Published online Mar 15, 2025. doi: 10.4239/wjd.v16.i3.95092
Revised: September 30, 2024
Accepted: December 23, 2024
Published online: March 15, 2025
Processing time: 295 Days and 1.3 Hours
Periodontitis, when exacerbated by diabetes, is characterized by increased M1 macrophage polarization and decreased M2 polarization. O-linked β-N-acetylglucosamine (O-GlcNAcylation), catalyzed by O-GlcNAc transferase (OGT), promotes inflammatory responses in diabetic periodontitis (DP). Additionally, p38 mitogen-activated protein kinase regulates macrophage polarization. However, the interplay between OGT, macrophage polarization, and p38 signaling in the progression of DP remains unexplored.
To investigate the effect of OGT on macrophage polarization in DP and its role in mediating O-GlcNAcylation of p38.
For in vivo experiments, mice were divided into four groups: Control, DP model, model + short hairpin (sh) RNA-negative control, and model + sh-OGT. Diabetes was induced by streptozotocin, followed by ligation and lipopolysaccharide (LPS) administration to induce periodontitis. The impact of OGT was assessed by injecting sh-OGT lentivirus. Maxillary bone destruction was evaluated using micro-computed tomography analysis and tartrate-resistant acid phosphatase staining, while macrophage polarization was determined through quantitative real-time polymerase chain reaction (qPCR) and immunohistochemistry. For in vitro experiments, RAW264.7 cells were treated with LPS and high glucose (HG) (25 mmol/L D-glucose) to establish a cell model of DP. OGT was inhibited by OGT inhibitor (OSMI4) treatment and knocked down by sh-OGT transfection. M1/M2 polarization was analyzed using qPCR, immunofluorescence, and flow cytometry. Levels of O-GlcNAcylation were measured using immunoprecipitation and western blotting.
Our results demonstrated that M1 macrophage polarization led to maxillary bone loss in DP mice, associated with elevated O-GlcNAcylation and OGT levels. Knockdown of OGT promoted the shift from M1 to M2 macrophage polarization in both mouse periodontal tissues and LPS + HG-induced RAW264.7 cells. Furthermore, LPS + HG enhanced the O-GlcNAcylation of p38 in RAW264.7 cells. OGT interacted with p38 to promote its O-GlcNAcylation at residues A28, T241, and T347, as well as its phosphorylation at residue Y221.
Inhibition of OGT-mediated p38 O-GlcNAcylation deactivates the p38 pathway by suppressing its self-phos
Core Tip: O-linked β-N-acetylglucosamine (O-GlcNAcylation) transferase (OGT) promotes the O-GlcNAcylation of p38 at S28, T241, and T347 sites and further activates the p38/mitogen-activated protein kinase pathway by phosphorylation itself at Y221. Inhibition of O-GlcNAcylation promotes M2-type polarization and inhibits M1-type polarization of macrophages, accelerating alveolar bone rebuild to decelerate diabetic periodontitis progression.
- Citation: Wu YK, Liu M, Zhou HL, He X, Wei J, Hua WH, Li HJ, Yuan QH, Xie YF. O-linked β-N-acetylglucosamine transferase regulates macrophage polarization in diabetic periodontitis: In vivo and in vitro study. World J Diabetes 2025; 16(3): 95092
- URL: https://www.wjgnet.com/1948-9358/full/v16/i3/95092.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i3.95092
Diabetes mellitus and periodontitis are prevalent chronic conditions affecting human health. Diabetes is a rapidly escalating disorder characterized by multiple complications that significantly impair quality of life and can lead to mortality[1]. Presently, periodontitis is recognized as the “sixth complication” of diabetes[2]. Globally, periodontitis affects approximately 10%-15% of adults, with diabetes exacerbating both the susceptibility and severity of the condition[3]. Uncontrolled hyperglycemia triggers a sustained inflammatory response, disrupts alveolar bone homeostasis within periodontal tissues, and alters the pathogenicity of the oral microbiome, contributing to the onset and progression of periodontitis[4]. Additionally, the inflammatory burden induced by periodontitis can compromise glycemic control and elevate the risk of further diabetic complications[5,6]. Consequently, elucidating the underlying pathological mechanisms linking diabetes and periodontitis is imperative to develop novel therapeutic strategies for diabetic periodontitis (DP).
Macrophages are key players in the innate immune system responsible for maintaining tissue homeostasis. Various stimuli, including infection and hypoxia, can polarize these cells into either an M1 or M2 phenotype, altering their func
The glucose flux generated by the metabolic pathways in diabetes provides a substrate for protein O-linked β-N-acetylglucosamine (O-GlcNAcylation)[13]. O-GlcNAcylation is a widely studied post-translational modification, catalyzed by O-GlcNAc transferase (OGT), which adds an O-GlcNAc moiety to the serine or threonine residues of proteins. This reversible modification can be removed by O-GlcNAcase (OGA)[14]. O-GlcNAcylated proteins undergo changes in stability, intracellular localization, and interactions with other proteins, influencing both physiological and pathological processes. Dysregulation of O-GlcNAcylation has been linked to various diseases, including diabetes and periodontitis[15-17]. Moreover, hyperglycemia can enhance O-GlcNAcylation, which in turn regulates macrophage polarization[18]. Therefore, investigating O-GlcNAcylation may contribute to a better understanding of the pathogenesis of DP.
Three major mitogen-activated protein kinase (MAPK) cascades have been identified in mammals. Among these, the p38 pathway is activated by environmental and genotoxic stress. P38 regulates multiple intracellular functions, playing a critical role in inflammation, cell survival, cell death, and tumorigenesis[19,20]. Accumulating evidence indicates that dysregulation of p38 is associated with macrophage polarization and apoptosis[21,22]. In DP, p38 activation responds to inflammatory cell infiltration and influences alveolar bone loss[23]. However, the role of O-GlcNAcylation in modulating p38 activity and its impact on macrophage polarization in DP remains unclear.
Collectively, the imbalance in M1/M2 macrophage polarization contributes to the pathogenesis of DP, a process associated with dysregulation of O-GlcNAcylation. Additionally, p38 regulates macrophage polarization. Despite this knowledge, the specific mechanisms by which O-GlcNAcylation regulates macrophage polarization in DP have not been well established. Based on these considerations, our study aimed to investigate the regulation of O-GlcNAcylation in macrophage polarization within the context of DP and the modification of p38. Our findings demonstrated that inhibition of OGT-mediated p38 O-GlcNAcylation suppresses p38 phosphorylation, thereby promoting the transition from M1 to M2 macrophage polarization, reducing the pathological damage caused by DP. These results suggested a novel regulatory mechanism in DP.
All animal experimental procedures were approved by the Ethics Committee of the Hospital of Chengdu University of Traditional Chinese Medicine and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (No. 2022-126). Male C57BL/6 mice (6-8 weeks old) were obtained from Charles River (Beijing, China). The animals were housed under a 12-hour light/dark cycle at a temperature of 22 ± 2 °C and humidity of 50%-60%, with ad libitum access to food and water.
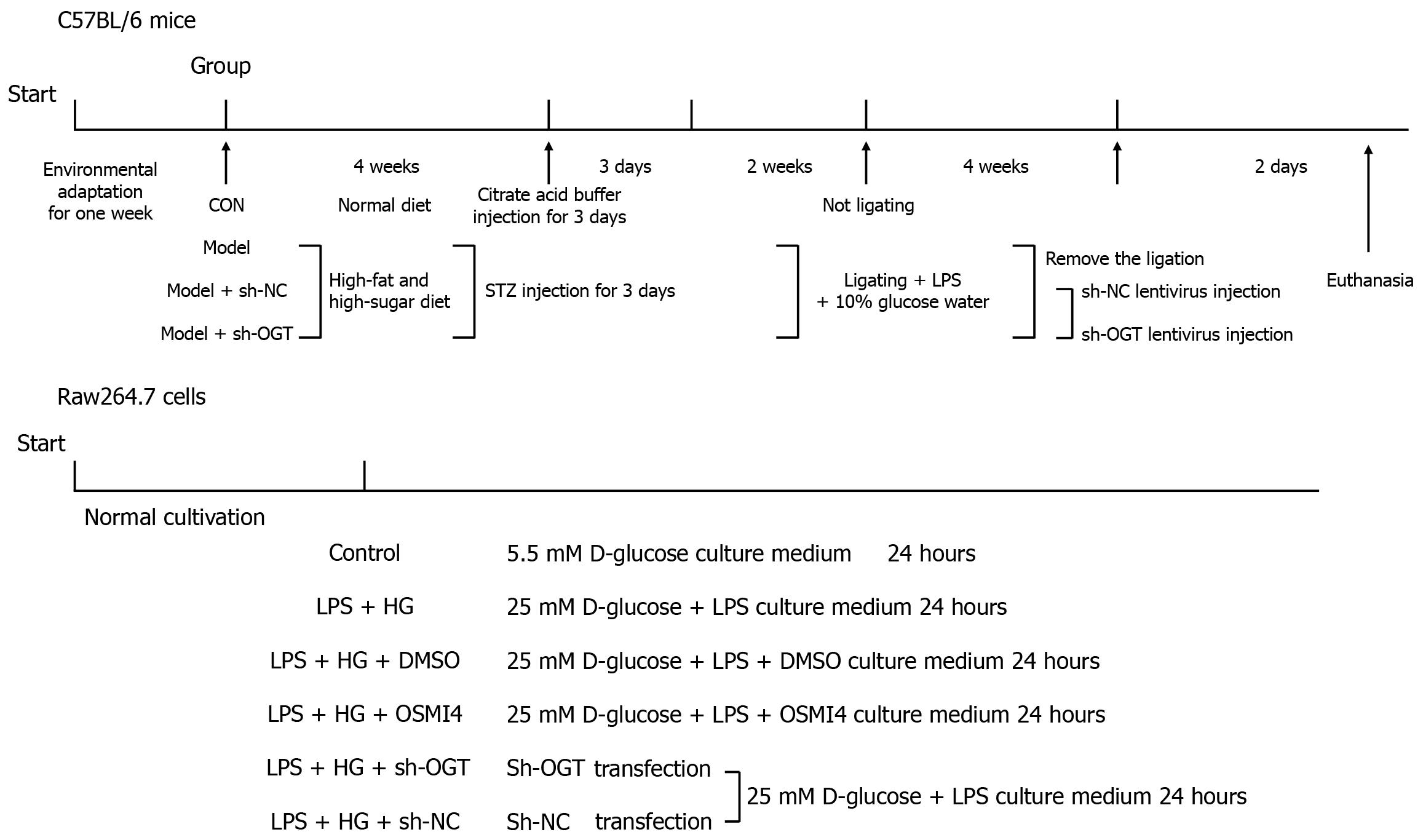
The experimental design is illustrated in Figure 1. Mice were randomly divided into four groups (n = 6 per group): Control, DP model, model + short hairpin (sh) RNA-negative control (NC), and model + sh-OGT. To induce diabetes, streptozotocin (STZ) (Sigma-Aldrich, St. Louis, MO, United States) was dissolved in freshly prepared 0.1 M citrate buffer (potential of hydrogen = 4.5). Mice were fed a continuous high-fat and high-sugar diet (Research Diets Inc., New Brunswick, NJ, United States) and intraperitoneally injected with 100 mg/kg STZ for three consecutive days[24]. The composition of the high-fat and high-sugar diet is detailed in Supplementary Table 1. After two weeks, mice with fasting blood glucose levels greater than 11.1 mmol/L and random blood glucose levels greater than 16.7 mmol/L were classified as diabetic. Each diabetic mouse was individually housed under standard environmental conditions and continued on the high-fat and high-sugar diet. Daily monitoring included weight, appetite, activity, and general health status. Blood glucose levels were measured every three days. Mice exhibiting severe pain or disease symptoms were promptly euthanized.
To induce periodontitis, diabetic mice were ligatured with 0.2 mm steel wire around the left maxillary second molar for four weeks, and received daily local inoculations of 20 μL lipopolysaccharide (LPS) (2 mg/mL) into the gingival crevices. They were also administered 10% glucose water as described previously[25] with slight modifications. Control group mice were injected with citrate buffer for three days and did not receive ligation.
To reduce OGT expression, 100 μL of sh-OGT lentivirus or sh-NC lentivirus was injected into the tail vein. Two days after the injection, mice were euthanized with 30 mg/kg sodium pentobarbital administered intraperitoneally. Maxillary bones were then isolated from all mice. Following micro-computed tomography (μCT) analysis, the excised maxillary bones were fixed in 4% paraformaldehyde for 48 hours and subsequently decalcified with 20% ethylene diamine tetraacetic acid for one month at 4 °C for further use.
Maxillae were placed in a container filled with sterile water and scanned using a μCT scanner (μCT80; Scanco Medical, Brüttisellen, Switzerland). The scanner settings were 70 kV, 200 mA, with an integration time of 300 ms and a voxel size of 10 μm. Three-dimensional images were reconstructed using intense pulsed light image-processing software (Scanco Medical) for quantitative and qualitative evaluation (threshold = 450).
To assess the severity of alveolar bone loss, the distance from the cemento-enamel junction (CEJ) to the alveolar bone crest (ABC) was measured at the mesial, middle, and distal sites on both the lingual and buccal sides (a total of six sites) of the maxillary left second molar using the three-dimensional images. The cumulative value of these six sites was calculated as the alveolar bone resorption for the maxillary left second molar.
Alveolar bone around the maxillary first molar was selected as the region of interest (ROI). The following micro-architectural parameters were assessed in the ROI images: Bone volume ratio (BV/TV); Trabecular thickness (Tb.Th); Trabecular separation (Tb.Sp); Trabecular number (Tb.N); Bone mineral density (BMD). The BV/TV value indicates the portion of mineralized tissue; Tb.Th, Tb.Sp and Tb.N provide detailed information on the amount, thickness and organization of trabecular bone; and BMD indicates the density of bone mineral.
Maxillary bones were embedded in paraffin following decalcification and sectioned into 5 μm-thick sagittal sections. A tartrate-resistant acid phosphate (TRAP) staining kit (BestBio, Shanghai, China) was used to detect osteoclast differentiation. Briefly, the paraffin sections were deparaffinized and rehydrated. Subsequently, the sections were stained with the staining buffer for 60 minutes in the dark. Hematoxylin was used to counterstain the sections for 1 minute. TRAP-positive cells (red particles in the cytoplasm) were quantified.
The levels of cluster of differentiation (CD) 86 and CD206 in the maxilla were measured using immunohistochemical (IHC) analysis. Paraffin sections were deparaffinized and rehydrated. Sections were then subjected to antigen retrieval by boiling in citrate buffer using a microwave and subsequently cooling to room temperature. After washing with distilled water, the sections were blocked with normal serum at 37 °C for 30 minutes.
Next, the sections were incubated overnight at 4 °C with primary antibodies against CD86 (91882; Cell Signaling Technology, Danvers, MA, United States) and CD206 (ab64693; Abcam, Cambridge, MA, United States). Following incubation, the sections were washed and incubated with horseradish peroxidase-conjugated secondary antibody (ab205718; Abcam) at 37 °C for 30 minutes. Color development was achieved using diaminobenzidine solution. Finally, the sections were sealed, dried, and examined under a microscope.
The IHC score was determined by multiplying the staining intensity score with the positive area score. Staining intensity was scored as follows: 0 (negative), 1 (weakly positive), 2 (moderately positive), and 3 (strongly positive). Positive area scores were assigned as: 0 (less than 5%), 1 (5%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (more than 75%).
Mouse macrophage cell line RAW264.7 was obtained from American type culture collection (Manassas, VA, United States). The cells were cultured in Dulbecco’s modified eagle medium (GIBCO BRL, Grand Island, NY, United States) supplemented with 10% fetal bovine serum (GIBCO BRL) and 1% penicillin-streptomycin (GIBCO BRL) in a humidified incubator at 37 °C with 5% carbon dioxide.
To establish an in vitro model mimicking DP, RAW264.7 cells were incubated in HG culture medium (25 mmol/L D-glucose; Sigma-Aldrich) supplemented with 100 ng/mL LPS (Sigma-Aldrich) for 24 hours. Cells in the control group were incubated in normal glucose medium (5.5 mmol/L D-glucose) without LPS for 24 hours.
The cells were divided into six groups: Control, LPS + HG, LPS + HG + dimethyl sulfoxide (DMSO), LPS + HG + OGT inhibitor (OSMI4), LPS + HG + sh-OGT, and LPS + HG + sh-NC. To inhibit O-GlcNAcylation, OSMI4 (10 μmol/L; MedChem Express, Monmouth Junction, NJ, United States) was used to treat Raw264.7 cells for 24 hours. Cells treated with DMSO served as the negative control. To knockdown OGT, sh-OGT and sh-NC designed and obtained from GenePharma (Shanghai, China) were transfected into Raw264.7 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States) for 48 hours in line with the manufacturer’s instructions. The experimental design is illustrated in Figure 1.
P38 mutants, including S28A, T241A, and T347A (where serine or threonine residues were mutated to alanine), were constructed using the quick mutation plus site-directed mutagenesis kit (Beyotime, Shanghai, China) according to the manufacturer’s protocols. These mutated plasmids and wild-type (WT) plasmids were transformed into Escherichia coli DH5α competent cells. Single colonies were selected, and the constructs were verified by sequencing.
Both the WT and mutated plasmids were transfected into the cells using lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions, with a duration of 48 hours.
Total RNA was extracted from maxillary bones and RAW264.7 cells using TRIzol reagent (Invitrogen, Carlsbad, CA, United States). RNA concentration and integrity were assessed using spectrophotometry and agarose gel electrophoresis, respectively. Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using the Goldenstar™ RT6 cDNA synthesis kit (TSINGKE, Nanjing, China). The synthesis reaction was carried out at 50 °C for 30 minutes followed by inactivation at 85 °C for 5 minutes. Quantitative real-time polymerase chain reaction (qPCR) was performed using the ArtiCanCEO SYBR qPCR mix (TSINGKE) on the PikoReal real-time PCR system (Thermo Fisher Scientific, Waltham, MA, United States) according to the manufacturer’s protocol. The reaction mix contained: SYBR qPCR mix (10 μL), forward primer (0.4 μL), reverse primer (0.4 μL), template (2 μL), and nuclease-free water (7.2 μL). The thermal cycling conditions were: Initial denaturation at 95 °C for 5 minutes, followed by 40 cycles of denaturation at 95 °C for 10 seconds and annealing/extension at 60 °C for 30 seconds.
Relative mRNA expression was calculated using the 2^-ΔΔCt method and expressed as fold changes relative to the control. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal reference gene. The primer sequences used were as follows: Inducible nitric oxide sythase (iNOS) forward: 5’-GTTCTCAGCCCAACAATACAAGA-3’; Reverse: 5’-GTTCTCAGCCCAACAATACAAGA-3’; Interleukin (IL)-6 forward: 5’-CTGCAAGAGACTTCCATCCAG-3’; Reverse: 5’-AGTGGTATAGACAGGTCTGTTGG-3’; IL-10 forward: 5’-GCTCTTACTGACTGGCATGAG-3’; Reverse: 5’-CGCAGCTCTAGGAGCATGTG-3’; Arginase (Arg)-1 forward: 5’-CTCCAAGCCAAAGTCCTTAGAG-3’; Reverse: 5’-GGAGCTGTCATTAGGGACATCA-3’; GAPDH forward: 5’-AGGTCGGTGTGAACGGATTTG-3’; Reverse: 5’-TGTAGACCATGTAGTTGAGGTCA-3’.
Total proteins were extracted from maxillary bones and RAW264.7 cells using Radioimmunoprecipitation assay buffer supplemented with protease inhibitors. Samples were centrifuged at 12000 g for 15 minutes, and the supernatants were collected. Protein concentrations were determined using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific).
Approximately 40 μg of protein was loaded onto 10% sodium dodecyl sulfate-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes. The membranes were blocked and incubated overnight at 4 °C with the following primary antibodies: Anti-O-GlcNAc (also known as RL2; ab2739; Abcam), anti-OGT (ab177941; Abcam), anti-OGA (ab124807; Abcam), anti-GAPDH (2118; Cell Signaling Technology), anti-phosphorylated-p38 (phospho Y221; ab76227; Abcam), and anti-p38 (ab170099; Abcam).
Subsequently, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (ab205719 or ab205718; Abcam) at room temperature for 1 hour. Protein bands were visualized using an enhanced chemiluminescence kit (Thermo Fisher Scientific). Band densities were quantified using ImageJ software (version 1.38e; NIH, Bethesda, MD, United States) and normalized to GAPDH levels.
Macrophage phenotypes were evaluated using flow cytometry. Briefly, after stimulation with LPS and HG, RAW264.7 cells were washed with phosphate-buffered saline (PBS) and blocked with an anti-CD16/32 antibody (NovoBiotechnology, Beijing, China). The cells were then washed again with PBS and incubated with phycoerythrin-conjugated anti-mouse CD86 antibody (E-AB-F0994D; Elabscience, Wuhan, China) and allophycocyanin-conjugated anti-mouse CD206 antibody (E-AB-F1135E; Elabscience) at 4 °C for 30 minutes.
Following incubation, the cells were resuspended in PBS, and the expression of surface markers was detected using a CytoFLEX flow cytometer (Beckman Coulter, Fullerton, CA, United States). Data analysis was performed using FlowJo software (version 10.9; FlowJo LLC, Ashland, OR, United States).
Cell slides were fixed in 4% paraformaldehyde for 15 minutes, permeabilized with 0.5% triton X-100 for 20 minutes, and blocked with normal serum for 30 minutes at room temperature. To measure the levels of macrophage markers, the slides were incubated overnight at 4 °C with primary antibodies against CD86 (91882; Cell Signaling Technology) and CD206 (ab64693; Abcam). The slides were then incubated with an Alexa Fluor 488-conjugated secondary antibody (ab150077; Abcam) for 1 hour at room temperature.
For double staining to observe intracellular localization, the slides were incubated with primary antibodies against OGT (AG4312; Beyotime) and p38 (ab170099; Abcam), followed by incubation with appropriate secondary antibodies (ab150113 or ab150083; Abcam). Nuclei were stained with 4’, 6-diamidino-2-phenylindole (Sigma-Aldrich). Fluorescence was observed under an LSM800 confocal microscope (Carl Zeiss AG, Jena, Germany). Relative fluorescent intensity was quantified using ImageJ software.
An immunoprecipitation (IP) kit (Beyotime) was used to perform IP and co-IP. Briefly, protein A + G magnetic beads were combined with the following antibodies at room temperature for 15 minutes: Anti-c-Jun N-terminal kinase (JNK) (ab76125; Abcam), anti-p38 (ab170099; Abcam), anti-inhibitor of κB alpha (IκBα) (ab32518; Abcam), anti-p65 (ab16502; Abcam), anti-nuclear factor (erythroid-derived 2)-like 2 (NRF2) (ab62352; Abcam), anti-heme oxygenase 1 (HO-1) (ab68477; Abcam), and IgG (ab172730; Abcam).
RAW264.7 cells were lysed using lysis buffer supplemented with a protease inhibitor cocktail. The lysates were centrifuged at 12000 g for 5 minutes, and the supernatant was collected. The supernatant was then incubated with the antibody-bead mixture at 4 °C overnight. Following incubation, the beads were isolated and washed several times with lysis buffer.
The bound proteins were eluted from the beads, and the O-GlcNAcylation levels of the precipitated proteins were assessed by Western blot using the RL2 antibody (ab2739; Abcam).
Data were analyzed and graphs were created using GraphPad Prism 8 software (GraphPad Software, La Jolla, CA, United States). Statistical significance was assessed using Student’s t-test for comparisons between two groups and one-way analysis of variance followed by Tukey’s post hoc test for comparisons among multiple groups. A P value less than 0.05 was considered statistically significant.
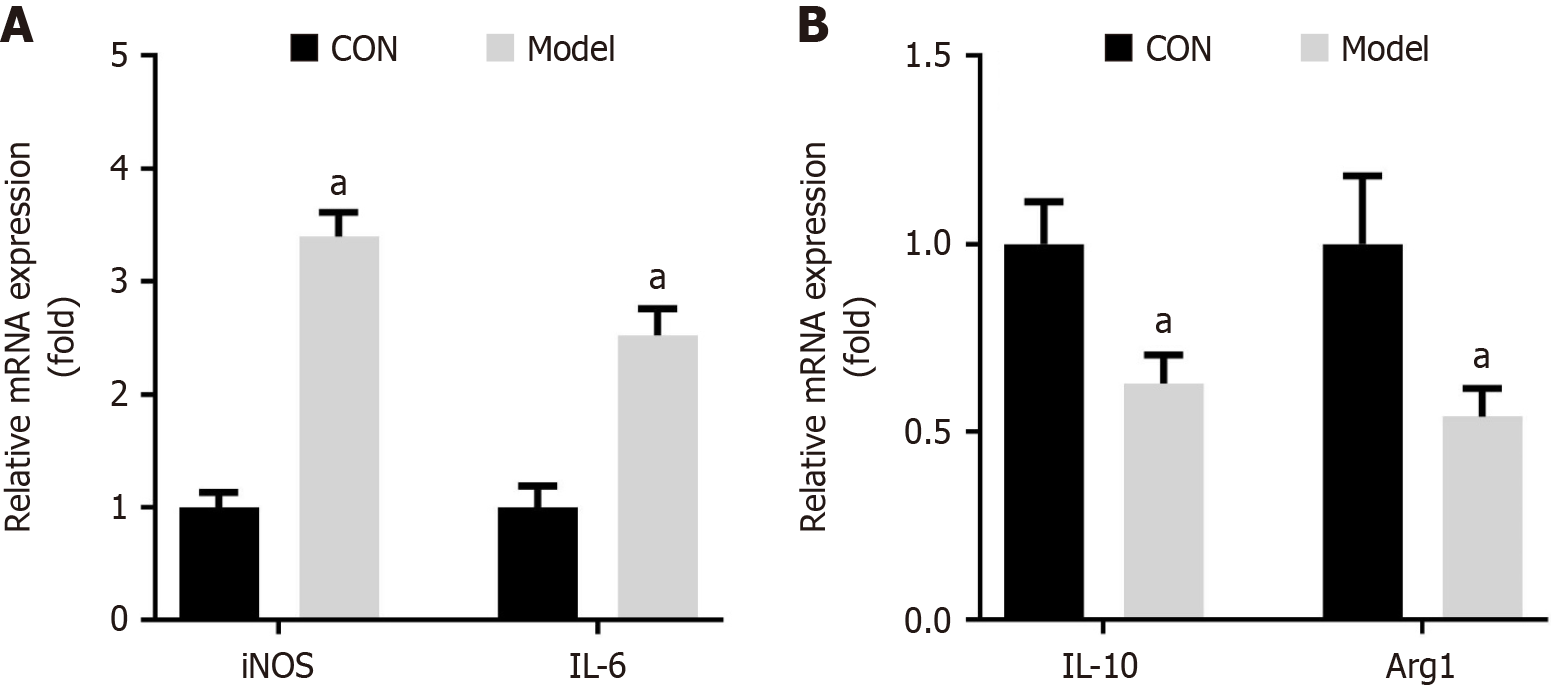
Macrophage polarization plays a critical role in various diseases, including diabetes and periodontitis. We assessed the expression of several polarization markers in DP model. The results demonstrated that the expression of M1 macrophage markers, such as iNOS and IL-6, was significantly upregulated in DP mice (Figure 2A). In contrast, the expression of M2-associated markers, including IL-10 and Arg1, was markedly downregulated in DP mice (Figure 2B). Collectively, these findings indicated that DP promoted M1 polarization while inhibiting M2 polarization.
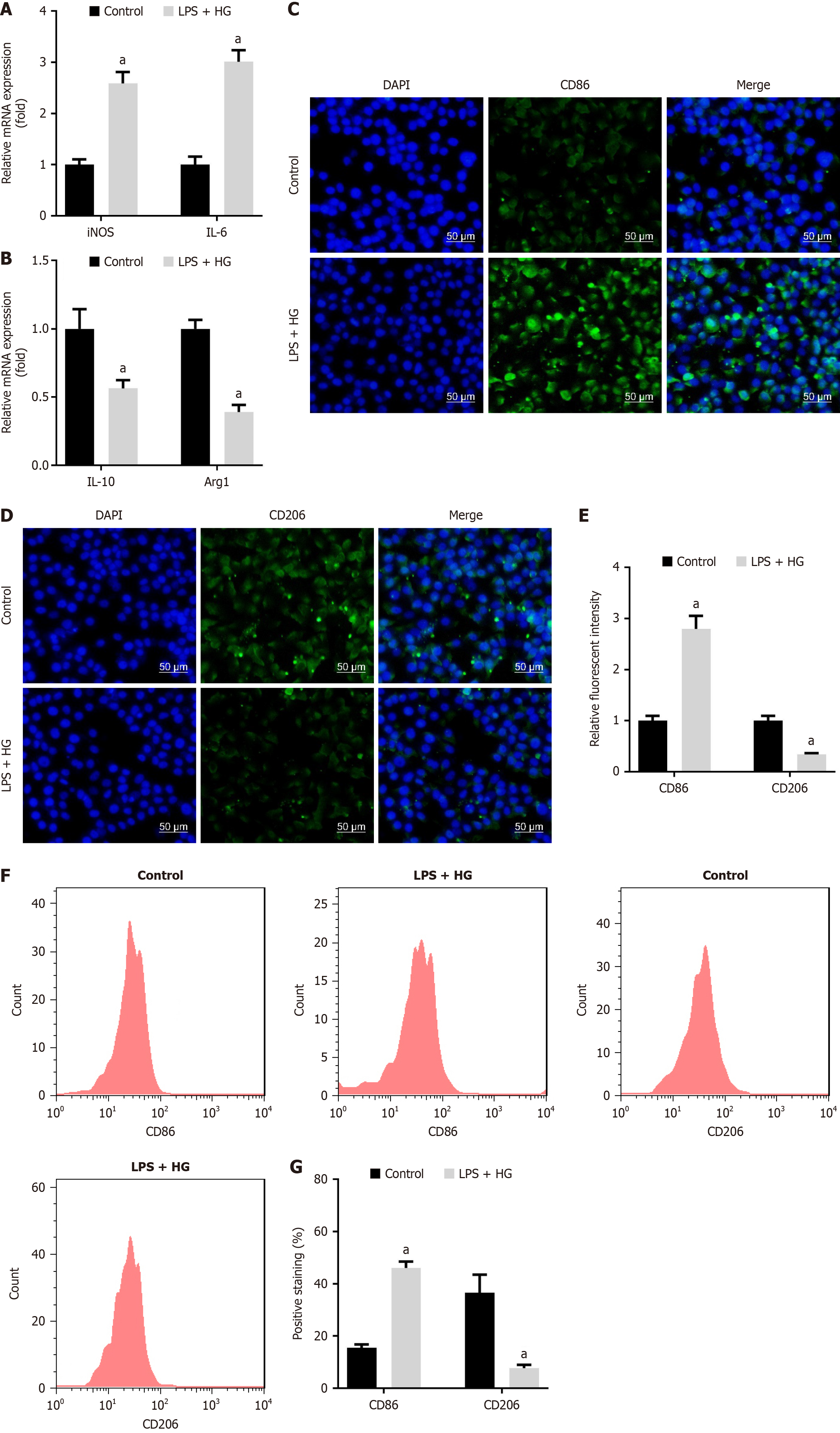
To evaluate the effect of LPS and HG on macrophage polarization, we assessed polarization markers in RAW264.7 cells treated with LPS and HG. Our results indicated that LPS and HG significantly elevated the levels of M1 macrophage markers, including iNOS and IL-6 (Figure 3A). Conversely, the levels of M2 macrophage markers, such as IL-10 and Arg1, were notably downregulated by LPS and HG (Figure 3B). Subsequently, CD86 levels were increased, and CD206 levels were reduced after LPS and HG treatment, which was analyzed using immunofluorescence analysis (Figure 3C-E). Similarly, flow cytometry results also indicated that LPS and HG treatment upregulated CD86 expression and downregulated CD26 expression (Figure 3F and G). These findings suggested that LPS promoted M1 polarization and inhibited M2 polarization under HG conditions.
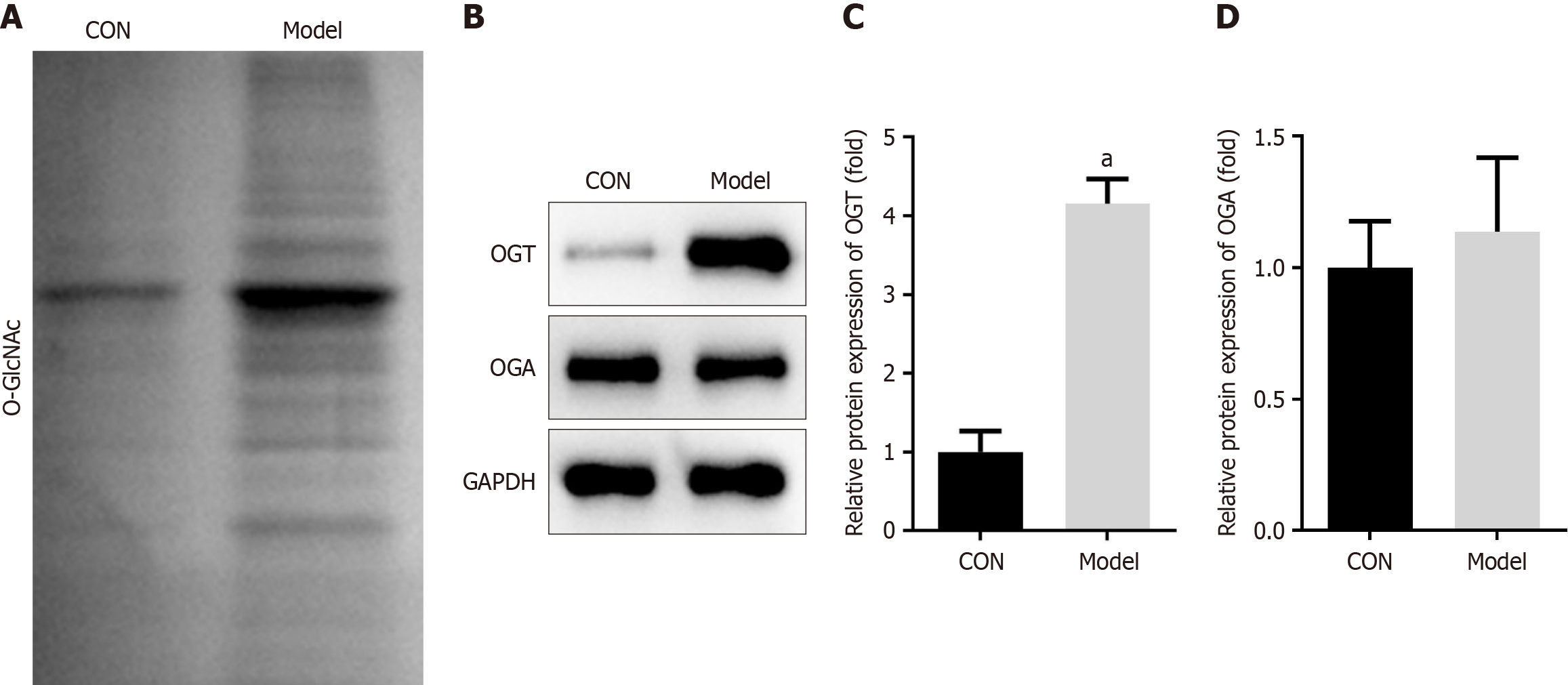
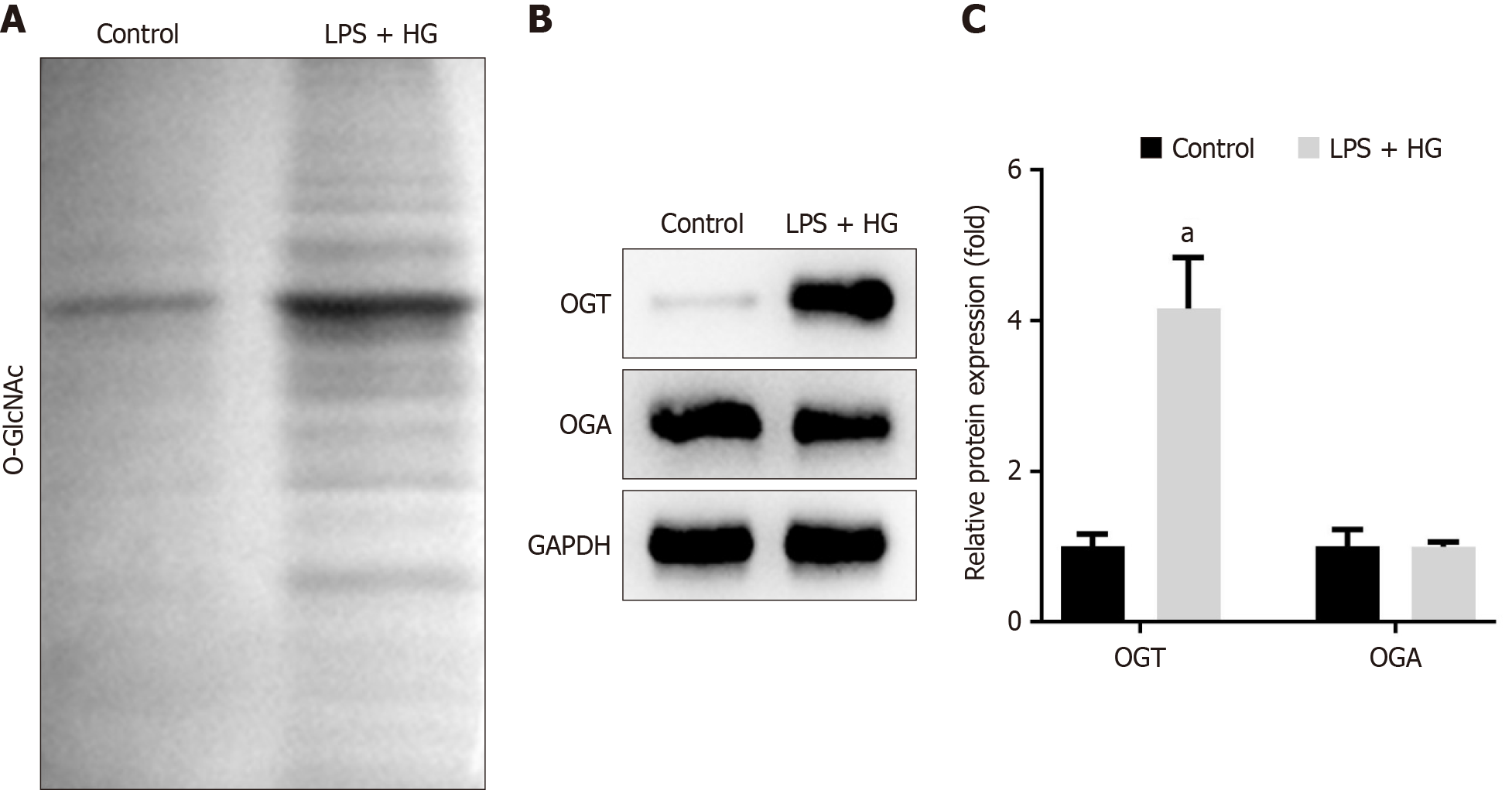
It is known that the concentration of extracellular glucose can alter the level of O-GlcNAcylation within cells[26]. Therefore, we investigated O-GlcNAcylation levels in DP mice and in cells treated with LPS and HG. Our results showed that total O-GlcNAcylation levels were significantly increased in the maxillary bones of DP mice (Figure 4A). Further analysis revealed that the expression of OGT was markedly upregulated in DP mice, whereas OGA levels did not show significant differences between the groups in the animal study (Figure 4B-D). Similarly, total O-GlcNAcylation levels were elevated in LPS + HG-treated RAW264.7 cells (Figure 5A). LPS and HG significantly increased OGT expression, but did not change OGA expression (Figure 5B and C). These findings indicated that OGT-mediated O-GlcNAcylation played an important role in the progression of DP.
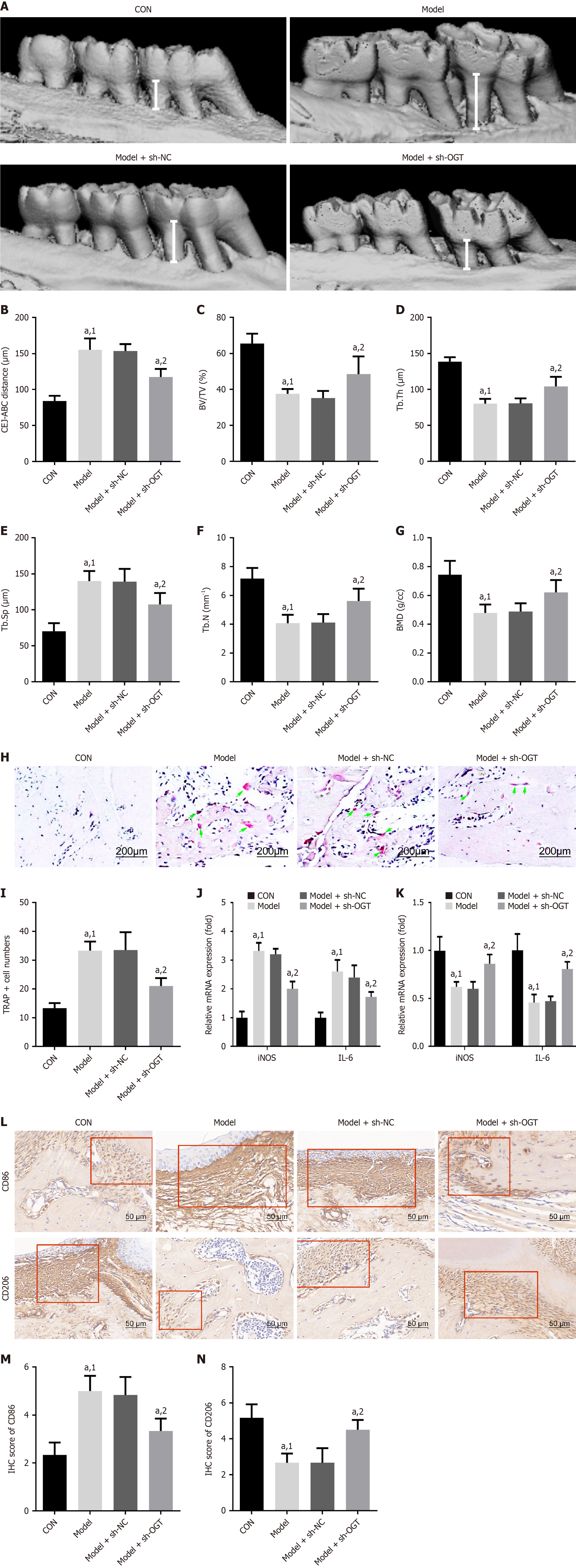
To explore the effects of OGT on bone loss and macrophage polarization in vivo, we injected sh-OGT and sh-NC lentivirus into mice. The results showed that DP significantly increased alveolar bone loss compared to control mice, but this increase was reversed by OGT knockdown (Figure 6A and B). Additionally, BV/TV, Tb.Th, Tb.N, and BMD were significantly reduced, while Tb.Sp was increased in the alveolar bones of DP mice compared to controls (Figure 6C-G). Knockdown of OGT significantly mitigated the increase in cementoenamel junction to alveolar bone crest distance and Tb.Sp, and the reduction in BV/TV, Tb.Th, Tb.N, and BMD parameters caused by DP (Figure 6A-G).
TRAP staining was performed to evaluate osteoclast differentiation. Consistent with the aforementioned trends, the number of TRAP-positive cells was significantly higher in the DP group than in the control group, and this increase was markedly reduced by OGT silencing (Figure 6H and I). Additionally, OGT knockdown significantly reduced the mRNA expression of iNOS and IL-6 and increased the mRNA expression of IL-10 and Arg1 (Figure 6J and K). Levels of CD86 were decreased, while levels of CD206 were increased in DP mice following OGT interference (Figure 6L-N). Collectively, these data suggested that DP induced alveolar bone loss, possibly through promoting M1 polarization and inhibiting M2 polarization.
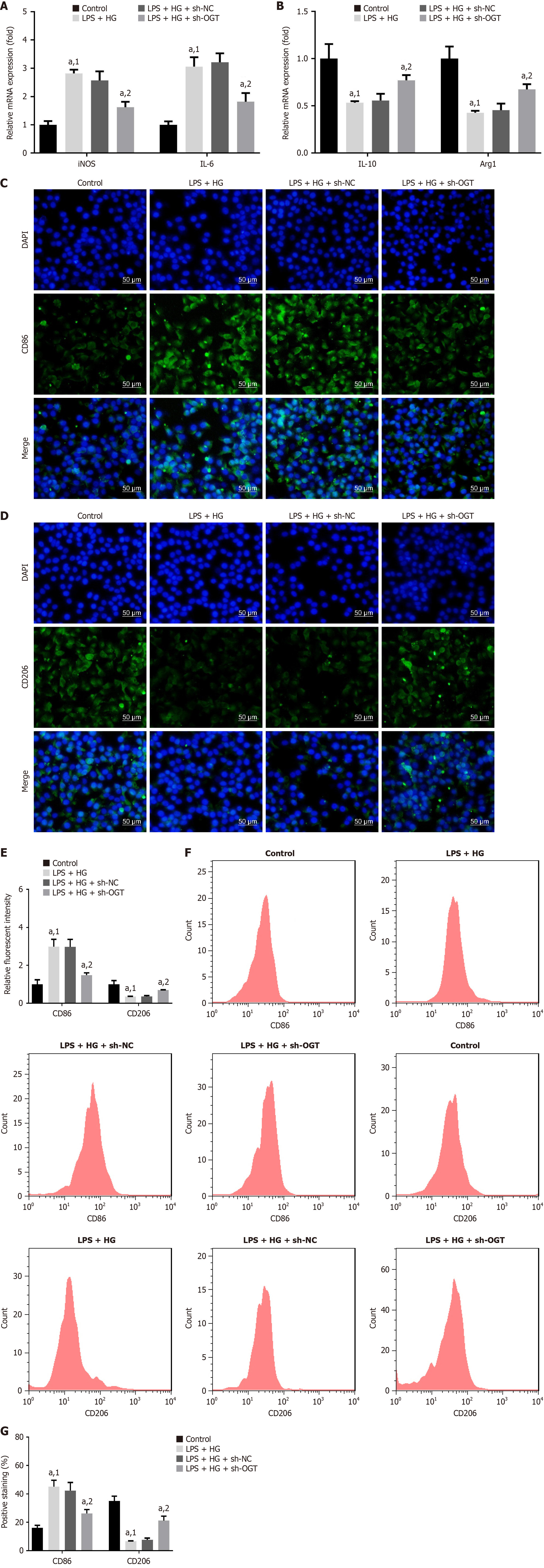
We further investigated the effect of OGT on macrophage polarization in vitro. Compared to sh-NC, silencing of OGT significantly downregulated the levels of iNOS, IL-6, and CD86 and upregulated the levels of IL-10, Arg1, and CD206 (Figure 7). These results demonstrated that OGT knockdown inhibited M1-type polarization and promotes M2-type polarization, thereby contributing to alveolar bone rebuilding and attenuation of DP.
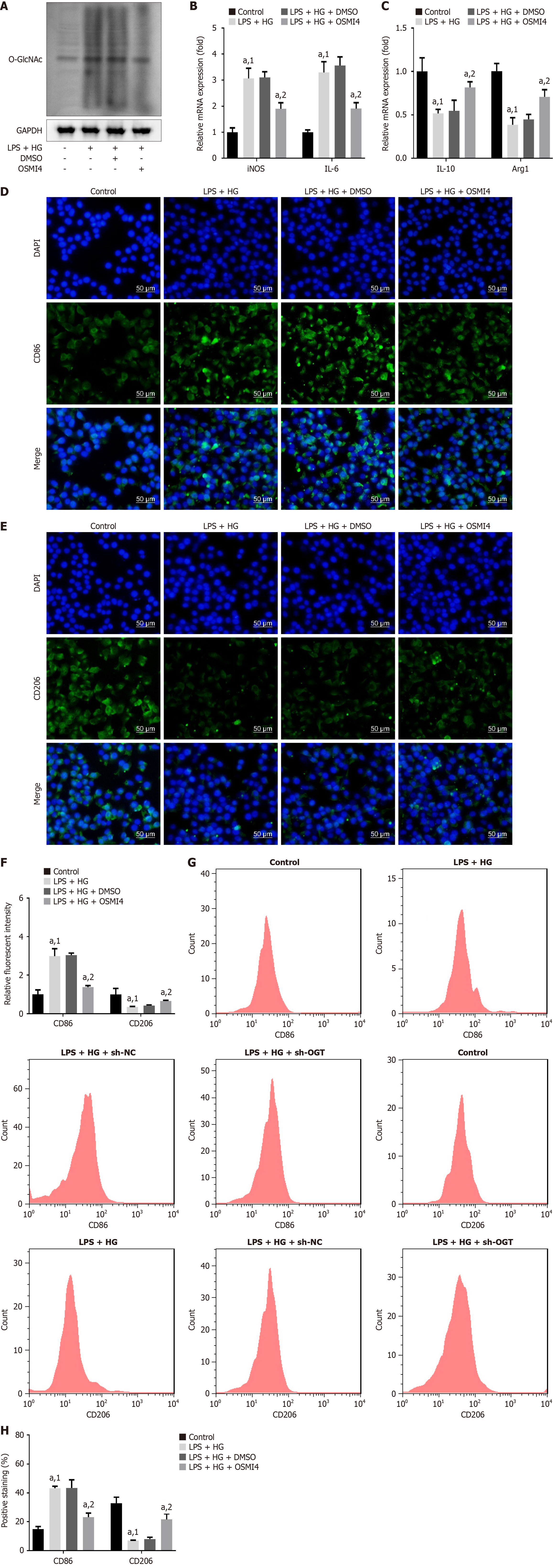
To further confirm the effect of O-GlcNAcylation on macrophage polarization, we utilized the OGT inhibitor OSMI4 to inhibit O-GlcNAcylation in macrophages. As anticipated, O-GlcNAcylation levels were significantly reduced after OSMI4 treatment (Figure 8A). In the LPS + HG cell model, iNOS, IL-6, and CD86 levels were significantly upregulated, while IL-10, Arg1, and CD206 levels were downregulated compared to the control (Figure 8B-H). Importantly, treatment with OSMI4 reversed these alterations, reducing the levels of iNOS, IL-6, and CD86 and increasing the levels of IL-10, Arg1, and CD206 under LPS and HG administration (Figure 8B-H).
Collectively, these findings indicated that OSMI4 promoted the shift of M1 macrophages toward an M2 phenotype by inhibiting O-GlcNAcylation.
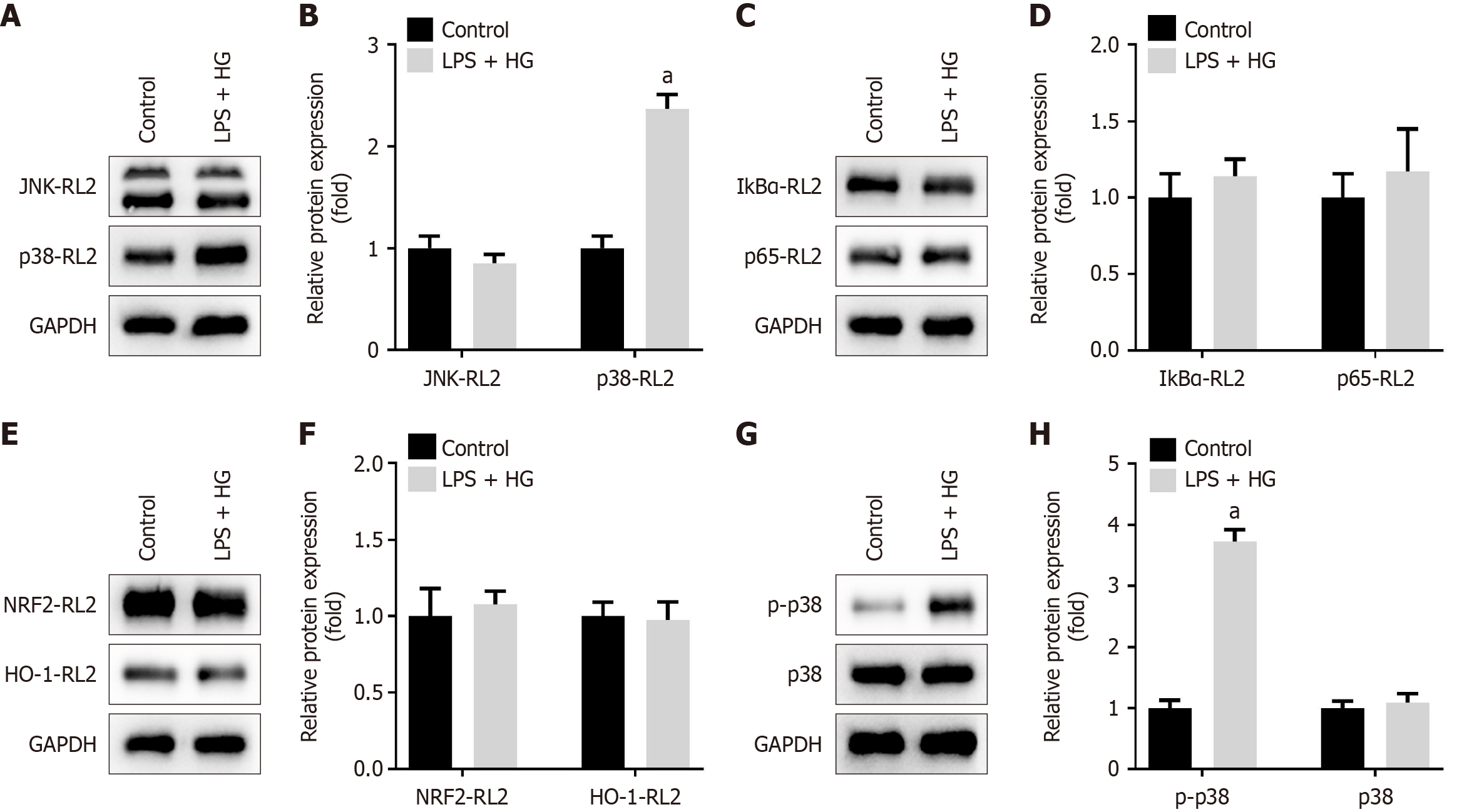
To identify the O-GlcNAcylated substrate proteins mediated by OGT, we examined key pathways involved in inflammation in macrophages, specifically the MAPK, nuclear factor kappa-B, and NRF2 pathways[27]. We measured the O-GlcNAcylation levels of factors within these pathways. As shown in Figure 9A-F, the O-GlcNAcylation levels of p38 were significantly increased in RAW264.7 cells treated with LPS and HG; however, the O-GlcNAcylation levels of JNK, IκBα, p65, NRF2, and HO-1 remained unchanged.
Given these findings, we focused on the p38/MAPK pathway. Our results indicated that LPS combined with HG significantly increased the phosphorylation levels of p38 without affecting the total protein levels of p38 (Figure 9G and H). In summary, LPS enhanced the O-GlcNAcylation of p38 under HG conditions, leading to increased phosphorylation levels and activation of the p38/MAPK pathway.
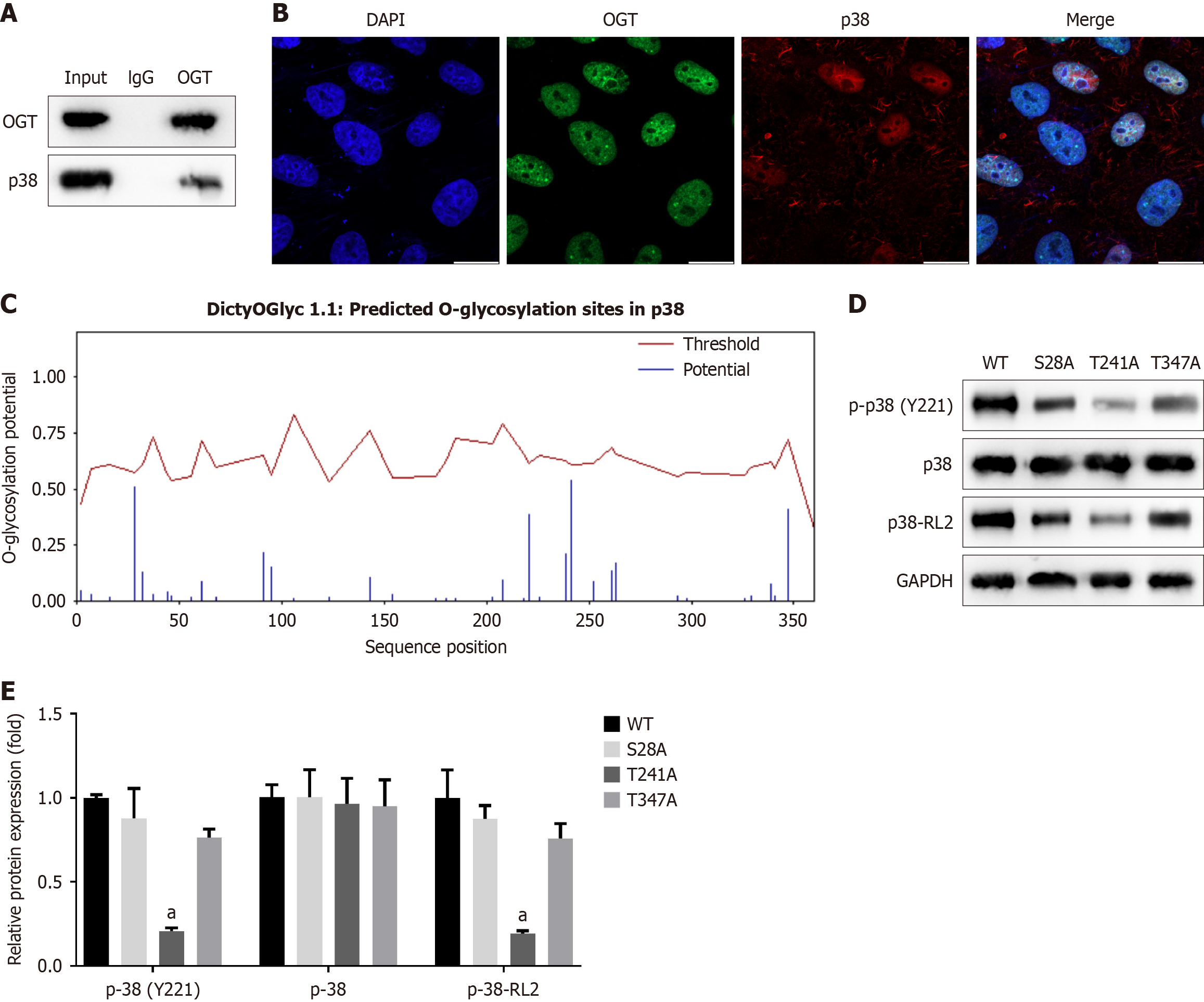
To further investigate the relationship between OGT and p38, we performed IP using an OGT antibody rather than IgG, indicating that OGT interacts with p38 (Figure 10A). Additionally, OGT and p38 exhibited partial colocalization in the cytoplasm (Figure 10B).
We then predicted potential O-GlcNAcylation sites on p38 and identified several candidate sites (Figure 10C). To determine which specific sites were O-GlcNAcylated, we constructed mutants targeting the three most likely sites: S28A, T241A, and T347A. Compared to the WT plasmid, each of these mutants S28A, T241A, and T347A significantly reduced the O-GlcNAcylation levels of p38 and correspondingly decreased the phosphorylation of p38 (Y221) (Figure 10D and E).
Taken together, these results suggest that the O-GlcNAcylation of p38 at residues S28, T241, and T347 promoted its phosphorylation at Y221, thereby activating the p38 pathway.
In the present study, we identified that OGT-mediated O-GlcNAcylation and phosphorylation of p38 contribute to alveolar bone destruction in DP by regulating macrophage polarization, thus revealing a novel pathological mechanism in DP.
Macrophages are known to polarize into M1 and M2 phenotypes. M1-polarized macrophages induce iNOS to produce nitric oxide, upregulate the inflammatory marker CD86, and secrete pro-inflammatory cytokines such as IL-6, IL-1β, IL-12, tumor necrosis factor-α, and IL-23[28,29]. In contrast, M2-polarized macrophages express markers such as CD36, CD163, and CD206, produce anti-inflammatory cytokines like IL-10 and transforming growth factor-β, and are associated with the metabolic enzyme Arg1[30]. A shift from an M2 to an M1-dominant state promotes insulin resistance[31]. Inhibition of inflammation by promoting M2 polarization may alleviate the progression of diabetes and its complications[32-34].
Accumulating evidence suggests that macrophage polarization influences bone regeneration and healing, particularly under HG conditions[35,36]. Therefore, we focused on the role of macrophage polarization in DP. The results of this study indicated that increased M1-type polarization and suppressed M2-type polarization are associated with alveolar bone loss, findings that align with previous research[12,37].
Subsequently, we observed that O-GlcNAcylation levels were elevated in DP, accompanied by increased OGT levels. Inhibition or downregulation of OGT promoted a shift from M1 to M2 macrophage polarization, thereby alleviating alveolar bone destruction. This suggested an important role for OGT-catalyzed O-GlcNAcylation in DP pathogenesis.
It is well established that excessive glucose alters intracellular O-GlcNAcylation levels through the hexosamine biosynthetic pathway. HG leads to increased O-GlcNAcylated proteins, inhibiting osteogenic differentiation and demonstrating that O-GlcNAcylation adversely affects bone health[38]. In mouse models of periodontitis, O-GlcNAcylation promotes osteoclast differentiation, and OGT deficiency increases bone density[39]. Therefore, inhibiting protein O-GlcNAcylation may represent a potential therapeutic strategy for treating DP.
To identify the proteins modified by O-GlcNAcylation, we further investigated the interaction between OGT and p38. Our findings indicated that OGT interacts with p38, and that p38 was O-GlcNAcylated at residues S28, T241, and T347 and phosphorylated at Y221 in the DP cell model. This suggests that O-GlcNAcylation of p38 activates the p38/MAPK pathway.
Previous studies have reported that the p38/MAPK pathway affects neutrophil apoptosis in periodontitis with diabetes[40,41]. P38 can be rapidly phosphorylated on serine and tyrosine residues under the stimulation of LPS, which regulates cell survival, proliferation, and differentiation[42]. O-GlcNAcylation can extensively interact with phosphorylation since these modifications often target the same amino acid residues within proteins[43]. In our study, the O-GlcNAcylation and phosphorylation sites in p38 were distinct, suggesting that these two modifications do not compete but may represent independent events. However, this hypothesis requires further exploration in future studies.
In conclusion, OGT promotes the O-GlcNAcylation of p38 at residues S28, T241, and T347, further activating the p38/MAPK pathway through its own phosphorylation at Y221. Inhibition of O-GlcNAcylation promotes M2-type polarization and inhibits M1-type polarization of macrophages, thereby accelerating alveolar bone rebuilding and decelerating the progression of DP.
| 1. | Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 1084] [Article Influence: 180.7] [Reference Citation Analysis (0)] |
| 2. | Nibali L, Gkranias N, Mainas G, Di Pino A. Periodontitis and implant complications in diabetes. Periodontol 2000. 2022;90:88-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 3. | Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, Taylor R. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 782] [Cited by in RCA: 1104] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 4. | Graves DT, Ding Z, Yang Y. The impact of diabetes on periodontal diseases. Periodontol 2000. 2020;82:214-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 5. | Polak D, Shapira L. An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. 2018;45:150-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 6. | Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 691] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 7. | Chen YN, Hu MR, Lei W, Chen WD. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 1722] [Article Influence: 287.0] [Reference Citation Analysis (0)] |
| 8. | Sun X, Li Y, Deng Q, Hu Y, Dong J, Wang W, Wang Y, Li C. Macrophage Polarization, Metabolic Reprogramming, and Inflammatory Effects in Ischemic Heart Disease. Front Immunol. 2022;13:934040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 9. | Cutolo M, Campitiello R, Gotelli E, Soldano S. The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis. Front Immunol. 2022;13:867260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 429] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 10. | Boutilier AJ, Elsawa SF. Macrophage Polarization States in the Tumor Microenvironment. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 1187] [Article Influence: 237.4] [Reference Citation Analysis (0)] |
| 11. | Almubarak A, Tanagala KKK, Papapanou PN, Lalla E, Momen-Heravi F. Disruption of Monocyte and Macrophage Homeostasis in Periodontitis. Front Immunol. 2020;11:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 12. | Zhang B, Yang Y, Yi J, Zhao Z, Ye R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J Periodontal Res. 2021;56:991-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 13. | Dassanayaka S, Readnower RD, Salabei JK, Long BW, Aird AL, Zheng YT, Muthusamy S, Facundo HT, Hill BG, Jones SP. High glucose induces mitochondrial dysfunction independently of protein O-GlcNAcylation. Biochem J. 2015;467:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Chang YH, Weng CL, Lin KI. O-GlcNAcylation and its role in the immune system. J Biomed Sci. 2020;27:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 15. | Nie H, Yi W. O-GlcNAcylation, a sweet link to the pathology of diseases. J Zhejiang Univ Sci B. 2019;20:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Li Y, Xie M, Men L, Du J. O-GlcNAcylation in immunity and inflammation: An intricate system (Review). Int J Mol Med. 2019;44:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Yang H, Xiao L, Wu D, Zhang T, Ge P. O-GlcNAcylation of NLRP3 Contributes to Lipopolysaccharide-Induced Pyroptosis of Human Gingival Fibroblasts. Mol Biotechnol. 2024;66:2023-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 18. | Rodrigues Mantuano N, Stanczak MA, Oliveira IA, Kirchhammer N, Filardy AA, Monaco G, Santos RC, Fonseca AC, Fontes M, Bastos CS Jr, Dias WB, Zippelius A, Todeschini AR, Läubli H. Hyperglycemia Enhances Cancer Immune Evasion by Inducing Alternative Macrophage Polarization through Increased O-GlcNAcylation. Cancer Immunol Res. 2020;8:1262-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Martínez-Limón A, Joaquin M, Caballero M, Posas F, de Nadal E. The p38 Pathway: From Biology to Cancer Therapy. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 303] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 20. | Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15:369-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 523] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 21. | Liu J, Wei Y, Jia W, Can C, Wang R, Yang X, Gu C, Liu F, Ji C, Ma D. Chenodeoxycholic acid suppresses AML progression through promoting lipid peroxidation via ROS/p38 MAPK/DGAT1 pathway and inhibiting M2 macrophage polarization. Redox Biol. 2022;56:102452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 102] [Reference Citation Analysis (0)] |
| 22. | Xu Y, Zhang Y, Xu Y, Zang G, Li B, Xia H, Yuan W. Activation of CD137 signaling promotes macrophage apoptosis dependent on p38 MAPK pathway-mediated mitochondrial fission. Int J Biochem Cell Biol. 2021;136:106003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Wang J, Li H, Li B, Gong Q, Chen X, Wang Q. Co-culture of bone marrow stem cells and macrophages indicates intermediate mechanism between local inflammation and innate immune system in diabetic periodontitis. Exp Ther Med. 2016;12:567-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Chen K, Yu B, Liao J. LncRNA SOX2OT alleviates mesangial cell proliferation and fibrosis in diabetic nephropathy via Akt/mTOR-mediated autophagy. Mol Med. 2021;27:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Gao R, Zhang W, Jiang Y, Zhai J, Yu J, Liu H, Li M. Eldecalcitol effectively prevents alveolar bone loss by partially improving Th17/Treg cell balance in diabetes-associated periodontitis. Front Bioeng Biotechnol. 2023;11:1070117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 26. | Sun C, Shang J, Yao Y, Yin X, Liu M, Liu H, Zhou Y. O-GlcNAcylation: a bridge between glucose and cell differentiation. J Cell Mol Med. 2016;20:769-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Li ST, Dai Q, Zhang SX, Liu YJ, Yu QQ, Tan F, Lu SH, Wang Q, Chen JW, Huang HQ, Liu PQ, Li M. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-κB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol Sin. 2018;39:1294-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 28. | Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front Immunol. 2019;10:1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 765] [Cited by in RCA: 1522] [Article Influence: 217.4] [Reference Citation Analysis (0)] |
| 29. | Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425-6440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 3659] [Article Influence: 457.4] [Reference Citation Analysis (0)] |
| 30. | Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. The Metabolic Signature of Macrophage Responses. Front Immunol. 2019;10:1462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 553] [Cited by in RCA: 1496] [Article Influence: 213.7] [Reference Citation Analysis (0)] |
| 31. | Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 2174] [Article Influence: 135.9] [Reference Citation Analysis (1)] |
| 32. | Liu J, Zhang Y, Sheng H, Liang C, Liu H, Moran Guerrero JA, Lu Z, Mao W, Dai Z, Liu X, Zhang L. Hyperoside Suppresses Renal Inflammation by Regulating Macrophage Polarization in Mice With Type 2 Diabetes Mellitus. Front Immunol. 2021;12:733808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 33. | Louiselle AE, Niemiec SM, Zgheib C, Liechty KW. Macrophage polarization and diabetic wound healing. Transl Res. 2021;236:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 446] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 34. | Zhang Y, Le X, Zheng S, Zhang K, He J, Liu M, Tu C, Rao W, Du H, Ouyang Y, Li C, Wu D. MicroRNA-146a-5p-modified human umbilical cord mesenchymal stem cells enhance protection against diabetic nephropathy in rats through facilitating M2 macrophage polarization. Stem Cell Res Ther. 2022;13:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 35. | Wu Z, Bai J, Ge G, Wang T, Feng S, Ma Q, Liang X, Li W, Zhang W, Xu Y, Guo K, Cui W, Zha G, Geng D. Regulating Macrophage Polarization in High Glucose Microenvironment Using Lithium-Modified Bioglass-Hydrogel for Diabetic Bone Regeneration. Adv Healthc Mater. 2022;11:e2200298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 36. | Shen X, Shen X, Li B, Zhu W, Fu Y, Xu R, Du Y, Cheng J, Jiang H. Abnormal macrophage polarization impedes the healing of diabetes-associated tooth sockets. Bone. 2021;143:115618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Li J, Liu Y, Lai W, Song L, Deng J, Li C, Jiang S. MicroRNA-126 regulates macrophage polarization to prevent the resorption of alveolar bone in diabetic periodontitis. Arch Oral Biol. 2023;150:105686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 38. | Gu H, Song M, Boonanantanasarn K, Baek K, Woo KM, Ryoo HM, Baek JH. Conditions Inducing Excessive O-GlcNAcylation Inhibit BMP2-Induced Osteogenic Differentiation of C2C12 Cells. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Taira TM, Ramos-Junior ES, Melo PH, Costa-Silva CC, Alteen MG, Vocadlo DJ, Dias WB, Cunha FQ, Alves-Filho JC, Søe K, Fukada SY. HBP/O-GlcNAcylation Metabolic Axis Regulates Bone Resorption Outcome. J Dent Res. 2023;102:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 40. | Kido R, Hiroshima Y, Kido JI, Ikuta T, Sakamoto E, Inagaki Y, Naruishi K, Yumoto H. Advanced glycation end-products increase lipocalin 2 expression in human oral epithelial cells. J Periodontal Res. 2020;55:539-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Tang Y, Liu J, Yan Y, Fang H, Guo C, Xie R, Liu Q. 1,25-dihydroxyvitamin-D3 promotes neutrophil apoptosis in periodontitis with type 2 diabetes mellitus patients via the p38/MAPK pathway. Medicine (Baltimore). 2018;97:e13903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1330] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 43. | van der Laarse SAM, Leney AC, Heck AJR. Crosstalk between phosphorylation and O-GlcNAcylation: friend or foe. FEBS J. 2018;285:3152-3167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/