TO THE EDITOR
Type 2 diabetes has become a global public health problem that can cause many complications and lead to increased mortality, and there are currently no effective drugs to prevent diabetes[1,2]. L-arginine (L-Arg) is a functional amino acid that can be found in any food that contains protein, such as meat, poultry, cheese products, and fish. It is often used as a dietary supplement and is found in large amounts in protamine. L-Arg can also be used as an oral supplement, such as L-Arg supplements, complex amino acid supplements, and functional foods, which play a vital role in animal maintenance, reproduction, growth, antiaging, and immunity[3]. L-Arg is a key substrate for the synthesis of nitric oxide (NO) and also a precursor for the synthesis of polyamines such as putrescine, spermidine, and spermine. Polyamines are involved in the regulation of relevant inflammations and have important effects on processes such as cell proliferation, growth, differentiation, and apoptosis[4]. L-Arg participates in various metabolic pathways in the body, including the urea cycle (which helps remove excess ammonia from the body), protein synthesis, etc. In the medical field, it helps prevent and treat cardiovascular diseases such as coronary heart disease and heart failure, and also has certain potential benefits for the prevention and treatment of diabetes[5,6].
Increasing clinical evidence suggests that dietary supplementation with L-Arg attenuates obesity, reduces arterial blood pressure, resists oxidation, and normalizes endothelial function, thereby reducing type 2 diabetes[7-9]. However, controversy remains regarding its effectiveness and safe dosage control, especially in diabetic patients[8]. Some studies suggest that supplementation with L-Arg may have a positive effect on insulin resistance in people with type 2 diabetes, and that L-Arg may help with blood sugar control by increasing the production of NO, which may improve insulin sensitivity. Although L-Arg may be beneficial to diabetic patients, when L-Arg is at a high dose, it will have adverse effects on diabetic patients, such as pro-inflammatory effects, which will aggravate the degree of myocardial cell damage. Specifically, myocardial cell damage is manifested as inflammatory cell infiltration, fat accumulation, and scar tissue formation (fibrosis) in the myocardium[10].
Administration level of L-Arg is associated with outcomes
Recently, Mansouri et al[11] published a study in the World Journal of Diabetes, which emphasized that high-dose L-Arg can damage myocardial structure through pro-oxidant and pro-inflammatory mechanisms, thereby aggravating myocardial injury in diabetic patients. This study investigated the effects of different concentrations of L-Arg (0.5, 1, and 1.5 g/kg daily) on diabetes. By measuring the blood glucose levels, total antioxidant capacity, and inflammatory mediator levels of eight rats via test kits, including ELISA and CK-MB, which are based on protocols previously described by Hortmann et al[10], it was found that blood glucose levels were slightly increased in the L-Arg group; however, after administration to diabetic rats, L-Arg decreased high-density lipoprotein levels and increased triglycerides, total cholesterol, low-density lipoprotein (LDL), and very low density lipoprotein levels. The significant increase in blood glucose levels in the diabetic group showed that L-Arg was able to enhance the mitochondrial function of brown adipose tissue with the help of gene expression to regulate blood glucose levels. In addition, the levels of inflammatory cytokines, NO, and malondialdehyde were significantly up-regulated in diabetic rats treated with 1 g/kg and 1.5 g/kg L-Arg, and there was an association between these indicators and blood glucose levels[12,13]. In diabetic rats treated with L-Arg at 1 g/kg and 1.5 g/kg, the concentrations of antioxidant capacity, superoxide dismutase, catalase, glutathione peroxidase, and glutathione all significantly decreased. Moreover, through immunohistochemistry and pathological analysis, it was found that after high-dose L-Arg (1 g/kg or 1.5 g/kg) treatment in diabetic rats, the protein level of Nrf-2 significantly decreased. Moreover, the degree of myocardial injury, fibrosis, and the upregulation of α-smooth muscle actin were all related to desmin-mediated myocardial damage, and these effects were not obvious in the treated normal rats. In addition, 0.5 g/kg L-Arg could reduce the body weight of diabetic rats and improve their blood lipid levels, but L-Arg at doses higher than 1 g/kg would significantly aggravate the degree of myocardial injury in diabetic rats. This study found that this increase might be due to the upregulation of NOS forms represented by inducible NO synthase (iNOS) and endothelial NO synthase (eNOS) under the action of hyperglycemia, both in terms of the quantity of gene expression and the scale of protein synthesis, thereby stimulating the production of NO and increasing the NO level in heart tissue of diabetic rats[14].
High-dose L-Arg causes cardiovascular damage through oxidative stress and cardiovascular remodeling
Although L-Arg shows benefits in some cases, it can also be associated with increased methylglyoxal in diabetic patients[15,16]. The precursors of late glycation end products produce harmful superoxide radicals, the accumulation of which causes oxidative stress to damage the cells[17]. Nrf-2 is a key transcription factor in the antioxidant mechanism against oxidative stress. By inducing and regulating the gene expression of the oxidant response element, it is beneficial for improving oxidative stress and maintaining the state of redox[18]. However, high doses of L-Arg significantly decrease the expression of Nrf-2, which controls the expression of antioxidant enzymes, including catalase and superoxide dismutase (SOD). The redox balance is disrupted, triggering oxidative stress, and lipid peroxidation causes severe damage to myocardial tissue.
Increased oxidative stress leads to an increase in oxidized LDL, which is elevated by L-Arg administration. Oxidized LDL further activates the NF-κB signaling pathway to trigger the expression of various cytokines, chemokines, interleukins, transforming growth factor-beta, and proinflammatory proteins[19,20].
NO is an important biological signaling molecule that is generated under normal physiological conditions by NOS, which catalyzes the conversion of L-Arg to L-carnitine[21]. When studying alveolar epithelial cells activated by cytokines, it was found that when the concentration of L-Arg was 60 μmol/L, the activity of iNOS reached half of its maximum value. This concentration level is equivalent to the medium serum arginine levels in rats and humans. Moreover, the expression level of iNOS is positively correlated with the dosage of L-Arg administered. The activity of iNOS largely depends on the supply of its substrate, L-Arg[22]. Specifically, in a diabetic rat model, after treatment with higher doses (1 g/kg or 1.5 g/kg) of L-Arg, the expression level of iNOS in myocardial tissue increased significantly, which in turn led to a substantial increase in the concentration of NO in myocardial tissue. ROS can also increase the generation of NO. NO serum level is associated with collagen production in the myocardium[23]. Thus, higher doses of L-Arg may also promote collagen production in heart muscle tissue and remodel cardiac tissue. Therefore, oxidative damage and fibrosis are the main contributors to cardiac injury after excess L-Arg administration.
Less than 0.5 g/kg L-ARG is safe for diabetes patients
It has been reported that 3 g/d L-Arg intervention significantly improves the ejection fraction and left ventricular function in ischemic heart failure patients[6]. To convert the dose of L-Argon in normal and type 2 diabetic rats to a human equivalent, this study applied the United States Food and Drug Administration 2005 conversion ratio (1:0.16, rat vs human), which is equivalent to a 70 kg person taking 5.6, 11.13, and 16.66 g L-Arg daily[9]. L-Arg can reduce the occurrence of oxidative stress by increasing the expression of endogenous antioxidant enzymes in type 2 diabetic rats, and the increased synthesis of glutathione leads to a decrease in ROS production, thereby enhancing the body's antioxidant response level[9,21]. Appropriate supplementation with Arg can increase the expression of genes involved in the antioxidative stress response in blood vessels, reduce lipid peroxidation in vascular epithelial cells, and effectively reduce myocardial injury and tissue O2- production in rats[22]. The study by Mansouri et al[11] demonstrated that, in diabetic rats, 0.5 g/kg is a safe dose that can be used without causing serious damage to the heart, and it is the maximum dose for L-Arg administration, improves the clearance of late nonenzymatic glycosylation products, and ultimately improves blood glucose levels in diabetic patients[23].
CONCLUSION
The study by Mansouri et al[11] advances the investigation of the safety of food supplements by exploring the effects of different doses of L-Arg on patients with type 2 diabetes, thereby clarifying the complexity and limitations of L-Arg supplementation. L-Arg is the substrate of eNOS and can generate NO. As a key signaling molecule, NO plays roles in vasodilation, antioxidation, and anti-inflammation in the body. Additionally, NO can protect the heart from damage by inhibiting myocardial hypertrophy and fibrosis. However, when high doses of L-Arg are ingested, NO reacts with superoxide anions to form peroxynitrite, a strong oxidant that can cause cell damage, and the accumulation of superoxide anion exacerbates oxidative stress, leading to cardiomyocyte damage and cardiac dysfunction. Excess L-Arg may increase the production of ROS by activating pathways such as NADPH oxidase, which can attack lipids, proteins, and DNA, leading to cellular dysfunction and tissue damage. L-Arg can enhance cellular antioxidant capacity by activating the Nrf2 pathway, increasing the expression of antioxidant enzymes such as glutathione peroxidase and SOD, thereby exerting a protective effect on the heart, and high doses of L-Arg can lead to cardiomyocyte damage, fibrosis, and cardiac remodeling, further aggravating cardiac dysfunction (Figure 1). Over 0.5 g/kg L-Arg is not safe for daily administration. It may aggravate myocardial damage by increasing blood glucose levels, the oxidative stress response, inflammatory mediator levels, and cardiac remodeling (Figure 2). L-Arg supplementation has shown some potential in clinical and experimental studies, especially in areas such as cardiovascular health, immune regulation, and athletic performance improvement. However, the effects of L-Arg were found to be dose-dependent, with low doses exerting a protective effect by generating NO, while high doses may lead to tissue damage by increasing oxidative stress and inflammatory responses. However, there are still some issues to be addressed in future studies, including further optimization of human studies, accurate improvement of the maximum safe treatment dose for patients with type 2 diabetes, and further investigations of the frequency of daily intake of L-Arg in patients with type 2 diabetes and the effects of different doses of L-Arg on patients with type 1 diabetes.
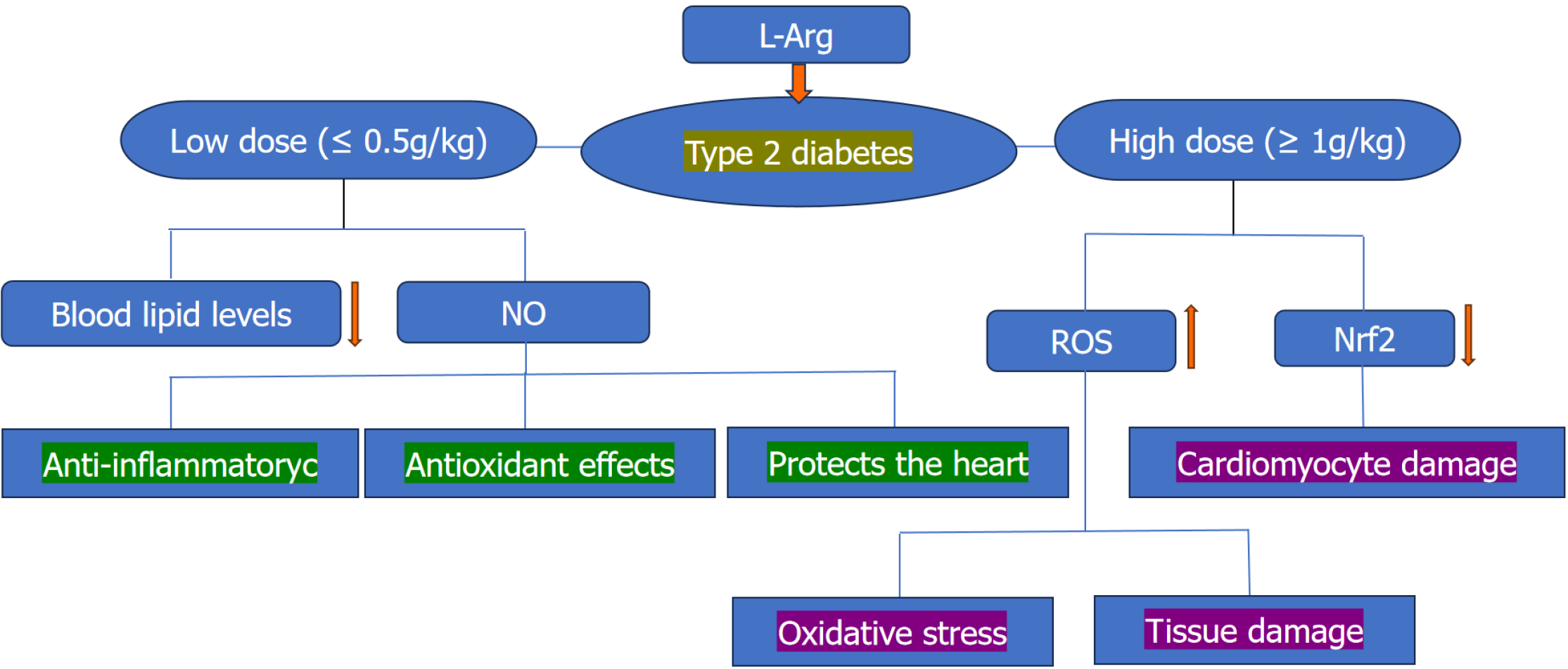
Figure 1 Effects of low-dose and high-dose L-arginine in patients with type 2 diabetes.
Low doses (≤ 0.5 g/kg) of L-arginine can improve blood lipid levels, whereas high doses (≥ 1 g/kg) of L-arginine can exacerbate oxidative stress and cause myocardial damage. NO: Nitric oxide; ROS: Reactive oxygen species.
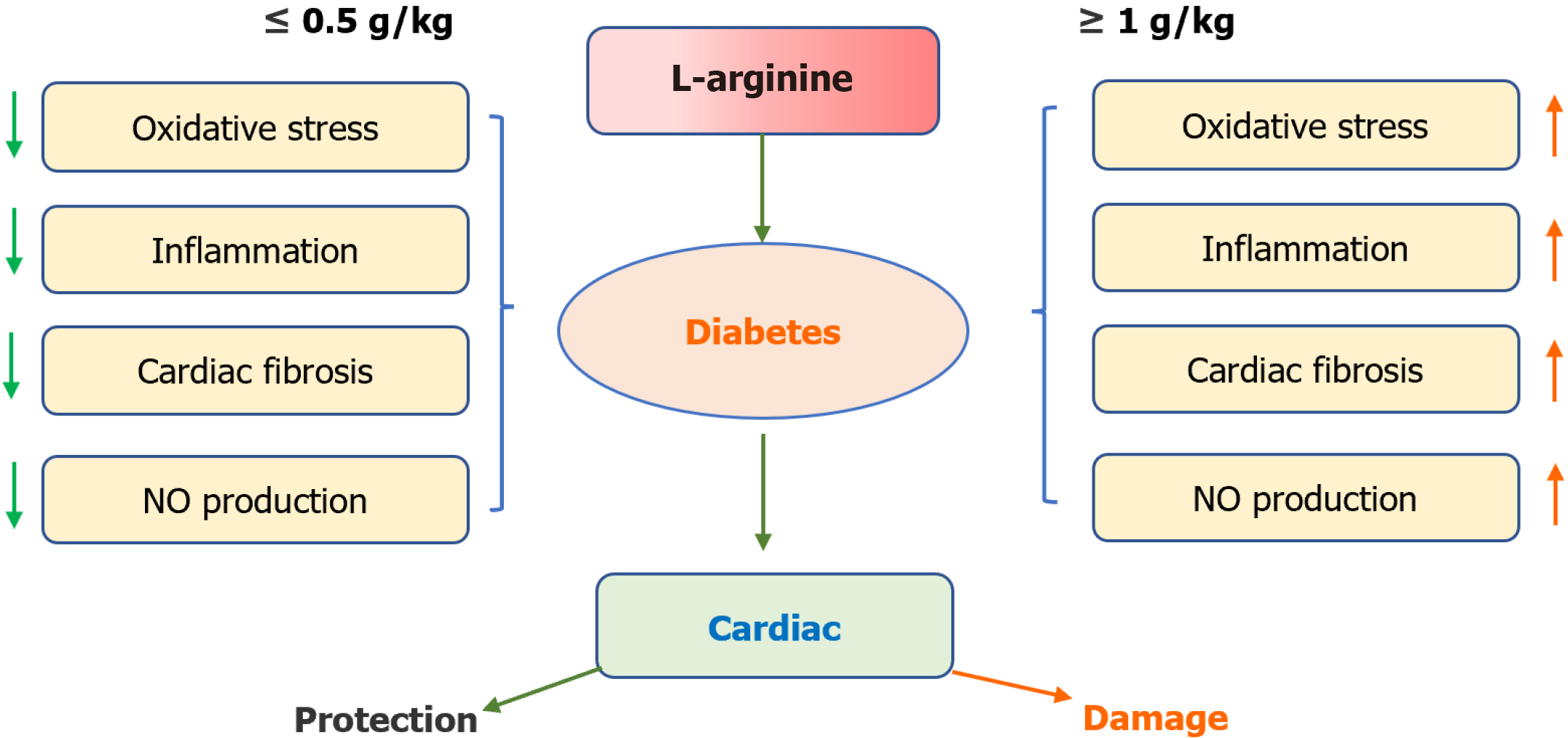
Figure 2 Excess L-arginine daily administration causes cardiac injury in diabetes mellitus patients.
0.5 g/kg L-arginine is beneficial for the treatment of diabetes and is the threshold of cardiac safety. NO: Nitric oxide.