Published online May 15, 2025. doi: 10.4239/wjd.v16.i5.101447
Revised: February 11, 2025
Accepted: March 25, 2025
Published online: May 15, 2025
Processing time: 148 Days and 0.6 Hours
Diabetes is characterized by insulin resistance as well as impaired insulin production, with β-cell dysfunction playing a critical role in disease progression. Exercise is known to improve insulin sensitivity, but its effects on pancreatic islet quality and function remain poorly understood. This work hypothesized that swimming training enhances glycemic control and insulin secretion by upregulating the insulin-like growth factor 1 (IGF-1)/phosphatidylinositol 3-kina
To investigate the effects of swimming on pancreatic islet quality and function in STZ-induced diabetic rats via the IGF-1/PI3K/AKT pathway.
Twenty-six Sprague-Dawley rats were grouped into diabetic and control groups, with each group further split into exercise and sedentary subgroups. Diabetic rats were induced with STZ. The exercise groups underwent swimming training for 60 minutes/day, 5 days/week, for 8 weeks. Body weight, food intake, blood glucose, insulin, lipids, and muscle glycogen were measured. Pancreatic islet morphology and the protein expression levels of IGF-1, PI3K, and AKT were analyzed. Data were analyzed using two-way repeated-measure ANOVA, followed by Tukey’s post-hoc test.
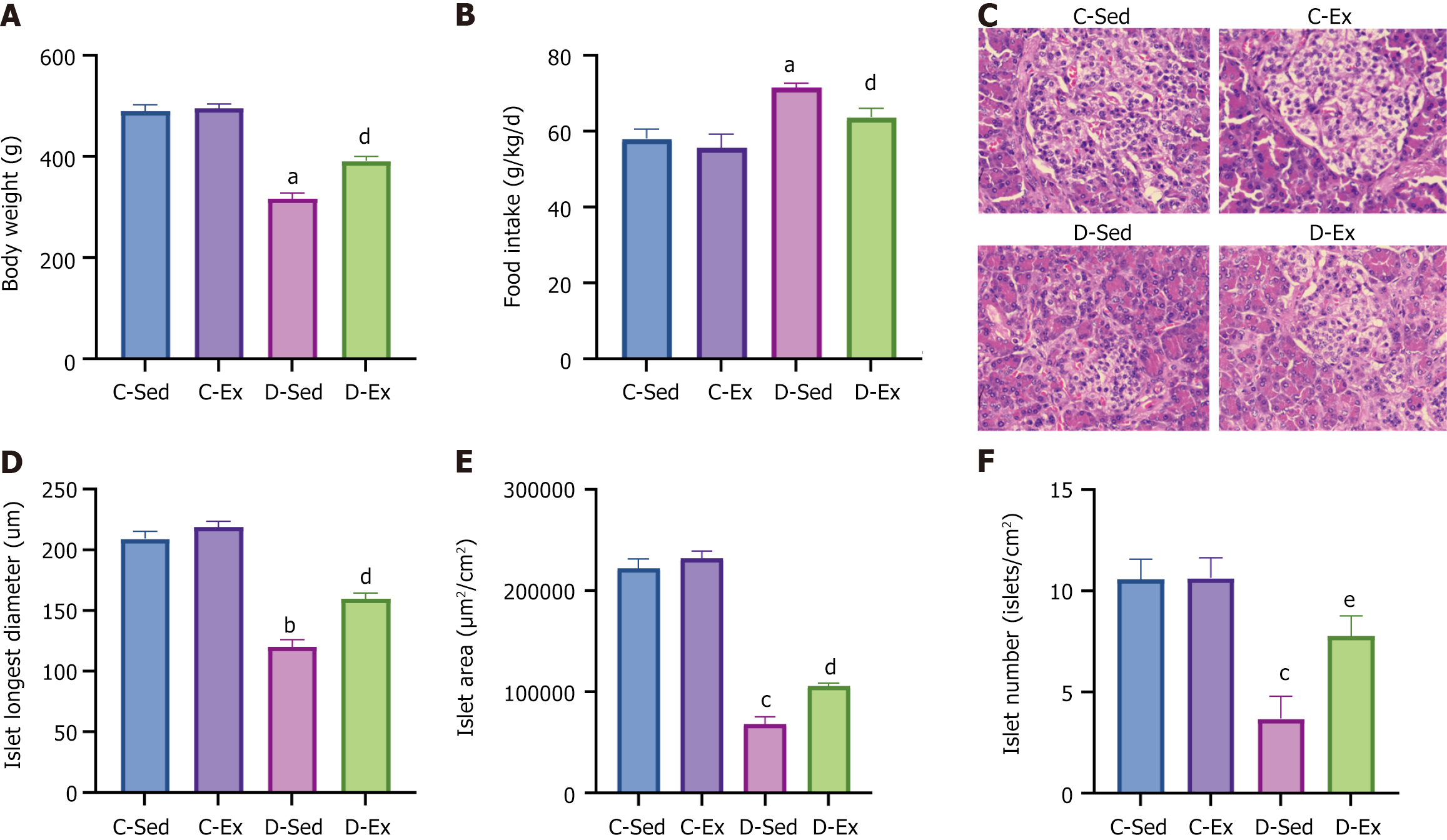
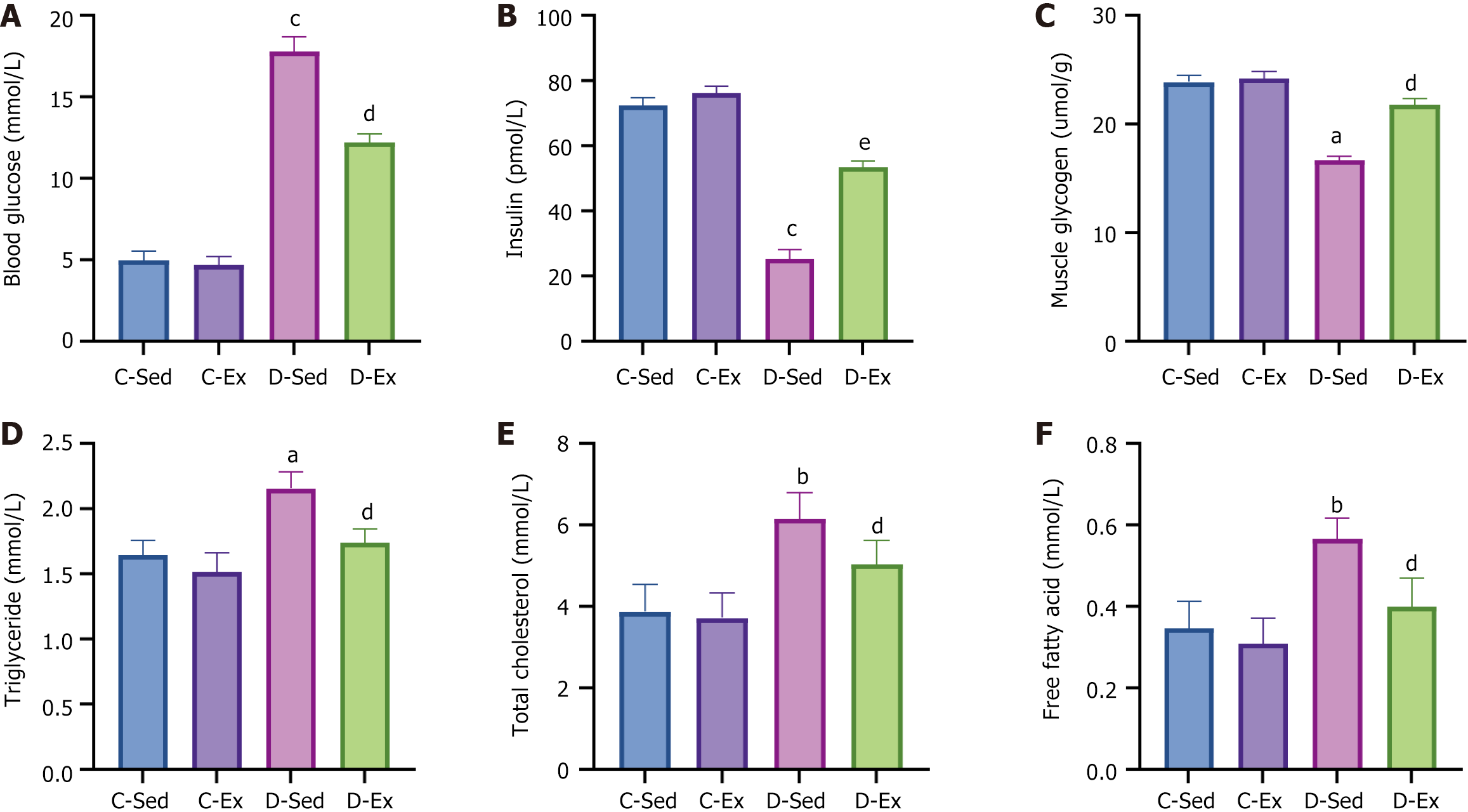
Exercise training significantly improved body weight [diabetic exercise group (D-Ex): 390.66 ± 50.14 g vs diabetic sedentary group (D-Sed): 315.89 ± 50.12 g, P < 0.05], reduced blood glucose (D-Ex: 12.21 ± 4.43 mmol/L vs D-Sed: 17.79 ± 2.05 mmol/L, P < 0.05), and increased insulin levels (D-Ex: 53.50 ± 15.31 pmol/L vs D-Sed: 25.31 ± 10.23 pmol/L, P < 0.05) in diabetic rats. It also enhanced islet morphology, increased IGF-1 expression, and activated the PI3K/AKT pathway (P < 0.05). In-vitro experiments confirmed that IGF-1 positively regulated insulin expression and inhibited β-cell apoptosis via the PI3K/AKT pathway.
Exercise training improves pancreatic islet quality and function in diabetic rats by modulating the
Core Tip: In this study, we intended to reveal the outcomes of swimming training on pancreatic islets, blood glucose, insulin, lipids, pancreatic beta-cells, insulin-like growth factor 1 (IGF-1), and the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway expression in diabetic rats. Our study elucidates how exercise training, via the modulation of the IGF-1-regulated PI3K/AKT pathway, improves islet quality and function in streptozotocin-induced diabetic rats.
- Citation: Wu YW, Wu CY, Lin F, Wu JY. Exercise training benefits pancreatic islet by modulating the insulin-like growth factor 1/phosphatidylinositol 3-kinase/protein kinase B pathway. World J Diabetes 2025; 16(5): 101447
- URL: https://www.wjgnet.com/1948-9358/full/v16/i5/101447.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i5.101447
The primary features of diabetes include insulin resistance and a lack of insulin production. Individuals with type 2 diabetes mellitus (T2DM) experience a gradual decline in β-cell functionality as the disease progresses[1]. In their study involving 2975 participants from China, Qian et al[2] discovered that the deterioration of β-cell function could be the primary factor leading to the progression of impaired glucose tolerance and fasting glucose into diabetes, rather than an exacerbation of insulin resistance. Research indicated that at the point of diagnosis, pancreatic islet function was approximately 50% of what is considered normal, and post-mortem examinations revealed a reduction of around 60% in β-cell mass[1]. Many Asian individuals diagnosed with T2DM frequently do not display signs of obesity and usually have serum insulin concentrations that are at or below the normal range[3,4]. This finding suggests that a reduced capacity for insulin secretion has a remarkable role in the incidence and advancement of T2DM. Recent findings suggest that the dysfunction of β cells as well as the reduction in β-cell mass can potentially be reversed, especially during the initial phases of the disease[1]. Therefore, early intervention with lowering the burden in β-cell mass and function can delay or reverse the progression of diabetes.
Many animal studies have shown that insulin secretion deficiency is closely related to a downregulation in islet β-cell proliferation and an increase in islet cell apoptosis in diabetes[5]. Temporary and mild insulin resistance can lead to the compensatory growth of β-cell mass[6], but long-term and severe insulin resistance may reduce β-cell proliferation[5]. Exercise increases insulin sensitivity and reduces insulin resistance in patients with diabetes and experimental animals[7]. Several studies found that exercise training diminished arginine- and glucose-stimulated insulin secretions[8]. Dela et al[9] discovered that in individuals with T2DM, physical training improved β-cell function under a moderate level of remaining secretory capacity; however, this enhancement was not observed when the secretory capacity was low. Majority of investigations focused on insulin resistance improved by exercise.
Insulin-like growth factor 1 (IGF-1) is requisite for the regulation of metabolic processes, growth, and developmental functions. Its influence is mediated through the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling cascade, which is crucial for various cellular functions, including growth, differentiation, and cell survival[10]. The PI3K/AKT pathway is known to be activated by physical exercise, suggesting a potential link between exercise-induced improvements in metabolic health and the activation of this pathway[11]. Previous studies have shown that exercise can modulate IGF-1 Levels and optimize glucose homeostasis in diabetic animals[12]. However, the precise mechanisms underlying these effects, particularly the interplay between IGF-1 as well as the PI3K/AKT pathway in response to exercise, remain to be fully elucidated. Understanding how exercise affects these pathways could provide insights into novel therapeutic strategies for diabetes management.
This study focused on revealing the outcomes of swimming training on pancreatic islets, blood glucose, insulin, lipids, pancreatic β-cells, IGF-1, and the PI3K/AKT pathway expression in diabetic rats. Swimming training was hypothesized to improve glycemic control and enhance insulin secretion through the upregulation of IGF-1 and arousal of the PI3K/AKT pathway. This study also sought to explore the relationship between IGF-1 and PI3K/AKT and the effect on islet function.
Twenty-six male Sprague-Dawley (SD) rats, aged 12 weeks old and weighing between 250 and 300 g, were obtained from Shanghai SLC Co (Shanghai, China). They were housed in plastic enclosures in a temperature-regulated environment set at 23 ± 3 °C, experiencing a light/dark cycle of 12 hours. They were also provided with unlimited food and water. This research was authorized by the Animal Ethics Committee at the First Hospital of Shanxi Medical University, confirming adherence to the required animal welfare guidelines.
The 26 SD rats were assigned randomly to four separate groups: The diabetic exercise group (D-Ex, n = 7), the diabetic sedentary group (D-Sed, n = 7), the control exercise group (C-Ex, n = 6), and the control sedentary group (C-Sed, n = 6). The 14 diabetic rats were successfully modeled by intraperitoneal injection with streptozotocin (STZ, 55 mg/kg) after fasting for 12 hours to induce partial insulin deficiency, in which blood glucose levels were above 16.7 mmol/L after 1 week[13]. Twelve of the diabetic rats were survived at the end of the experiment, and they were distributed into D-Ex (n = 6) and D-sed (n = 6) groups. Another 12 of the SD rats without injection of any medication were survived at the end of the experiment. The D-Ex and C-Ex groups swam concurrently without any additional weight for 60 minutes every day, 5 days a week, over a period of 8 weeks. They were placed in a barrel filled with water, kept at a temperature between
All rats were placed under anesthesia via intraperitoneal injection of sodium thiopental, administered at a dosage of 40 mg/kg of body weight 8-16 hours after the final exercise session[16]. Blood was drawn from the tail veins, followed by centrifugation at 1100 × g for 10 minutes to isolate the serum. The levels of serum glucose, triglycerides, total cholesterol, as well as free fatty acids were detected applying an autoanalyzer (RT-1904C; Rayto, Shenzhen, China). Serum insulin concentrations were assessed using a radioimmunoassay technique described in previous studies[17].
Blood was collected from rats that fasted for 12 hours and then centrifuged at 2500 g for 10 minutes. The resulting serum was then deposited at -80 °C for further analysis. The pancreas was isolated through a ventral midline incision. The whole pancreas was dissected free from fat and connective tissue immediately. The pancreas was placed in fixative (44% paraformaldehyde, 47% distilled water, and 9% glacial acetic acid) for 48 hours, and then 70% ethanol was used to clean and maintain for Hematoxylin and eosin (HE) staining[18]. The presence of islet β-cells in the tissue sections was identified utilizing anti-insulin antibodies derived from guinea pigs.
The pancreatic β-cell area was detected by testing all distinct images obtained from two insulin-stained sections of every rats while employing a Zeiss Axiovert microscope (Carl Zeiss Microimaging, Thornwood, NY, United States). The quantification outcomes for β-cells were conveyed as a percentage of the total examined area that displayed insulin-positive cells, with analysis performed using IP Lab Spectrum software (Scanalytics Inc., Fairfax, VA, United States). The pancreatic β-cell mass was tested by multiplying the proportion of the area positive for insulin by the bigness of the relevant pancreatic section. The dimensions of individual β-cells were found by dividing the area marked by insulin staining by the number of nuclei found within the correlative insulin-positive structures in randomly selected sections stained via immunofluorescence.
Following fixation in 4% paraformaldehyde solution (pH 7.2), the pancreases of all rats were embedded in paraffin blocks, from which serial sections measuring 5 μm were composed and located on slides. Every sixth or seventh section was chosen for morphological analysis after the tissue was rehydrated to avoid selecting the same islet section multiple times. Sections were captured using an Olympus BX51 camera (Japan). Islet area, perimeter, and the longest diameter were quantified with Image-Pro Plus (version 6.0) software. An eyepiece micrometer was employed to count the number of islets. The area of each section was measured using a Vernier caliper. On the basis of these measurements, the density of islets per square centimeter and the islet area per square centimeter in the sections were determined[19].
Total RNA was isolated applying TRIzol reagent, after which the precipitate was cleansed with 75% (v/v) ethanol and resuspended in distilled water free of RNase. The RNA’s purity and concentration were evaluated through ultraviolet spectrophotometry. Following the manufacturer’s instructions for the FastKing OneStep ProbeReal-time quantitative polymerase chain reaction (RT-qPCR) MasterMix, a 20 μL reaction mixture was prepared for reverse transcription, cultivated at 42 °C for 15 minutes in a T100 thermalcycler (BIO-RAD, Hercules, CA, United States), and heated at 95 °C for 3 minutes to synthesize cDNA, which was subsequently chilled on ice. An additional 80 μL reaction mixture was created by combining SuperReal Premix Plus, RNase-free distilled water, cDNA samples, and forward as well as reverse RNA primers. The amplification process was conducted in the CFX Connect Real-Time System (BIO-RAD, Hercules, CA, United States) under the following parameters: 95 °C for 15 minutes and 40 cycles of 10 seconds at 95 °C, 30 seconds at
The concentration of protein in homogenates was tested via Bradford assay, utilizing bovine serum albumin as the calibration standard[21]. For analysis, 80 μg of protein from each sample was loaded onto a 10% polyacrylamide gel. The proteins were subsequently moved onto nitrocellulose membranes, which were then blocked using a 5% skimmed milk powder mixture in TBS-T for one hour. Following this, the membranes were treated with primary antibodies at room temperature for a duration of two hours againsting phosphorylated PI3K, p-PI3K, p-AKT, AKT, IGF-1, and insulin, all at a dilution of 1:1000. Following this incubation, the membranes were laundered and incubated with a goat anti-rat horseradish peroxidase-conjugated secondary antibody, diluted either at 1:1500 or 1:3000. The signal was amplified using ECL detection reagents, and images were captured with a Gel-Imaging System (Tanon 4200, Shanghai, China). The optical density for each band was quantified using Alpha-Ease TM FC 32-bit software.
The INS-1E rat β-cell line, derived from pancreatic islets, was maintained at 37 °C within a controlled atmosphere comprising 95% humidity and 5% carbon dioxide. The cells were cultivated in Dulbecco’s Modified Eagle Medium (DMEM) and, enriched with 10% fetal bovine serum (Life Technologies, Carlsbad, CA, United States). The culture included 100 µg/mL of penicillin-streptomycin, 100 µg/mL of L-glutamine, and 0.05 µg/mL of β-mercaptoethanol (Gibco Invitrogen, Carlsbad, CA, United States) for optimal growth conditions. Once approximately 65% of the cells adhered, they were synchronized by incubating in serum-free medium for 8 hours. The antibody utilized in this investigation was TCF7 L2 from Cell Signaling Technology (United States).
Islet β-cells were seeded into six-well plates (Corning, Inc., Corning, NY, United States) at a density of 1 × 106 cells per well and then incubated at 37 °C for 24 hours. IGF-1 cDNA was synthesized and cloned into the pLVX-IRESneo vector to induce IGF-1 overexpression. For the transfection process, 4 µg of siRNA designed to specifically target IGF-1, PI3K, or control siRNA (sourced from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, United States) were mixed with 250 µL of serum-free DMEM. Simultaneously, 10 µL of Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, United States) was combined with 250 µL of serum-free DMEM. This mixture was gently swirled and then incubated at room temperature for 5 minutes. The si-RNA suspension was then combined with the Lipofectamine 2000 solution and allowed to incubate at room temperature for an additional 20 minutes. This final mixture was applied to the six-well plates, gently mixed, and placed in an incubator at 37 °C. After 6 hours of transfection, the culture medium was replaced with 2 mL of fresh medium in each well. The cells were collected for further experiments after 48 hours incubation period, with non-transfected cells utilized as negative controls. Western blot analysis was performed to confirm the transfection’s effectiveness.
First, INS-1E cells were washed two times with PBS. Next, 2.5 μL of Annexin V and 2.5 μL of propidium iodide were added to each tube, mixed by vortex, and incubated at room temperature in the dark for 15 minutes. Afterwards, 1 mL of PBS was added to each tube, followed by centrifugation at 1500 rpm for 5 minutes. This washing step was repeated two times before analysis using a flow cytometer (Cyto FLEX, BECKMAN COULTER, United States) to determine the percentage of apoptotic cells[22].
The findings are conveyed as mean values accompanied by their standard deviations. Statistical evaluations were conducted utilizing SPSS software (version 17.6, SPSS, Chicago, IL, United States). Two-way repeated-measure ANOVA was employed to analyze the data, followed by Tukey’s test for post-hoc assessments to pinpoint specific differences. One-way ANOVA was performed for group comparisons, establishing a significance threshold at P < 0.05, as indicated by Tukey’s test results.
The D-Sed rats experienced a notable reduction in body weight compared with the C-Sed group, whereas the D-Ex rats demonstrated a marked increase in body weight relative to the D-Sed rats (Figure 1A). Food intake exhibited an opposite pattern across the groups (Figure 1B). Observations of HE-stained pancreatic sections from the four groups revealed that the C-Sed rat sections showed regular islet shapes with round nuclei stained blue and abundant cytoplasm stained pink. The C-Ex rat sections displayed regular islet shapes with slightly increased islet area. The D-Sed rat sections revealed severe islet atrophy, irregular shapes, vacuolar degeneration, and extensive exocrine gland invasion. By contrast, the D-Ex rat sections showed mild islet atrophy, relatively regular shapes, and minimal exocrine gland invasion (Figure 1C). Comparison of islet characteristics showed that the D-Sed rats had a significant decrease in the longest islet diameter, area, and quantity compared with the C-Sed rats. By contrast, the D-Ex rats had significantly larger islet diameters, areas, and numbers than the D-Sed rats (Figure 1D-F).
The D-Sed rats had higher blood glucose levels, lower insulin levels, and decreased muscle glycogen than the C-Sed rats. However, compared with the D-Sed rats, the D-Ex rats exhibited a significant decrease in blood glucose, a significant increase in insulin, and a significant increase in muscle glycogen (Figure 2A-C). The D-Sed rats exhibited a considerable increase in triglycerides, total cholesterol, and free fatty acids compared with the C-Sed group. Conversely, the D-Ex rats demonstrated a notable reduction in triglycerides, total cholesterol, and free fatty acids compared with the D-Sed rats (Figure 2D-F).
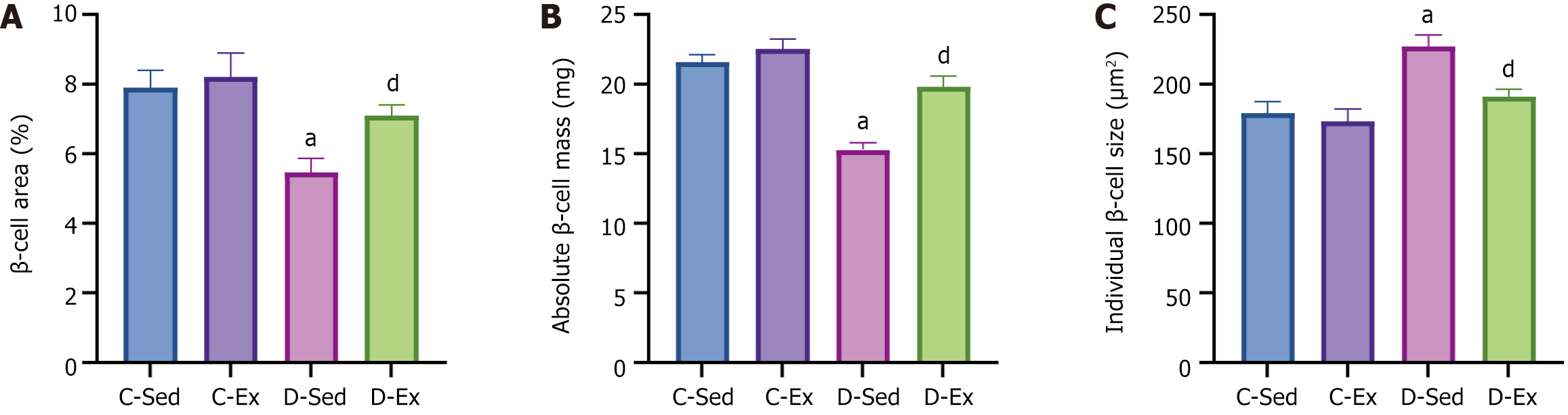
Compared with the C-Sed rats, the D-Sed rats exhibited a significant decrease in the percentage of islet β-cell area relative to the total pancreatic area in the sections. However, compared with the D-Sed rats, the D-Ex rats had a significant increase in the percentage of islet β-cell area relative to the total section area (Figure 3A). Given that no differences were observed in pancreatic weight across the groups (data not presented), the pancreatic β-cell mass - determined by multiplying the β-cell area by the pancreatic weight - reflected similar trends to those seen in the area measurements (Figure 3B). Compared with the C-Sed rats, the D-Sed rats showed a significant increase in individual β-cell size. However, compared with the D-Sed rats, the D-Ex rats had a significant decrease in individual β-cell size (Figure 3C).
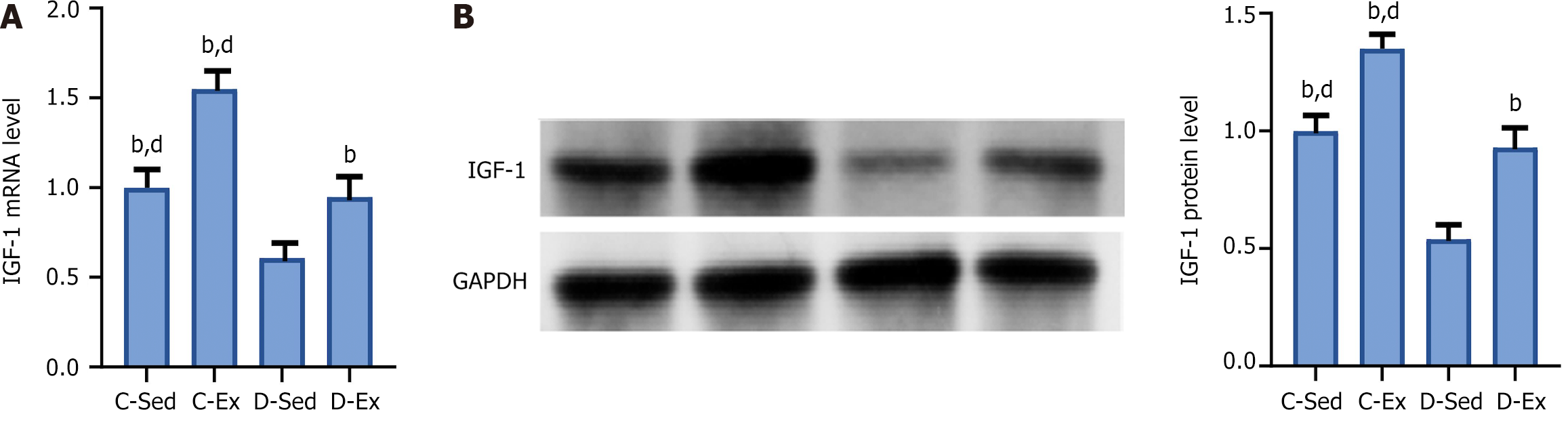
Compared with the C-Sed rats, the D-Sed rats had a significant decrease in IGF-1 mRNA and protein levels. However, compared with the D-Sed rats, the D-Ex rats showed a significant increase in IGF-1 mRNA and protein levels (Figure 4).
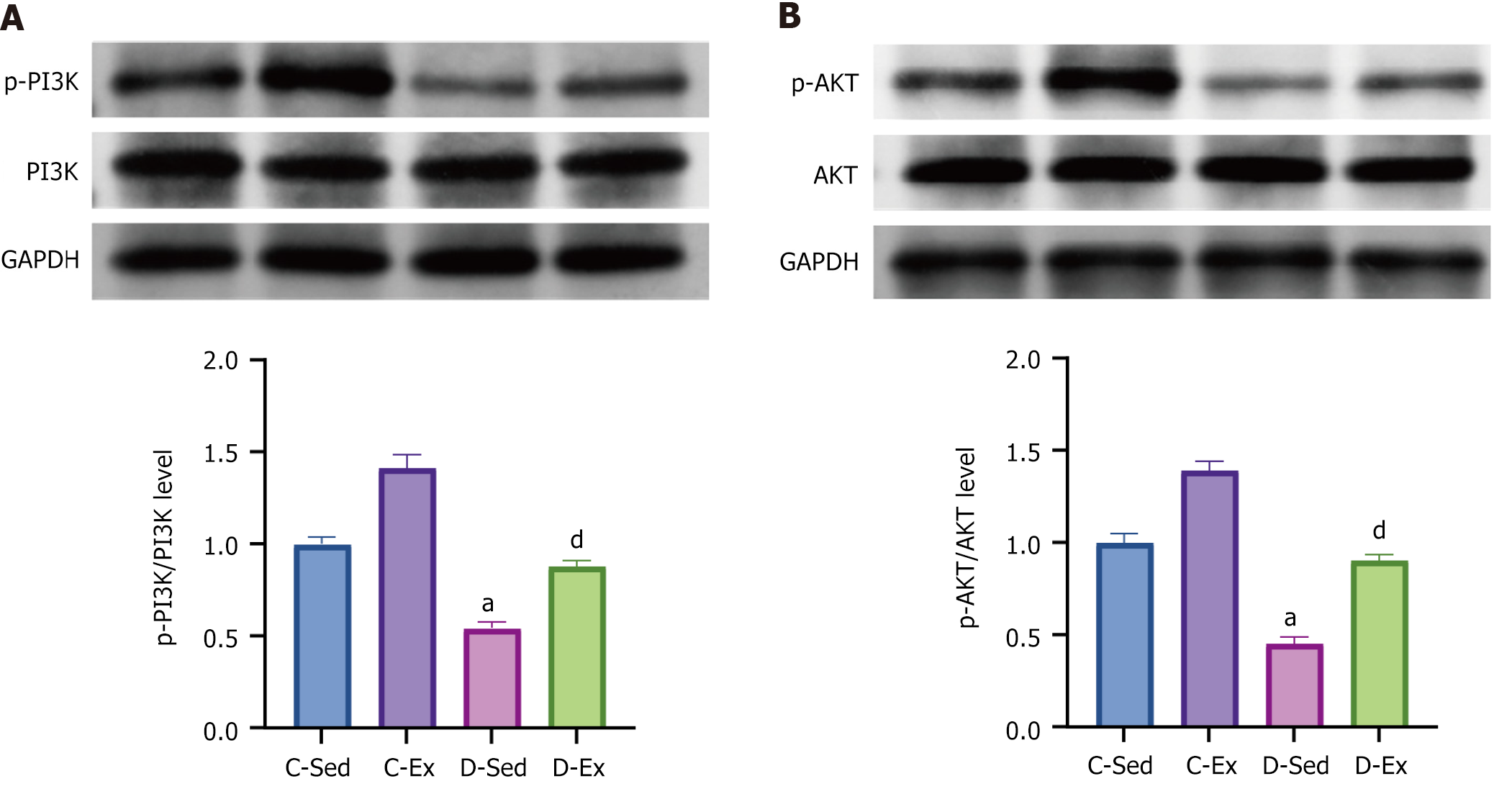
Compared with the C-Sed rats, the D-Sed rats showed a significantly reduced ratio of p-PI3K/PI3K and p-AKT/AKT protein levels. However, compared with the D-Sed rats, the D-Ex rats exhibited a significant increase in the ratios of p-PI3K/PI3K and p-AKT/AKT (Figure 5).
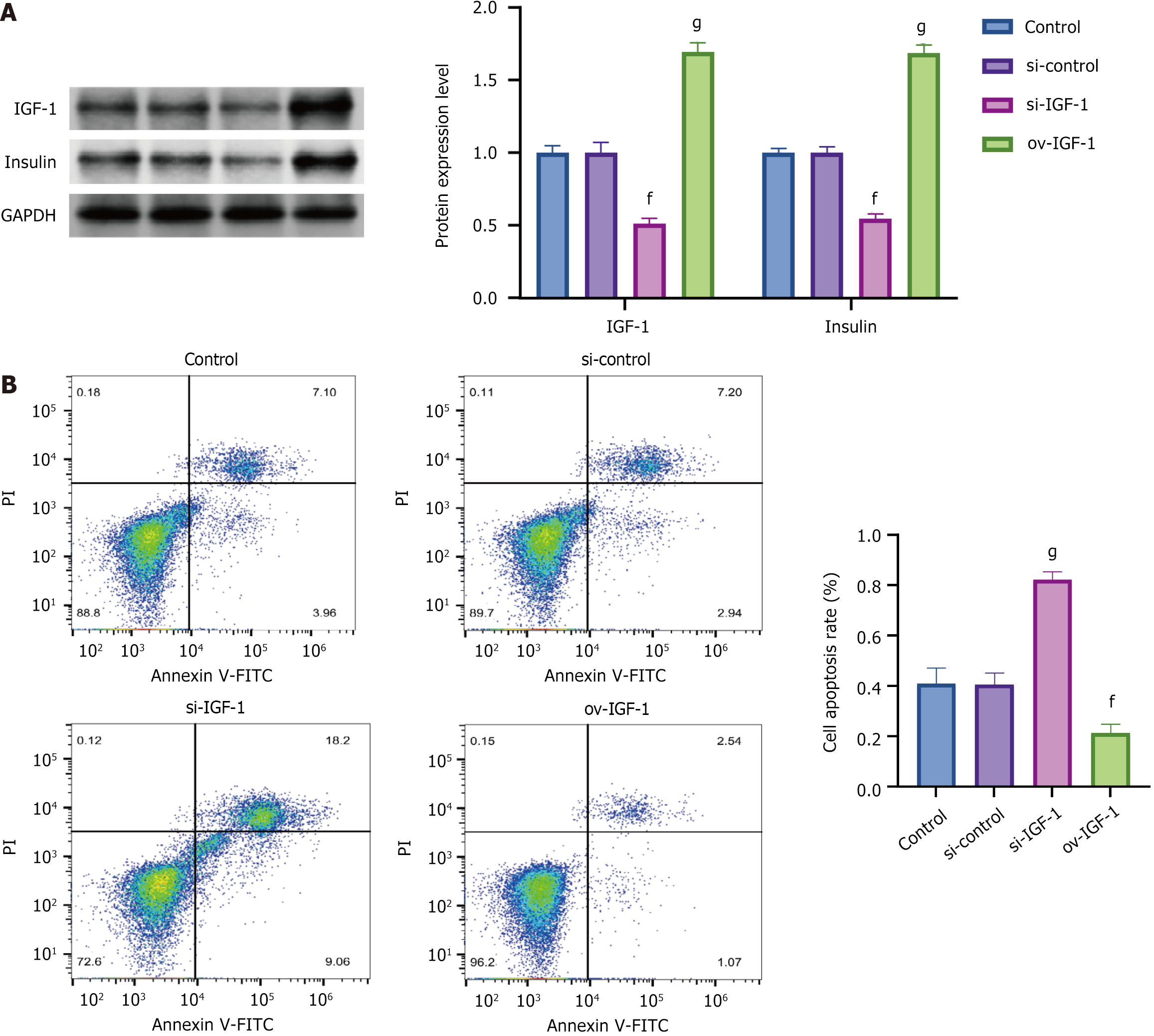
After IGF-1 was knocked down, the insulin expression levels significantly decreased, and the rate of cell apoptosis markedly increased. Conversely, after IGF-1 was overexpressed, the insulin expression levels significantly increased, and the rate of cell apoptosis substantially decreased (Figure 6).
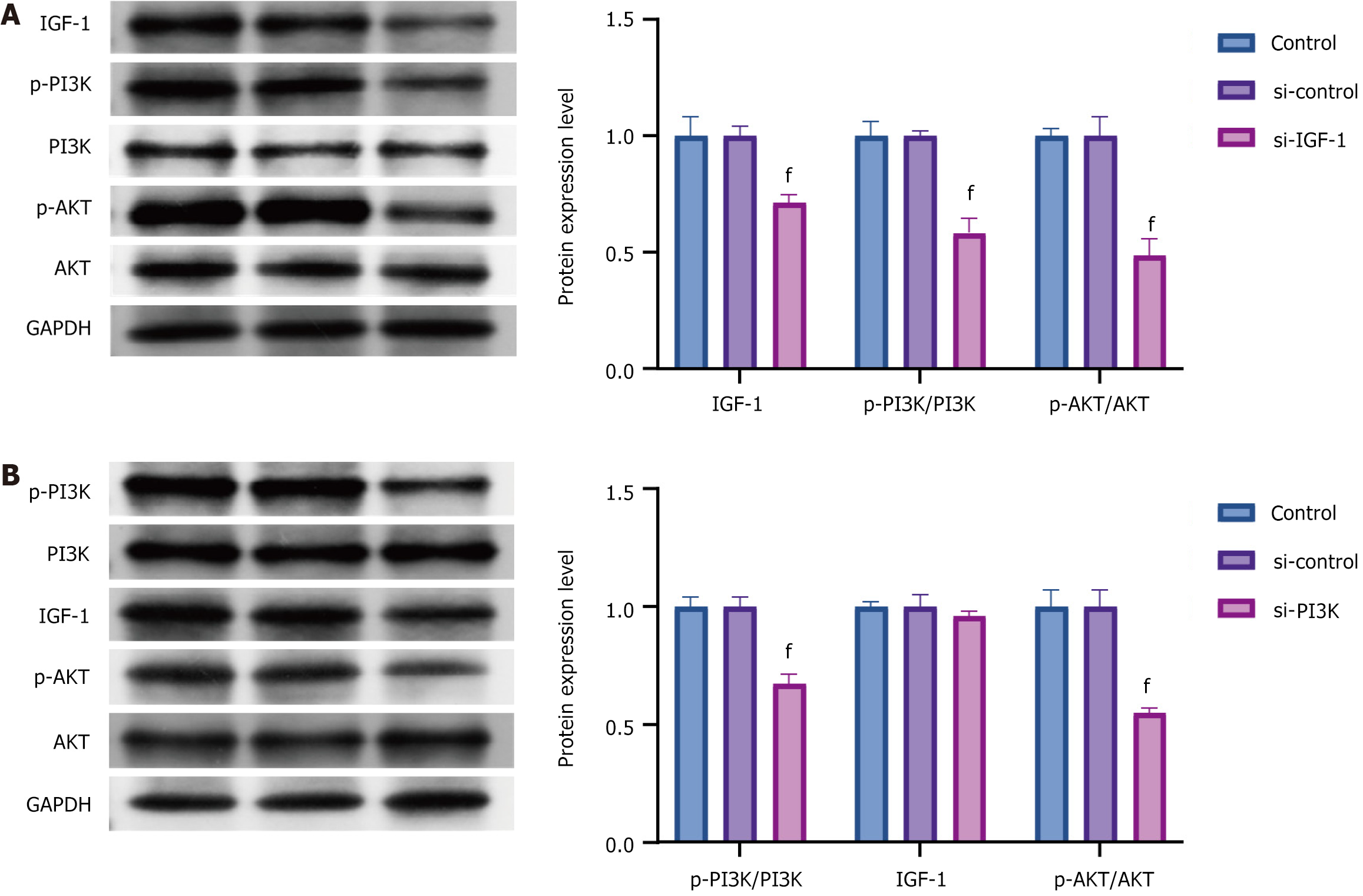
The silencing of IGF-1 Led to a marked decrease in the expression levels of p-PI3K/PI3K and p-AKT/AKT. Conversely, when PI3K was knocked down, no significant change was noted in the IGF-1 expression, but the expression levels of p-AKT/AKT decreased significantly (Figure 7).
Exercise has long been recognized for its expansive health benefits, extending its utility into the realm of metabolic disorders such as diabetes[23,24]. The present study aimed to clarify how exercise training influences islet mass and functionality in rats with diabetes induced by STZ. The findings demonstrated that exercise training ameliorates various structural and functional abnormalities induced by diabetes, highlighting its potential therapeutic efficacy.
The positive long-term effect of consistent exercise on individuals with T2DM is well documented[25,26]. Engaging in regular aerobic exercise leads to reductions in visceral fat and body weight while preserving lean muscle mass. Additionally, it enhances insulin sensitivity and helps improve blood glucose levels[27]. Numerous studies have shown that physical activity promotes glucose regulation by increasing glucose absorption in skeletal muscles and adipose tissue[28,29]. A recent study reported that exercise training improved b-cell function in adults who were sedentary and overweight and with moderate dyslipidemia[30]. However, limited research has indicated that physical activity influences the mass and functionality of pancreatic β-cells in diabetic animal models. A STZ-induced diabetic rat model was utilized in the present study to explore the primary effects of exercise on pancreatic β-cell function. The typical pathological changes in islets induced by STZ are islet atrophy, islet shape irregular, and some islet cell necrosis with unstructured eosinophilic material. Park et al[3] used 90% pancreatectomized diabetic model with serum glucose levels of 9.4-11.8 mmol/L and insulin deficiency. Some morphologic parameters in the pancreatectomy diabetic model were better than those in the sham group because the remaining pancreas was intact. The compensatory growth of the remaining β-cell mass may gradually restore insulin secretary capability over time. However, the STZ-induced diabetic model had a characteristic of progressive deterioration in functions and morphology of islet and adapted to the purpose of this study.
STZ-induced diabetic rats often display chronic wasting state, which affects the experimental process. Hoybergs et al[31] found that the tolerance to identical dose of STZ was higher in low-body-weight rats than in medium- and high-body-weight rats. The present study used rats weighing less than 300 g to establish a diabetic model. The results found that the body weights in diabetic rats remarkably increased after 8 weeks, in accordance with the results of Hoybergs et al[31]. During the course of the current study, one rat from the D-Sed group died after 5 weeks as a result of a gradual decrease in body weight. Another rat from the D-Ex group, which maintained stable body weight throughout the experiment, drowned. The results suggest that exercise training could improve chronic wasting state caused by diabetes. Consistent with the results of the present study, Park et al[3] reported that exercise did not affect the body weight of non-STZ-induced rats.
The STZ-treated rats exhibited clear abnormalities in pancreatic function related to diabetes. A notable decrease was found in pancreatic β-cell mass, assessed by the proportion of the total area occupied by insulin-positive cells, indicating significant β-cell loss or impaired function in the diabetic rats. Consistent with these findings, previous studies reported that hyperglycemia and insulin resistance induced oxidative stress and inflammatory responses, precipitating β-cell apoptosis and functional deterioration. Conversely, exercise markedly mitigated these pathological features. Notably, the D-Ex rats demonstrated a significant preservation of β-cell mass compared with their sedentary counterparts. This finding suggests a protective role of exercise, potentially mediated through multiple mechanisms including enhanced insulin sensitivity, reduced systemic inflammation, and augmented β-cell resilience.
The role of IGF-1 in maintaining β-cell mass and function emerged as a crucial aspect of this study. IGF-1 is known to exert mitogenic and anti-apoptotic effects on β-cells, enhancing their survival and functional capacity[32]. Diabetic sedentary rats exhibited significantly reduced IGF-1 mRNA and protein levels, correlating with β-cell dysfunction. Remarkably, exercise training increased the IGF-1 Levels in diabetic rats, suggesting that physical activity stimulates IGF-1 expression, which, in turn, may bolster β-cell function and resilience. IGF-1 pathway activation likely attenuates diabetes-induced apoptosis and enhances β-cell proliferation, contributing to the observed preservation of islet architecture and function in D-Ex rats.
Another pivotal discovery pertains to the PI3K/AKT signaling pathway, which is integral to cellular growth, survival, and metabolism[33]. Diabetes was associated with a reduction in p-PI3K/PI3K and p-AKT/AKT ratios, indicating impaired pathway activity, which likely contributes to β-cell dysfunction and apoptosis. Exercise training restored the activity of the PI3K/AKT pathway in diabetic rats, evidenced by increased p-PI3K/PI3K and p-AKT/AKT ratios. This resurrection of PI3K/AKT signaling in D-Ex rats underscores a mechanism through which exercise exerts its β-cell protective effects. Enhanced PI3K/AKT signaling may stimulate β-cell proliferation, improve glucose metabolism, and resist apoptotic triggers, hence preserving β-cell mass and function.
The interplay between IGF-1 and the PI3K/AKT pathway was further delineated in vitro. Knocking down IGF-1 Led to a significant reduction in p-PI3K/PI3K and p-AKT/AKT levels, whereas PI3K knockdown did not affect the IGF-1 Levels but significantly diminished AKT phosphorylation. These findings propose that IGF-1 acts upstream to modulate the PI3K/AKT signaling, catalyzing a cascade of downstream effects that promote β-cell survival and function. This result is consistent with those of previous studies demonstrating that IGF-1 activates the PI3K/AKT pathway to protect β-cells from apoptosis under stress conditions[34]. Notably, the results of the present study align with those of Lin et al[35], who reported that IGF-1 deficiency impaired AKT phosphorylation and exacerbated β-cell dysfunction in diabetic models. However, the present study extends these findings by demonstrating that exercise-induced upregulation of IGF-1 can activate the PI3K/AKT pathway, thereby mitigating diabetes-induced β-cell damage. This finding adds a novel dimension to the existing literature because few studies have explored the role of exercise in modulating IGF-1/PI3K/AKT signaling in the context of diabetes.
The findings also shed light on the systemic benefits of exercise, including improved lipid profiles and glycemic control. Exercise significantly reduced the increased levels of triglycerides, total cholesterol, and free fatty acids in diabetic rats. This amelioration of dyslipidemia likely reduces lipid-induced toxicity (glucolipotoxicity) on β-cells, preventing functional deterioration. These results are in line with those of previous studies showing that exercise improves lipid metabolism and reduces glucolipotoxicity in T2DM[36]. For instance, Krause et al[37] demonstrated that aerobic exercise decreased circulating free fatty acids and improved β-cell function in diabetic rodents. The enhanced muscle glycogen content and improved insulin sensitivity observed in D-Ex rats can synergistically alleviate the metabolic burden on pancreatic β-cells, promoting their viability and function. This finding was supported by studies indicating that exercise enhances insulin sensitivity and glucose uptake in skeletal muscle, thereby reducing the demand for insulin secretion from β-cells[38,39].
From a broader perspective, this study highlights the multifaceted benefits of exercise training in diabetes management. Beyond glycemic control, exercise fosters a conducive environment for pancreatic health, counteracting the deleterious effects of hyperglycemia and dyslipidemia. Mechanistically, exercise-induced upregulation of IGF-1 and consequent PI3K/AKT pathway activation emerge as crucial mediators of β-cell protection and functional preservation. These molecular insights underscore the necessity of incorporating physical activity as a cornerstone in diabetes management, extending potential benefits to pancreatic resilience and overall metabolic health.
While this study provides valuable insights into the beneficial effects of exercise on islet mass and function in STZ-induced diabetic rats, several limitations must be acknowledged. First, the use of a rodent model limits the direct translatability of the findings to human diabetes because metabolic and physiological differences exist between species. Furthermore, the limited number of participants in each experimental group may hinder the applicability of the findings to broader populations. Another limitation is the exclusive focus on male rats, given that sex-dependent differences in metabolic responses to exercise and diabetes are documented in literature. Thus, the findings may not fully represent the effects in female rats. Furthermore, the key molecular pathways involved in β-cell preservation were identified in a controlled laboratory setting, and different exercise regimens or intensities were not explored, which could provide more comprehensive insights. Finally, the research failed to consider various confounding variables, including dietary differences and stress levels, which may affect the results. To improve the robustness and generalizability of future research, investigations should focus on overcoming these limitations by incorporating larger, more varied participant groups, examining different types of exercise, and including both male and female subjects.
This study illuminates the profound effect of exercise training on islet mass and function in STZ-induced diabetic rats. The protective effects of exercise are mediated through multiple pathways, prominently involving IGF-1 and the PI3K/AKT signaling cascade. The preservation of β-cell mass, improved pancreatic morphology, and enhanced systemic metabolic profiles underscore the therapeutic potential of exercise in diabetes. These findings advocate for the integration of regular physical activity into clinical management strategies, aiming to mitigate β-cell dysfunction and halt the progression of diabetes. Further research is warranted to explore the translational applicability of these findings and optimize exercise protocols for maximal therapeutic benefit in diabetic individuals.
| 1. | Wajchenberg BL. beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 538] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 2. | Qian L, Xu L, Wang X, Fu X, Gu Y, Lin F, Peng Y, Li G, Luo M. Early insulin secretion failure leads to diabetes in Chinese subjects with impaired glucose regulation. Diabetes Metab Res Rev. 2009;25:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Park S, Hong SM, Lee JE, Sung SR. Exercise improves glucose homeostasis that has been impaired by a high-fat diet by potentiating pancreatic beta-cell function and mass through IRS2 in diabetic rats. J Appl Physiol (1985). 2007;103:1764-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Min HK. Non-insulin-dependent diabetes mellitus (NIDDM) in Korea. Diabet Med. 1996;13:S13-S15. [PubMed] [DOI] [Full Text] |
| 5. | Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53 Suppl 3:S16-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 795] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 6. | Jetton TL, Lausier J, LaRock K, Trotman WE, Larmie B, Habibovic A, Peshavaria M, Leahy JL. Mechanisms of compensatory beta-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes. 2005;54:2294-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Perrini S, Henriksson J, Zierath JR, Widegren U. Exercise-induced protein kinase C isoform-specific activation in human skeletal muscle. Diabetes. 2004;53:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Dela F, Mikines KJ, Tronier B, Galbo H. Diminished arginine-stimulated insulin secretion in trained men. J Appl Physiol (1985). 1990;69:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 9. | Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance beta-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E1024-E1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Liu C, Liu S, Wang S, Sun Y, Lu X, Li H, Li G. IGF-1 Via PI3K/Akt/S6K Signaling Pathway Protects DRG Neurons with High Glucose-induced Toxicity. Open Life Sci. 2019;14:502-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Feng L, Li B, Xi Y, Cai M, Tian Z. Aerobic exercise and resistance exercise alleviate skeletal muscle atrophy through IGF-1/IGF-1R-PI3K/Akt pathway in mice with myocardial infarction. Am J Physiol Cell Physiol. 2022;322:C164-C176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 12. | Żebrowska A, Sikora M, Konarska A, Zwierzchowska A, Kamiński T, Robins A, Hall B. Moderate intensity exercise in hypoxia increases IGF-1 bioavailability and serum irisin in individuals with type 1 diabetes. Ther Adv Endocrinol Metab. 2020;11:2042018820925326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Koya V, Lu S, Sun YP, Purich DL, Atkinson MA, Li SW, Yang LJ. Reversal of streptozotocin-induced diabetes in mice by cellular transduction with recombinant pancreatic transcription factor pancreatic duodenal homeobox-1: a novel protein transduction domain-based therapy. Diabetes. 2008;57:757-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Morifuji M, Sanbongi C, Sugiura K. Dietary soya protein intake and exercise training have an additive effect on skeletal muscle fatty acid oxidation enzyme activities and mRNA levels in rats. Br J Nutr. 2006;96:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 15. | Qiu S, Wu C, Lin F, Chen L, Huang Z, Jiang Z. Exercise training improved insulin sensitivity and ovarian morphology in rats with polycystic ovary syndrome. Horm Metab Res. 2009;41:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Sipahi E, Ustün H, Niyazi Ayoglu F. Acute effects of pentobarbital, thiopental and urethane on lung oedema induced by alpha-naphthythiourea (ANTU). Pharmacol Res. 2002;45:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Dakic TB, Markelic MB, Ruzicic AA, Jevdjovic TV, Lakic IV, Djordjevic JD, Vujovic PZ. Hypothalamic insulin expression remains unaltered after short-term fasting in female rats. Endocrine. 2022;78:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 18. | Pospisilik JA, Martin J, Doty T, Ehses JA, Pamir N, Lynn FC, Piteau S, Demuth HU, McIntosh CH, Pederson RA. Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes. 2003;52:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 274] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 19. | Sahloul R, Yaqub N, Driscoll HK, Leidy JW Jr, Parkash J, Matthews KA, Chertow BS. Noninsulinoma pancreatogenous hypoglycemia syndrome: quantitative and immunohistochemical analyses of islet cells for insulin, glucagon, somatostatin, and pancreatic and duodenal homeobox protein. Endocr Pract. 2007;13:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Bong D, Sohn J, Lee SV. Brief guide to RT-qPCR. Mol Cells. 2024;47:100141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 21. | Pillai-Kastoori L, Schutz-Geschwender AR, Harford JA. A systematic approach to quantitative Western blot analysis. Anal Biochem. 2020;593:113608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 22. | Manohar SM, Shah P, Nair A. Flow cytometry: principles, applications and recent advances. Bioanalysis. 2021;13:181-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 23. | Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, Crespo CJ, Kirwan JP, Zierath JR. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med Sci Sports Exerc. 2022;54:353-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 485] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 24. | Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84:S15-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 264] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 25. | Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, Belloni E, Canu T, Terruzzi I, Scifo P, Del Maschio A, Luzi L. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Sampath Kumar A, Maiya AG, Shastry BA, Vaishali K, Ravishankar N, Hazari A, Gundmi S, Jadhav R. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann Phys Rehabil Med. 2019;62:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 27. | Colberg SR, Grieco CR. Exercise in the treatment and prevention of diabetes. Curr Sports Med Rep. 2009;8:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr. 2007;85:662-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 117] [Reference Citation Analysis (0)] |
| 29. | Berggren JR, Hulver MW, Houmard JA. Fat as an endocrine organ: influence of exercise. J Appl Physiol (1985). 2005;99:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Slentz CA, Tanner CJ, Bateman LA, Durheim MT, Huffman KM, Houmard JA, Kraus WE. Effects of exercise training intensity on pancreatic beta-cell function. Diabetes Care. 2009;32:1807-1811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Hoybergs YM, Biermans RL, Meert TF. The impact of bodyweight and body condition on behavioral testing for painful diabetic neuropathy in the streptozotocin rat model. Neurosci Lett. 2008;436:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Cui F, He X. IGF-1 ameliorates streptozotocin-induced pancreatic β cell dysfunction and apoptosis via activating IRS1/PI3K/Akt/FOXO1 pathway. Inflamm Res. 2022;71:669-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 33. | Wang C, Sun Y, Cong S, Zhang F. Insulin-Like Growth Factor-1 Promotes Human Uterine Leiomyoma Cell Proliferation via PI3K/AKT/mTOR Pathway. Cells Tissues Organs. 2023;212:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006;147:2643-2649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Lin KN, Zhang K, Zhao W, Huang SY, Li H. Insulin-like Growth Factor 1 Promotes Cell Proliferation by Downregulation of G-Protein-Coupled Receptor 17 Expression via PI3K/Akt/FoxO1 Signaling in SK-N-SH Cells. Int J Mol Sci. 2022;23:1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 36. | Sullivan PW, Morrato EH, Ghushchyan V, Wyatt HR, Hill JO. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000-2002. Diabetes Care. 2005;28:1599-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 257] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 37. | Krause MP, Riddell MC, Hawke TJ. Effects of type 1 diabetes mellitus on skeletal muscle: clinical observations and physiological mechanisms. Pediatr Diabetes. 2011;12:345-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1608] [Cited by in RCA: 1753] [Article Influence: 175.3] [Reference Citation Analysis (1)] |
| 39. | Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 938] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/