-0.90, 3.74) | 0.18 | -4.33 (-5.57, | -0.60 | -2.62 (-3.78, | -0.40 | 2.09 (0.89, 3.30) | 0.27 | 2.38 (1.22, 3.55) | 0.33 |
| P value | 0.229 | < 0.001a | < 0.001a | 0.001a | < 0.001a | |||||
| TC | 1.42 (-4.04, 6.87) | 0.06 | -16.44 (-20.36, | -0.70 | -0.39 (-3.98, 3.19) | -0.00 | -0.20 (-3.33, 2.93) | -0.00 | 2.74 (-0.86, 6.33) | 0.12 |
| P value | 0.609 | 0.001a | 0.829 | 0.898 | 0.135 | |||||
| PG | -0.03 (-0.63, 0.58) | -0.00 | -0.03 (-0.63, 0.58) | -0.00 | -0.03 (-0.63, 0.58) | -0.00 | -0.03 (-0.63, 0.58) | -0.00 | -0.03 (-0.63, 0.58) | -0.00 |
| P value | 0.933 | 0.933 | 0.933 | 0.933 | 0.933 | |||||
| TX | -0.09 (-0.17, 0.00) | -0.30 | -0.09 (-0.18, | -0.30 | -0.08 (-0.15, 0.01) | -0.30 | -0.09 (-0.18, | -0.30 | -0.08 (-0.15, 0.01) | -0.30 |
| P value | 0.041a | 0.038a | 0.036a | 0.038a | 0.036a | |||||
The correlation coefficients in Table 3 demonstrate substantial interrelationships among TG, HDL, TC, PG, and TX, with statistically significant connections discovered at P < 0.05. TG showed a strong positive association with TC (r = 0.69, P < 0.05) and a moderate positive correlation with TX (r = 0.38, P < 0.05), showing that greater TG levels are connected with increasing amounts of these molecules. Conversely, TG was negatively linked with HDL (r = -0.55, P < 0.05), suggesting an inverse association where elevated TG corresponded to reduced HDL levels. HDL was highly negatively correlated with TC (r = -0.79, P < 0.05) and moderately negatively correlated with TX (r = -0.29, P < 0.05), suggesting that increased HDL levels may be linked to reduced cholesterol and TX concentrations. TC exhibited a small but statistically significant negative association with PG (r = -0.09, P < 0.05) and a positive correlation with TX (r = 0.34, P < 0.05). Additionally, PG and TX demonstrated a modest negative connection (r = -0.14, P < 0.05), indicating a subtle inverse link.
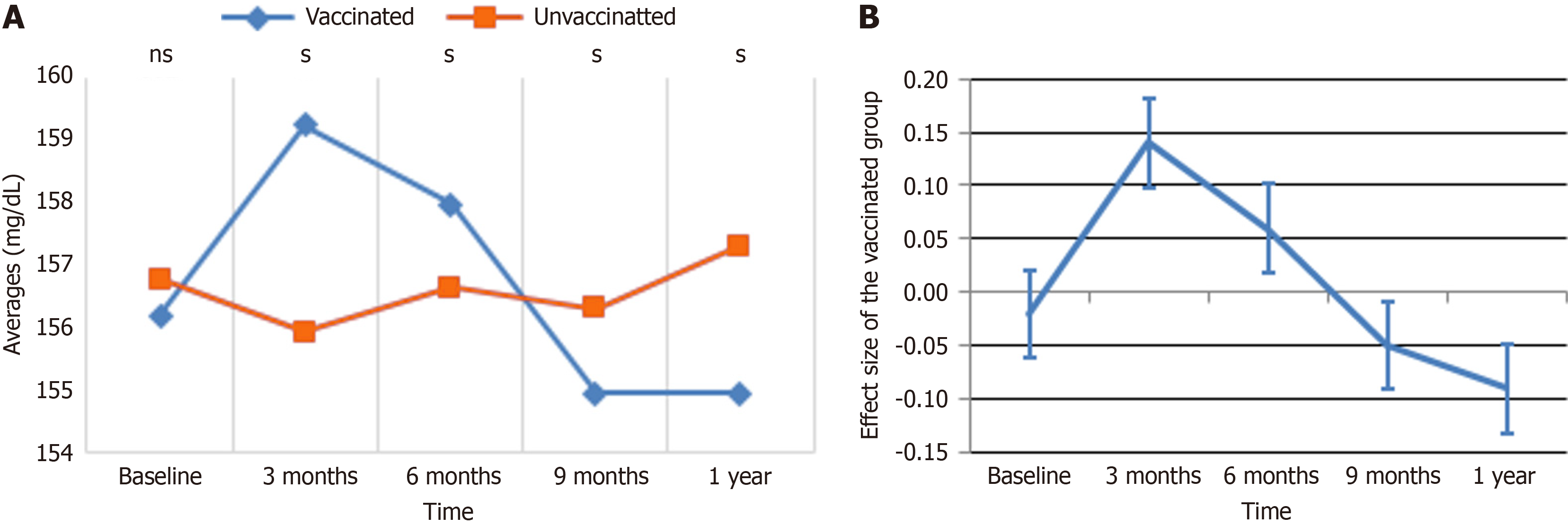
TG: Figure 1 displays the trends in TG levels and the related ESs over time for vaccinated and unvaccinated groups, providing complementary viewpoints on the observed changes. In Figure 1A, both groups started with comparable TG levels at baseline. Over the coming months, a significant divergence emerges: The vaccinated group exhibited a fast increase, peaking at 159 after 3 months, while the unvaccinated group remained relatively stable. After this high, TG levels in the vaccinated group fell sharply, continuing to decrease until reaching a minimum at 9 months, at which point the unvaccinated group began to show a tiny increase. By 1 year, TG levels settled in both groups, with the vaccinated group retaining lower levels than the unprotected group. Figure 1B supplements these data by displaying the effect magnitude of immunization on TG levels over time. At baseline, the ES was close to zero, indicating no meaningful difference between the groups. By 3 months, the ES climbed sharply, peaking at roughly 0.2, reflecting the considerable increase in TG levels in the vaccinated group. This was followed by a continuous fall, with the impact size nearing zero by 6 months and reaching a minimum of -0.1 at 9 months. By 1 year, the impact size stabilized at a negative value, corresponding with lower TG levels seen in the vaccinated group compared to the unvaccinated group. Together, these panels demonstrate a dynamic response in TG levels following immunization, characterized by an early spike, a subsequent fall, and final stabilization below baseline levels.
The GEE multiple regression analysis for TG levels are shown in Table 4. Vaccination status showed no significant connection with TG levels in both crude (β = 0.07, P = 0.959) and adjusted models (β = 1.46, P = 0.263). Additionally, whereas time was substantially linked with TG levels (P < 0.001), no significant changes in TG levels were found at 3 months, 6 months, 9 months, or 1 year compared to baseline. The interaction between vaccination and time likewise did not provide significant effects, with all interaction terms exhibiting non-significant p-values (P > 0.05). Age was revealed as a key predictor, with people aged ≤ 40 years having lower TG levels compared to those older than 40 years (β = -13.29, P < 0.001). Similarly, females showed considerably lower TG levels than males (β = -8.68, P < 0.001). Alcohol usage was related with considerably reduced TG levels (β = -8.77, P < 0.001), but smoking and chat exposure showed no significant relationships. In terms of pharmaceuticals, metformin was substantially linked with elevated TG levels in the crude analysis (β = 7.08, P < 0.001), although this association was attenuated in the adjusted model (β = 2.75, P = 0.176). Insulin usage and combination therapy did not show significant effects. These results indicate that TG levels are influenced primarily by age, sex, alcohol consumption, and medication, with no significant impacts from vaccination status or time-related interactions.
| Variables (n = 224) | Categories | Crude effect | Adjusted effect | ||
| β (95%CI) | P value | β (95%CI) | P value | ||
| Vaccination status | Vaccinated | 0.07 (-2.76, 2.91) | 0.9591 | 1.46 (-1.09, 4.02) | 0.263 |
| Unvaccinated | Reference | Reference | |||
| Time | < 0.00a | < 0.00b | |||
| 3 months | 0.15 (-3.40, 3.71) | 0.933 | 0.15 (-3.40, 3.71) | 0.933 | |
| 6 months | 0.35 (-3.62, 4.32) | 0.862 | 0.35 (-3.62, 4.32) | 0.862 | |
| 9 months | -0.67 (-2.84, 1.49) | 0.542 | -0.67 (-2.84, 1.49) | 0.542 | |
| 1 year | 0.07 (-2.09, 2.24) | 0.948 | 0.07 (-2.09, 2.24) | 0.948 | |
| Base line | Reference | Reference | |||
| Group timeb | 0.070 | ||||
| Vaccinated 3 months | 3.87 (-2.99, 10.72) | 0.269 | |||
| Vaccinated 6 months | 1.90 (-5.29, 9.10) | 0.604 | |||
| Vaccinated 9 months | -0.77 (-6.49, 4.96) | 0.793 | |||
| Vaccinated 1 year | -1.77 (-7.49, 3.96) | 0.546 | |||
| Vaccinated baseline | Reference | ||||
| Age group | ≤ 40 years | -12.92 (-16.27, -9.57) | < 0.001a | -13.29 (-16.70, -9.88) | < 0.001b |
| > 40 years | Reference | Reference | |||
| Sex | Female | -6.40 (-10.33, -2.48) | 0.001a | -8.68 (-12.41, -4.95) | < 0.001b |
| Male | Reference | Reference | |||
| Alcohol exposure | Yes | -6.38 (-10.42, -2.35) | 0.002a | -8.77 (-12.64, -4.89) | < 0.001b |
| No | Reference | Reference | |||
| Chat exposure | Yes | 2.45 (-3.18, 8.09) | 0.394 | ||
| No | Reference | ||||
| Smoking exposure | Yes | 3.32 (-7.28, 13.93) | 0.539 | ||
| No | Reference | ||||
| Medications | 0.003a | 0.394 | |||
| Insulin | 1.16 (-4.15, 6.47) | 0.668 | 0.79 (-3.97, 5.56) | 0.745 | |
| Metformin | 7.08 (2.73, 11.44) | 0.001 | 2.75 (-1.23, 6.72) | 0.176 | |
| Both | Reference | Reference | |||
| Intercept | 167.68 | ||||
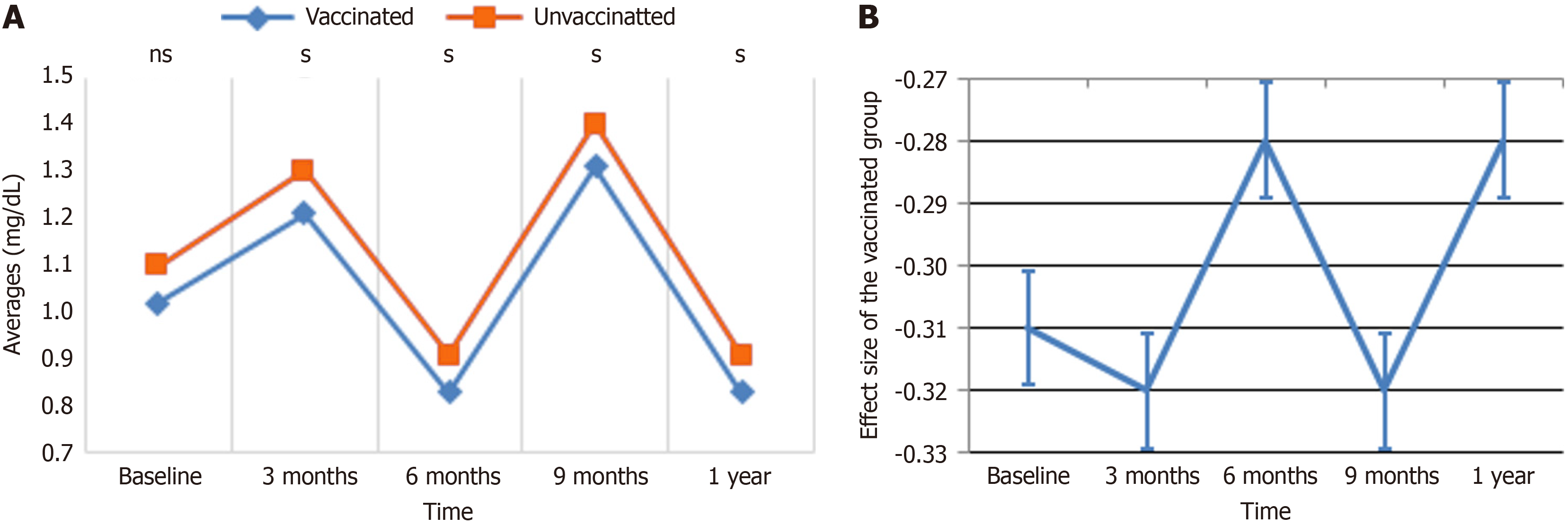
HDL: Figure 2 displays the average value trends of HDL levels over time for vaccinated and unvaccinated populations. At baseline, both groups displayed similar HDL levels, with the vaccinated group having slightly higher values. By the 3-month mark, HDL levels in the vaccinated group decreased significantly, whereas the unvaccinated group exhibited a small decline. At 6 months, HDL levels in the vaccinated group begin to recover but stayed below baseline, whereas the unvaccinated group showed a slight increase. By the 9-month point, HDL levels in both groups converged, after which the vaccinated group had a precipitous fall by 1 year, reaching a value much lower than the unvaccinated group, which maintained a more consistent trajectory. These observations indicate that vaccination may alter HDL levels, with a considerable reduction occurring in the latter months.
The GEE analysis demonstrated that changes in HDL levels were strongly impacted by time, vaccination, and demographic characteristics, as shown in Table 5. Vaccination status alone did not reveal a significant effect on HDL levels in the adjusted analysis (β = 1.30, P = 0.210). Over time, HDL levels decreased significantly at 3 months (β = -2.23, P = 0.004), 9 months (β = -3.60, P < 0.001), and 1 year (β = -3.19, P < 0.001) compared to baseline, while the interaction between vaccination and time indicated significant reductions in HDL levels at 3 months (β = -5.75, P < 0.001) and 6 months (β = -4.04, P = 0.002), with no significant changes at 9 months (β = 0.67, P = 0.553) or 1 year (β = 0.96, P = 0.457). Younger ones (≤ 40 years) showed greater HDL levels compared to those older than 40 (β = 2.23, P < 0.001), while females displayed higher HDL levels than males (β = 1.33, P = 0.008). Other covariates, including alcohol exposure (β = -0.57, P = 0.348), chat use (β = 0.23, P = 0.734), smoking (β = 0.06, P = 0.934), and medication use (P = 0.749), did not demonstrate significant relationships with HDL levels. These findings demonstrate that time, age, and sex are key predictors of HDL changes, with considerable reductions in the vaccinated group during the early follow-up period.
| Variables (n = 224) | Categories | Crude effect | Adjusted effect | ||
| β (95%CI) | P value | β (95%CI) | P value | ||
| Vaccination status | Vaccinated | -0.21 (-1.02, 0.59) | 0.6091 | 1.30 (-0.74, 3.34) | 0.210 |
| Unvaccinated | Reference | Reference | |||
| Time | < 0.001a | < 0.001b | |||
| 3 months | -3.69 (-4.96, -2.42) | < 0.001 | -2.23 (-3.74, -0.72) | 0.004b | |
| 6 months | -2.22 (-3.55, -0.89) | 0.001 | -1.19 (-2.82, 0.43) | 0.150 | |
| 9 months | -3.43 (-4.37, -2.49) | < 0.001 | -3.60 (-4.67, -2.54) | < 0.001b | |
| 1 year | 2.95 (--4.26, -1.64) | < 0.001 | -3.19 (-4.82, -1.57) | < 0.001b | |
| Baseline | Reference | Reference | |||
| Group timeb | Vaccinated 3 months | -5.75 (-8.18, -3.33) | < 0.001b | ||
| Vaccinated 6 months | -4.04 (-6.58, -1.49) | 0.002b | |||
| Vaccinated 9 months | 0.67 (-1.55, 2.90) | 0.553 | |||
| Vaccinated 1 year | 0.96 (-1.58, 3.50) | 0.457 | |||
| Vaccinated baseline | Reference | ||||
| Age group | ≤ 40 years | 2.15 (1.16, 3.14) | < 0.001a | 2.23 (1.21, 3.25) | < 0.001b |
| > 40 years | Reference | Reference | |||
| Sex | Female | 1.23 (0.22, 2.23) | 0.017a | 1.33 (.35, 2.31) | 0.008b |
| Male | Reference | Reference | |||
| Alcohol exposure | Yes | -0.57 (-1.76, 0.62) | 0.348 | ||
| No | Reference | ||||
| Chat exposure | Yes | 0.23 (-1.08, 1.53) | 0.734 | ||
| No | Reference | ||||
| Smoking exposure | Yes | 0.06 (-1.41, 1.53) | 0.934 | ||
| No | Reference | ||||
| Medications | 0.081 | 0.749 | |||
| Insulin | 0.23 (-1.19, 1.64) | 0.753 | 0.29 (-1.08, 1.67) | 0.676 | |
| Metformin | -1.03 (-2.09, 0.03) | 0.057 | -0.27 (-1.36, 0.82) | 0.628 | |
| Both | Reference | Reference | |||
| Intercept | 57.58 | ||||
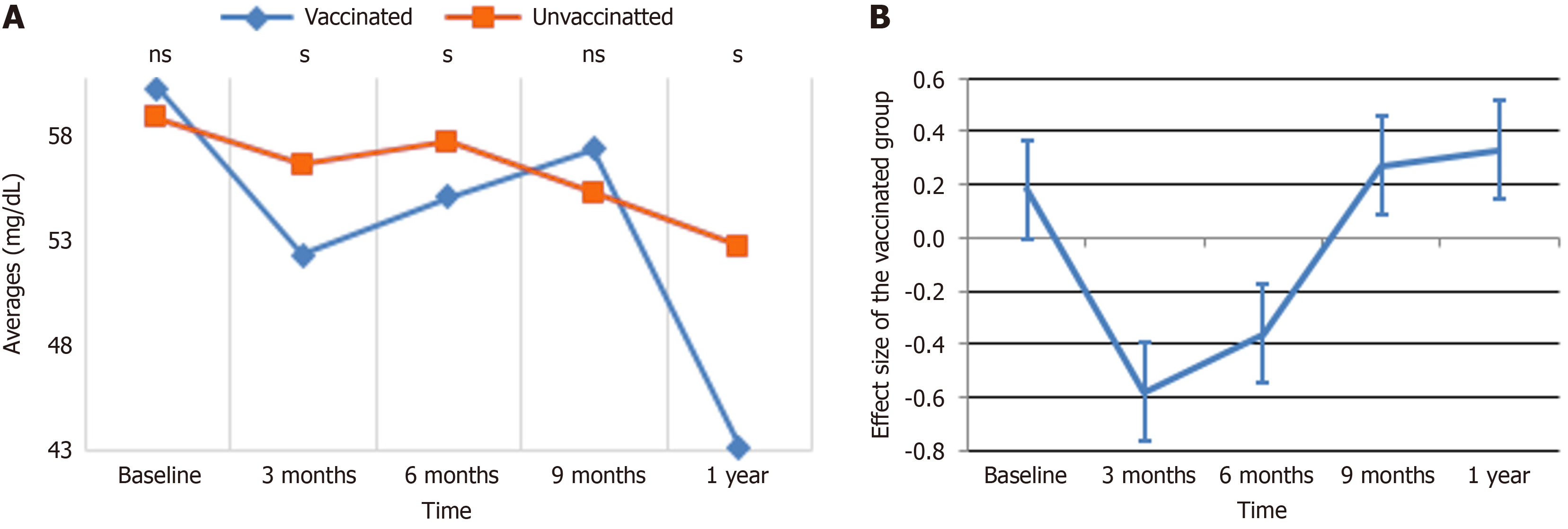
TC: The patterns and ESs of TC levels over time are presented in Figure 3A and B. In Figure 3A, the average TC levels for vaccinated and unvaccinated groups are compared throughout time. At baseline, both groups exhibit similar TC levels. At 3 months, the unvaccinated group exhibited a dramatic increase, peaking at roughly 201, while the vaccinated group remained reasonably steady. By 6 months, the TC levels in the unvaccinated group declined considerably, aligning closely with those of the vaccinated group. From 9 months to 1 year, the unvaccinated group exhibited a small rising trend, but the vaccinated group maintained rather steady levels throughout the follow-up period. Figure 3B depicts the ES of vaccination on TC levels over time. At baseline, the ES was around zero, indicating no major difference between the groups. By 3 months, the impact size reduced sharply to roughly -0.8, reflecting the pronounced increase in TC levels in the unvaccinated group compared to the vaccinated group. At 6 months, the ES began to recover, moving toward zero, and maintained this trend through 9 months and 1 year, stabilizing at a slightly positive number. These data indicate that immunization helps stabilize TC levels, minimizing the volatility observed in the unvaccinated group over time.
As indicated in Table 6, the study demonstrated that chronological, demographic, and behavioral factors significantly influenced TC levels. Vaccination status alone did not significantly affect TC levels after adjustment (β = 2.28, P = 0.347); however, the interaction between vaccination and time demonstrated a significant reduction in TC levels at 3 months for the vaccinated group compared to the unvaccinated group (β = -17.85, P < 0.001), with no significant changes at later time points. Younger participants (≤ 40 years) had considerably lower TC levels than older participants (β = -6.19, P < 0.001), while females had lower levels compared to males (β = -4.90, P = 0.007). Behavioral characteristics, including alcohol use (β = -5.09, P = 0.005) and smoking (β = -4.47, P = 0.020), were related with reduced TC levels, but chat exposure showed no significant impact. Although metformin use appeared to influence TC levels in the crude analysis, this effect was not significant after adjustment. These findings show the dynamic impacts of immunization, time, demographic variables, and behaviors on TC changes.
| Variables (n = 224) | Categories | Crude effect | Adjusted effect | ||
| β (95%CI) | P value | β (95%CI) | P value | ||
| Vaccination status | Vaccinated | -2.58 (-4.93, -0.22) | 0.032a | 2.28 (-2.47, 7.04) | 0.347 |
| Unvaccinated | Reference | Reference | |||
| Time | < 0.001a | < 0.001b | |||
| 3 months | 12.42 (8.61, 16.22) | < 0.001 | 16.96 (12.26, 21.66) | < 0.001b | |
| 6 months | -0.32 (-4.08, 3.45) | 0.869 | 0.14 (-4.71, 4.99) | 0.954 | |
| 9 months | -0.19 (-1.83, 1.44) | 0.814 | 0.22 (-1.43, 1.87) | 0.798 | |
| 1 year | 0.35 (-3.42, 4.12) | 0.856 | 0.01 (-4.85, 4.87) | 0.996 | |
| Baseline | Reference | Reference | |||
| Group timeb | Vaccinated 3 months | -17.85 (-24.17, -11.53) | < 0.001b | ||
| Vaccinated 6 months | -1.81 (-8.16, 4.54) | 0.577 | |||
| Vaccinated 9 months | -1.62 (-6.16, 2.92) | 0.485 | |||
| Vaccinated 1 year | 1.32 (-5.04, 7.68) | 0.684 | |||
| Vaccinated baseline | Reference | ||||
| Age group | ≤ 40 years | -6.14 (-9.43, -2.85) | < 0.001a | -6.19 (-9.59, -2.81) | < 0.001b |
| > 40 years | Reference | Reference | |||
| Sex | Female | -3.39 (-6.65, -0.56) | 0.041a | -4.90 (-8.47, -1.33) | 0.007b |
| Male | Reference | Reference | |||
| Alcohol exposure | Yes | -3.85 (-7.14, 0.62) | 0.022a | -5.09 (-8.65, -1.53) | 0.005b |
| No | Reference | Reference | |||
| Chat exposure | Yes | 0.80 (-3.48, 5.09) | 0.714 | ||
| No | Reference | ||||
| Smoking exposure | Yes | -2.99 (-6.38, 0.39) | 0.083a | -4.47 (-8.26, -0.69) | 0.020b |
| No | Reference | Reference | |||
| Medications | 0.024a | 0.437 | |||
| Insulin | 1.67 (-3.04, 6.38) | 0.486 | 1.13 (-3.43, 5.70) | 0.627 | |
| Metformin | 4.71 (1.28, 8.14) | 0.007 | 2.26 (-1.21, 5.72) | 0.202 | |
| Both | Reference | ||||
| Intercept | 192.41 | ||||
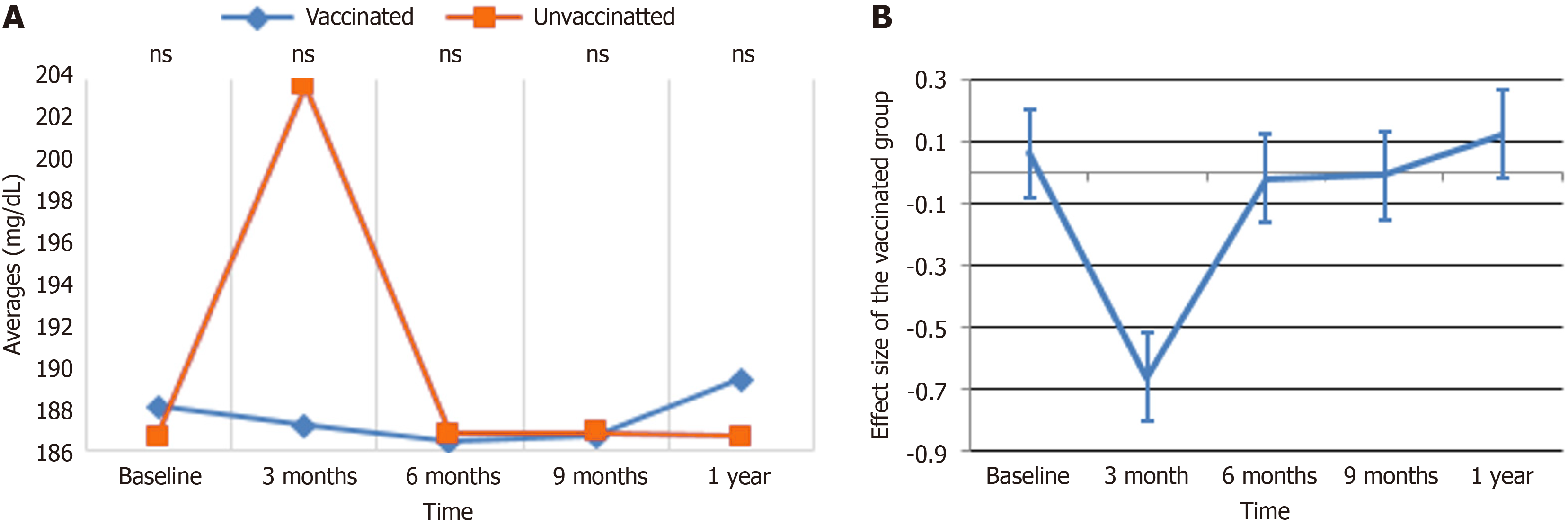
PG: Figure 4 depicts the impact of immunization on PG levels over time. In Figure 4A, the vaccinated group displayed a rapid and prolonged fall in PG levels by the 3-month mark, which remained consistently low through 6 months, 9 months, and 1 year, while the unvaccinated group maintained relatively steady levels with just a modest decline after 6 months. Figure 4B further underlines that the ES of PG levels in the vaccinated group remained around zero across all time points, showing minimal variability and a uniform reduction across individuals. These findings combined indicate that vaccination considerably reduces PG levels in a consistent and continuous manner, whereas PG levels in the unvaccinated group remained mostly unaltered.
The results indicated in Table 7, resulting from GEE multiple regression analysis, revealed that age and metformin use are significant predictors of PG levels among the 224 persons studied. After controlling for covariates, participants aged ≤ 40 years were shown to have substantially lower PG levels compared to those aged > 40 years (β = -0.68, P = 0.010). It was also shown that metformin administration was related with a considerable drop in PG levels (β = -1.22, P < 0.001) compared to other drug regimens. Although females were shown to have higher PG levels in the crude analysis (β = 0.83, P = 0.001), this connection was not significant after correction (β = 0.45, P = 0.118). Vaccination status apparently did not have a significant effect, as vaccinated people showed an adjusted β of -0.04 (P = 0.880) relative to unvaccinated persons. Similarly, alcohol exposure (β = -0.33, P = 0.281), chat exposure (β = 0.31, P = 0.407), and smoking exposure (β = 0.58, P = 0.488) were not significantly linked with PG levels. These findings indicate that whereas age and metformin use significantly influence PG levels, other factors, including vaccination status and behavioral exposures, appear to have limited or no impact.
| Variables (n = 224) | Crude effect | Adjusted effect | |||
| β (95%CI) | P value | β (95%CI) | P value | ||
| Vaccination status | Vaccinated | -0.03 (-0.62, 0.57) | 0.9331 | -0.04 (-0.56, 0.48) | 0.880 |
| Unvaccinated | Reference | Reference | |||
| Age group | ≤ 40 years | -0.53 (-1.07, 0.01) | 0.055a | -0.68 (-1.20, -0.17) | 0.010b |
| > 40 years | Reference | Reference | |||
| Sex | Female | 0.83 (0.32, 1.33) | 0.001a | 0.45 (-0.11, 1.01) | 0.118 |
| Male | Reference | Reference | |||
| Alcohol exposure | Yes | -0.53 (-1.17, 0.10) | 0.101a | -0.33 (-0.94, 0.27) | 0.281 |
| No | Reference | Reference | |||
| Chat exposure | Yes | 0.31 (-0.42, 1.04) | 0.407 | ||
| No | Reference | ||||
| Smoking exposure | Yes | 0.58 (-1.07, 2.24) | 0.488 | ||
| No | Reference | ||||
| Medications | 0.001a | < 0.001b | |||
| Insulin | -0.33 (-1.01, 0.34) | 0.335 | -0.37 (-1.03, 0.29) | 0.278 | |
| Metformin | -1.12 (-1.71, -0.53) | < 0.001 | -1.22 (-1.79, -0.65) | < 0.001b | |
| Both | Reference | ||||
| Intercept | 26.51 | ||||
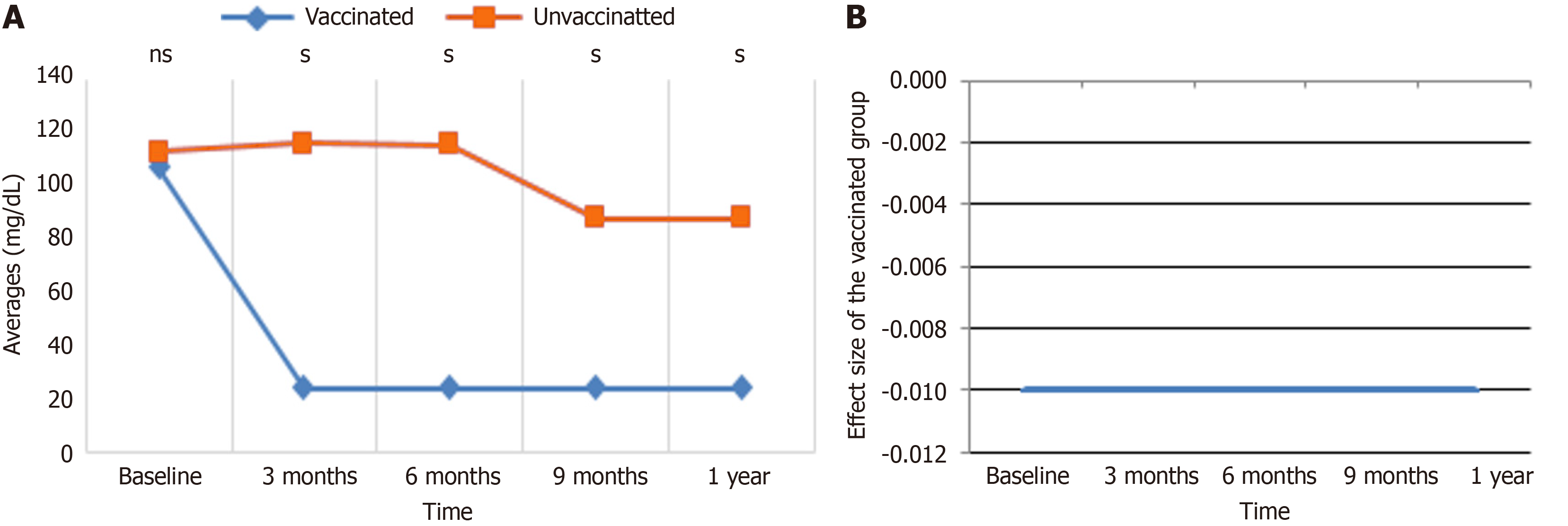
TX average value trend and ES: Figure 5 indicates the patterns and influence of vaccination on TX levels over time. In Figure 5A, the average TX levels are displayed for vaccinated and unvaccinated groups at baseline, 3 months, 6 months, 9 months, and 1 year. Both groups displayed similar TX levels at baseline, but the unvaccinated group showed higher variability, with noticeable peaks at 3 and 9 months, while the vaccinated group demonstrated a steadier trend with lesser changes. By 1 year, TX levels in both groups converged to similar averages. Figure 5B displays the ES of vaccination on TX levels throughout the same time points, revealing a consistently negative effect ranging between -0.27 and -0.33. Although the effect magnitude fluctuated slightly, notably at 6 and 9 months, it indicates that vaccination is associated with a moderate reduction in TX levels. Collectively, these findings indicate that immunization stabilizes TX levels throughout time and contributes to a modest reduction compared to the unvaccinated group.
Table 8 summarize the GEE multiple regression analysis assessing the correlations between various variables and TX levels across 224 participants. Vaccination status was substantially associated with lower TX levels, as vaccinated individuals had a crude β of -0.09 (P = 0.032) and an adjusted β of -0.07 (P = 0.034) compared to unvaccinated persons, showing a protective effect of vaccination. Time was a significant predictor, with subjects > 40 years continuously displaying greater TX levels (adjusted β = 0.19, P < 0.001), demonstrating an age-related rise in TX. Sex was also a significant determinant, as females showed lower TX levels (adjusted β = -0.19, P < 0.001), while males displayed greater amounts (adjusted β = 0.29, P < 0.001). Regarding drugs, insulin use was related with a significant drop in TX levels (adjusted β = -0.34, P < 0.001) compared to metformin. The combination of both drugs revealed a slightly smaller but still substantial reduction (adjusted β = -0.13, P < 0.001). However, lifestyle factors such as alcohol exposure, conversation exposure, and smoking exposure did not significantly influence TX levels. The interaction between group and time was not significant (P = 0.247), suggesting that the combined influence of both variables on TX levels was minimal. Overall, the data indicate the strong impact of vaccination, age, sex, and medication use, notably insulin, on TX levels, while lifestyle exposures appear to have negligible influence.
| Variables (n = 224) | Crude effect | Adjusted effect | |||
| β (95%CI) | P value | β (95%CI) | P value | ||
| Vaccination status | Vaccinated | -0.09 (-0.16, -0.01) | 0.032a | -0.07 (-0.13, -0.01) | 0.034b |
| Unvaccinated | Reference | Reference | |||
| Time | < 0.001a | < 0.001b | |||
| 3 months | 0.19 (0.19, 0.20) | < 0.001 | 0.19 (0.19, 0.20) | < 0.001b | |
| 6 months | -0.19 (-0.20, -0.19) | < 0.001 | -0.19 (-0.20, -0.19) | < 0.001b | |
| 9 months | 0.29 (0.29, 0.30) | < 0.001 | 0.29 (0.29, 0.30) | < 0.001b | |
| 1 year | -0.19 (-0.20, -0.19) | < 0.001 | -0.19 (-0.20, -0.19) | < 0.001b | |
| Baseline | Reference | Reference | |||
| Group timeb | Vaccinated 3 months | -0.00 (-0.01, 0.00) | 0.247 | ||
| Vaccinated 6 months | 0.01 (-0.01, 0.02) | 0.247 | |||
| Vaccinated 9 months | -0.00 (-0.01, 0.00) | 0.247 | |||
| Vaccinated 1 year | 0.01 (-0.01, 0.02) | 0.247 | |||
| Vaccinated baseline | Reference | ||||
| Age group | ≤ 40 years | -0.34 (-0.39, -0.28) | < 0.001a | -0.34 (-0.39, -0.29) | < 0.001b |
| > 40 years | Reference | Reference | |||
| Sex | Female | -0.12 (-0.19, -0.04) | 0.002a | -0.13 (-0.19, -0.07) | < 0.001b |
| Male | Reference | Reference | |||
| Alcohol exposure | Yes | -0.02 (-0.11, 0.07) | 0.706 | ||
| No | Reference | ||||
| Chat exposure | Yes | -0.01 (-0.14, 0.12) | 0.885 | ||
| No | Reference | ||||
| Smoking exposure | Yes | -0.02 (-0.19, 0.16) | 0.853 | ||
| No | Reference | ||||
| Medications | < 0.001a | 0.222 | |||
| Insulin | 0.07 (-0.01, 0.16) | 0.094 | 0.05 (-0.02, 0.12) | 0.139 | |
| Metformin | 0.16 (0.08, 0.24) | < 0.001 | 0.05 (-0.02, 0.13) | 0.151 | |
| Both | Reference | ||||
| Intercept | 1.29 | ||||
This study assessed the long-term effects of the J&J COVID-19 vaccination on metabolic markers, including TG, HDL, TC, PG, and TX, in type 2 diabetic patients. The data showed different patterns of biomarker changes, indicating the vaccination's immunometabolic impact and its importance to both vaccine safety and efficacy.
TG stability: TG levels remained steady at all time points post-vaccination, suggesting negligible influence of the vaccine on TG metabolism. This stability coincides with studies by Alghamdi et al[25], who observed little changes in TG levels post-COVID-19 vaccination. By contrast, TG variability is more pronounced after acute SARS-CoV-2 infection, typically suggesting systemic inflammation and metabolic dysregulation[26]. The lack of significant TG modifications in this trial highlights the vaccine's safety in preserving lipid stability, particularly crucial for diabetic persons at higher risk of dyslipidemia. A potential mechanism for this stability may involve the vaccine's targeted immune activation, which does not provoke widespread systemic inflammation or alter hepatic lipid synthesis pathways, thus maintaining TG homeostasis.
HDL dynamics: HDL levels revealed a biphasic trend, with significant reductions at 3- and 6-months post-vaccination followed by recovery and stabilization at 9 months and 1 year. Similar patterns of temporary HDL decline during early immunological activation have been described, demonstrating the redistribution of lipoproteins as part of the inflammatory response[15]. HDL's anti-inflammatory and antioxidative capabilities are well-established, and its recovery post-vaccination indicates resolution of the immunological response without long-term deleterious effects[27]. These findings also correlate with studies associating low baseline HDL levels to worse outcomes in COVID-19, underlining HDL's potential as a biomarker for both disease severity and vaccine efficacy[14]. The transient HDL reduction may be attributed to acute-phase responses that modulate lipid metabolism, where pro-inflammatory cytokines temporarily suppress apolipoprotein A-I synthesis, leading to reduced HDL levels before subsequent homeostatic recovery.
TC trends: The observed drop in TC levels 3 months post-vaccination, with stabilization thereafter, supports a temporary immune activation altering lipid metabolism. This trend matches alterations seen in acute COVID-19, when TC levels drop in response to heightened inflammation and lipid redistribution[28]. Unlike the persistent dyslipidemia reported in post-COVID conditions, the transient nature of TC changes post-vaccination reflects the vaccine’s targeted immune activation and minimal disruption to long-term lipid homeostasis[28-31]. This phenomenon may result from vaccine-induced inflammatory cytokines influencing hepatic cholesterol synthesis and clearance, leading to a temporary decline in circulating cholesterol levels before restoration of metabolic equilibrium.
PG stability: PG levels were unaltered throughout the trial, contrasting with findings from other vaccines, such as the Bacillus Calmette-Guérin vaccine, which has been found to modify eicosanoid pathways[32]. This stability may indicate that the J&J vaccine generates a more targeted immunological response, with reduced activation of broad inflammatory mediators like PG. Such specificity minimizes the danger of systemic inflammation, adding to the vaccine's good safety profile[33,34]. One possible explanation for this stability is that the vaccine-induced immune response may not ex
TX reduction and thrombosis risk: TX levels revealed consistent decreases across all time periods post-vaccination, a comforting conclusion considering early concerns regarding vaccine-induced thrombotic events. TX are essential mediators of platelet activation and thrombogenesis, and their lowering predicts a lowered tendency for thrombotic problems post-vaccination[17]. These findings accord with studies indicating no substantial increase in thrombotic risk following COVID-19 vaccination and emphasize the vaccine's involvement in reducing TX-mediated prothrombotic states[35,36]. The observed TX reduction may be driven by a vaccine-induced shift in platelet function, potentially through modulation of endothelial nitric oxide and prostacyclin production, which counteract TX synthesis and promote vascular homeostasis.
The correlation analysis revealed extensive interactions across lipid and eicosanoid indicators, underlining their linked involvement in metabolic and inflammatory processes. TG favorably associated with TC and TX but negatively with HDL, consistent with recognized lipid metabolic relationships[37]. HDL's negative connection with TX underlines its protective effect against thrombogenesis, reinforcing its importance in cardiovascular health[38]. These findings agree with broader discoveries that lipid and eicosanoid dynamics can serve as markers of inflammatory and metabolic responses, both in infection and post-vaccination contexts[39]. The interplay between these markers may be mediated by vaccine-induced immunomodulation, wherein inflammatory cytokines transiently influence lipid transport and meta
In summary, this study shows the nuanced effects of the J&J vaccine on lipid and eicosanoid biomarkers, indicating temporary alterations compatible with immune activation and resolution without long-term deleterious effects. The stability of TG and PG, the recovery of HDL, the temporary fall in TC, and the prolonged decrease in TX synergistically emphasize the vaccine's metabolic safety and prospective cardiovascular advantages. These findings provide a platform for further study into the molecular pathways behind biomarker dynamics and their implications for vaccine efficacy and safety in diabetic and larger populations.
Although this study incorporated statistical adjustments to address differences between the vaccinated and unvaccinated groups, the possibility of selection bias cannot be completely eliminated. Future research would benefit from the implementation of propensity score matching to improve the comparability of the groups by effectively balancing observed covariates. Furthermore, we recognize that conducting additional sensitivity analyses could yield more comprehensive insights into the robustness of our findings. While our present analytical strategy is statistically valid, employing these advanced methodologies could significantly enhance the rigor and precision of subsequent investigations in this field.
This study shows the transitory and reversible effects of the J&J COVID-19 vaccination on lipid and eicosanoid bio
The authors express their gratitude to the staff of Adama Hospital Medical College for their invaluable support throughout this study. They also extend their heartfelt thanks to all participants for their involvement and cooperation.
| 1. | Addissouky T, Ali M, El Sayed IET, Wang Y. Revolutionary Innovations in Diabetes Research: From Biomarkers to Genomic Medicine. Iranian J Diabet Obes. 2023;15:; 228-242. [DOI] [Full Text] |
| 2. | Deng L, Jia L, Wu XL, Cheng M. Association Between Body Mass Index and Glycemic Control in Type 2 Diabetes Mellitus: A Cross-Sectional Study. Diabetes Metab Syndr Obes. 2025;18:555-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Zhao X, An X, Yang C, Sun W, Ji H, Lian F. The crucial role and mechanism of insulin resistance in metabolic disease. Front Endocrinol (Lausanne). 2023;14:1149239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 265] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 4. | Abel ED, Gloyn AL, Evans-Molina C, Joseph JJ, Misra S, Pajvani UB, Simcox J, Susztak K, Drucker DJ. Diabetes mellitus-Progress and opportunities in the evolving epidemic. Cell. 2024;187:3789-3820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 122] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 5. | Drucker DJ. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: the end of the beginning. Cell Metab. 2021;33:479-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 6. | Li R, Shen M, Yang Q, Fairley CK, Chai Z, McIntyre R, Ong JJ, Liu H, Lu P, Hu W, Zou Z, Li Z, He S, Zhuang G, Zhang L. Global Diabetes Prevalence in COVID-19 Patients and Contribution to COVID-19- Related Severity and Mortality: A Systematic Review and Meta-analysis. Diabetes Care. 2023;46:890-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 7. | Yu B, Li C, Sun Y, Wang DW. Insulin Treatment Is Associated with Increased Mortality in Patients with COVID-19 and Type 2 Diabetes. Cell Metab. 2021;33:65-77.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 8. | Pal R, Bhadada SK, Misra A. COVID-19 vaccination in patients with diabetes mellitus: Current concepts, uncertainties and challenges. Diabetes Metab Syndr. 2021;15:505-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 9. | Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Van Dromme I, Spiessens B, Vingerhoets J, Custers J, Scheper G, Robb ML, Treanor J, Ryser MF, Barouch DH, Swann E, Marovich MA, Neuzil KM, Corey L, Stoddard J, Hardt K, Ruiz-Guiñazú J, Le Gars M, Schuitemaker H, Van Hoof J, Struyf F, Douoguih M; ENSEMBLE Study Group. Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S. N Engl J Med. 2022;386:847-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 10. | Corchado-Garcia J, Zemmour D, Hughes T, Bandi H, Cristea-Platon T, Lenehan P, Pawlowski C, Bade S, O'Horo JC, Gores GJ, Williams AW, Badley AD, Halamka J, Virk A, Swift MD, Wagner T, Soundararajan V. Analysis of the Effectiveness of the Ad26.COV2.S Adenoviral Vector Vaccine for Preventing COVID-19. JAMA Netw Open. 2021;4:e2132540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Lewis NM, Self WH, Gaglani M, Ginde AA, Douin DJ, Keipp Talbot H, Casey JD, Mohr NM, Zepeski A, Ghamande SA, McNeal TA, Shapiro NI, Gibbs KW, Files DC, Hager DN, Shehu A, Prekker ME, Erickson HL, Gong MN, Mohamed A, Johnson NJ, Srinivasan V, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Busse LW, Lohuis CCT, Duggal A, Wilson JG, Gordon AJ, Qadir N, Chang SY, Mallow C, Rivas C, Babcock HM, Kwon JH, Exline MC, Lauring AS, Halasa N, Chappell JD, Grijalva CG, Rice TW, Rhoads JP, Jones ID, Stubblefield WB, Baughman A, Womack KN, Lindsell CJ, Hart KW, Zhu Y, Adams K, Patel MM, Tenforde MW; IVY Network Collaborators. Effectiveness of the Ad26.COV2.S (Johnson & Johnson) Coronavirus Disease 2019 (COVID-19) Vaccine for Preventing COVID-19 Hospitalizations and Progression to High Disease Severity in the United States. Clin Infect Dis. 2022;75:S159-S166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Livingston EH, Malani PN, Creech CB. The Johnson & Johnson Vaccine for COVID-19. JAMA. 2021;325:1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 13. | Davinelli S, Intrieri M, Corbi G, Scapagnini G. Metabolic indices of polyunsaturated fatty acids: current evidence, research controversies, and clinical utility. Crit Rev Food Sci Nutr. 2021;61:259-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Agouridis AP, Pagkali A, Zintzaras E, Rizos EC, Ntzani EE. High-density lipoprotein cholesterol: A marker of COVID-19 infection severity? Atheroscler Plus. 2021;44:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Stepanova N, Rysyev A, Snisar L. Low high-density lipoprotein level is a risk factor for postvaccination COVID-19 in hemodialysis patients. Atherosclero. 2023;379:S134. [DOI] [Full Text] |
| 16. | Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ, Alexiou A, Mukerjee N, Batiha GE. Prostaglandins and non-steroidal anti-inflammatory drugs in Covid-19. Biotechnol Genet Eng Rev. 2024;40:3305-3325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Chiang KC, Raghavan R, Gupta A. SARS-CoV-2 vaccination induced cerebral venous sinus thrombosis: Do megakaryocytes, platelets and lipid mediators make up the orchestra? Free Neuropathol. 2021;2:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Ahmed M, Suhrawardy A, Olszewski A, Rahman T, Makhni EC. Overlapping Surgery in Orthopaedics: A Review of Efficacy, Surgical Costs, Surgical Outcomes, and Patient Safety. J Am Acad Orthop Surg. 2024;32:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Bhutta ZA, Salam RA, Gomber A, Lewis-Watts L, Narang T, Mbanya JC, Alleyne G. A century past the discovery of insulin: global progress and challenges for type 1 diabetes among children and adolescents in low-income and middle-income countries. Lancet. 2021;398:1837-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Diggle PJ, Heagerty PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford: Oxford Academic, 2003: 181. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Irún P, Gracia R, Piazuelo E, Pardo J, Morte E, Paño JR, Boza J, Carrera-Lasfuentes P, Higuera GA, Lanas A. Serum lipid mediator profiles in COVID-19 patients and lung disease severity: a pilot study. Sci Rep. 2023;13:6497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 22. | Wickremsinhe E, Fantana A, Berthier E, Quist BA, Lopez de Castilla D, Fix C, Chan K, Shi J, Walker MG, Kherani JF, Knoderer H, Regev A, Harding JJ. Standard Venipuncture vs a Capillary Blood Collection Device for the Prospective Determination of Abnormal Liver Chemistry. J Appl Lab Med. 2023;8:535-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 23. | Ćurić ŽB, Masle AM, Kibel A, Selthofer-Relatić K, Stupin A, Mihaljević Z, Jukić I, Stupin M, Matić A, Kozina N, Šušnjara P, Juranić B, Kolobarić N, Šerić V, Drenjančević I. Effects of n-3 Polyunsaturated Fatty Acid-Enriched Hen Egg Consumption on the Inflammatory Biomarkers and Microvascular Function in Patients with Acute and Chronic Coronary Syndrome-A Randomized Study. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 24. | World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16597] [Cited by in RCA: 20261] [Article Influence: 1558.5] [Reference Citation Analysis (9)] |
| 25. | Alghamdi A, Wani K, Alnaami AM, Al-Daghri NM. Dose Intervals and Time since Final Dose on Changes in Metabolic Indices after COVID-19 Vaccination. Vaccines (Basel). 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Arutyunov GP, Tarlovskaya EI, Arutyunov AG, Polyakov DS, Grigorieva NY, Gubareva IV, Kamilova UK, Kim ZF, Kuznetsova AS, Kuznetsova TY, Ruzanov DY, Svarovskaya AV, Smirnova EА, Sugraliev AB, Frolova IA, Aimakhanova GT, Batluk TI, Bashkinov RA, Bikushova IV, Gordeychuk ED, Gubareva EY, Evdokimov DS, Zakirova GA, Loginova AO, Melnikov ES, Moiseenko NB, Trubnikova MA, Shcherbakov SY. Lipid profile changes after the acute COVID-19 period. Sub-analysis of the International Registry "Dynamics Analysis of Comorbidities in SARS-CoV-2 Survivors" (AKTIV SARS-CoV-2)" (12-month follow-up). Russ J Cardiol. 2024;29:5716. [DOI] [Full Text] |
| 27. | Chidambaram V, Kumar A, Sadaf MI, Lu E, Al'Aref SJ, Tarun T, Galiatsatos P, Gulati M, Blumenthal RS, Leucker TM, Karakousis PC, Mehta JL. COVID-19 in the Initiation and Progression of Atherosclerosis: Pathophysiology During and Beyond the Acute Phase. JACC Adv. 2024;3:101107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 28. | Chidambaram V, Shanmugavel Geetha H, Kumar A, Majella MG, Sivakumar RK, Voruganti D, Mehta JL, Karakousis PC. Association of Lipid Levels With COVID-19 Infection, Disease Severity and Mortality: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2022;9:862999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Bellavite P, Ferraresi A, Isidoro C. Immune Response and Molecular Mechanisms of Cardiovascular Adverse Effects of Spike Proteins from SARS-CoV-2 and mRNA Vaccines. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 30. | Jang J, Oh HJ, Lee EK. Despite the pandemic: upward trajectories of medication adherence and persistence in patients with dyslipidemia. Front Pharmacol. 2024;15:1488452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Tavartkiladze A, Simonia G, Kasradze D, Okrostsvaridze N, Revazishvili P, Maisuradze M, Andronikashvili I, Tavartkiladze G, Dundua G, Egiazarov D, Gabadadze S, Potskhoraia T, Japaridze T, Mamukishvili T. Impact of Persistent Biochemical Alterations in Post-COVID Syndrome Patients on Cancer Risk, Cardiovascular Health, and Dyslipidemia A Comparative Study with Control Group. J Cancer Res. 2024;2:1-22. |
| 32. | Nathella PK, Padmapriyadarsini C, Nancy A, Karunanithi K, Selvaraj N, Renji RM, Shrinivasa BM, Babu S. BCG vaccination is associated with longitudinal changes in systemic eicosanoid levels in elderly individuals: A secondary outcome analysis. Heliyon. 2024;10:e32643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Bitounis D, Jacquinet E, Rogers MA, Amiji MM. Strategies to reduce the risks of mRNA drug and vaccine toxicity. Nat Rev Drug Discov. 2024;23:281-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 118] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 34. | Lavelle EC, McEntee CP. Vaccine adjuvants: Tailoring innate recognition to send the right message. Immunity. 2024;57:772-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 35. | Hippisley-Cox J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, Zaccardi F, Watkinson P, Shankar-Hari M, Doidge J, Harrison DA, Griffin SJ, Sheikh A, Coupland CAC. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 36. | Jain K, Tyagi T, Gu SX, Faustino EVS, Hwa J. Demographic diversity in platelet function and response to antiplatelet therapy. Trends Pharmacol Sci. 2025;46:78-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Zinellu A, Paliogiannis P, Fois AG, Solidoro P, Carru C, Mangoni AA. Cholesterol and Triglyceride Concentrations, COVID-19 Severity, and Mortality: A Systematic Review and Meta-Analysis With Meta-Regression. Front Public Health. 2021;9:705916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Sotoudeheian M. Neutrophil-to-High-Density Lipoprotein Cholesterol Ratio and Metabolic Dysfunction-Associated Steatotic Liver Disease. 2025 Preprint. [DOI] [Full Text] |
| 39. | Richter FC, Alrubayyi A, Teijeira Crespo A; Oxford-Cardiff COVID-19 Literature Consortium, Hulin-Curtis S. Impact of obesity and SARS-CoV-2 infection: implications for host defence - a living review. Oxf Open Immunol. 2021;2:iqab001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/