Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.103520
Revised: March 4, 2025
Accepted: April 10, 2025
Published online: June 15, 2025
Processing time: 204 Days and 2.8 Hours
Diabetic foot ulcers (DFUs) are a significant challenge in diabetic care, and the efficacy of negative pressure wound therapy (NPWT) in treating them remains a subject of continuous investigation.
To provide a comprehensive meta-analysis of the role of NPWT in the manage
A systematic review was performed based on Preferred Reporting Items for Sys
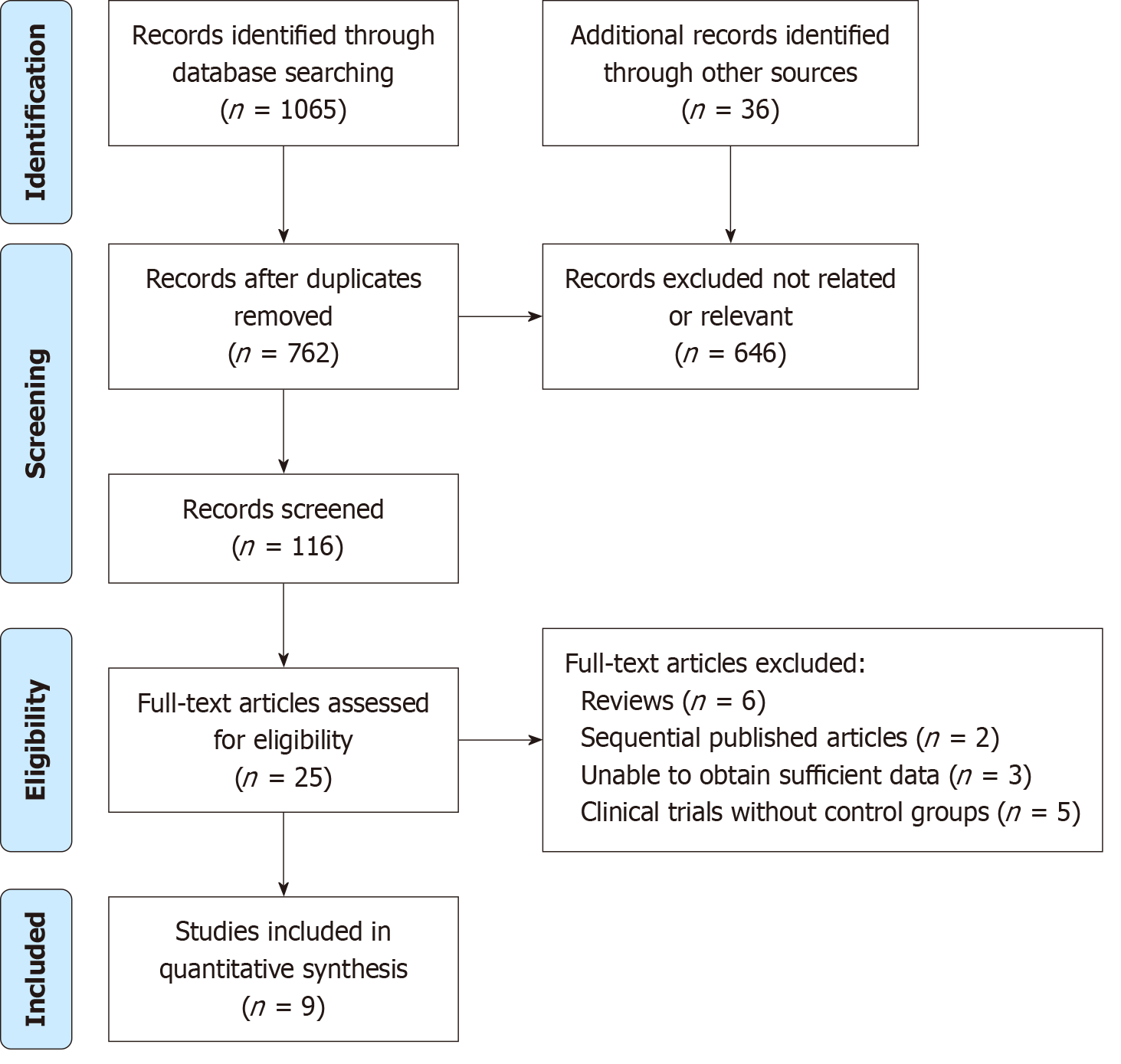
Of the 1101 identified articles, 9 RCTs were selected for meta-analysis. Studies spanned from 2005 to 2020 and originated from countries including the United States, Chile, Pakistan, Italy, India, and Germany. Meta-analysis demonstrated a significant improvement in wound healing rate [risk ratio (RR) = 1.46, 95%CI: 1.22-1.76, P < 0.01] and a reduction in amputation rate (RR = 0.69, 95%CI: 0.50-0.96, P = 0.006) with NPWT. Furthermore, the time for granulation tissue formation was significantly reduced by an average of 19.54 days. However, the incidence of adverse events did not significantly differ between NPWT and control treatments.
NPWT significantly improves wound healing rates and reduces amputation rates in DFUs. It also hastens the formation of granulation tissue. However, the therapy does not significantly alter the risk of adverse events compared to alternate treatments.
Core Tip: This comprehensive meta-analysis synthesizes data from randomized controlled trials to evaluate the impact of negative pressure wound therapy (NPWT) on diabetic foot ulcers, a prevalent and challenging complication in diabetic care. Our analysis not only reaffirms the efficacy of NPWT in enhancing wound healing rates and reducing amputation risks but also emphasizes its role in accelerating granulation tissue formation without increasing adverse events.
- Citation: Deng YX, Wang XC, Xia ZY, Wan MY, Jiang DY. Efficacy and safety of negative pressure wound therapy for the treatment of diabetic foot ulcers: A meta-analysis. World J Diabetes 2025; 16(6): 103520
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/103520.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.103520
Diabetes mellitus (DM) is a metabolic disorder resulting from inadequate insulin secretion or functional deficiency. It is a significant global public health issue that is increasing in incidence[1]. Diabetic foot ulcers (DFUs) are among the most prevalent and serious consequences of diabetes, impacting 12% to 25% of patients with the condition. The majority of these patients are at high risk for recurrence within 5 years post-treatment, adversely affecting their quality of life and imposing a considerable strain on healthcare systems[2]. Multiple etiological factors contribute to the onset of DFUs, including inadequate glycemic management, trauma, callus formation, foot deformities, unsuitable footwear, dry skin, peripheral neuropathy, and compromised circulation. Chronic, non-healing DFUs may ultimately result in limb amputa
In recent years, our expanding knowledge of the cellular and molecular mechanisms of wound healing has enabled the development of numerous new wound treatment methods. These encompass hyperbaric oxygen therapy, the use of topical growth factors, the use of bioengineered skin and tissue replacements, and negative pressure wound therapy (NPWT)[2]. NPWT has garnered significant attention owing to its distinctive mechanism of action and effectiveness. Although the exact underlying mechanisms of NPWT activity are not fully understood, it is thought that there are several synergistic effects. NPWT is thought to create a vacuum at the wound site, influencing microvascular hemodynamics to enhance blood flow and consequently improve the delivery of oxygen and nutrients, which are critical for wound healing[5]. Minimizing wound exudate can diminish local inflammation and related consequences. Moreover, the enhancement of granulation tissue, an essential component of the healing process, is another proposed consequence of NPWT. The therapy may also limit bacterial contamination in the wound environment, hence minimizing the likelihood of wound infection and associated consequences[6]. Moreover, NPWT is thought to facilitate wound closure by employing mecha
Notwithstanding the recognized efficacy of NPWT in promoting wound healing, a substantial disparity exists between theoretical and empirical evidence and the results obtained in practical settings. This mismatch underscores the necessity for additional exploration to fully harness the potential of NPWT and enhance its application in clinical environments. Systematic reviews and meta-analyses offer rigorous approaches to synthesize findings from numerous studies, im
Throughout the course of this research, standards set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[9] were rigorously followed. On September 28, 2024, comprehensive searches were conducted across four digital repositories: PubMed, Embase, Web of Science, and the Cochrane Library, without any temporal restrictions. The search terminology and query structure were meticulously tailored to each individual database's specifications. The specific search terms of PubMed were: ("negative pressure wound therapy" OR "NPWT") AND ("diabetic foot" OR "diabetic foot ulcer" OR "DFU") AND ("randomized" OR "clinical trial"). No language limitation was applied. Reference lists of relevant articles were screened manually for possible additional records.
The inclusion criteria were as follows: (1) Studies involving patients with DFUs regardless of the severity or grade; (2) Experimental group must receive intervention using various negative pressure devices; (3) Control group must be treated using different wound dressings; (4) Must be a prospective randomized controlled trial (RCT); and (5) Outcome measures should include wound healing time, wound healing rate, granulation tissue formation time, amputation rate, and the incidence of adverse events.
The exclusion criteria were as follows: (1) Studies in which NPWT was not used properly, for instance, studies where instances of leakage were not addressed promptly; (2) Studies with incomplete data or where data could not be accessed; or (3) Case reports, commentaries, expert opinion and narrative reviews.
During review, two independent assessors undertook the tasks of literature screening and data extraction. Following this, mutual verification was performed. If any disparities arose, a collaborative discussion between the reviewers was conducted. If a consensus remained elusive, a third evaluator was consulted for arbitration. The extraction process encompassed critical data points from the selected articles, including the primary author, date of publication, research setting and participant count, study methodology, ulcer severity, and in-depth details concerning the intervention-ranging from application methods and distinctive features of the NPWT apparatus to the specific treatments admini
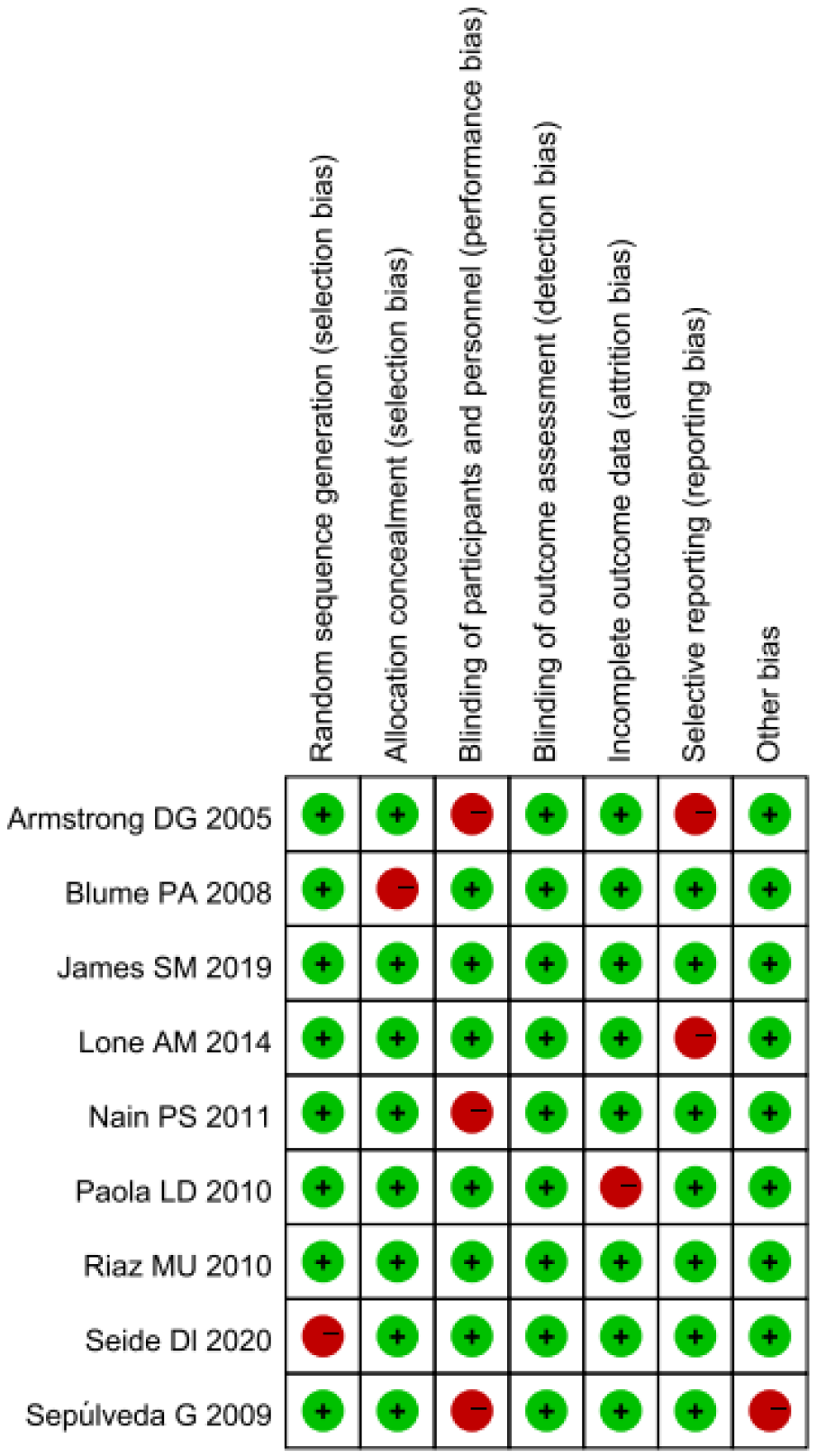
In evaluating the quality of the studies incorporated, the Cochrane Collaboration's risk of bias instrument[10] was utilized. Two independent evaluators examined several domains: random sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective reporting, and other potential bias sources. Each domain was classified as having a low, uncertain, or high risk of bias. Discrepancies in evaluations were resolved through discussion, and a third reviewer was consulted when necessary.
Statistical heterogeneity of the included studies was assessed using χ2 statistics, and the extent of heterogeneity was measured by the I2 value. In instances where the I2 value was below 50% and the corresponding P value was ≥ 0.10, this indicated a lack of significant heterogeneity, leading to the application of the fixed-effect model to calculate the overall effect size. If the I2 value was ≥ 50% or the corresponding P value was < 0.10, this indicated considerable heterogeneity. In instances of observed statistical heterogeneity, the random-effects model was utilized to ascertain the composite effect size. The weighted mean difference and its 95%CI were utilized as the effect size for the study of continuous variables. The risk ratio (RR) with its 95%CI was the chosen metric for effect size in dichotomous variables. The symmetry of the funnel plot was examined to identify any possible publishing bias. A balanced distribution of data points on both sides of the funnel plot's peak indicated lower probability of publication bias affecting the meta-analysis results. Egger's linear regression test was utilized as a quantitative method to identify the existence of publication bias. All statistical analyses were performed using a two-tailed approach. A P value less than 0.05 was considered statistically significant. All data analyses were conducted utilizing Stata software, version 17 (StataCorp, College Station, TX, United States).
In the preliminary search across the electronic databases, a total of 1101 pertinent articles were identified. Upon eli
The characteristics of studies included in this systematic review are presented in Table 1. The meta-analysis compiles studies spanning from 2005 to 2020 originating from the United States, Chile, Pakistan, Italy, India, and Germany. While study grades and durations varied, treatment modalities predominantly focused on NPWT, vacuum-assisted closure, vacuum sealing drainage, and negative pressure sealed drainage dressing. The treatment durations, where specified, ranged up to 16 weeks, and follow-ups extended up to 10 months. Predominant observational indices across these studies were 90% granulation tissue formation time, wound healing rate, amputation rate, and the incidence of adverse events, though the depth of detail varied among studies.
| Ref. | Study site | Year | Grade | Duration | Treatment | Treatment time | Follow-up time | Observational indices |
| Armstrong et al[11] | United States | 2005 | TX University 2-3 | Exp (1.2 ± 3.9) months, Con (1.8 ± 5.9) months | NPWT 50-200 mmHg | 16 weeks | 16 weeks | 90% granulation tissue formation time, wound healing rate, incidence of adverse events |
| Blume et al[12] | United States | 2008 | Wagener 2-3 | Exp (198.3 ± 323.5) days, Con (206.03 ± 365.9) days | VAC 100 mmHg | Exp (63.6 ± 36.57) days, Con (78.1 ± 39.29) days | 16 weeks | 90% granulation tissue formation time, wound healing rate, amputation rate, incidence of adverse events |
| Sepúlveda et al[13] | Chile | 2009 | Not given | Not given | VAC | Not given | Not given | 90% granulation tissue formation time |
| Riaz et al[14] | Pakistan | 2010 | Not given | 3-5 months | VAC 50-125 mmHg | Not given | 6 months | 90% granulation tissue formation time |
| Paola et al[15] | Italy | 2010 | TX University 2-3 | Not given | VSD 125 mmHg | 8 weeks | 8 weeks | 90% granulation tissue formation time, amputation rate |
| Nain et al[19] | India | 2011 | Not given | Not given | Negative pressure sealed drainage dressing 15.96-59.85 kPa | Exp 2345 days, Con 46145 days | 3 months | Wound healing rate |
| Lone et al[16] | India | 2014 | Armstrong 2-3 | Not given | NPWT 80-125 mmHg | 8 weeks | 6-10 months | 90% granulation tissue formation time, amputation rate |
| James et al[17] | India | 2019 | ≥ Wagener 2 | Exp (51.4 ± 36.3) days, Con (52.6 ± 27.6) days | NPWT 125 mmHg | Not given | 1 week, 2 weeks, 1 month, 2 months | Amputation rate |
| Seidel et al[18] | Germany | 2020 | Wagener 2-4 | > 4 weeks | VAC | 16 weeks | 6 months | 90% granulation tissue formation time, wound healing rate, amputation rate, incidence of adverse events |
The evaluation of bias risk was conducted across multiple domains in the 9 studies that were included. Two studies demonstrated a low risk of bias in all categories, indicating a high level of methodological rigor. However, 33% of the studies were found to have a high risk of bias in the domain of blinding of participants and personnel. This suggests that the potential for performance bias might have influenced the outcomes in these studies. Furthermore, in 21% of the included randomized controlled trials, a high risk of selective reporting bias was observed. This indicates that the possi
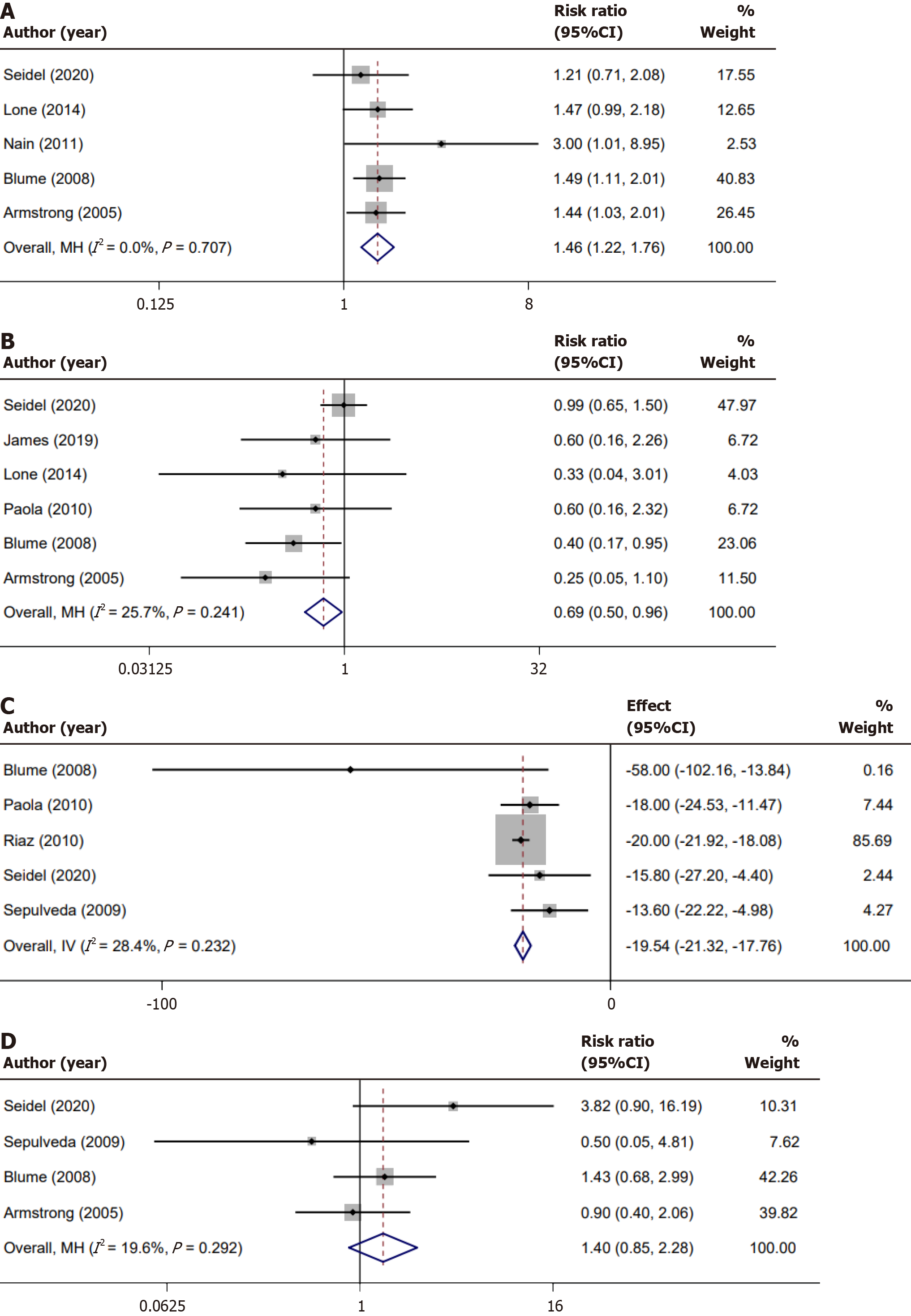
A total of 5 studies reported on wound healing rate. The combined effect size indicated no significant heterogeneity amongst these studies (I2 = 0%, P = 0.707). Utilizing the fixed-effects model for meta-analysis, we identified a statistically significant difference (RR = 1.46, 95%CI: 1.22-1.76, P < 0.01). A Forest plot of the results across the studies is shown in Figure 3A.
We conducted a meta-analysis on the amputation rate reported across studies, with all included studies exclusively documenting minor amputation events. Among the studies reviewed, six provided data regarding these minor ampu
In the realm of wound healing, the time taken for granulation tissue formation is a pivotal marker. The endpoint for granulation tissue formation is typically defined as the percentage of the wound surface covered by healthy, vascularized granulation tissue. In clinical studies, a wound is often considered to have achieved adequate granulation tissue forma
One of the key evaluative criteria in assessing the safety and effectiveness of any intervention is understanding the incidence of adverse events. Four studies included in our meta-analysis shed light on the incidence of specific adverse events, namely edema, infection, pain, and bleeding. Notably, among these adverse outcomes, infection emerged as the most frequently reported complication. Upon evaluating the consistency of results across these studies, our meta-analysis found a relatively low level of heterogeneity, with an I2 value of 19.6% and a corresponding P value of 0.292. Such a result underscores a general alignment in the methodologies and outcomes of these studies, enhancing the credibility of our collective conclusions. However, when we pooled the results using a fixed-effects model, the observed differences in the incidence of adverse events were not statistically significant. Specifically, the RR was 1.40 with a 95%CI spanning from 0.85-2.28 (P = 0.16; Figure 3D). This indicates that the intervention being evaluated did not significantly alter the risk of experiencing adverse events compared to the control or alternate treatments.
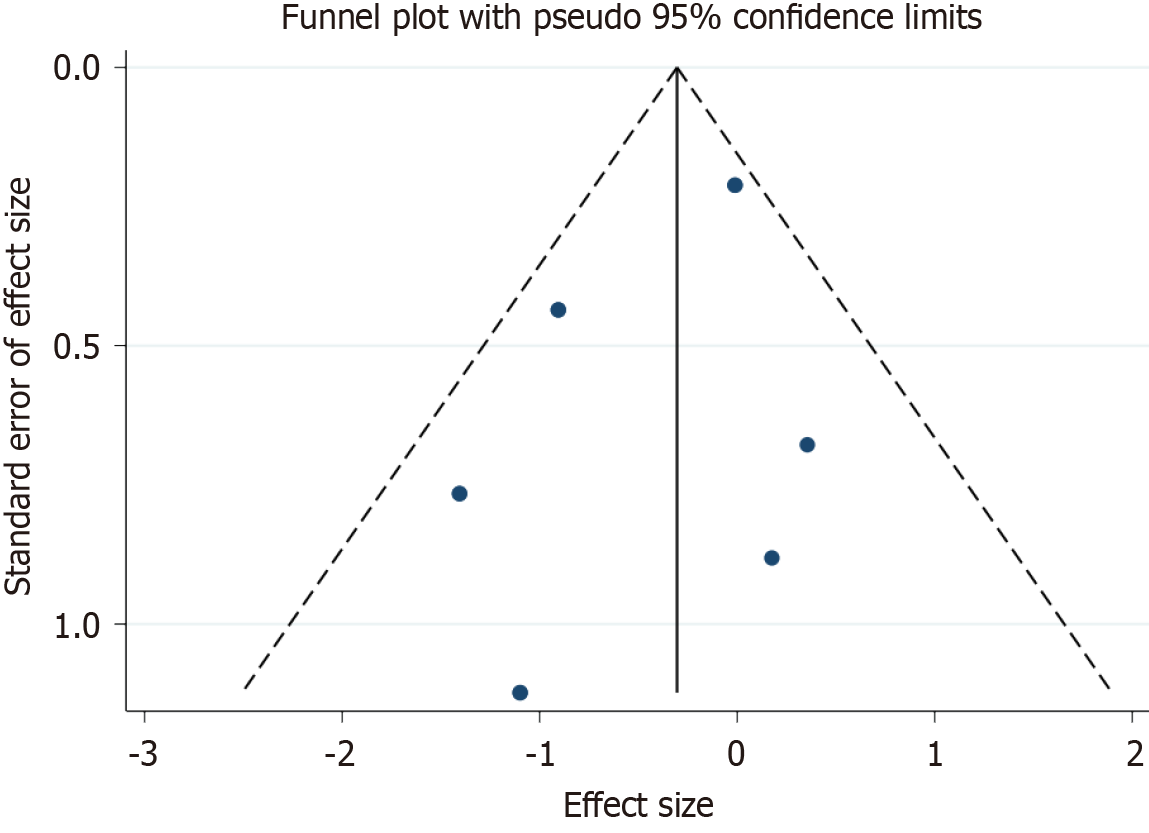
The funnel plot, visualized from the data of the incorporated studies, demonstrated a symmetrical distribution, under
DM patients exhibit histological and cellular alterations in skin tissue even when structural integrity appears intact. Chronic hyperglycemia induces abnormal cell proliferation, persistent inflammation, endothelial dysfunction, and microenvironmental imbalances, all of which impair wound healing. These pathophysiological complexities make DFUs particularly challenging to manage. NPWT has demonstrated significant therapeutic efficacy in DFUs, potentially through mechanisms involving differential gene expression and microvascular modulation[20]. This meta-analysis represents a significant advancement in understanding the clinical efficacy and safety of NPWT in DFU management. By integrating data from nine RCTs conducted across diverse geographic regions, our study demonstrates that NPWT significantly enhances wound healing rates, expedites granulation tissue formation, and reduces the risk of minor amputations, all without a substantial increase in adverse events[21]. Notably, these findings highlight the multimodal benefits of NPWT, which align with the TIME strategy, thereby reinforcing its comprehensive role in optimizing wound care. Clinically, these results provide robust evidence supporting the incorporation of NPWT into standard treatment protocols for DFUs, potentially improving patient outcomes and reducing the socio-economic burden associated with diabetic foot complications. This insight into NPWT's specific molecular mechanisms has the potential to revolutionize clinical applications and spearhead new therapeutic innovations. NPWT facilitates wound healing through a multimodal approach, including enhanced microvascular perfusion, mechanical stress-induced cellular proliferation, exudate management, and bacterial load reduction[22]. Additionally, it promotes granulation tissue formation and epithelialization by modulating local cytokine and growth factor expression, reinforcing its role in optimizing wound healing conditions. While NPWT effectively manages wound exudate and promotes debridement through negative pressure, it does not inherently eliminate the need for manual removal of necrotic or built-up tissue during dressing changes. Regular assessment and debridement remain essential components of wound care to ensure optimal healing outcomes[23]. However, comprehensive validation through further research is necessary.
Traditional dressings and treatment approaches for DFUs frequently fail to address the underlying problems of the wound. Despite continuous advancements in dressing materials, they often adhere to the tissue, potentially damaging the newly produced granulation tissue during changes. Furthermore, extended arid conditions at the wound site can impede the migratory activity of epithelial cells, hence delaying wound healing. Conversely, NPWT is essential for enhancing wound microcirculation and the wound bed structure, promoting the favorable regeneration of fibroblasts and endothe
Although the standardization of endpoints in chronic wound care studies remains limited, several investigations have reported complete wound closure as a key outcome measure. This variability in endpoint definitions, ranging from the achievement of approximately 80% granulation tissue coverage to complete epithelialization, poses challenges when attempting to directly compare study outcomes. In some studies, complete wound closure is defined as the full re-establishment of an intact epidermis across the entire wound surface, whereas others consider the threshold of 80% granulation tissue formation as a significant marker of progress toward healing[26-28]. Such heterogeneity in endpoint definitions can influence the interpretation of treatment efficacy and may contribute to inconsistencies in reported outcomes across the literature. However, our meta-analysis focused on the most consistently reported endpoints-namely, granulation tissue formation and overall wound healing rates-which have been more uniformly documented across the selected studies. By concentrating on these endpoints, we were able to provide meaningful insights into the intervention's effectiveness while minimizing the impact of variable endpoint criteria. First and foremost, the findings underscored a clear enhancement in wound healing rate, as evidenced by data pooled from five studies that consistently showed significant improvements. This was further bolstered by the lack of significant heterogeneity among these studies, thereby bolstering the reliability of this outcome. In terms of amputation rates, a critical endpoint in wound care, the analysis revealed promising results. The data from six studies not only indicated a statistically significant reduction in amputation rates for patients under the given intervention, but also emphasized moderate consistency across these studies. The 31% reduction in amputation risk elucidates the intervention's potential to revolutionize patient care in this domain.
Further, the duration of granulation tissue formation, a key marker for wound healing progression, has shown re
This meta-analysis has several limitations that warrant consideration. First, the heterogeneity in outcome definitions across studies may compromise comparability and uniform interpretation of results. Additionally, potential residual confounding factors-unaccounted for in individual studies and publication lag could limit the comprehensiveness of our findings. Variability in NPWT devices, including differences in negative pressure settings and operating modes, further complicates uniform data synthesis. To address these gaps, future research should incorporate prospective, multicenter trials with standardized outcome definitions and detailed reporting of NPWT device parameters. The use of individual patient-level data could mitigate residual confounding, while stratified analyses would help elucidate the relationship between NPWT treatment duration and clinical outcomes, including differentiation between partial (minor) and full (major) amputations. Moreover, standardized adverse event reporting, especially regarding infection rates, is crucial to better delineate the safety profile of NPWT. These improvements are expected to benefit clinicians and researchers by providing robust, reproducible evidence that can optimize NPWT protocols in DFU management, thereby enhancing patient outcomes. Ultimately, such studies will help resolve current uncertainties and inform future therapeutic stra
In summary, NPWT demonstrably enhances wound healing and reduces amputation incidents in DFUs. It accelerates granulation tissue formation, a crucial healing phase. Nonetheless, its impact on the incidence of adverse events remains neutral, underscoring the necessity for meticulous clinical judgment when considering its implementation.
We thank all the participants for their efforts.
| 1. | Hossain MJ, Al-Mamun M, Islam MR. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci Rep. 2024;7:e2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 334] [Article Influence: 167.0] [Reference Citation Analysis (0)] |
| 2. | Antar SA, Ashour NA, Sharaky M, Khattab M, Ashour NA, Zaid RT, Roh EJ, Elkamhawy A, Al-Karmalawy AA. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomed Pharmacother. 2023;168:115734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 212] [Reference Citation Analysis (0)] |
| 3. | Akkus G, Sert M. Diabetic foot ulcers: A devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World J Diabetes. 2022;13:1106-1121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (7)] |
| 4. | Ansari P, Akther S, Khan JT, Islam SS, Masud MSR, Rahman A, Seidel V, Abdel-wahab YHA. Hyperglycaemia-Linked Diabetic Foot Complications and Their Management Using Conventional and Alternative Therapies. Appl Sci. 2022;12:11777. [DOI] [Full Text] |
| 5. | Ha JH, Jin H, Park JU. Association between socioeconomic position and diabetic foot ulcer outcomes: a population-based cohort study in South Korea. BMC Public Health. 2021;21:1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Crocker RM, Palmer KNB, Marrero DG, Tan TW. Patient perspectives on the physical, psycho-social, and financial impacts of diabetic foot ulceration and amputation. J Diabetes Complications. 2021;35:107960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Gallo L, Gallo M, Chin B, Copeland A, Avram R, McRae M, McRae M, Thoma A, Coroneos CJ, Voineskos SH. Closed Incision Negative Pressure Therapy Versus Traditional Dressings for Low Transverse Abdominal Incisions Healing by Primary Closure: A Systematic Review and Meta-Analysis. Plast Surg (Oakv). 2023;31:390-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Alam W, Hasson J, Reed M. Clinical approach to chronic wound management in older adults. J Am Geriatr Soc. 2021;69:2327-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 9. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51476] [Article Influence: 10295.2] [Reference Citation Analysis (2)] |
| 10. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 26276] [Article Influence: 1751.7] [Reference Citation Analysis (4)] |
| 11. | Armstrong DG, Lavery LA; Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366:1704-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 597] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 12. | Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care. 2008;31:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 377] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 13. | Sepúlveda G, Espíndola M, Maureira M, Sepúlveda E, Ignacio Fernández J, Oliva C, Sanhueza A, Vial M, Manterola C. [Negative-pressure wound therapy versus standard wound dressing in the treatment of diabetic foot amputation. A randomised controlled trial]. Cir Esp. 2009;86:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Riaz MU, Khan MUR, Akbar A. Comparison of vacuum assisted closure versus normal saline dressing in healing diabetic wounds. Pak J Med Health Sci. 2010;4:308-313. |
| 15. | Paola LD, Carone A, Ricci S, Russo A, Ceccacci T, Ninkovic S. Use of Vacuum Assisted Closure Therapy in the Treatment of Diabetic Foot Wounds. J Diabet Foot Complic. 2010;2:3-44. |
| 16. | Lone AM, Zaroo MI, Laway BA, Pala NA, Bashir SA, Rasool A. Vacuum-assisted closure versus conventional dressings in the management of diabetic foot ulcers: a prospective case-control study. Diabet Foot Ankle. 2014;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | James SMD, Sureshkumar S, Elamurugan TP, Debasis N, Vijayakumar C, Palanivel C. Comparison of Vacuum-Assisted Closure Therapy and Conventional Dressing on Wound Healing in Patients with Diabetic Foot Ulcer: A Randomized Controlled Trial. Niger J Surg. 2019;25:14-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Seidel D, Storck M, Lawall H, Wozniak G, Mauckner P, Hochlenert D, Wetzel-Roth W, Sondern K, Hahn M, Rothenaicher G, Krönert T, Zink K, Neugebauer E. Negative pressure wound therapy compared with standard moist wound care on diabetic foot ulcers in real-life clinical practice: results of the German DiaFu-RCT. BMJ Open. 2020;10:e026345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Nain PS, Uppal SK, Garg R, Bajaj K, Garg S. Role of negative pressure wound therapy in healing of diabetic foot ulcers. J Surg Tech Case Rep. 2011;3:17-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I. Diabetic Wound-Healing Science. Medicina (Kaunas). 2021;57:1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 448] [Article Influence: 89.6] [Reference Citation Analysis (2)] |
| 21. | Mieczkowski M, Mrozikiewicz-Rakowska B, Kowara M, Kleibert M, Czupryniak L. The Problem of Wound Healing in Diabetes-From Molecular Pathways to the Design of an Animal Model. Int J Mol Sci. 2022;23:7930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Normandin S, Safran T, Winocour S, Chu CK, Vorstenbosch J, Murphy AM, Davison PG. Negative Pressure Wound Therapy: Mechanism of Action and Clinical Applications. Semin Plast Surg. 2021;35:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 23. | Kolimi P, Narala S, Nyavanandi D, Youssef AAA, Dudhipala N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells. 2022;11:2439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 258] [Reference Citation Analysis (0)] |
| 24. | Burhan A, Khusein NBA, Sebayang SM. Effectiveness of negative pressure wound therapy on chronic wound healing: A systematic review and meta-analysis. Belitung Nurs J. 2022;8:470-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Mamun AA, Shao C, Geng P, Wang S, Xiao J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front Immunol. 2024;15:1395479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |
| 26. | Chattopadhyay D, Sinha M, Kapoor A, Kumar M, Singh K, Mathew-Steiner SS, Sen CK. Deficient functional wound closure as measured by elevated trans-epidermal water loss predicts chronic wound recurrence: An exploratory observational study. Sci Rep. 2024;14:23593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 27. | Weigelt MA, Lev-Tov HA, Tomic-Canic M, Lee WD, Williams R, Strasfeld D, Kirsner RS, Herman IM. Advanced Wound Diagnostics: Toward Transforming Wound Care into Precision Medicine. Adv Wound Care (New Rochelle). 2022;11:330-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Pan Y, Yang D, Zhou M, Liu Y, Pan J, Wu Y, Huang L, Li H. Advance in topical biomaterials and mechanisms for the intervention of pressure injury. iScience. 2023;26:106956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/