Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.104482
Revised: February 5, 2025
Accepted: February 26, 2025
Published online: May 15, 2025
Processing time: 122 Days and 17.3 Hours
Early kidney damage is a significant complication in children with newly diag
To investigate the association between SII, NLR, PLR, and early kidney damage in newly diagnosed T1DM children without pre-existing albuminuria, assessing their utility as predictive biomarkers.
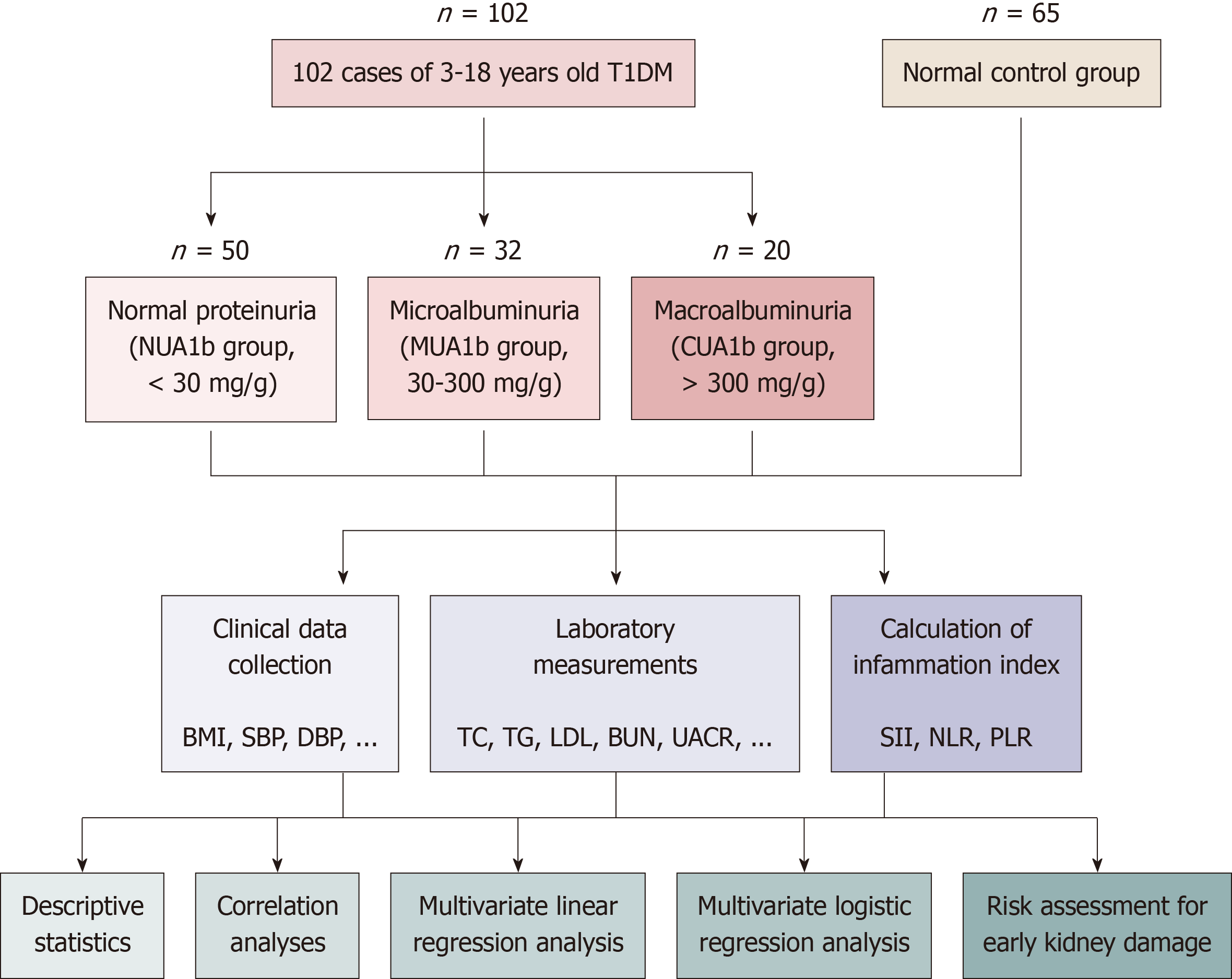
A longitudinal cohort study was conducted on 102 children aged 3-18 years with newly diagnosed T1DM [baseline urinary albumin-to-creatinine ratio (UACR) < 30 mg/g] recruited between January 2020 and June 2023. Participants were fo
SII emerged as a significant independent predictor of early kidney damage [odds ratio = 1.002, 95% confidence interval (CI): 1.0008-1.0033, P = 0.0016], with an area under the curve of 0.719 (95%CI: 0.612-0.826, P < 0.001). Using an SII threshold of ≥ 624.015 achieved a sensitivity of 59.6% and specificity of 92%. Combining SII with NLR and PLR improved predictive accuracy (area under the curve = 0.787), with sensitivity and specificity of 63.5% and 96%, respectively. Correlation analyses revealed significant associations between SII, metabolic markers (triglycerides, glycated hemoglobin), and UACR.
SII is a reliable biomarker for early kidney damage in T1DM children, offering high specificity for identifying at-risk patients. Combining SII with NLR and PLR enhances diagnostic precision, supporting its integration into clinical practice. Longitudinal monitoring of these markers may facilitate early interventions to mitigate renal complications in pediatric T1DM.
Core Tip: This study highlights the predictive value of systemic inflammatory markers - systemic immune inflammation index (SII), neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio - for early kidney damage in children with newly diagnosed type 1 diabetes mellitus. SII emerged as a significant independent predictor of renal injury, with a high specificity threshold. The combination of SII with neutrophil-to-lymphocyte ratio or platelet-to-lymphocyte ratio further enhanced diagnostic accuracy. These simple, cost-effective markers provide valuable tools for early detection and in
- Citation: Cao LF, Xu QB, Yang L. Systemic immune indicators for predicting renal damage in newly diagnosed type 1 diabetic children. World J Diabetes 2025; 16(5): 104482
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/104482.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.104482
Type 1 diabetes mellitus (T1DM) is an autoimmune disease characterized by the destruction of insulin-producing beta cells in the pancreas, leading to absolute insulin deficiency. While the disease primarily affects glucose metabolism, it is increasingly recognized that systemic inflammation plays a significant role in the early complications observed in T1DM, particularly in renal dysfunction[1]. Chronic diabetic complications are major determinants of long-term health outcomes in children with diabetes. Among these, diabetic nephropathy is particularly significant, as it contributes to approximately 50% of cases of end-stage renal disease in this population[2]. Diabetic renal damage, however, remains the most prevalent and debilitating of these, underscoring the importance of early detection and management in preventing irreversible renal impairment. The identification of early biomarkers for renal damage is crucial for timely interventions to prevent the progression of diabetic nephropathy in children.
In recent years, various inflammatory markers have been implicated in the pathogenesis of T1DM and its complications. Early renal damage in T1DM has been increasingly recognized as being closely associated with systemic inflammation and oxidative stress[3,4]. Numerous studies have demonstrated that both systemic immune inflammation and localized inflammatory responses play critical roles in the pathogenesis of diabetic kidney damage, particularly in the early stages of the disease. Recent evidence suggests that inflammatory markers, such as those derived from routine blood tests, may serve as valuable indicators of renal injury in T1DM patients. Among these, the systemic immune inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) have gained attention as potential biomarkers of systemic inflammation[5]. Blood cell counts, routinely obtained in clinical practice, provide a simple and cost-effective means to evaluate these inflammatory indices, making them highly relevant for early detection and intervention in diabetic nephropathy. These markers are easy to measure and have been associated with the severity of various inflammatory conditions, including metabolic disorders and cardiovascular diseases[6]. However, the specific role of these markers in predicting early kidney injury in children with newly diagnosed T1DM remains underexplored.
SII, calculated as the product of platelet count and neutrophil count divided by lymphocyte count, has been shown to correlate with inflammatory activity in several chronic diseases, including T2DM[7,8]. NLR and PLR are simple inflammatory indices derived from routine blood counts and have been used to predict systemic inflammation and complications in conditions such as hypertension, cardiovascular disease, and metabolic syndrome. Previous studies have suggested that these markers may be useful in predicting kidney involvement in adult populations with diabetes. However, there is limited data on their utility in pediatric T1DM, particularly in the context of early renal dysfunction. We hypothesize that systemic inflammatory markers, specifically SII, NLR, and PLR, are associated with early renal damage in newly diagnosed T1DM children. Furthermore, we propose that SII has superior predictive value compared to NLR and PLR. This study aims to investigate the longitudinal relationship between these inflammatory markers and early kidney injury, as assessed by the urinary albumin-to-creatinine ratio (UACR), in children with newly diagnosed T1DM who are free of albuminuria at baseline. In addition, we explore the correlation between these inflammatory markers and key metabolic parameters, such as glycated hemoglobin (HbA1c), lipid profiles, and renal function indices, to better understand the underlying pathophysiological mechanisms. By examining the predictive value of SII, NLR, and PLR, this research seeks to contribute to the development of effective monitoring and management strategies for early renal complications in pediatric T1DM patients.
This study utilized a longitudinal cohort design to investigate the relationship between systemic inflammatory markers and early renal injury in newly diagnosed T1DM children without pre-existing albuminuria. A total of 102 participants aged 3-18 years were recruited from the Department of Endocrinology and Genetic Metabolism at our hospital between January 2020 and June 2023. All participants met the diagnostic criteria outlined in the 2020 Chinese Children Expert Consensus on Standardized Diagnosis and Treatment of T1DM. Inclusion criteria were: (1) Newly diagnosed T1DM patients with a UACR < 30 mg/g at baseline; and (2) Written informed consent provided by guardians. Exclusion criteria included: (1) Acute injurious diseases or major surgeries within six months; (2) Autoimmune or other endocrine disorders; (3) Recent use of drugs affecting renal function (within three months); (4) Malignant tumors or existing liver/kidney diseases; and (5) Incomplete clinical data. The study was approved by the Jiangxi Children’s Hospital Ethics Committee (Approval No: JXSETYY-YXKY-20240117). Details of patient recruitment and study design are shown in Figure 1. The sample size was determined based on prior studies[9] that reported associations between inflammatory markers and renal outcomes in diabetic populations. A power analysis was conducted using an expected medium effect size (Cohen’s f2 = 0.15) for multiple regression analyses, with an alpha level of 0.05 and a power of 0.8. This required a minimum sample size of 91 participants. To account for potential attrition, we recruited 102 participants.
Demographic information such as age, gender, and family history of diabetes was recorded for each participant. Clinical parameters, including body mass index, systolic blood pressure (SBP), diastolic blood pressure (DBP), and HbA1c, were measured during the initial consultation. Blood pressure measurements were taken in a standardized manner using a calibrated sphygmomanometer after 5 minutes of rest.
Fasting blood and midstream urine samples were collected and processed on the same day. Biochemical and inflammatory markers were measured using the following methods: (1) Routine blood parameters: Measured using the AU5831 Automatic Biochemical Analyzer (Beckman Coulter, United States), including total cholesterol (TC), triglycerides (TG), low-density lipoprotein, high-density lipoprotein, blood urea nitrogen, serum creatinine, alanine aminotransferase, and aspartate aminotransferase; (2) Insulin and C-peptide: Assayed using chemiluminescence immunoassays; (3) HbA1c: Quantified using the MQ6000 Analyzer (ARKRAY Inc., Japan) following National Glycohemoglobin Standardization Program guidelines; and (4) UACR: Calculated as urinary albumin (mg/L) divided by urinary creatinine (g/L), measured using the BA400 Automatic Specific Protein Analyzer (BioSystems, Spain). The estimated glomerular filtration rate (eGFR) was calculated using the Schwartz equation[10]: eGFR (mL/minute/1.73 m2) = K × height (cm) × 88.4/serum creatinine (μmol/L), where K is a constant dependent on age and sex (K = 0.413 for children ≥ 2 years). C-reactive protein (CRP), a key marker of systemic inflammation, was measured by high-sensitivity enzyme-linked immunosorbent assay. The SII, NLR, and PLR were calculated as follows: SII = platelet count × neutrophil count/lymphocyte count; NLR = neutrophil count/lymphocyte count; PLR = platelet count/lymphocyte count. Fasting insulin levels and C-peptide were measured by chemiluminescence immunoassay, and HbA1c was determined using high-performance liquid chromatography.
Participants were followed every six months for up to three years, with repeat measurements of clinical and laboratory parameters. The primary endpoint was the development of significant albuminuria (UACR ≥ 30 mg/g). For the assessment of early kidney damage, participants were stratified into two groups based on their UACR: The “no damage” group (UACR < 30 mg/g) and the “damage” group (UACR ≥ 30 mg/g). Logistic regression analyses were conducted to evaluate the association between systemic inflammatory markers (SII, NLR, PLR) and the risk of early kidney damage. Longitudinal data were analyzed using logistic regression to assess the associations between baseline inflammatory markers and subsequent renal outcomes. Receiver operating characteristic curves determined the predictive value of these markers for early nephropathy. The results are presented as odds ratios with 95% confidence intervals (CIs), area under the curve (AUC) values, sensitivity, and specificity.
The data were analyzed using SPSS version 25 (IBM, Armonk, NY, United States). Descriptive statistics were used to summarize the demographic and clinical characteristics of the study population. Categorical variables were presented as frequencies and percentages, while continuous variables were expressed as means ± SD. Group comparisons were performed using one-way analysis of variance for continuous variables and χ2-tests for categorical variables. Post-hoc analyses with Bonferroni correction were conducted to identify intergroup differences when analysis of variance revealed statistical significance. Correlation analyses were performed using Pearson’s or Spearman’s correlation coefficients (depending on the distribution of the data) to explore associations between systemic inflammatory markers (SII, NLR, and PLR) and clinical parameters, including metabolic and renal biomarkers. A P value of < 0.05 was considered statistically significant. To identify predictors of systemic inflammation, multiple regression analyses were performed. The regression models included UACR, TG, HbA1c, and other relevant clinical variables as independent predictors of SII, NLR, and PLR. The results were expressed as estimates with 95%CIs.
The cohort included patients newly diagnosed with T1DM, free from albuminuria at baseline (UACR < 30 mg/g), and followed them longitudinally to evaluate the progression of renal complications. Baseline demographic and clinical characteristics are summarized in Table 1. No significant differences were observed in age distribution, gender, or family history of diabetes among the groups (P > 0.05). Body mass index, SBP, and DBP showed minor variations that were not statistically significant (P > 0.05). Significant differences were noted in TG and low-density lipoprotein levels (P < 0.001 and P = 0.002, respectively), suggesting metabolic alterations. Similarly, HbA1c, C-peptide, fasting insulin, and eGFR displayed significant intergroup differences (P < 0.001 for all), indicating their relevance in systemic inflammation and renal function. These findings align with the hypothesis that metabolic and inflammatory changes contribute to early renal dysfunction in children with T1DM.
| Indicator | NC group | NUA1b group | MUA1b group | CUA1b group | F/H/χ2 | P value |
| Age, months | 112 (106, 116) | 119 (77, 150) | 123 (79, 155) | 131 (82, 145) | 0.005 | 0.995 |
| Family history | 65 (30, 35) | 50 (23, 27) | 32 (15, 17) | 20 (11, 9) | 0.489 | 0.783 |
| BMI, kg/m² | 16.79 (15.01, 19.54) | 16.40 (15.38, 18.95) | 15.70 (14.75, 17.25) | 17.15 (14.73, 19.65) | 3.76 | 0.153 |
| SBP, mmHg | 104 ± 11 | 106 ± 13 | 108 ± 10 | 109 ± 15 | 0.473 | 0.625 |
| DBP, mmHg | 66 (59, 73) | 64 (60, 72) | 65 (60, 70) | 67 (60, 78) | 0.758 | 0.471 |
| Blood urea, mmol/L | 4.03 (3.45, 4.68) | 4.39 (3.86, 5.88) | 4.90 (4.08, 6.43) | 5.20 (4.14, 7.97)a | 3.348 | 0.187 |
| Blood creatinine, μmol/L | 37 (34, 42) | 35 (30, 45) | 33 (26, 44) | 41 (35, 48)c | 2.006 | 0.14 |
| C-reactive protein, mg/L | 4.1 (0.1, 5.7) | 3.4 (0.0, 5.2) | 2.4 (0.7, 4.4) | 5.0 (2.3, 11.2) | 1.002 | 0.059 |
| ALT, U/L | 29 (11, 37) | 32 (24, 41) | 36 (27, 51) | 40 (22, 49) | 0.177 | 0.838 |
| AST, U/L | 32 (20, 36) | 31 (22, 46) | 34 (26, 44) | 41 (19, 53) | 0.007 | 0.993 |
| Total cholesterol, mmol/L | 3.89 (3.34, 4.39) | 4.31 (3.79, 5.08)a | 4.91 (4.50, 5.41)a,b | 5.68 (4.19, 6.65)a,b | 8.708 | 0.013 |
| Triglycerides, mmol/L | 0.91 (0.71, 1.30) | 1.05 (0.72, 2.15)a | 1.70 (1.19, 2.91)a,b | 2.83 (1.86, 3.79)a,b,c | 20.631 | < 0.001 |
| HDL, mmol/L | 1.35 (1.18, 1.53) | 1.25 (1.07, 1.54) | 1.23 (1.00, 1.47) | 1.31 (1.06, 1.44) | 0.244 | 0.784 |
| LDL, mmol/L | 2.06 (1.76, 2.51) | 1.94 (1.60, 2.61) | 2.98 (2.20, 3.50)a,b | 2.44 (2.00, 3.31)b | 6.593 | 0.002 |
| C-peptide, nmol/L | 0.80 (0.55, 1.10) | 0.25 (0.09, 0.48)a | 0.24 (0.15, 0.29)a | 0.20 (0.12, 0.32)a | 76.498 | < 0.001 |
| Insulin, mU/L | 13.50 (8.50, 19.75) | 2.46 (1.05, 5.62)a | 2.05 (1.07, 3.63)a | 2.79 (0.85, 4.22)a | 100.35 | < 0.001 |
| eGFR, mL/minute/1.73 m² | 194.36 ± 44.67 | 202.63 ± 74.54 | 207.92 ± 54.58 | 164.64 ± 33.71a,b,c | 3.376 | 0.038 |
| UACR, mg/g | 8 (4, 14) | 6 (3, 17) | 76 (46, 158)a,b | 565 (366, 820)a,b,c | 85.232 | < 0.001 |
| HbA1c, % | 5.0 (4.7, 5.3) | 9.9 (7.0, 13.2)a | 12.9 (11.4, 16.0)a,b | 14.8 (13.4, 15.3)a,b | 19.238 | < 0.001 |
| Systemic inflammation index, 109/L | 278 (188.89, 331.47) | 366.76 (254.14, 458.30)a | 331.12 (202.53, 999.19)a | 1459.92 (1214.27, 2838.30)a,b,c | 37.354 | < 0.001 |
| Neutrophil-to-lymphocyte ratio | 1.12 (0.69, 1.46) | 1.42 (1.02, 1.95)a | 1.80 (1.17, 2.59)a | 5.54 (2.58, 9.55)a,b,c | 24.207 | < 0.001 |
| Platelet-to-lymphocyte ratio | 83.17 (59.83, 105.49) | 104.05 (79.23, 115.83)a | 113.51 (85.76, 149.95)a | 130.13 (118.99, | 20.53 | < 0.001 |
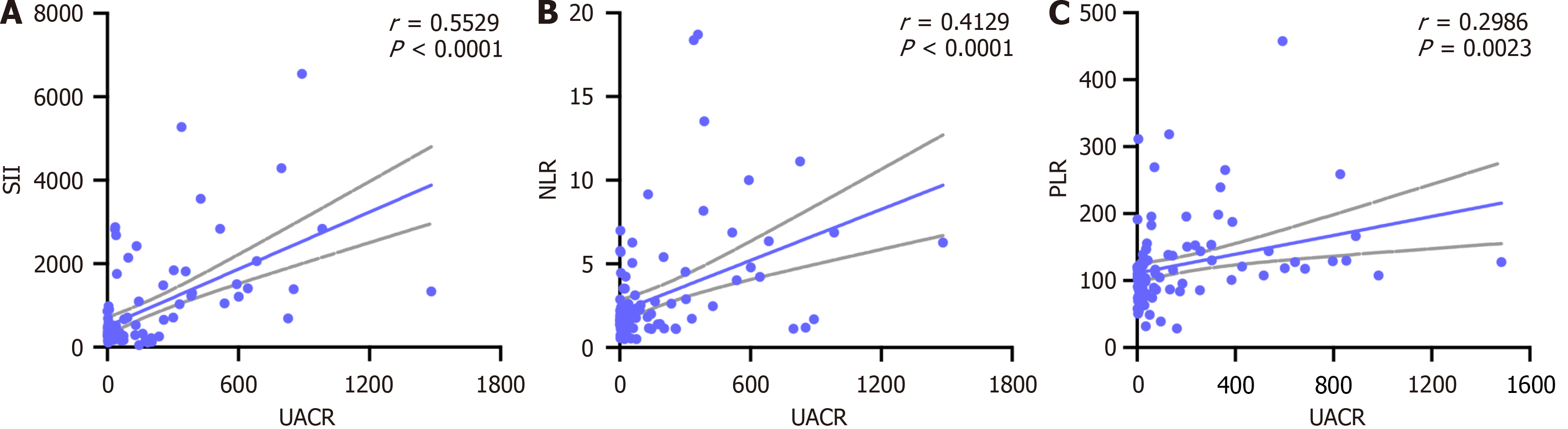
Table 2 outlines the correlation between systemic inflammatory markers (SII, NLR, and PLR) and clinical parameters. UACR demonstrated a strong positive correlation with all three inflammatory indices (P < 0.001) (Table 2, Figure 2), highlighting its potential role in early renal injury. TG and HbA1c were positively correlated with SII and PLR (P = 0.006 and P = 0.044, respectively), while TC showed a moderate correlation with PLR (P = 0.006). SBP and DBP exhibited weaker associations, and eGFR showed non-significant negative trends with the inflammatory markers. CRP correlated moderately with SII but not with NLR or PLR. These results emphasize the interplay between systemic inflammation, lipid metabolism, and renal function in T1DM.
| Indicator | SII, r | SII, P | NLR, r | NLR, P | PLR, r | PLR, P |
| Age, months | 0.12 | 0.228 | 0.055 | 0.585 | 0.084 | 0.4 |
| SBP, mmHg | 0.121 | 0.226 | 0.145 | 0.147 | 0.093 | 0.35 |
| DBP, mmHg | 0.119 | 0.232 | 0.200a | 0.044 | 0.048 | 0.634 |
| Blood urea, mmol/L | 0.121 | 0.226 | 0.092 | 0.36 | 0.129 | 0.195 |
| Blood creatinine, μmol/L | 0.089 | 0.372 | 0.168 | 0.091 | 0.117 | 0.24 |
| TC, mmol/L | 0.135 | 0.177 | 0.104 | 0.297 | 0.268b | 0.006 |
| TG, mmol/L | 0.270b | 0.006 | 0.188 | 0.058 | 0.227a | 0.022 |
| HDL, mmol/L | -0.091 | 0.362 | 0.092 | 0.358 | 0.154 | 0.123 |
| LDL, mmol/L | 0.111 | 0.266 | 0.056 | 0.573 | 0.177 | 0.075 |
| HbA1c, % | 0.200a | 0.044 | 0.129 | 0.197 | 0.176 | 0.077 |
| eGFR, mL/minute/1.73 m² | -0.064 | 0.521 | -0.183 | 0.066 | -0.054 | 0.588 |
| UACR, mg/g | 0.552b | < 0.001 | 0.413b | < 0.001 | 0.300b | < 0.001 |
| ALT, U/L | -0.076 | 0.451 | 0.045 | 0.65 | -0.064 | 0.522 |
| AST, U/L | 0.012 | 0.908 | -0.067 | 0.501 | 0.13 | 0.194 |
| BMI, kg/m² | -0.008 | 0.933 | -0.003 | 0.973 | -0.031 | 0.755 |
| C-peptide, nmol/L | 0.063 | 0.529 | -0.151 | 0.129 | -0.084 | 0.401 |
| Insulin, mU/L | -0.052 | 0.604 | -0.036 | 0.716 | 0.045 | 0.653 |
| Family history | 0.086 | 0.391 | 0.032 | 0.753 | 0.054 | 0.592 |
| C-reactive protein, mg/L | 0.151 | 0.131 | 0.034 | 0.738 | 0.063 | 0.529 |
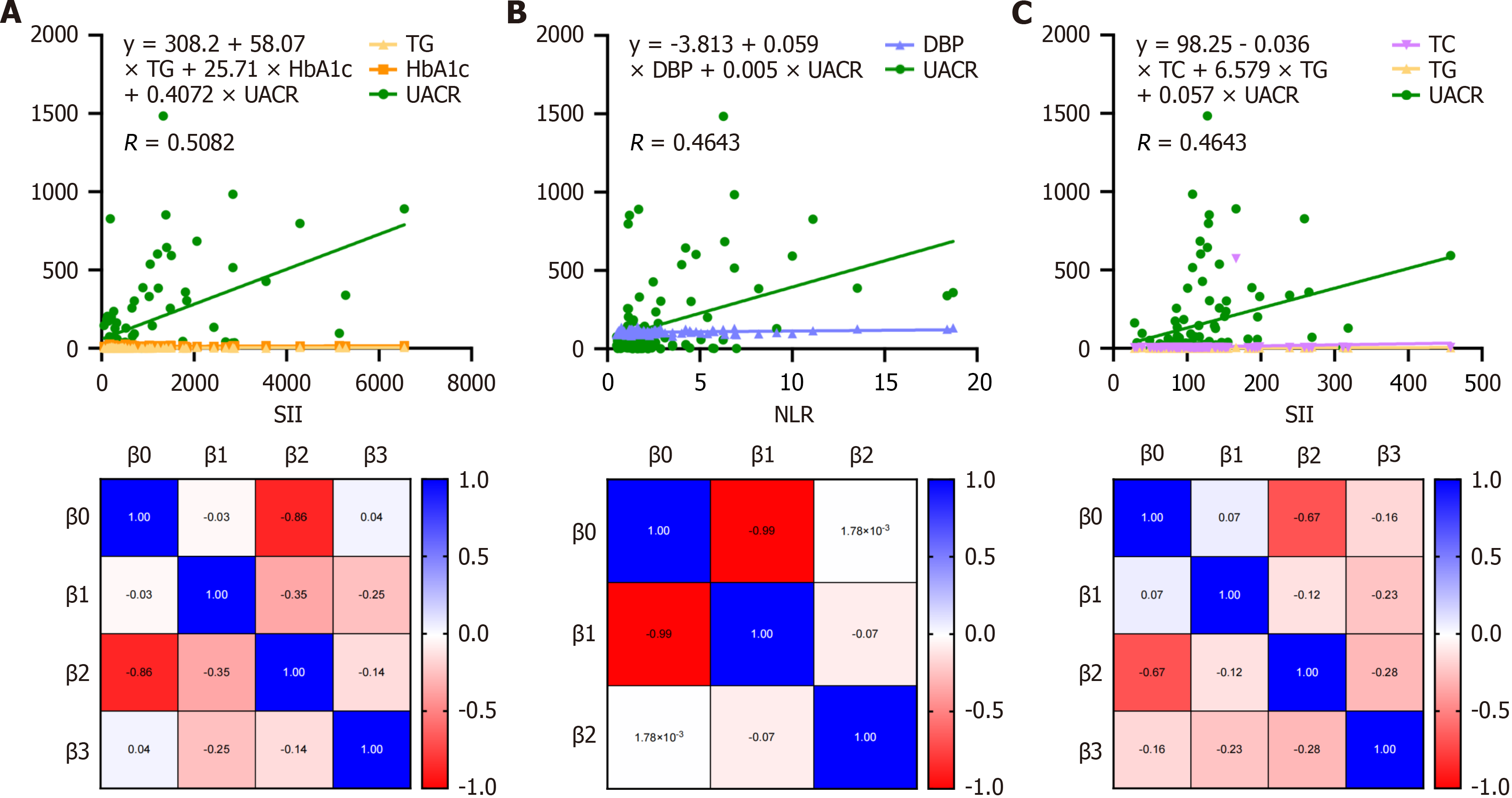
The regression analysis identified UACR as the strongest predictor of SII levels (Figure 3A, Supplementary Table 1). With an estimate of 1.955 (P < 0.0001), UACR was strongly associated with increased SII levels. For each unit increase in UACR, SII levels rose by approximately 1.955 units, underscoring the pivotal role of renal dysfunction in systemic inflammation. Although TG exhibited a positive estimate (80.01), its P value (P = 0.171) suggested no statistically significant contribution to predicting SII levels at conventional thresholds. A positive estimate (3.19) was observed for HbA1c, but it was not statistically significant (P = 0.902), indicating minimal predictive influence on SII levels in this model. Figure 3B presents the regression analysis for NLR levels. DBP showed a statistically significant positive association with NLR levels, with an estimate of 0.056 (P = 0.019). This indicates that each unit increase in DBP corresponds to a 0.056-unit rise in NLR, suggesting blood pressure’s role in systemic inflammation. A strong positive estimate (0.005, P < 0.001) confirmed UACR as a significant contributor to NLR, emphasizing the link between renal function and inflammation. Figure 3B and Supplementary Table 2 underscore the importance of monitoring DBP and UACR as indicators of inflammatory responses in newly diagnosed T1DM children. Figure 3C and Supplementary Table 3 provide insights into the factors influencing PLR levels. The intercept was highly significant (P < 0.0001), with an estimate of 98.25, suggesting a strong baseline relationship with PLR levels. TG exhibited a statistically significant positive association with PLR levels, supporting its relevance in systemic inflammation. UACR was also significantly associated with PLR, emphasizing the interplay between renal function and inflammatory responses. TC did not demonstrate a statistically significant impact on PLR levels in this analysis. These results highlight the significant roles of TG and UACR in influencing PLR, further confirming the close relationship between lipid metabolism, renal function, and systemic inflammation in the studied cohort. The findings from regression analyses across SII, NLR, and PLR underscore the importance of UACR as a consistent and significant predictor of systemic inflammation. Additionally, DBP and TG contribute variably to inflammatory indices, while other parameters such as HbA1c and TC exhibit limited predictive influence. These insights provide a foundation for further exploration of systemic inflammation’s role in early complications among children with newly diagnosed T1DM.
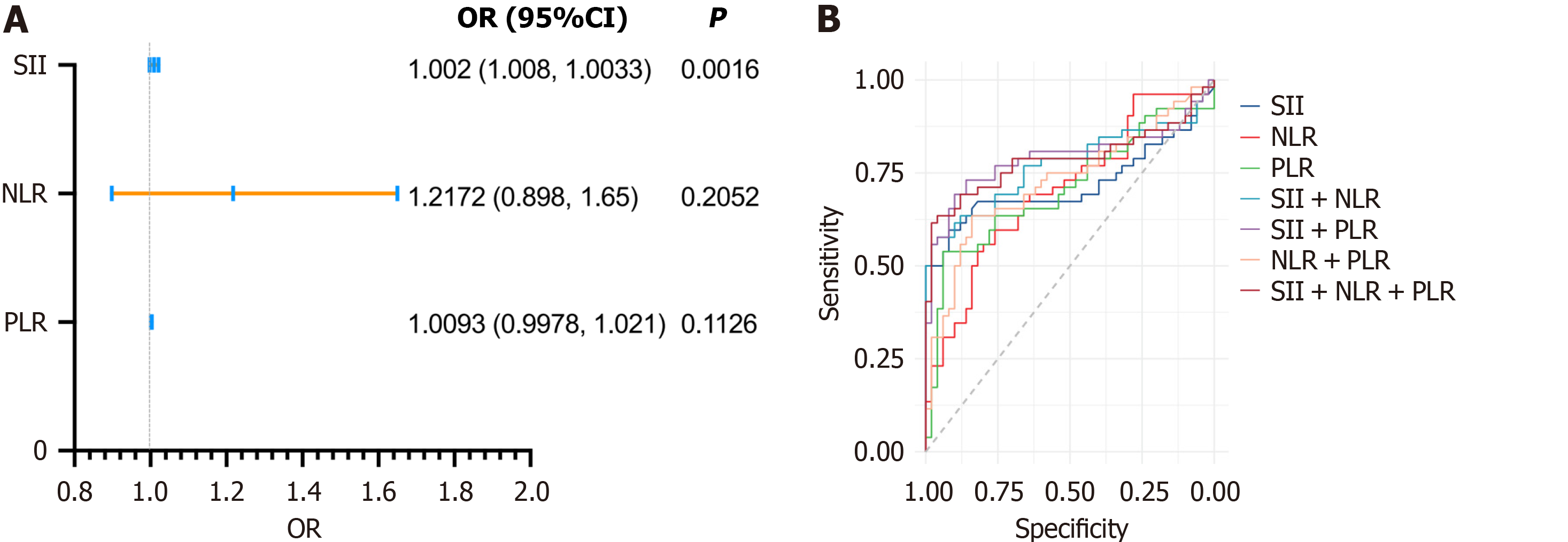
Logistic regression analysis demonstrated that systemic inflammatory markers were significantly associated with the progression of albuminuria in children with T1DM. Among these markers, the SII emerged as the most significant predictor, with an odds ratio of 1.002 (95%CI: 1.0008-1.0033, P = 0.0016). However, NLR and PLR exhibited limited predictive utility when assessed individually (P > 0.05 for both) (Figure 4A). The predictive ability of SII was further validated through receiver operating characteristic analysis, which revealed an AUC of 0.719 (P < 0.001). This cor
| Indicator combination | AUC | 95% confidence interval | P value | Threshold | Sensitivity, % | Specificity, % |
| SII | 0.72 | 0.612-0.826 | < 0.001 | 624.015 | 59.6 | 92 |
| NLR | 0.7 | 0.603-0.804 | < 0.001 | 2.17 | 55.8 | 80 |
| PLR | 0.71 | 0.604-0.811 | < 0.001 | 127.51 | 53.8 | 94 |
| SII + NLR | 0.77 | 0.676-0.869 | < 0.001 | 0.645 | 55.8 | 98 |
| SII + PLR | 0.79 | 0.695-0.887 | < 0.001 | 0.502 | 69.2 | 90 |
| NLR + PLR | 0.73 | 0.627-0.828 | < 0.001 | 0.466 | 63.5 | 84 |
| SII + NLR + PLR | 0.79 | 0.690-0.883 | < 0.001 | 0.604 | 63.5 | 96 |
T1DM is one of the most prevalent endocrine disorders in childhood, and its incidence has been rising globally in recent years. The development of microvascular complications in T1DM begins early in the disease course and can manifest within a few years of diagnosis, even in children[11]. Among these complications, diabetic kidney damage is of particular concern, as it significantly impacts long-term health outcomes. This study investigated the longitudinal association between systemic inflammatory markers - SII, NLR, and PLR - and the progression of early kidney damage in children newly diagnosed with T1DM. By following patients without albuminuria at baseline, we aimed to explore how inflammation contributes to the development of albuminuria over time. Our results demonstrated that elevated SII levels were strongly associated with progression to significant albuminuria, highlighting the potential of this marker as an early in
Elevated inflammatory markers have been shown to be predictive of poor outcomes in both adult and pediatric po
The NLR, a marker of the balance between innate and adaptive immune responses, has gained attention as a potential biomarker of systemic inflammation in T1DM. Studies have shown that NLR correlates with disease severity in au
Furthermore, the study found that elevated TG and TC levels, particularly in the microalbuminuria and macroalbuminuria groups, were positively correlated with PLR, highlighting the interplay between lipid metabolism and inflammation in T1DM. Inflammatory cells, including neutrophils and platelets, are known to interact with lipids and promote atherosclerosis, which can further aggravate renal damage through increased glomerular endothelial cell dysfunction and monocyte infiltration[24]. Studies have shown that in metabolic disorders like obesity and dyslipidemia, impaired adipocyte function leads to enhanced release of pro-inflammatory cytokines, such as tumor necrosis factor α and in
Despite the strengths, our study has several limitations. First, while our longitudinal design allows for temporal analysis, the relatively short follow-up period may not capture the full spectrum of nephropathy progression. Extending the follow-up duration in future studies will provide more robust insights into long-term outcomes. Second, while SII was identified as a significant predictor, the underlying molecular mechanisms were not explored. Future research should focus on elucidating the pathways linking systemic inflammation to renal injury, including the role of oxidative stress and endothelial dysfunction. Additionally, while our study provides strong evidence of the relationship between systemic inflammation and kidney function, it did not explore the underlying mechanisms of inflammation in detail. Future studies should aim to elucidate the pathways by which inflammation contributes to renal injury in T1DM, such as the role of oxidative stress, endothelial dysfunction, and immune dysregulation. Furthermore, while we assessed several potential predictors of systemic inflammation, other factors such as genetic predisposition and environmental influences were not considered. Exploring these factors could provide a more comprehensive understanding of the interplay between systemic inflammation and early kidney injury.
This study highlights the critical role of systemic inflammation, as reflected by SII, in the progression of early kidney damage in children with newly diagnosed T1DM. Our findings underscore the potential of SII as a reliable and practical biomarker for identifying at-risk individuals. Future longitudinal research with extended follow-up and expanded biomarker panels will be essential to validate these findings and advance our understanding of diabetic nephropathy pathogenesis.
| 1. | Kolseth IB, Reine TM, Parker K, Sudworth A, Witczak BJ, Jenssen TG, Kolset SO. Increased levels of inflammatory mediators and proinflammatory monocytes in patients with type I diabetes mellitus and nephropathy. J Diabetes Complications. 2017;31:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Muntean C, Starcea IM, Banescu C. Diabetic kidney disease in pediatric patients: A current review. World J Diabetes. 2022;13:587-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 3. | Liu P, Zhang Z, Cai Y, Li Z, Zhou Q, Chen Q. Ferroptosis: Mechanisms and role in diabetes mellitus and its complications. Ageing Res Rev. 2024;94:102201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 79] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 4. | Pérez-Morales RE, Del Pino MD, Valdivielso JM, Ortiz A, Mora-Fernández C, Navarro-González JF. Inflammation in Diabetic Kidney Disease. Nephron. 2019;143:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 5. | Gao Y, Lu RX, Tang Y, Yang XY, Meng H, Zhao CL, Chen YL, Yan F, Cao Q. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio in patients with type 2 diabetes at different stages of diabetic retinopathy. Int J Ophthalmol. 2024;17:877-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 6. | Scutca AC, Jugănaru I, Nicoară DM, Brad GF, Bugi MA, Asproniu R, Cristun LI, Mărginean O. Systemic Inflammatory Response Index (SIRI) as a Predictive Marker for Adverse Outcomes in Children with New-Onset Type 1 Diabetes Mellitus. J Clin Med. 2024;13:2582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Guo W, Song Y, Sun Y, Du H, Cai Y, You Q, Fu H, Shao L. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011-2018. Front Endocrinol (Lausanne). 2022;13:1071465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 222] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 8. | Huang P, Mai Y, Zhao J, Yi Y, Wen Y. Association of systemic immune-inflammation index and systemic inflammation response index with chronic kidney disease: observational study of 40,937 adults. Inflamm Res. 2024;73:655-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 9. | Altıncık SA, Yıldırımçakar D, Avcı E, Özhan B, Girişgen İ, Yüksel S. Plasma leucine-rich α-2-glycoprotein 1 - a novel marker of diabetic kidney disease in children and adolescents with type 1 diabetes mellitus? Pediatr Nephrol. 2023;38:4043-4049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Blufpand HN, Westland R, van Wijk JA, Roelandse-Koop EA, Kaspers GJ, Bökenkamp A. Height-independent estimation of glomerular filtration rate in children: an alternative to the Schwartz equation. J Pediatr. 2013;163:1722-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Mamilly L, Mastrandrea LD, Mosquera Vasquez C, Klamer B, Kallash M, Aldughiem A. Evidence of Early Diabetic Nephropathy in Pediatric Type 1 Diabetes. Front Endocrinol (Lausanne). 2021;12:669954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Tang L, Xu GT, Zhang JF. Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy. Neural Regen Res. 2023;18:976-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 13. | Gómez-Jaramillo L, Cano-Cano F, Sánchez-Fernández EM, Ortiz Mellet C, García-Fernández JM, Alcalá M, Álvarez-Gallego F, Iturregui M, González-Montelongo MDC, Campos-Caro A, Arroba AI, Aguilar-Diosdado M. Unravelling the Inflammatory Processes in the Early Stages of Diabetic Nephropathy and the Potential Effect of (S(s))-DS-ONJ. Int J Mol Sci. 2022;23:8450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Wang FJ, Ding HX. [Interpretation of the Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition)]. Hebei Yike Daxue Xuebao. 2024;45:1241-1246. [DOI] [Full Text] |
| 15. | Zhang R, Chen J, Xiong Y, Wang L, Huang X, Sun T, Zha B, Wu Y, Yan C, Zang S, Zhou Q, Huang Z, Liu J. Increased neutrophil count Is associated with the development of chronic kidney disease in patients with diabetes. J Diabetes. 2022;14:442-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Jung CY, Yoo TH. Pathophysiologic Mechanisms and Potential Biomarkers in Diabetic Kidney Disease. Diabetes Metab J. 2022;46:181-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 17. | Mahajan M, Prasad MK, Ashok C, Guria RT, Marandi S, Vidyapati, Subrat S, Chowdhury A. The Correlation of the Neutrophil-to-Lymphocyte Ratio With Microvascular Complications in Patients With Diabetes Mellitus. Cureus. 2023;15:e44601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Wang RH, Wen WX, Jiang ZP, Du ZP, Ma ZH, Lu AL, Li HP, Yuan F, Wu SB, Guo JW, Cai YF, Huang Y, Wang LX, Lu HJ. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. 2023;14:1115031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 224] [Reference Citation Analysis (0)] |
| 19. | Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The Platelet-to-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann Lab Med. 2019;39:345-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 403] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 20. | Wan H, Wang Y, Fang S, Chen Y, Zhang W, Xia F, Wang N, Lu Y. Associations between the Neutrophil-to-Lymphocyte Ratio and Diabetic Complications in Adults with Diabetes: A Cross-Sectional Study. J Diabetes Res. 2020;2020:6219545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Akase T, Kawamoto R, Ninomiya D, Kikuchi A, Kumagi T. Neutrophil-to-lymphocyte ratio is a predictor of renal dysfunction in Japanese patients with type 2 diabetes. Diabetes Metab Syndr. 2020;14:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Li X, Wang L, Liu M, Zhou H, Xu H. Association between neutrophil-to-lymphocyte ratio and diabetic kidney disease in type 2 diabetes mellitus patients: a cross-sectional study. Front Endocrinol (Lausanne). 2023;14:1285509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 49] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 23. | Li J, Wang X, Jia W, Wang K, Wang W, Diao W, Ou F, Ma J, Yang Y. Association of the systemic immuno-inflammation index, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio with diabetic microvascular complications. Front Endocrinol (Lausanne). 2024;15:1367376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 24. | Chen X, Zhang X, Gong Z, Yang Y, Zhang X, Wang Q, Wang Y, Xie R. The link between diabetic retinal and renal microvasculopathy is associated with dyslipidemia and upregulated circulating level of cytokines. Front Public Health. 2022;10:1040319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 25. | Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Valko M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol. 2023;97:2499-2574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 1192] [Article Influence: 397.3] [Reference Citation Analysis (0)] |
| 26. | Xiong P, Zhang F, Liu F, Zhao J, Huang X, Luo D, Guo J. Metaflammation in glucolipid metabolic disorders: Pathogenesis and treatment. Biomed Pharmacother. 2023;161:114545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/