Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.102141
Revised: January 4, 2025
Accepted: February 26, 2025
Published online: May 15, 2025
Processing time: 197 Days and 18.1 Hours
Type 2 diabetes mellitus (T2DM) is a prevalent metabolic disorder increasingly linked with hypertension, posing significant health risks. The need for a predi
To develop and validate a nomogram prediction model for hypertension in T2DM patients.
A retrospective observational study was conducted using data from 26850 T2DM patients from the Anhui Provincial Primary Medical and Health Information Management System (2022 to 2024). The study included patients aged 18 and above with available data on key variables. Exclusion criteria were type 1 diabetes, gestational diabetes, insufficient data, secondary hypertension, and abnormal liver and kidney function. The Least Absolute Shrinkage and Selection Operator regression and multivariate logistic regression were used to construct the nomogram, which was validated on separate datasets.
The developed nomogram for T2DM patients incorporated age, low-density lipoprotein, body mass index, diabetes duration, and urine protein levels as key predictive factors. In the training dataset, the model demonstrated a high discriminative power with an area under the receiver operating characteristic curve (AUC) of 0.823, indicating strong predictive accuracy. The validation dataset confirmed these findings with an AUC of 0.812. The calibration curve analysis showed excellent agreement between predicted and observed outcomes, with absolute errors of 0.017 for the training set and 0.031 for the validation set. The Hosmer-Lemeshow test yielded non-significant results for both sets (χ2 = 7.066, P = 0.562 for training; χ2 = 6.122, P = 0.709 for validation), suggesting good model fit.
The nomogram effectively predicts hypertension risk in T2DM patients, offering a valuable tool for personalized risk assessment and guiding targeted interventions. This model provides a significant advancement in the management of T2DM and hypertension comorbidity.
Core Tip: This study focused on type 2 diabetes mellitus complicated by hypertension. It used a large dataset and statistical methods to identify key risk factors like age, low-density lipoprotein, body mass index, diabetes duration, and urine protein. A validated nomogram was built for personalized risk assessment.
- Citation: Zhao JY, Dou JQ, Chen MW. Construction of a risk prediction model for hypertension in type 2 diabetes: Independent risk factors and nomogram. World J Diabetes 2025; 16(5): 102141
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/102141.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.102141
Type 2 diabetes mellitus (T2DM), a metabolic disorder hallmarked by insulin resistance and relative insulin deficiency, has ascended to the forefront of global health concerns[1]. Its escalating prevalence, coupled with the potential for devastating complications, poses a significant threat to public health. The confluence of T2DM and hypertension is particularly concerning, as it amplifies the risk for cardiovascular diseases, renal failure, and other conditions that can severely compromise patients' quality of life (QOL)[2]. Given the high prevalence of this comorbidity, there is an urgent need for effective predictive instruments to identify individuals at elevated risk, thereby enabling targeted intervention and preventive strategies.
A multitude of studies have delved into the intricate relationship between T2DM and hypertension, highlighting age, disease duration, body mass index (BMI), glucose metabolism parameters, and blood pressure levels as pivotal factors in the development of T2DM with hypertensive complications[3-5]. Despite these insights, the variability in patient demographics and the diverse methodologies employed across studies have resulted in a dearth of a unified, pragmatic risk prediction model. Currently, several existing hypertension assessment tools are accessible in the medical field. However, they frequently exhibit notable limitations when employed to evaluate the hypertension risk among patients with T2DM. For instance, traditional tools like the Framingham Risk Score, which has been widely used for predicting cardiovascular risks including those related to hypertension in the general population, primarily rely on broad population-based data[6,7]. It was initially developed without specific considerations for the unique pathophysiological interactions that occur between diabetes and hypertension in T2DM patients. As a result, it may underestimate or overestimate the actual risk of hypertension in this specific patient cohort. Some models might be developed based on relatively small sample sizes or specific regional populations, lacking the necessary generalizability to diverse T2DM patient groups[8]. Many existing prediction models do not comprehensively incorporate all the key factors that are relevant to T2DM patients with hypertension[6]. For instance, some may overlook the impact of certain metabolic markers such as specific lipid profiles or the dynamic changes in glucose control over time, which are known to have a significant influence on the development and progression of hypertension in these patients[9,10]. Consequently, in clinical practice, these limitations pose significant challenges for clinicians. They often find it difficult to make precise individualized risk assessments for T2DM patients with hypertension which can have negative impacts on patient outcomes and the efficient use of healthcare resources.
Nomograms, as graphical representations of statistical models, offer a unique advantage in quantifying the cumulative impact of multiple risk factors. They provide a visual and accessible means of estimating the likelihood of an event, such as the onset of a disease[11]. Over the past decade, nomograms have been increasingly utilized in various medical contexts, showcasing their potential to enhance clinical prediction accuracy and facilitate personalized patient care[12,13]. However, the development and validation of nomograms tailored to T2DM complicated by hypertension have been relatively scarce, with existing models often lacking the rigor of external validation and a thorough evaluation of their performance metrics.
The co-morbidity of T2DM and hypertension not only significantly reduces patients' QOL but also substantially increases the risk of life-threatening complications like stroke and myocardial infarction. In daily clinical practice, healthcare providers are constantly faced with the challenge of accurately assessing the hypertension risk in T2DM patients. Existing models fall short in offering comprehensive and precise evaluations, often resulting in sub - optimal treatment decisions. Given this dire situation and the pressing need in clinical settings, there is an urgent call for a more accurate and targeted prediction model. This study aims to bridge this gap, driven by the imperative to improve clinical management, safeguard patients' health, and reduce the overall burden of these two prevalent diseases.
The study employed a retrospective, observational design, harvesting data from the Anhui Provincial Primary Medical and Health Information Management System spanning the years 2022 to 2024. The dataset comprised 26,850 patients diagnosed with T2DM, including 10343 concurrent diabetes with hypertension (DH).
Inclusion criteria were as follows: (1) Confirmed diagnosis of T2DM, Specifically, the diagnosis was based on one of the following conditions: A fasting plasma glucose (FBG) level equal to or exceeding 7.0 mmol/L, with the necessity of reexamination on another day for those lacking typical diabetes symptoms; or the presence of the classic “polyuria, polydipsia, polyphagia, and weight loss” symptoms of diabetes in conjunction with a random blood glucose or venous plasma glucose level of no less than 11.1 mmol/L; or an oral glucose tolerance test result showing a 2-hour blood glucose level of at least 11.1 mmol/L, with reexamination required for those without typical symptoms; or a glycated hemoglobin (HbA1c) level reaching or surpassing 6.5%; (2) Age 18 years or older at the time of diagnosis; and (3) Available data on key variables, including age, gender, disease duration, BMI, FBG, two-hour plasma glucose, HbA1c, systolic blood pressure (SBP), and diastolic blood pressure (DBP). The observed indicators were determined in accordance with the risk factors reported in previous research studies.
Exclusion criteria included: (1) Patients with type 1 diabetes or other forms of diabetes; (2) Patients with a history of gestational diabetes; (3) Insufficient data for risk factor assessment; (4) Secondary hypertension due to underlying medical conditions; and (5) Abnormal liver and kidney function.
Data collection strictly complies with privacy and ethical guidelines. Personally identifiable information is anonymized to ensure confidentiality. The study protocol was approved by the Ethics Committee of Chaohu Hospital Affiliated to Anhui Medical University (No. KYXM-202312-007), and no informed consent was required for retrospective analysis. To ensure data reliability, the system data were regularly updated and verified by medical professionals. The data were double-checked and entered by two individuals to ensure accuracy and reliability.
The outcome variable was defined as the incidence of hypertension among the training group patients. Blood pressure measurements were conducted following standardized clinical procedures using calibrated sphygmomanometers. Hypertension was diagnosed when the SBP repeatedly equaled or exceeded 140 mmHg and/or the DBP repeatedly equaled or exceeded 90 mmHg, in line with established diagnostic guidelines.
The variables under consideration were selected based on their established association with hypertension risk factors as reported in the extant literature. These included: (1) Gender, coded as 1 for male and 2 for female, to facilitate statistical analysis; (2) Age, recorded in years, as a continuous variable; (3) Total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (TG), all measured in milligrams per deciliter (mg/dL) and treated as continuous variables; (4) BMI, calculated as weight in kilograms divided by the square of height in meters, and con
The measurement of these variables was conducted in accordance with standardized clinical protocols to ensure accuracy and consistency, allowing for a reliable assessment of the relationship between these factors and the incidence of hypertension.
For the analysis, we utilized SPSS 23.0 and R 4.2.2 software. Quantitative data with normal distribution were presented as mean ± SD, with group comparisons made using t-tests for parametric distributions and rank sum tests for non-parametric distributions. Categorical data were expressed as percentages (%) and compared using χ2 tests.
We selected the Least Absolute Shrinkage and Selection Operator (LASSO) regression for variable selection due to its effectiveness in handling multicollinearity and its ability to perform both variable selection and regularization[14]. LASSO regression is based on the L1 penalty, which shrinks some coefficients to zero, thus selecting a subset of variables that are most predictive of the outcome. This method is particularly useful when the number of potential predictors is large relative to the number of observations, as it can help to prevent overfitting and improve model interpretability. The combination of LASSO regression and multivariable analysis was employed to refine the variable selection process. LASSO regression was first used to reduce the dimensionality of the data by identifying the most influential variables, which were then included in a multivariable logistic regression model. This two-step approach leverages the strengths of both methods, enhancing the model's predictive performance.
The dataset was divided into training and validation sets in an 8:2 ratio to assess the model's predictive accuracy and generalizability. The training set, comprising 80% of the data, was used to develop the model, while the remaining 20% served as the validation set to test the model's performance on new data. This partitioning strategy is a standard practice in machine learning and statistical modeling, ensuring that the model is developed on a large portion of the data and then tested on a separate portion to assess its predictive performance.
In the training set, we applied LASSO regression to 12 candidate variables, including gender (coded as 1 for male and 2 for female), age, TC, HDL, LDL, BMI, duration of illness, creatinine levels, HbA1c, GSP, and TG as continuous variables, and urine protein levels (coded as negative: -, positive: +) as a categorical variable. LASSO regression reduced the 12 candidate indicators to 5 potential predictive indicators, which were then included in a multivariable logistic regression model to construct a nomogram prediction model for the risk of developing DH. The nomogram model was developed using the rms package in R software and was evaluated using the area under the receiver operating characteristic curve (AUC), calibration curve, and Hosmer-Lemeshow test. The model's predictive accuracy was further assessed using decision curve analysis (DCA) to evaluate its clinical utility at different threshold probabilities. Statistical significance was set at P < 0.05, indicating that any observed differences or associations were statistically meaningful.
Table 1 compares the general information between the no DH group (n = 16507) and the DH group (n = 10343). Significant differences were found in gender distribution (P < 0.001), with a slight male predominance in both groups. The DH group had a higher mean age (61.51 vs 52.21 years), TC (4.02 vs 4.42 mmol/L), LDL (2.54 vs 2.76 mmol/L), BMI (27.03 vs 25.11 kg/m²), duration of diabetes (10.23 vs 6.99 years), creatinine (76.29 vs 66.96 μmol/L), HbA1c (8.57% vs 8.81%), and GSP (2.60 vs 2.79 mmol/L), all with P < 0.001. No significant differences were observed in HDL and TG levels. Urine protein levels were significantly higher in the DH group, with more patients showing positive results and higher grades of proteinuria. These results highlight the distinct clinical profiles associated with DH, including older age, dyslipidemia, increased BMI, and proteinuria.
| Item | No DH group (n = 16507) | DH group (n = 10343) | P value |
| Gender [n (%)] | < 0.001 | ||
| Male | 9887 (59.90) | 6099 (58.97) | |
| Female | 6620 (40.10) | 4244 (41.03) | |
| Age (years) | 52.21 ± 11.64 | 61.51 ± 12.01 | < 0.001 |
| TC (mmol/L) | 4.42 ± 1.19 | 4.02 ± 1.34 | < 0.001 |
| HDL (mmol/L) | 1.14 ± 0.42 | 1.11 ± 0.36 | 0.168 |
| LDL (mmol/L) | 2.76 ± 0.84 | 2.54 ± 0.92 | < 0.001 |
| BMI (kg/m²) | 25.11 ± 3.29 | 27.03 ± 3.86 | < 0.001 |
| Duration of diabetes (years ± SD) | 6.99 ± 6.54 | 10.23 ± 7.93 | < 0.001 |
| Creatinine (μmol/L) | 66.96 ± 21.36 | 76.29 ± 48.46 | < 0.001 |
| HbA1c (%) | 8.81 ± 2.19 | 8.57 ± 2.02 | < 0.001 |
| GSP (mmol/L) | 2.79 ± 0.75 | 2.60 ± 0.71 | < 0.001 |
| TG (mmol/L) | 2.38 ± 2.55 | 2.35 ± 2.80 | 0.491 |
| Urine protein [n (%)] | < 0.001 | ||
| Negative | 15464 (93.68) | 8274 (80.00) | |
| + | 496 (3.00) | 596 (5.76) | |
| ++ | 282 (1.71) | 617 (5.97) | |
| +++ | 265 (1.61) | 866 (8.37) |
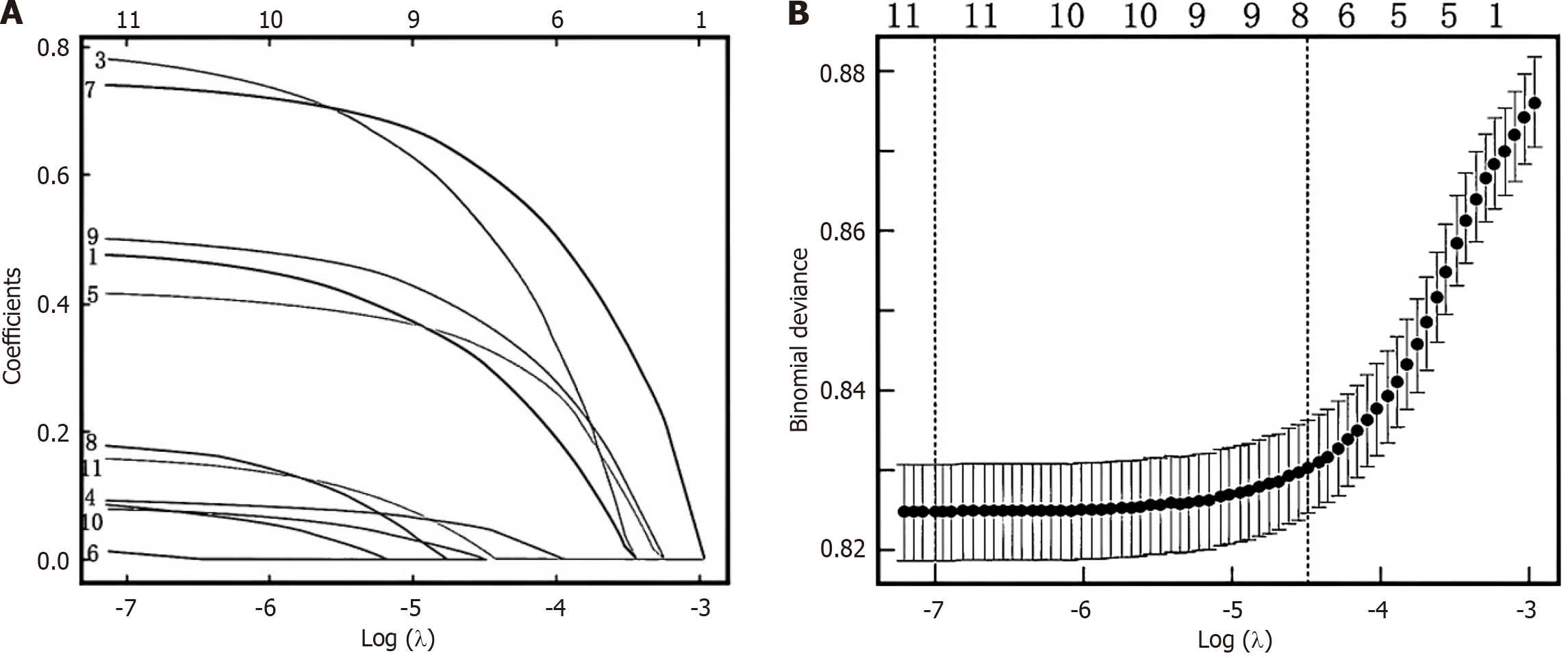
The variables with significance in the univariate analysis were included in the Lasso regression analysis for dimensionality reduction. With the change of the penalty coefficient λ, the independent variables initially included in the model gradually decreased, and finally the λ value of the most simple model was obtained within a variance range of lambda.min, i.e., lambda.lse. At this time, the included predictive variables included age, LDL, BMI, Duration of Diabetes and Urine Protein, as shown in Figure 1.
Table 2 presents the results of a multivariate logistic regression model analysis, highlighting significant predictors of an outcome. The constant term is highly significant with a β of -10.25 and a P value of 0.001, leading to an OR of 0.001. Age is positively associated with the outcome, with a β of 0.08, a P value of 0.001, and an OR of 1.09 (95%CI: 1.07-1.10). LDL also shows a significant association with the outcome, with a β of 0.23, a P value of 0.001, and an OR of 1.04 (95%CI: 1.02-1.06). BMI has a β of 0.25, a P value of 0.001, and an OR of 1.28 (95%CI: 1.24-1.31). The duration of diabetes is significantly related to the outcome, with a β of 0.03, a P value of 0.001, and an OR of 1.03 (95%CI: 1.02-1.05). Urine Protein levels, categorized as "+", "++", and "+++", exhibit increasing significance with escalating levels, with the "+++" category showing the strongest association, having a β of 2.82, a P value of 0.001, and an OR of 16.48 (95%CI: 8.36-36.58). All variables with the exception of the constant term are statistically significant at the P < 0.001 Level, indicating their importance in predicting the outcome.
| Item | Coefficient (β) | SD | Z-statistic (Z) | P value | OR | 95%CI |
| Constant | -10.25 | 0.56 | -18.55 | 0.001 | 0.001 | - |
| Age | 0.08 | 0.008 | 15.56 | 0.001 | 1.09 | 1.07-1.10 |
| LDL | 0.23 | 0.05 | 4.32 | 0.001 | 1.04 | 1.02-1.06 |
| BMI | 0.25 | 0.012 | 16.03 | 0.001 | 1.28 | 1.24-1.31 |
| Duration of diabetes | 0.03 | 0.006 | 3.98 | 0.001 | 1.03 | 1.02-1.05 |
| Urine Protein | ||||||
| + | 0.72 | 0.25 | 2.71 | 0.001 | 2.03 | 1.25-3.48 |
| ++ | 1.38 | 0.34 | 4.11 | 0.001 | 4.00 | 2.12-8.02 |
| +++ | 2.82 | 0.36 | 7.54 | 0.001 | 16.48 | 8.36-36.58 |
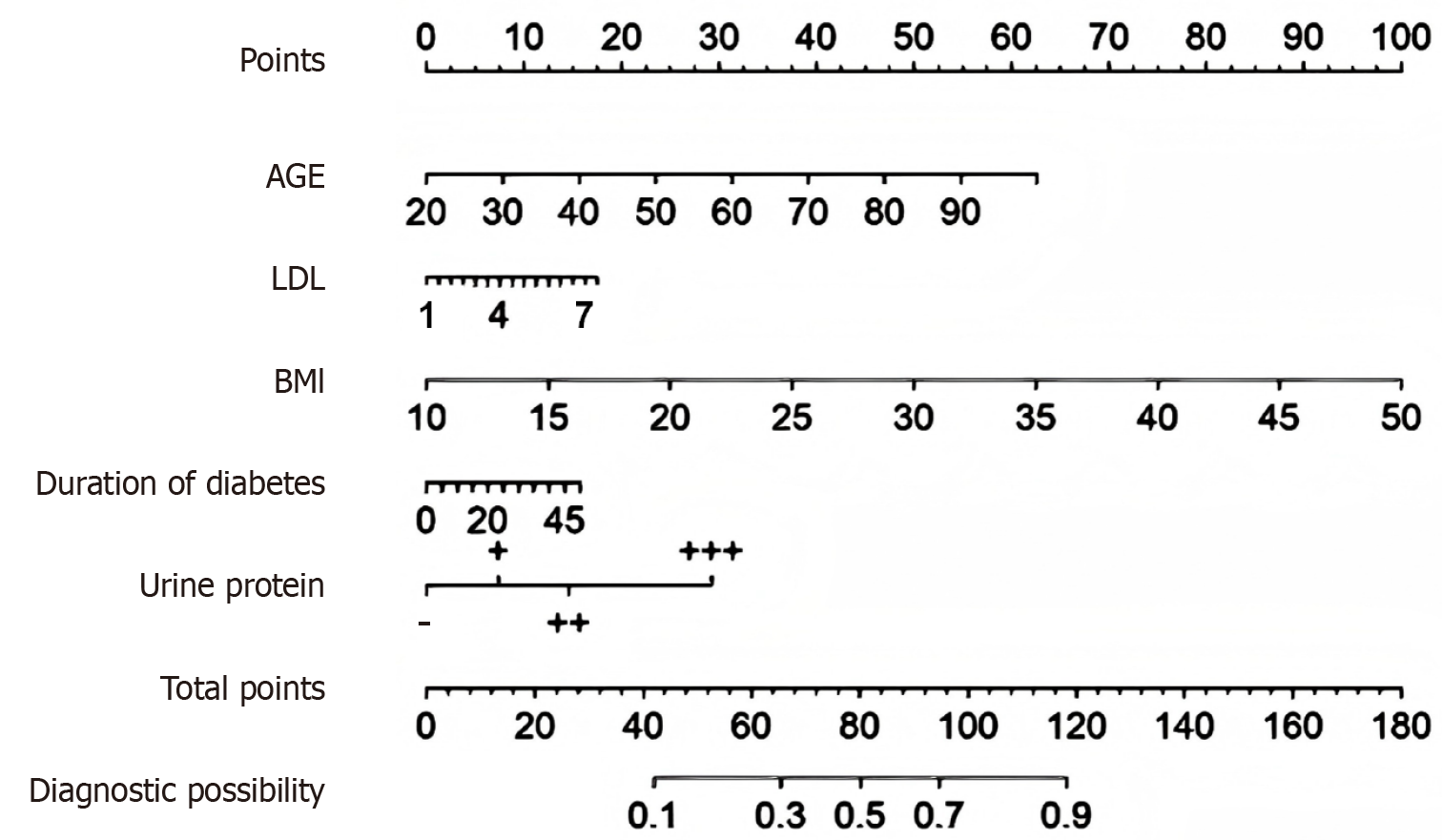
Age, the duration of diabetes, LDL levels, BMI, and urine protein levels have been established as independent risk factors for the onset of DH and have been effectively integrated into a nomogram model, as depicted in Figure 2. The nomogram includes a scale for age, with points assigned as you move up the scale from 20 to 90 years. LDL cholesterol levels are also accounted for, with points increasing from 1 to 7 mmol/L. BMI contributes to the point total, with a scale from 10 to 50 kg/m². The duration of diabetes is represented with a scale from 0 to 45 years, and urine protein levels are categorized and scored as follows: "-" for 0 points, "++" for 15 points, and "+++" for 30 points. The total points accrued from these variables are then plotted on the total points scale, which ranges from 0 to 180. Finally, the total points are translated into a diagnostic possibility, ranging from 0.1 to 0.9, providing a probability estimate of the condition in question. This tool allows for a visual and quantitative assessment of risk based on individual patient characteristics.
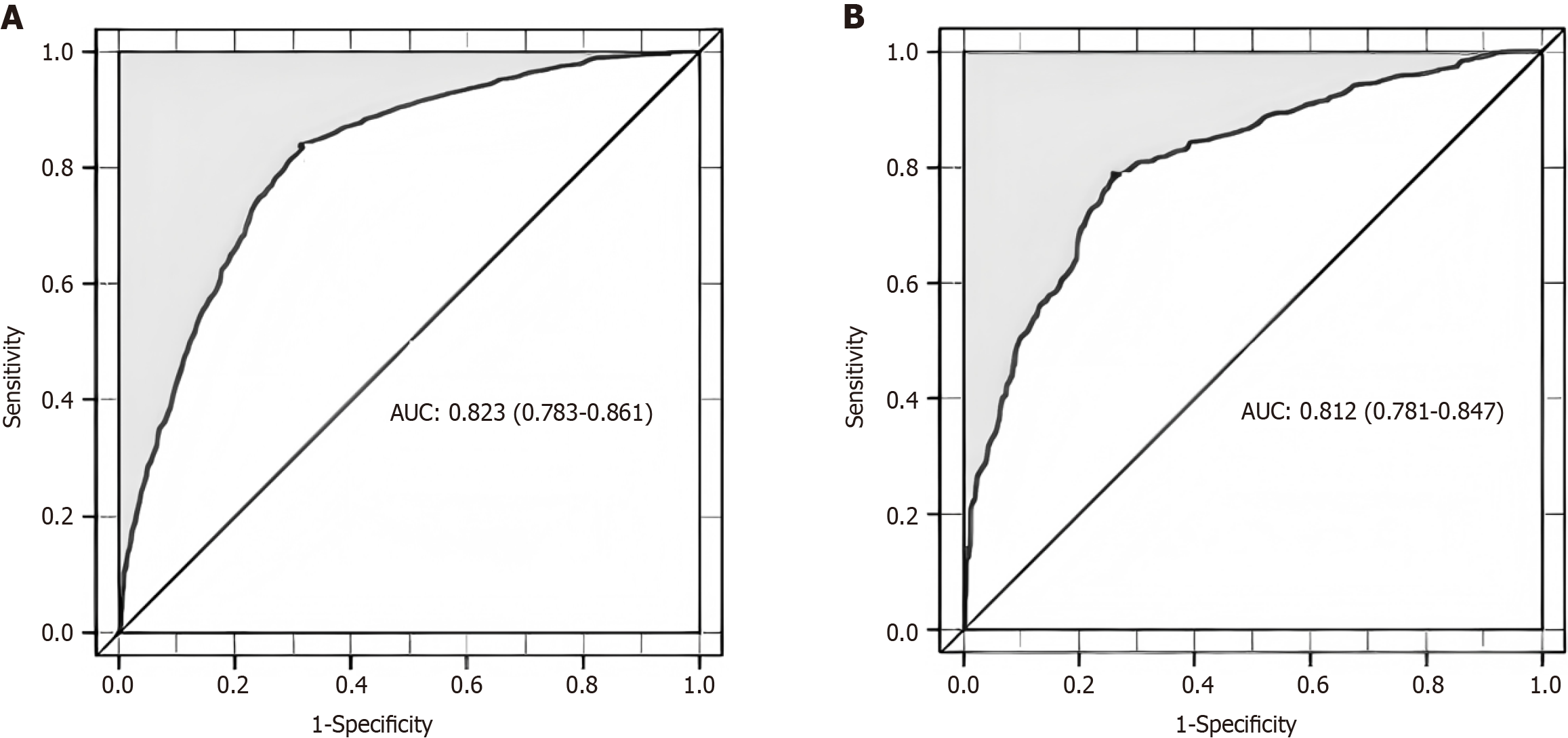
The training group's predictive nomogram model boasts an AUC of 0.823, within a 95%CI of 0.79 to 0.85, as illustrated in Figure 3A. Subsequent validation using a distinct dataset confirmed the robustness of the DH predictive model, with an AUC of 0.812, ranging from 0.781 to 0.847, as seen in Figure 3B. Notably, the AUC for the validation group's predictive nomogram was marginally reduced relative to the training group, affirming the model's consistent discriminative power across both cohorts.
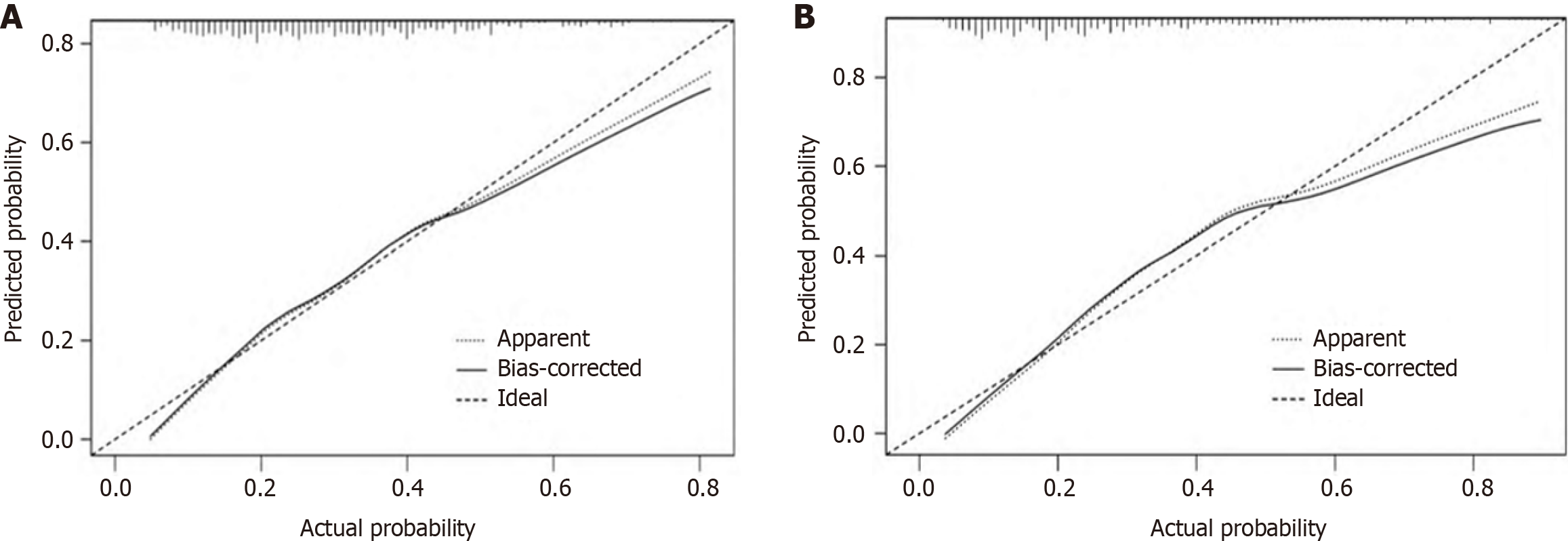
The calibration curve analysis, validated through bootstrapping for both the training and validation sets, demonstrated minimal absolute errors of 0.017 and 0.031, respectively. This suggests a close alignment between the predicted and observed outcomes, affirming the model's accuracy. Furthermore, the Hosmer-Lemeshow test yielded non-significant χ2 values of 7.066 (P = 0.562) for the training set and 6.122 (P = 0.709) for the validation set. These results indicate a strong calibration of the model, with no significant discrepancy between predicted and actual values, as depicted in Figure 4.
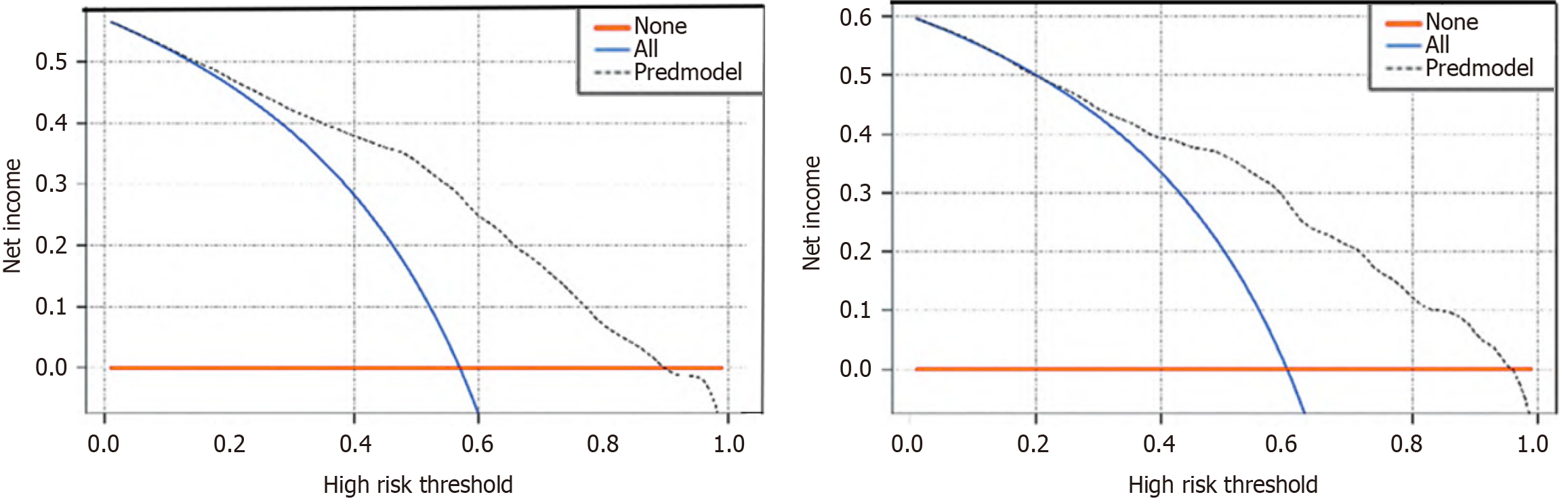
Delving deeper into the model's utility, the DCA, featured in Figure 5, provides a visual representation of the model's net benefit across various threshold probabilities. The analysis demonstrates that the DH nomogram yields a higher net benefit over a simple strategy of assuming a uniform risk across all patients when the individual's threshold probability for developing DH exceeds 1%. Note: The gray dotted line represents the model, the blue line indicates that all samples are positive and all patients are intervened; the red line indicates that all samples are negative and all patients are not intervened.
The intricate relationship between T2DM and hypertension is well-documented and poses a significant public health challenge. Globally, T2DM has reached epidemic proportions, with an estimated 463 million individuals affected in 2019, a number anticipated to surge to 700 million by 2045[15]. Hypertension, a prevalent condition affecting over one billion people worldwide, is also a leading contributor to mortality, accounting for approximately 7.5 million deaths annually[16]. The co-occurrence of these two conditions amplifies the risk of cardiovascular events and kidney disease, leading to a substantial decrease in patients' QOL[17].
The coexistence of T2DM and hypertension, a scenario observed in a significant proportion of patients, varies across different populations and is influenced by a myriad of factors, including genetics, lifestyle, and environmental exposures. Sun et al[18] utilized a population-based prospective cohort study to establish a bidirectional causal relationship between T2DM and hypertension , demonstrating that T2DM is likely to causally affect hypertension, while the reverse relationship is not supported, and highlighted the importance of maintaining optimal glycemic levels and blood pressure (especially systolic) in T2DM patients. Studies from diverse geographical regions, such as Asia, Europe, and North America, consistently report a higher prevalence of hypertension among individuals with T2DM compared to the general population[8].
Shared risk factors for T2DM and hypertension, such as advancing age, obesity, physical inactivity, sedentary lifestyle, and unhealthy dietary habits, further intertwine these conditions. This study demonstrated a significant correlation between BMI and hypertension in T2DM patients, with statistically significant differences (P < 0.05). Consistent with prior research by Aronow[19], who reported a 2 to 6-fold higher incidence of hypertension in obese individuals, and Zhang et al[20], who identified obesity as an independent risk factor for DH, our findings underscore the importance of BMI in assessing patient health. Higher BMIs, indicative of greater adiposity, are associated with elevated LDL levels, which contribute to vascular sclerosis and are a key factor in atherosclerosis, as cited in references[21,22]. Zhang et al[23] revealed that young adult LDL cholesterol levels, particularly those ≥ 100 mg/dL, are significantly associated with a 64% increased risk for coronary heart disease later in life, independent of cholesterol levels in later adulthood, underscoring the importance of early management of lipid levels for long-term cardiovascular health.
For T2DM patients, it is crucial to engage in regular exercise, maintain a balanced diet, and manage weight to mitigate these risks[24]. Additionally, rigorous control of blood pressure, lipids, and glucose is essential to prevent complications[25]. Urine protein, a normal urine constituent, becomes pathological when its levels exceed normal ranges, a condition known as proteinuria. Proteinuria in individuals with normal blood pressure can signal kidney disease, which is both a regulator of blood pressure and a target organ for hypertension-induced damage[26]. Conversely, uncontrolled hypertension can lead to kidney disease and proteinuria, termed hypertensive nephropathy[27]. The association between urine protein increases and hypertension risk is well-documented[28,29], aligning with our study's outcomes. In the context of T2DM, aggressive management of both hypertension and kidney disease is imperative to minimize the impact of DH. The nomogram, a valuable clinical research tool, estimates disease occurrence probabilities by integrating risk factors. It is renowned for its reliability, practicality, and ease of understanding, making it a staple in disease prediction and prognosis research[30]. Our study developed a nomogram based on five risk factors identified through Lasso regression and multivariate logistic regression analysis. By tallying the scores of these risk factors, the nomogram provides a DH risk probability. This nomogram offers a superior, personalized risk prediction and assessment compared to other statistical methods[31], delivering intuitive and visual insights. The study's results indicate that both the training and validation groups exhibited strong discrimination, accuracy, and clinical utility, affirming the nomogram's value for healthcare professionals in assessing individual risks.
Previous studies have made strides in unraveling the complex web of risk factors associated with T2DM complicated by hypertension and in developing predictive models. Yang et al[32] developed and validated a nomogram model incorporating age, BMI, diabetic nephropathy, and diabetic retinopathy as independent risk factors to predict the individual risk of developing hypertension in patients with T2DM, demonstrating good accuracy and clinical applicability, however, the model's generalizability may be limited due to the specific patient population. Wu et al[33] developed a nomogram prediction model using age, waist-height ratio, and HDL-cholesterol to predict the risk of comorbid diabetes in hypertensive patients, showing good discrimination and clinical utility, but the model's applicability may be constrained to the specific community population and requires further validation in diverse settings. The results of this study showed that the prevalence of hypertension in T2DM patients was 55.7%, which is basically consistent with the results of domestic and foreign studies. This study combined Lasso regression with multifactorial logistic regression model analysis, and the results showed that age, LDL, BMI, duration of diabetes, and urine protein were risk factors for DH. The risk of DH increased with the increase of age, LDL, BMI, duration of diabetes, and urine protein.
This study extends the current literature by incorporating a large, recent dataset and applying rigorous statistical methods to develop a nomogram that is both accurate and externally validated. The nomogram fills a gap in the existing prediction tools, providing a user-friendly and evidence-based approach for clinicians to identify high-risk patients and tailor prevention and intervention strategies. The potential impact of the nomogram extends beyond the clinical setting, as it can inform public health interventions and policy development. By identifying high-risk populations, resources can be effectively allocated to target preventive measures and health education programs, ultimately contributing to the global efforts in reducing the burden of T2DM and hypertension.
However, several caveats should be acknowledged. The study is limited by its retrospective nature and reliance on electronic health record data, which may contain measurement errors or missing data. Regarding blood pressure measurement, as the data comes from a public source, details on standardization are lacking. We don't know about the time of measurement, the subject's posture, the measurement technique, or its relation to meals. Given the high prevalence of postprandial hypotension in type 2 diabetes patients, non-standardized measurements could misclassify hypertension cases and affect the accuracy of the risk prediction model. Future prospective studies are needed to validate the nomogram's performance in real-world clinical settings. Additionally, the model could be further refined by incorporating novel biomarkers or emerging risk factors, such as genetic predispositions or lifestyle factors, to enhance its predictive power.
In conclusion, this study addressed the significant public health challenge posed by the coexistence of T2DM and hypertension. By leveraging LASSO regression and multivariable analysis, it identified age, LDL levels, BMI, duration of diabetes, and urine protein levels as independent risk factors for DH in T2DM patients and constructed a nomogram-based predictive model. The model demonstrated good predictive accuracy, robustness, and clinical utility in both the training and validation groups, filling a gap in existing prediction tools and offering a user-friendly approach for personalized risk assessment and intervention strategy tailoring. However, limited by its retrospective nature and reliance on electronic health record data, future prospective studies and incorporation of additional novel biomarkers or risk factors are needed to further refine and validate the model in diverse populations, aiming to optimize patient care and contribute more effectively to reducing the burden of this prevalent comorbidity in the context of precision medicine.
We would like to express our sincere gratitude to all the colleagues and friends who provided valuable suggestions and support during the research process. Their insights and assistance were crucial in helping us complete this study and shape the manuscript.
| 1. | DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, Simonson DC, Testa MA, Weiss R. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 1456] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 2. | Song DK, Hong YS, Sung YA, Lee H. Risk factor control and cardiovascular events in patients with type 2 diabetes mellitus. PLoS One. 2024;19:e0299035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Kamalumpundi V, Shams E, Tucker C, Cheng L, Peterson J, Thangavel S, Ofori O, Correia M. Mechanisms and pharmacotherapy of hypertension associated with type 2 diabetes. Biochem Pharmacol. 2022;206:115304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (1)] |
| 4. | Chakraborty S, Verma A, Garg R, Singh J, Verma H. Cardiometabolic Risk Factors Associated With Type 2 Diabetes Mellitus: A Mechanistic Insight. Clin Med Insights Endocrinol Diabetes. 2023;16:11795514231220780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (1)] |
| 5. | Elhefnawy ME, Ghadzi SMS, Noor Harun S. Predictors Associated with Type 2 Diabetes Mellitus Complications over Time: A Literature Review. J Vasc Dis. 2022;1:13-23. [DOI] [Full Text] |
| 6. | Ahmad A, Lim LL, Morieri ML, Tam CH, Cheng F, Chikowore T, Dudenhöffer-Pfeifer M, Fitipaldi H, Huang C, Kanbour S, Sarkar S, Koivula RW, Motala AA, Tye SC, Yu G, Zhang Y, Provenzano M, Sherifali D, de Souza RJ, Tobias DK; ADA/EASD PMDI, Gomez MF, Ma RCW, Mathioudakis N. Precision prognostics for cardiovascular disease in Type 2 diabetes: a systematic review and meta-analysis. Commun Med (Lond). 2024;4:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Abohelwa M, Kopel J, Shurmur S, Ansari MM, Awasthi Y, Awasthi S. The Framingham Study on Cardiovascular Disease Risk and Stress-Defenses: A Historical Review. J Vasc Dis. 2023;2:122-164. [DOI] [Full Text] |
| 8. | Naseri MW, Esmat HA, Bahee MD. Prevalence of hypertension in Type-2 diabetes mellitus. Ann Med Surg (Lond). 2022;78:103758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 9. | Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014;5:444-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 472] [Cited by in RCA: 594] [Article Influence: 49.5] [Reference Citation Analysis (12)] |
| 10. | Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can J Cardiol. 2018;34:575-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1040] [Cited by in RCA: 1119] [Article Influence: 139.9] [Reference Citation Analysis (1)] |
| 11. | Jian X, Du S, Zhou X, Xu Z, Wang K, Dong X, Hu J, Wang H. Development and validation of nomograms for predicting the risk probability of carbapenem resistance and 28-day all-cause mortality in gram-negative bacteremia among patients with hematological diseases. Front Cell Infect Microbiol. 2022;12:969117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 12. | Li X, Xu Q, Gao C, Yang Z, Li J, Sun A, Wang Y, Lei H. Development and validation of nomogram prognostic model for predicting OS in patients with diffuse large B-cell lymphoma: a cohort study in China. Ann Hematol. 2023;102:3465-3475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Toma M, Wei OC. Predictive Modeling in Medicine. Encyclopedia. 2023;3:590-601. [DOI] [Full Text] |
| 14. | Vasquez MM, Hu C, Roe DJ, Chen Z, Halonen M, Guerra S. Least absolute shrinkage and selection operator type methods for the identification of serum biomarkers of overweight and obesity: simulation and application. BMC Med Res Methodol. 2016;16:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 15. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 6418] [Article Influence: 916.9] [Reference Citation Analysis (12)] |
| 16. | Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1423] [Cited by in RCA: 2312] [Article Influence: 385.3] [Reference Citation Analysis (3)] |
| 17. | Hezam AAM, Shaghdar HBM, Chen L. The connection between hypertension and diabetes and their role in heart and kidney disease development. J Res Med Sci. 2024;29:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 18. | Sun D, Zhou T, Heianza Y, Li X, Fan M, Fonseca VA, Qi L. Type 2 Diabetes and Hypertension. Circ Res. 2019;124:930-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 19. | Aronow WS. Association of obesity with hypertension. Ann Transl Med. 2017;5:350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Zhang Y, Zhang WQ, Tang WW, Zhang WY, Liu JX, Xu RH, Wang TD, Huang XB. The prevalence of obesity-related hypertension among middle-aged and older adults in China. Front Public Health. 2022;10:865870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 21. | Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ Res. 2016;118:1786-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 485] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 22. | Maksimovic M, Vlajinac H, Radak D, Marinkovic J, Maksimovic J, Jorga J. Association of overweight and obesity with cardiovascular risk factors in patients with atherosclerotic diseases. J Med Biochem. 2020;39:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Vittinghoff E, Pletcher MJ, Allen NB, Zeki Al Hazzouri A, Yaffe K, Balte PP, Alonso A, Newman AB, Ives DG, Rana JS, Lloyd-Jones D, Vasan RS, Bibbins-Domingo K, Gooding HC, de Ferranti SD, Oelsner EC, Moran AE. Associations of Blood Pressure and Cholesterol Levels During Young Adulthood With Later Cardiovascular Events. J Am Coll Cardiol. 2019;74:330-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 24. | Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84:S15-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 264] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 25. | Hu H, Hori A, Nishiura C, Sasaki N, Okazaki H, Nakagawa T, Honda T, Yamamoto S, Tomita K, Miyamoto T, Nagahama S, Uehara A, Yamamoto M, Murakami T, Shimizu C, Shimizu M, Eguchi M, Kochi T, Imai T, Okino A, Kuwahara K, Kashino I, Akter S, Kurotani K, Nanri A, Kabe I, Mizoue T, Kunugita N, Dohi S; Japan Epidemiology Collaboration on Occupational Health Study Group. Hba1c, Blood Pressure, and Lipid Control in People with Diabetes: Japan Epidemiology Collaboration on Occupational Health Study. PLoS One. 2016;11:e0159071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Cravedi P, Remuzzi G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br J Clin Pharmacol. 2013;76:516-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Ameer OZ. Hypertension in chronic kidney disease: What lies behind the scene. Front Pharmacol. 2022;13:949260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 28. | Lee H, Park MS, Kang MK, Song TJ. Association between Proteinuria Status and Risk of Hypertension: A Nationwide Population-Based Cohort Study. J Pers Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 29. | Currie G, Delles C. Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis. 2013;7:13-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Wang X, Liu H, Wang P, Wang Y, Yi Y, Li X. A nomogram for analyzing risk factors of poor treatment response in patients with autoimmune hepatitis. Eur J Gastroenterol Hepatol. 2024;36:113-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 31. | Zhang J, Yang W, Lian C, Zhao Q, Ming WK, Ip CC, Mu HH, Ching Tom K, Lyu J, Deng L. A nomogram for predicting survival in patients with skin non-keratinizing large cell squamous cell carcinoma: A study based on the Surveillance, Epidemiology, and End Results database. Front Med (Lausanne). 2023;10:1082402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Yang J, Wang X, Jiang S. Development and validation of a nomogram model for individualized prediction of hypertension risk in patients with type 2 diabetes mellitus. Sci Rep. 2023;13:1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Wu Y, Tan W, Liu Y, Li Y, Zou J, Zhang J, Huang W. Development and validation of a nomogram prediction model for hypertension-diabetes comorbidity based on chronic disease management in the community. Lipids Health Dis. 2023;22:135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/