Published online Apr 15, 2025. doi: 10.4239/wjd.v16.i4.100467
Revised: January 6, 2025
Accepted: January 17, 2025
Published online: April 15, 2025
Processing time: 195 Days and 5.6 Hours
Platelet indices (PIs) including high mean platelet volume (MPV), plateletcrit (PLC), and platelet distribution width (PLDW) are associated with poor glycemic control. In addition, they can indicate prothrombotic and procoagulation risk am
Core Tip: Platelet indices (PIs) have been mentioned in the literature as parameters of glucoregulation. However, their clinical implications in real-world diabetes care are unclear. PIs if estimated within 2 h of blood collection could be useful and cheap indicators of glycemic control. PIs are nonspecific and affected by various patients characters. Therefore, combination with blood glucose measurement are crucial. PIs have the advantage of reflecting the previous 2 wk glycemic control compared to the glycated hemoglobin (120 d). PIs could be useful before surgery, and during pregnancy mean platelet volume and plateletcrit are more affected by confounders, and because of that platelet large cell ratio is more specific.
- Citation: Mirghani HO. Platelets indices clinical implications in diabetes mellitus: A broader insight. World J Diabetes 2025; 16(4): 100467
- URL: https://www.wjgnet.com/1948-9358/full/v16/i4/100467.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i4.100467
The diabetes mellitus epidemic is rising worldwide with a significant impact on patients, healthcare and the community due to its microvascular and macrovascular complications. Diabetes mellitus affects all age groups with type 2 constituting 90%–95% of cases in adults, and type 1 diabetes being the most common metabolic abnormality in children[1].
Diabetes mellitus and cardiovascular disease are major determinants of morbidity and mortality. Poor glycemic control is the major cause of microvascular diseases including nephropathy, neuropathy and retinopathy. There is a close connection between the blood as a connective tissue and blood glucose, and red blood cell survival and other blood indices are disturbed by high blood glucose in diabetes mellitus[2]. Importantly, the hemoglobin is nonenzymatically glycated to form glycated hemoglobin (HbA1c), which reflects the blood glucose status throughout the red blood cell lifespan[3]. The American Diabetes Association approved HbA1c as a measure of glycemic control and recommended a HbA1c of < 7 to avoid diabetes microvascular complications. However, the test is inaccurate among patients with hemoglobinopathy and renal impairment[4,5]. In addition, HbA1c measures glycemic control over the red blood cell lifespan (4 mo). Therefore, a rapid test that reflects glycemic control over a shorter period is attractive. Platelet indices (PIs) are easy to measure, cheap and fulfill the above needs in diabetes mellitus.
Platelets that are important for homeostasis are produced in large amounts by megakaryocytes (100 billion/d), and their lifespan varies between 8 and 10 d. There is a direct association between increased platelet activity, diabetes, procoagulability and thrombosis risk, and evidence that patients with diabetes are at high risk of cardiac and cerebral disorders[6,7]. However, the clinical implications of mean platelet volume (MPV), plateletcrit (PLC), and platelet distribution width (PLDW) in diabetes management are lacking. Therefore, there is increasing interest in the role of PIs in glycemic control and homeostatic changes in diabetes[8].
We read with interest the article published by Regassa et al[9] who found that MPV, PLC, PLDW and platelet large cell ratio (PLCR) were associated with poor glycemic control and microvascular complications among patients with diabetes. Although previous studies were conducted on the same patients, they showed contradictory findings[10-13]. In addition, Regassa et al[9] adopted strict inclusion criteria to assess the PIs among healthy controls and diabetic patients with good/poor control. The importance of the Regassa et al[9] study is that it was conducted in a low-income country where measurement of HbA1c was not available or cost-effective. Therefore, their study is highly relevant.
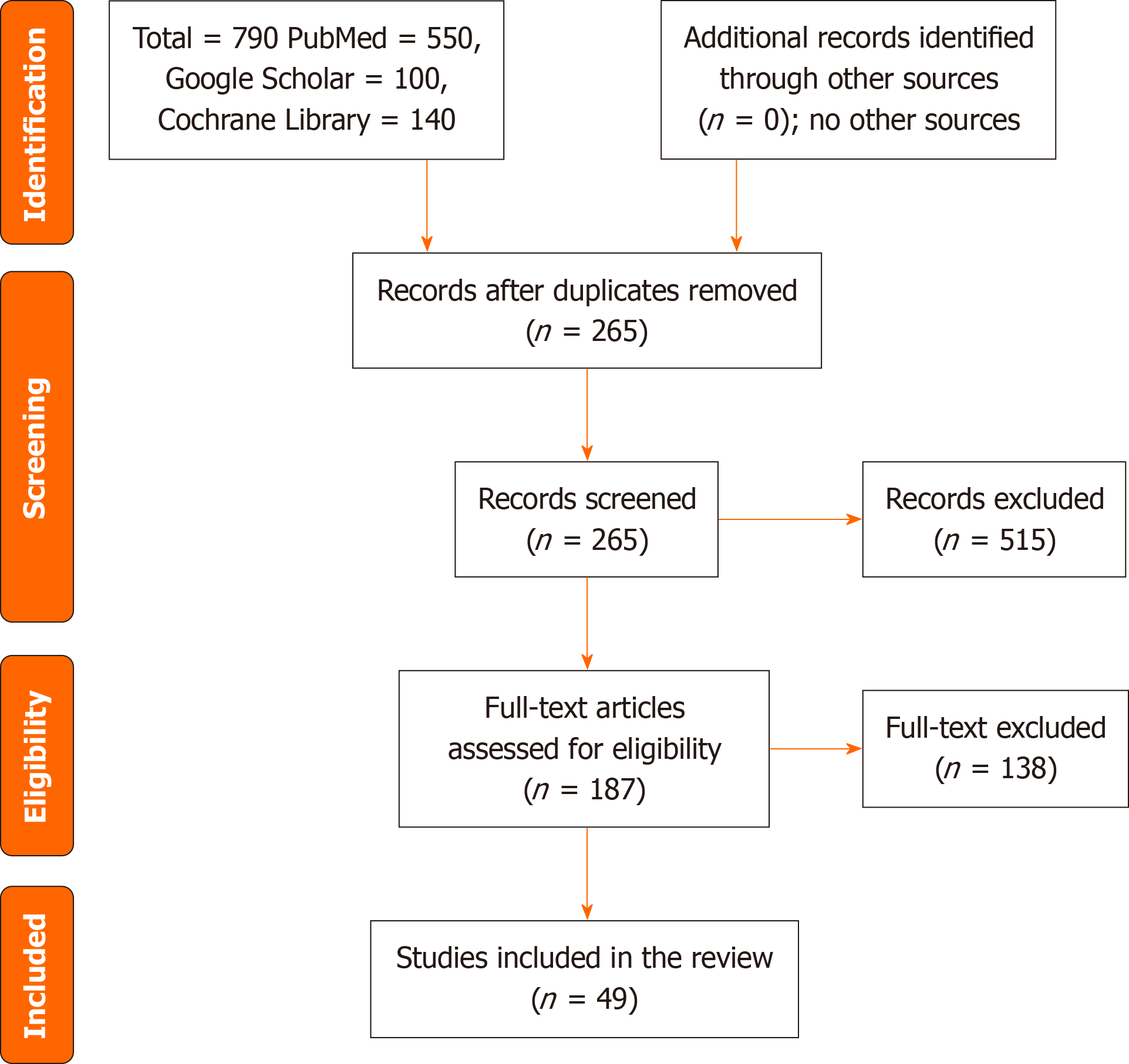
The subject of PIs is complex, and to address the important issues raised by Regassa et al[9], we searched PubMed/MEDLINE, Cochrane Library and Google Scholar for relevant articles from inception to August 17, 2024. The terms used were mean platelet volume, PLC, PLDW, PLCR, HbA1c, PIs, platelet activity, diabetes mellitus and HbA1c. Out of the 790 articles retrieved, 187 full texts were reviewed, and 49 were included (Figure 1).
Type 2 diabetes: The first retrieved article was published in Turkey[14]. The authors found a high MPV among patients with diabetes compared to healthy subjects. Additionally, MPV was higher among patients with poor glycemic control. MPV returned to normal following good glycemic control. In contrast, Singer et al[15] observed no improvement in platelet activity with improving HbA1c, which could be explained by the higher baseline HbA1c, long duration of diabetes, and statin and aspirin consumption. Wu et al[16] found a correlation between HbA1c and MPV, but no correlation was found with platelet count (PLT). A plausible explanation is that the PDW and PLT are influenced by the preceding 15–20 d (lifespan of platelets) rather than the glycemic period of 30–120 d [17]. Importantly, MPV is not an independent predictor of diabetes and glycemic control, because it is positively associated with treatment with angiotensin receptor blockers (ARBs), age, and hemoglobin levels, and negatively correlated with hypercholesterolemia[18].
Platelets play an important role in homeostasis. The interplay between platelets and adhesive glycoproteins after endothelial damage initiates platelet aggregation, leading to thrombosis. Importantly, large platelets are more active and secrete more dense granules, because big platelets (high MPV) significantly contribute to platelet activation processes, and lead to thrombosis and acute arterial events[19,20]. Another plausible explanation of the association between platelet volume and microvascular complications could be their association with poor glycemic control (a predictor of diabetic microvascular complications)[14,15].
Large platelets in patients with diabetes contain dense granules, high enzymatic activity, and a high potential for thrombosis leading to thrombosis and diabetes microvascular complications[21]. A systemic review and meta-analysis[22] concluded the association between PIs and microvascular complications. The capillary non-perfusion, releases of platelet-driven growth factors, and endothelin-1 activation are proposed mechanisms.
PLC, mean platelet component, mean platelet dry mass, and MPV were associated with glycemic parameters in Serbia[2]. Further research confirmed the association between blood glucose, low-density lipoproteins, high-low-density lipoproteins, high cholesterol, and increased total white blood cells. In addition, MPV and PDW were increased. The authors noticed low platelets and high interferon (IFN) among patients with poor glycemic control[23]. The above changes resolved/decreased with hyperglycemia treatment. The quantitative and qualitative abnormalities in the blood are well-known among patients with diabetes[24]. However, thrombocytopenia could be a more beneficial parameter for hyperglycemia if combined with MPV and other PIs.
The high IFN, and inflammatory markers in diabetes in particular poor glycemic control, are associated with increased platelet volume and thrombocytopenia[25]. The effects of hyperglycemia on platelet activity could be part of disturbed homeostasis in diabetes mediated by inflammation, increasing adhesion molecules, and circulating selectins[26]. The association of inflammatory markers, including C-reactive protein and platelet hyperactivity, was observed by Jabeen and colleagues in 2013[26]. In addition, protein kinase C activation, nonenzymatic glycation of the platelet surface proteins, and osmotic changes lead to platelet membrane rigidity. Activated platelets in diabetes form psuedopodia, and change their shape from the usual biconcave to spheres, affecting the RDW[9].
Platelet hyperactivity was evident in newly diagnosed and known patients with diabetes and correlated with HbA1c. Because of that, PIs could be a better alternative to HbA1c in areas where the facilities for HbA1c measurement are not available and among patients with hemoglobin disorders[27]. However, PIs, including MPV, are increased among patients with renal impairment and have an inverse relation with glomerular filtration rate[28]. Therefore, platelet activity has the same limitation as HbA1c.
Type 1 diabetes mellitus: Studies conducted on type 1 diabetes are scarce. Söbü et al[28] found that MPV was associated with poor glycemic control and duration of diabetes; however, no significant differences were evident in MPV between patients with good glycemic control and healthy controls. Venkatesh et al[29] assessed PLT, MPV, PLDW, PLCR and PLC and found higher MPV, PDW and PLCR, and lower PLC among children with poor glycemic control compared to control subjects and others with optimal HbA1c. Only PDW was high among newly diagnosed patients, pointing to the effect of diabetes duration. In another study published by Ma et al[30], MPV and PDW were significantly higher among type 1 diabetes with ketoacidosis.
Platelets indices and vascular disease: One of the major objectives in the treatment of diabetes is cardiovascular disease (microvascular and macrovascular complications, and cardiovascular risk factors) prevention and treatment[31]. Importantly, HbA1c can predict microvascular but not macrovascular and cardiovascular risk factors. Therefore, cardiovascular risk assessment at diagnosis is mandatory in diabetes care for the proper treatment and prescription of novel diabetic medications with cardiac and renal protection[32]. PIs and other blood parameters are easy to measure using impedance technology which is cost-effective, easy and accurate [33]. PIs can predict microvascular complications[34,35], coronary syndrome, glycemic control and thrombotic events in low-income countries where the facilities for HbA1c measurement are not available or not affordable[36,37].
Studies investigating calmodulin glycation in platelets as a measure of glycemic control showed significance only when correlated with serum fructosamine. However, the role of fructosamine as a glycemic parameter is controversial[38]. Because of that, platelet glycation adds no benefit compared to HbA1c and other glycemic control measures.
Regassa et al[9] found that PLDW, MPV, PLCR and PLC were higher among patients with type 2 diabetes, and suggested these as glucoregulators for follow-up in low-income countries. Regassa et al[9] applied strict inclusion and exclusion criteria. They excluded patients with cancer, infection and some drugs, and evaluated the controls for the same. Therefore, their results were robust because drugs[39], infection[40] and cancer[41] have been shown to affect PIs. However, the authors' strict measures are difficult to apply in the real world, because patients with diabetes are more prone to infection[42] and cancer[43]. Another limitation of the Regassa et al[9] study was that they did not screen the controls for undiscovered diabetes mellitus, and prediabetes, which are common and can alter PIs[44,45]. In addition, Regassa et al[9] used fasting blood glucose and mean blood glucose as a measure of glycemic control, but HbA1c measurement might not be available. However, fasting blood glucose and mean blood glucose do not reflect the glycemic status and continuous blood glucose measurements are more accurate. In the context of the short platelet lifespan, it is more accurate to use continuous blood glucose monitoring, because it has the advantage of recording the time in the range which is more beneficial than fasting blood glucose and mean blood glucose taken at different points[46].
Diabetes mellitus is a chronic lifelong disease with cardiovascular complications and a high economic burden worldwide, particularly in developing countries. As a result, affordable and readily available diagnostic methods are needed for diagnosis and monitoring of microvascular complications of diabetes[21].
The bidirectional relationship between PIs and diabetes mellitus in terms of endothelial dysfunction and microvascular complications has attracted researchers to the use of PIs as a cheap, easy-to-conduct method of diabetes follow-up. Previous studies showed the association between PIs, diabetes glycemic control and microvascular complications[14-30]. However, the correlation and the significance varied considerably between the studies, and the type of the measured PIs[17].
MPV, PLDW, P-LCR and PLC are sensitive measures of glycemic control (82%); however, they are less specific (54.5%)[33]. The PIs are not independent predictors of glycemic control and cardiac complications but are influenced by age, diabetes duration, and drugs, including ARBs, aspirin and lipid-lowering drugs, which are widely prescribed to patients with diabetes[24,47]. In addition, glucagon-like peptide-1 receptor agonists, and DPP-4 inhibitors affect platelet ag
PLC is measured from PLT and MPV (PLC = PLT × MPV/10 000); therefore, they are discussed together. Age, duration of diabetes, drugs including ARBs, statins and aspirin, hypercholesterolemia, and hemoglobin could affect MPV and PLC[16,18]. PIs including MPV are increased among patients with renal impairment and show an inverse relation with glomerular filtration rate. Therefore, platelet activity has the same limitation as HbA1c[28]. However, PLDW is influenced by the preceding 15–20 d (lifespan of platelets)[16] and is less likely influenced by duration of diabetes[31]. Therefore, it might be more accurate as a glucoregulator because it is useful among newly diagnosed patients.
PLCR is a new index that needs special equipment (Sysmex analyzer) that might not be available in remote areas, and because of that more studies are suggested[9].
PIs, although associated with glycemic control and microvascular complications, can predict macrovascular disease among patients with diabetes, but they are not specific because the evidence was based mostly on observational studies. Therefore, a cause and effect cannot be concluded. Additionally, PI results should be viewed in the face of many confounders including demographic factors, pregnancy, renal failure, medication, hemoglobin and duration of diabetes.
PIs are sensitive, rapid, readily available and affordable measures of glycemic control, and can predict microvascular complications among patients with type 1 and type 2 diabetes. PLDW is more specific if performed promptly; good patient selection is needed to rule out confounders such as components of the metabolic syndrome, demographic characteristics and drugs.
The study limitations were the reliance on observational studies that were prone to bias, and we could not control for various confounders, including demographic factors and comorbidities.
PIs when measured promptly and within 2 h could be short-term pointers to glycemic control in the life span of the platelets (2 wk). They are cheap and easy to measure; therefore, PIs are useful in remote areas with limited facilities where measurement of HbA1c is not available or cost-effective. However, good patient selection is needed to rule out confounding variables including drugs, other comorbidities and demographic factors. PIs could be implemented with daily blood glucose to inform doctors in low-income countries about their patients' glycemic control and cardiovascular risk. An important application might be when blood glucose control is needed in a short period (before elective surgery) due to the short lifespan of platelets. Larger multicenter studies are needed to assess the most relevant PIs for prediction of glycemic control and microvascular complications.
We would like to acknowledge the Saudi Digital Library for free accessing the data.
| 1. | Satti SA, Saadeldin IY, Dammas AS. Diabetic Ketoacidosis in children admitted to Pediatric Intensive Care Unit of King Fahad Hospital, Al-Baha, Saudi Arabia: Precipitating factors, epidemiological parameters and clinical presentation. Sudan J Paediatr. 2013;13:24-30. [PubMed] |
| 2. | Milosevic D, Panin VL. Relationship Between Hematological Parameters and Glycemic Control in Type 2 Diabetes Mellitus Patients. J Med Biochem. 2019;38:164-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 3. | Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 4. | Ziaee A, Ghorbani A, Kalbasi S, Hejrati A, Moradi S. Association of hematological indices with prediabetes: A cross-sectional study. Electron Physician. 2017;9:5206-5211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Vigersky RA. Going beyond HbA1c to understand the benefits of advanced diabetes therapies. J Diabetes. 2019;11:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Batten L, Sathyapalan T, Palmer TM. Molecular Mechanisms Linking Diabetes with Increased Risk of Thrombosis. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 7. | Josefsson EC. Platelet intrinsic apoptosis. Thromb Res. 2023;231:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Tsikas D, Bollenbach A, Hanff E, Kayacelebi AA. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine (hArg): the ADMA, SDMA and hArg paradoxes. Cardiovasc Diabetol. 2018;17:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Regassa DA, Berihun GA, Habtu BF, Haile WB, Nagaash RS, Kiya GT. Platelet indices as predictors of poor glucoregulation in type 2 diabetes mellitus adults at Bishoftu General Hospital, Ethiopia. World J Diabetes. 2024;15:1889-1902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (3)] |
| 10. | Kizilgul M, Sencar E, Ucan B, Beysel S, Ozcelik O, Ozbek M, Cakal E. Components of the Complete Blood Count in Type 2 Diabetes Mellitus with Inadequate Glycemic Control. Dicle Tıp Dergisi. 2018;45:113-120. [RCA] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 11. | N PR, Gopinath M, Hafeez M, An R. To Study the Platelet Indices as a Predictor of Microvascular Complications in Type 2 Diabetes Mellitus. J Assoc Physicians India. 2022;70:11-12. [PubMed] |
| 12. | Kadić D, Hasić S, Spahić E. Mean platelet volume predicts the glycemic control deterioration in diabetes mellitus type 2 patients. Med Glas (Zenica). 2016;13:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Hussein HA, Elmagzoub FA, Babikir DM, Samaan MA. The Effect of Metformin and Glimepiride on Platelet Count and Indices Among Diabetic Patients were Attending Jaber Abu Aliz Diabetic Center in Khartoum State. J Biomed Pharm Res. 2021;10:93-98. [DOI] [Full Text] |
| 14. | Demirtunc R, Duman D, Basar M, Bilgi M, Teomete M, Garip T. The relationship between glycemic control and platelet activity in type 2 diabetes mellitus. J Diabetes Complications. 2009;23:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Singer J, Weissler Snir A, Leshem-Lev D, Rigler M, Kornowski R, Lev EI. Effect of intensive glycemic control on platelet reactivity in patients with long-standing uncontrolled diabetes. Thromb Res. 2014;134:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Wu M, Xiao L, Yang X. Positive Relationship of Platelet Volume Indices with HbA1c in Unselected Type-2 Diabetes Mellitus Patients. Clin Lab. 2019;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Reddy KS, Bentoor SN, Sakthivadivel V. Platelet indices as an accouterment for monitoring short-term glycemic levels and as an economical alternative to HbA1c. J Family Med Prim Care. 2023;12:561-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Verdoia M, Schaffer A, Barbieri L, Cassetti E, Nardin M, Bellomo G, Marino P, Sinigaglia F, De Luca G; Novara Atherosclerosis Study (NAS) group. Diabetes, glucose control and mean platelet volume: a single-centre cohort study. Diabetes Res Clin Pract. 2014;104:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Aktas F, Aktuglu MB. Evaluation of the relation between HBA1C and MPV, PDW levels of patients with Type 2 diabetes admitted in internal medicine polyclinics. North Clin Istanb. 2023;10:681-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Mi AE, Abdallah N, Eldars W. Mean Platelet Volume and Platelet Distribution Width Correlate with Microvascular Complications in Egyptian People with Type 2 Diabetes Mellitus. Curr Diabetes Rev. 2021;17:e080621193947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 21. | Dev A, R NK, K A, Sivan G, Rajesh Lenin R, Kumar JS. Correlation of Mean Platelet Volume and Red Cell Distribution Width With HbA1c and Its Association With Microvascular Complications in Type 2 Diabetes Mellitus: A Cross-Sectional Study at a Tertiary Hospital in India. Cureus. 2024;16:e66139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 22. | Ji S, Zhang J, Fan X, Wang X, Ning X, Zhang B, Shi H, Yan H. The relationship between mean platelet volume and diabetic retinopathy: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Abdel-Moneim A, Semmler M, Abdel-Reheim ES, Zanaty MI, Addaleel W. Association of glycemic status and interferon-γ production with leukocytes and platelet indices alterations in type2 diabetes. Diabetes Metab Syndr. 2019;13:1963-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Filippatos T, Tsimihodimos V, Pappa E, Elisaf M. Pathophysiology of Diabetic Dyslipidaemia. Curr Vasc Pharmacol. 2017;15:566-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Palella E, Cimino R, Pullano SA, Fiorillo AS, Gulletta E, Brunetti A, Foti DP, Greco M. Laboratory Parameters of Hemostasis, Adhesion Molecules, and Inflammation in Type 2 Diabetes Mellitus: Correlation with Glycemic Control. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Jabeen F, Fawwad A, Rizvi HA, Alvi F. Role of platelet indices, glycemic control and hs-CRP in pathogenesis of vascular complications in type-2 diabetic patients. Pak J Med Sci. 2013;29:152-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Gulia M, Gupta M, Singh Lehl S, Singla M, Tahlan A, Kaur J. Mean platelet volume and glycaemic control in patients with new-onset Type 2 diabetes mellitus. J R Coll Physicians Edinb. 2022;52:105-109. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Söbü E, Demir Yenigürbüz F, Özçora GDK, Köle MT. Evaluation of the Impact of Glycemic Control on Mean Platelet Volume and Platelet Activation in Children with Type 1 Diabetes. J Trop Pediatr. 2022;68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Venkatesh V, Kumar R, Varma DK, Bhatia P, Yadav J, Dayal D. Changes in platelet morphology indices in relation to duration of disease and glycemic control in children with type 1 diabetes mellitus. J Diabetes Complications. 2018;32:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Ma SG, Yang LX, Qiu XQ. Assessment of the platelet parameters and serum butyrylcholinesterase activity in type 1 diabetes patients with ketoacidosis. Platelets. 2013;24:544-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S20-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 1066] [Article Influence: 533.0] [Reference Citation Analysis (4)] |
| 32. | Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, Tunnicliffe D, Ruospo M, Natale P, Saglimbene V, Nicolucci A, Johnson DW, Tonelli M, Rossi MC, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque LI, Lloyd A, Ahmad N, Liu Y, Tiv S, Millard T, Gagliardi L, Kolanu N, Barmanray RD, McMorrow R, Raygoza Cortez AK, White H, Chen X, Zhou X, Liu J, Rodríguez AF, González-Colmenero AD, Wang Y, Li L, Sutanto S, Solis RC, Díaz González-Colmenero F, Rodriguez-Gutierrez R, Walsh M, Guyatt G, Strippoli GFM. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 446] [Article Influence: 89.2] [Reference Citation Analysis (1)] |
| 33. | Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14:28-32. [PubMed] |
| 34. | Rasoulinejad SA. Is there an association between mean platelet volume and diabetic retinopathy? A case-control study. Caspian J Intern Med. 2021;12:129-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Ünübol M, Ayhan M, Güney E. The relationship between mean platelet volume with microalbuminuria and glycemic control in patients with type II diabetes mellitus. Platelets. 2012;23:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Venkata B, M SM. A Study of Platelets Indices in Patients with Diabetic Retinopathy and without Diabetic Retinopathy & the Effect of Hyperglycemia on Platelet Indices. J Assoc Physicians India. 2022;70:11-12. [PubMed] |
| 37. | Vivas D, García-Rubira JC, Bernardo E, Angiolillo DJ, Martín P, Calle-Pascual A, Núñez-Gil I, Macaya C, Fernández-Ortiz A. Influence of HbA1c levels on platelet function profiles associated with tight glycemic control in patients presenting with hyperglycemia and an acute coronary syndrome. A subanalysis of the CHIPS Study ("Control de HIperglucemia y Actividad Plaquetaria en Pacientes con Síndrome Coronario Agudo"). J Thromb Thrombolysis. 2013;35:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Muruganandam A, Romsa GJ, Thibert RJ, Cheung RM, Draisey TF, Mutus B. Glycated calmodulin from platelets as an index of glycemic control. Clin Chem. 1993;39:815-819. [PubMed] |
| 39. | Çalapkulu M, Sencar ME, Unsal IO, Sakiz D, Ozbek M, Cakal E. Effect of Exenatide Therapy on Platelet Function in Type 2 Diabetes Mellitus. J Coll Physicians Surg Pak. 2021;31:1035-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Xu F, Qu S, Wang L, Qin Y. Mean platelet volume (MPV): new diagnostic indices for co-morbidity of tuberculosis and diabetes mellitus. BMC Infect Dis. 2021;21:461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Song X, Zhu H, Pei Q, Tan F, Li C, Zhou Z, Zhou Y, Yu N, Li Y, Pei H. Significance of inflammation-based indices in the prognosis of patients with non-metastatic colorectal cancer. Oncotarget. 2017;8:45178-45189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Polk C, Sampson MM, Roshdy D, Davidson LE. Skin and Soft Tissue Infections in Patients with Diabetes Mellitus. Infect Dis Clin North Am. 2021;35:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 589] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 44. | Mansour AA, Al-Maliky AA, Kasem B, Jabar A, Mosbeh KA. Prevalence of diagnosed and undiagnosed diabetes mellitus in adults aged 19 years and older in Basrah, Iraq. Diabetes Metab Syndr Obes. 2014;7:139-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (2)] |
| 45. | Braester A, Shturman A, Raviv B, Dorosinsky L, Rosental E, Atar S. What a Family Doctor Should Know about Incidental Finding of High Mean Platelet Volume, Metabolic Syndrome, and Pre-diabetes. Isr Med Assoc J. 2021;23:699-702. [PubMed] |
| 46. | Riddlesworth TD, Beck RW, Gal RL, Connor CG, Bergenstal RM, Lee S, Willi SM. Optimal Sampling Duration for Continuous Glucose Monitoring to Determine Long-Term Glycemic Control. Diabetes Technol Ther. 2018;20:314-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 205] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 47. | Jia G, Aroor AR, Sowers JR. Glucagon-Like Peptide 1 Receptor Activation and Platelet Function: Beyond Glycemic Control. Diabetes. 2016;65:1487-1489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Yucel K, Disci SI, Yucel M. Effect of Glycemic Control on Platelet Indices in Children with Type 1 Diabetes Mellitus. Sisli Etfal Hastan Tip Bul. 2024;58:139-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Lancé MD, van Oerle R, Henskens YM, Marcus MA. Do we need time adjusted mean platelet volume measurements? Lab Hematol. 2010;16:28-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/