Published online Mar 15, 2025. doi: 10.4239/wjd.v16.i3.100580
Revised: November 26, 2024
Accepted: December 27, 2024
Published online: March 15, 2025
Processing time: 153 Days and 22.6 Hours
Autonomous cortisol secretion (ACS) is linked to a higher prevalence of metabolic abnormalities and an increased risk of major adverse cardiovascular events.
To evaluate glucose and bone metabolism in patients with ACS using a conti
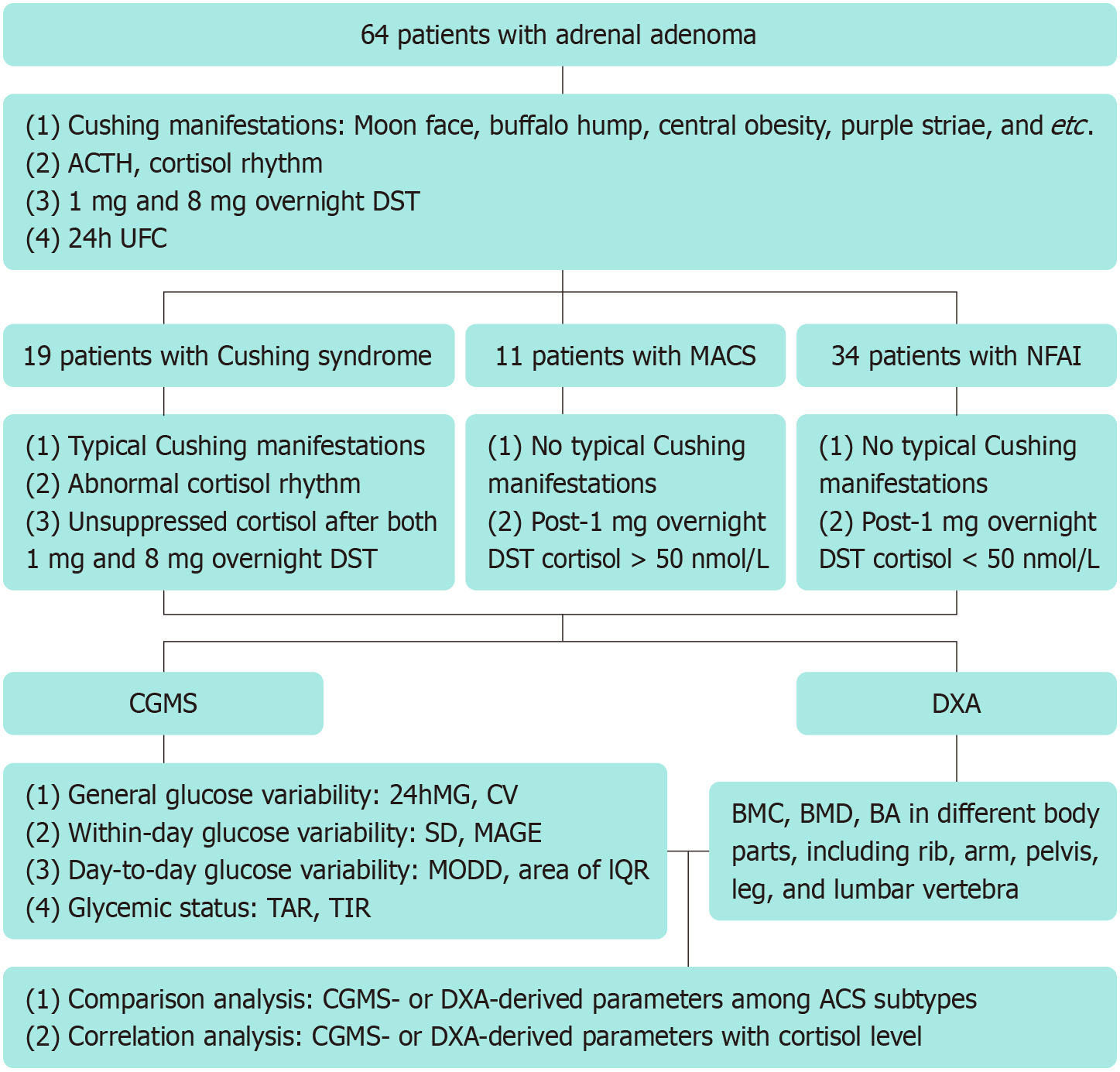
Patients diagnosed with ACS, including Cushing syndrome, mild ACS (MACS), and nonfunctional adrenal incidentaloma (NFAI), were recruited for this study. Glucose variability and glycemic status were assessed using CGMS. Regional bone mineral content (BMC), bone mineral density (BMD), and bone area (BA) were evaluated using DXA. CGMS- and DXA-derived parameters were compared across the subgroups of ACS. Correlation analysis was performed to examine relationships between varying degrees of cortisol secretion, measured by cortisol after 1 mg overnight dexamethasone suppression test (DST) or 24-hour urine free cortisol (24h UFC), and CGMS- or DXA-derived parameters.
A total of 64 patients with ACS were included in this study: 19 with Cushing syndrome, 11 with MACS, and 34 with NFAI. Glucose variability, time above range (TAR), and time in range (TIR) along with specific areal BMC, BMD, and BA, differed significantly between groups of Cushing syndrome and NFAI. A significant positive correlation was observed between glucose variability or TAR and cortisol after 1 mg overnight DST or 24h UFC. By contrast, TIR, along with regional BMC, BMD, and BA, were negatively correlated with varying degrees of cortisol secretion.
Glucose and bone metabolism impairments are on a continuum alteration from NFAI to MACS and Cushing syndrome. Prompt attention should be given to these patients with ACS, especially those with mild hormone secretion. Parameters of glucose variability and glycemic status along with bone condition in regions rich in cancellous bone will provide valuable information.
Core Tip: Many years elapsed before glucose and bone metabolic disorders in autonomous cortisol secretion (ACS) were studied in clinical and experimental studies. The current study is moving the field forward, highlighting a continuum of glucose and bone metabolism impairment from nonfunctional adrenal incidentaloma to mild ACS and Cushing syndrome. Prompt attention should be given to these patients with ACS, especially those with mild hormone secretion. Given that ACS has a great impact on bone and glucose metabolic disorders with ensuing cardiovascular diseases and fracture, a multidisciplinary effort is needed to choose a patient-tailored treatment and foster the strategies against adverse outcomes.
- Citation: Han MM, Cao XM, Liu ZA, Zhang Y, Liu YF. Continuum of glucose and bone metabolism impairment across autonomous cortisol secretion: A cross-sectional study. World J Diabetes 2025; 16(3): 100580
- URL: https://www.wjgnet.com/1948-9358/full/v16/i3/100580.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i3.100580
Vital human activities are exquisitely regulated through the collaboration of multiple systems in the body, wherein the endocrine system plays a pivotal role in maintaining homeostasis, particularly in metabolism, water balance, and electrolyte regulation. The hypothalamus-pituitary-adrenal axis, which regulates rhythmic cortisol secretion, is fundamental to these processes. Autonomous cortisol secretion (ACS), usually caused by adrenal adenoma, tips the balance of the hypothalamus-pituitary-adrenal axis, resulting in metabolic disorders and increasing the risks of cardiovascular events[1-3].
According to the guidelines, 1 mg overnight dexamethasone suppression test (DST) is the gold standard for diagnosing ACS. Cushing syndrome, a form of ACS, typically presents with Cushing manifestations, such as moon face, buffalo bump, central obesity, and hirsutism[4]. Mild ACS (MACS) is diagnosed based on morning cortisol exceeding 50 nmol/L after 1 mg overnight DST and lack of classical Cushing features[5]. Patients with adrenal adenomas who have morning cortisol level below 50 nmol/L after 1 mg overnight DST were categorized as nonfunctional adrenal incidentaloma (NFAI). The data accumulated to date suggest that subtle, undetectable, and intermittent ACS may also occur in patients with NFAI[6,7].
Multiple lines of evidence have demonstrated that ACS is involved in the occurrence and progression of glucose and bone metabolic disorders. The data collected in the literature on this issue suggest that prolonged exposure to cortisol excess leads to increased prevalence of diabetes and osteoporosis, which in turn increases the risk of cardiovascular event and mortality in these patients[8-10]. It is worth mentioning that removing ACS through adrenolectomy achieves amelioration of either glucose or bone metabolism[11,12].
Generally, bone remodeling occurs in an exquisite equilibrium, balancing bone formation and bone resorption. ACS breaks this balance, giving rise to decreased bone formation and increased bone resorption. Disorders of bone remodeling commonly manifest as decreased bone mineral density (BMD) or bone mineral content (BMC). Another indication is decreased bone area (BA), reflecting compromised periosteal bone formation and appositional growth[13]. Several converging lines of data suggest reduction of regional BMC, BMD, and BA in patients with Cushing syndrome compared to matched healthy controls as measured by dual-energy X-ray absorptiometry (DXA; Hologic device Discovery® QDR Series, Waltham, MA, United States)[13-17]. DXA was commonly used in clinical practice, providing an accurate assessment of regional BMC, BMD, and BA.
Over the past few decades, advancements in glucose monitoring by wireless method, sensor techniques, and interstitial glucose measurements have enabled the field moving towards continuous glucose monitoring. Continuous glucose monitoring system (CGMS), a risk-free and efficient method, provides a comprehensive 24-hour glucose profile, surpassing the limited scope of traditional capillary glucose testing or glycosylated hemoglobin (HbA1c), which offers only a general assessment of glycemic condition[18]. Preliminary studies conducted by our team have explored glucose variability and glycemic status in patients with adrenal adenomas using CGMS, revealing increased glucose variability and decreased time in range (TIR)[19].
Based on the accurate assessment of glucose and bone metabolism by CGMS and DXA, this study evaluated the metabolic profile across ACS subtypes. Additionally, the correlation between metabolic parameters and cortisol levels was evaluated to provide a basis for expeditious abnormalities detection and early clinical intervention.
This study was conducted at the First Hospital of Shanxi Medical University (Shanxi, China) between December 2018 and January 2024. The study protocol was approved by the ethics committee at the First Hospital of Shanxi Medical University and written conformed consent was obtained from all recruited patients (2018K006).
Patients diagnosed with Cushing syndrome, ACS, or NFAI were recruited for the study. It was recommended that all participants undergo adrenal function assessments. According to the recent Endocrine Society guidelines[4,5], Cushing syndrome was diagnosed based on the following criteria: Typical Cushing manifestations, elevated midnight cortisol, suppressed adrenocorticotropic hormone, increased 24h UFC, and unsuppressed cortisol after both 1 mg overnight DST and 8 mg overnight DST. MACS was diagnosed by cortisol > 50 nmol/L after 1 mg overnight DST without classical Cushing features. NFAI was diagnosed by a post-1 mg overnight DST cortisol < 50 nmol/L.
The exclusion criteria were as follows: A history of exogenous glucocorticoids exposure; administration of medications or presence of diseases affecting the hypothalamus-pituitary-adrenal axis, glucose metabolism, or bone metabolism; adrenal carcinoma; autonomous aldosterone or catecholamine secretion; and severe comorbidities that renders patients unable to participate in the study.
Glucose profile was assessed using CGMS in all recruited patients prior to operation or medication therapy. The sensor was placed on the radial side of the upper arm. Patients were instructed to scan for a real-time glucose value every six to eight hours to ensure data integrity.
Based on CGMS monitoring data, indicators of glucose variability and glycemic status were calculated with reference to our previously published article[19]. Sensor glucose values within the range of 3.9-7.8 mmol/L are considered normal. Glucose variability is classified into three categories: General glucose variability, within-day glucose variability, and day-to-day glucose variability. Glycemic status is assessed by time above range (TAR) and TIR. Specifically, general glucose variability includes 24-hour mean glucose (24hMG) and coefficient of variation (CV). Within-day glucose variability consists of standard deviation of sensor glucose (SD) and mean amplitude of glucose excursion (MAGE). Day-to-day glucose variability comprises mean of daily difference (MODD) and area of interquartile range (IQR). TAR is defined as the duration during which sensor glucose values > 7.8 mmol/L throughout the day. TIR is defined as the duration during which sensor glucose values falls within 3.9-7.8 mmol/L throughout the day.
Bone condition was assessed in all patients using DXA prior to operation or medication treatment. On the day of examination, the patients were instructed to wear suitable, loose hospital clothing and to avoid wearing any ornaments containing metal. There was one technician in charge of examination, who was blinded to patients’ information. DXA provided information of areal BMC, BMD, and BA from various parts of the body, including head, arms, rib, pelvis, legs, and lumbar vertebrae (L).
A schematic diagram of study design and data analysis is shown in Figure 1. Parameters derived from CGMS and DXA were compared among groups of Cushing syndrome, MACS, and NFAI. Additional, correlation between CGMS- or DXA-derived parameters and cortisol after 1 mg overnight DST or 24h UFC were analyzed using appropriate statistical methods.
For CGMS data, the Kruskal-Wallis rank sum test with Dunn’s test was responsible for comparison analysis, while Spearman’s correlation analysis was used for assessing the relationship between variables. For DXA data, comparisons were conducted using analysis of covariance with Bonferroni method, and partial correlation analysis was performed after adjusting for sex, age, and body mass index (BMI).
SPSS version 19.0 (SPSS Inc., Chicago, IL, United States) and R software version 4.3.3 (R Foundation for Statistical Computing, Vienna, Austria) were applied for data analyses and graph plotting. P < 0.05 was considered statistically significant. Correlation coefficient, denoted as r, was interpreted as follows: r < 0 indicated a negative correlation, while r > 0 indicated a positive correlation.
Sixty-four patients were included in this study, including nineteen patients with Cushing syndrome, eleven with MACS, and thirty-four with NFAI. The recruited patients across the different subtypes of ACS were matched for age and sex. The clinical and hormonal profiles in groups of Cushing syndrome, MACS, and NFAI are shown in Table 1.
| Parameters | Cushing syndrome | MACS | NAFI | Normal range |
| Number of cases | 19 | 11 | 34 | - |
| Sex (male:female) | 10:9 | 6:5 | 17:17 | - |
| Age (years old) | 54.48 ± 11.86 | 56.72 ± 8.29 | 53.26 ± 11.39 | - |
| BMI (kg/m2) | 25.63 ± 3.05 | 27.93 ± 2.51 | 27.07 ± 5.65 | - |
| ACTH (pmol/L) | 0.61 ± 0.37 | 5.09 ± 1.42 | 6.20 ± 2.09 | 1.6-13.9 |
| Cortisol at 8 am (nmol/L) | 582.14 ± 206.59 | 347.83 ± 96.07 | 335.40 ± 119.13 | 171-536 |
| Cortisol at 4 pm (nmol/L) | 575.61 ± 223.27 | 212.33 ± 59.95 | 210.54 ± 96.75 | 64-327 |
| Cortisol at 0 am (nmol/L) | 550.95 ± 207.78 | 135.95 ± 42.71 | 121.81 ± 49.57 | - |
| 24h UFC (nmol/24 h) | 942.47 ± 210.76 | 282.12 ± 95.27 | 186.54 ± 56.69 | 100-279 |
| ACTH after 1 mg overnight DST (pmol/L) | 0.52 (0.22, 0.74) | 1.09 (0.44, 1.20) | 1.12 (0.79, 1.29) | |
| Cortisol after 1 mg overnight DST (nmol/L) | 595.47 ± 209.973 | 84.21 ± 26.46 | 25.94 ± 8.41 | - |
| ACTH after 8 mg overnight DST (pmol/L) | 0.4 (0.22, 0.66) | - | - | |
| Cortisol after 8 mg overnight DST (nmol/L) | 598.29 ± 207.99 | - | - | - |
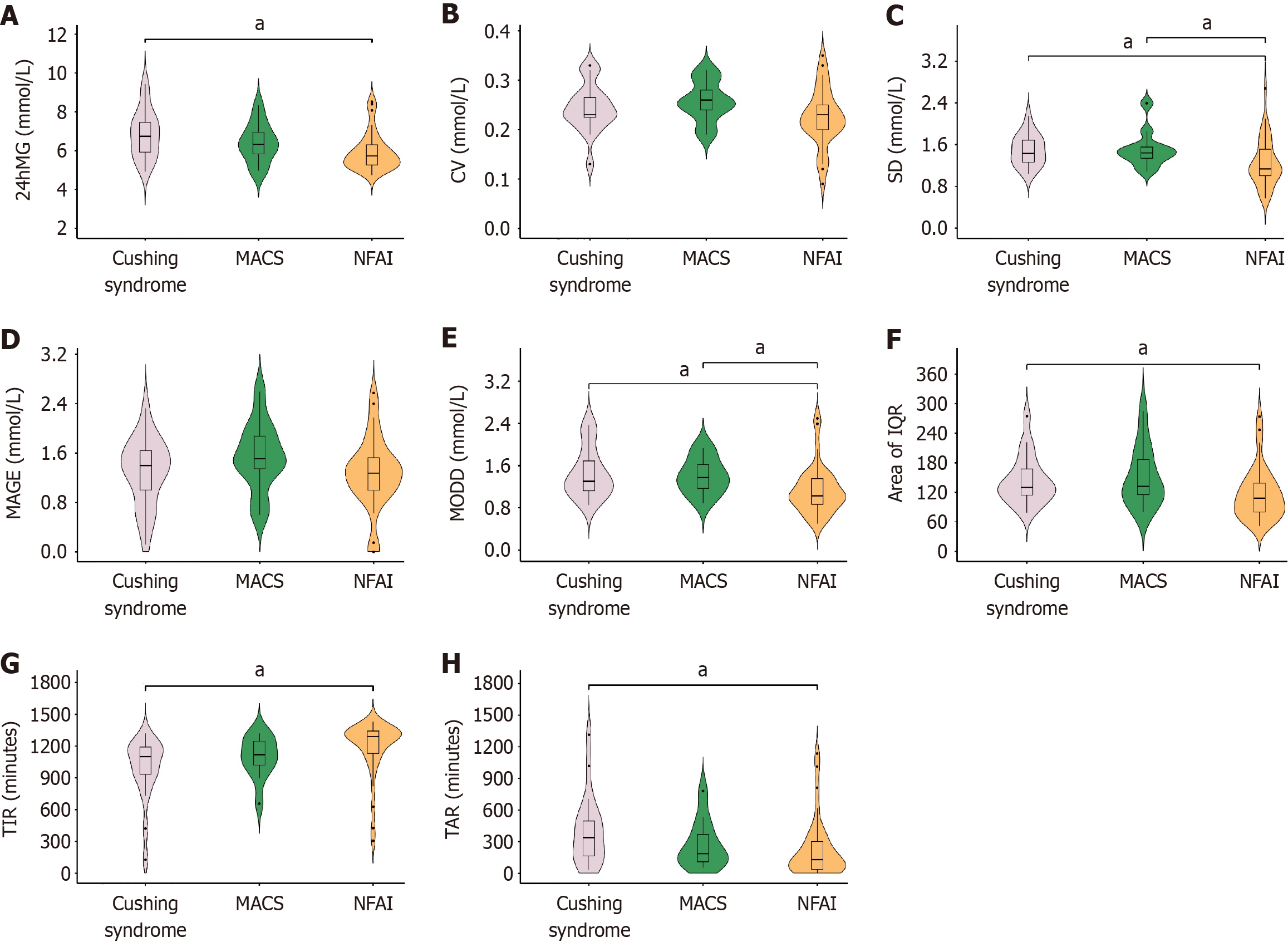
CGMS-derived parameters: The parameters of glucose variability and glycemic status differed significantly among groups of Cushing syndrome, MACS, and NFAI (Figure 2). Specifically, for general glucose variability, 24hMG was significantly different between the groups of Cushing syndrome and NFAI. For within-day glucose variability, SD showed significant difference between groups of Cushing syndrome and NFAI, as well as between groups of MACS and NFAI. For day-to-day glucose variability, both MODD and area of IQR were significantly different between groups of Cushing syndrome and NFAI, while MODD also showed significant difference between groups of MACS and NFAI. For glycemic status, significant differences in TAR and TIR were observed between groups of Cushing syndrome and NFAI. There is no parameter showing significant difference between groups of Cushing syndrome and MACS (Figure 2).
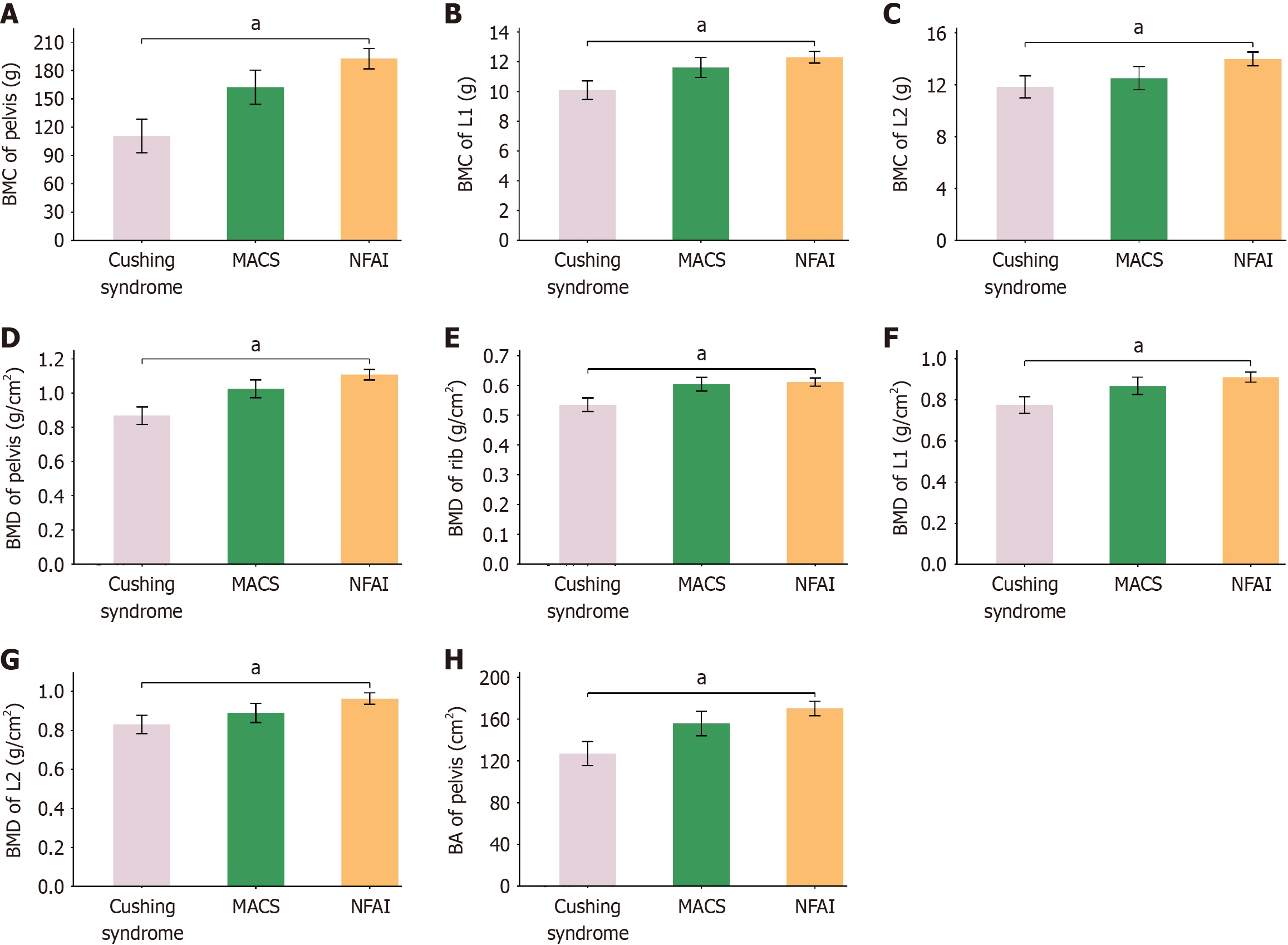
DXA-derived parameters: BMC, BMD, and BA of specific regions were significantly different between groups of Cushing syndrome and NFAI (Figure 3). To be specific, for BMC, significant difference was observed in pelvis, L1, and L2; for BMD, significant difference was found in rib, pelvis, L1, and L2; and for BA, significant difference was identified in pelvis. No significant difference in BMC, BMD, or BA was observed in other regions of the body (Supplementary Table 1). There were no parameters with significance when compared between groups of Cushing syndrome and MACS, or MACS and NFAI (Figure 3 and Supplementary Table 1).
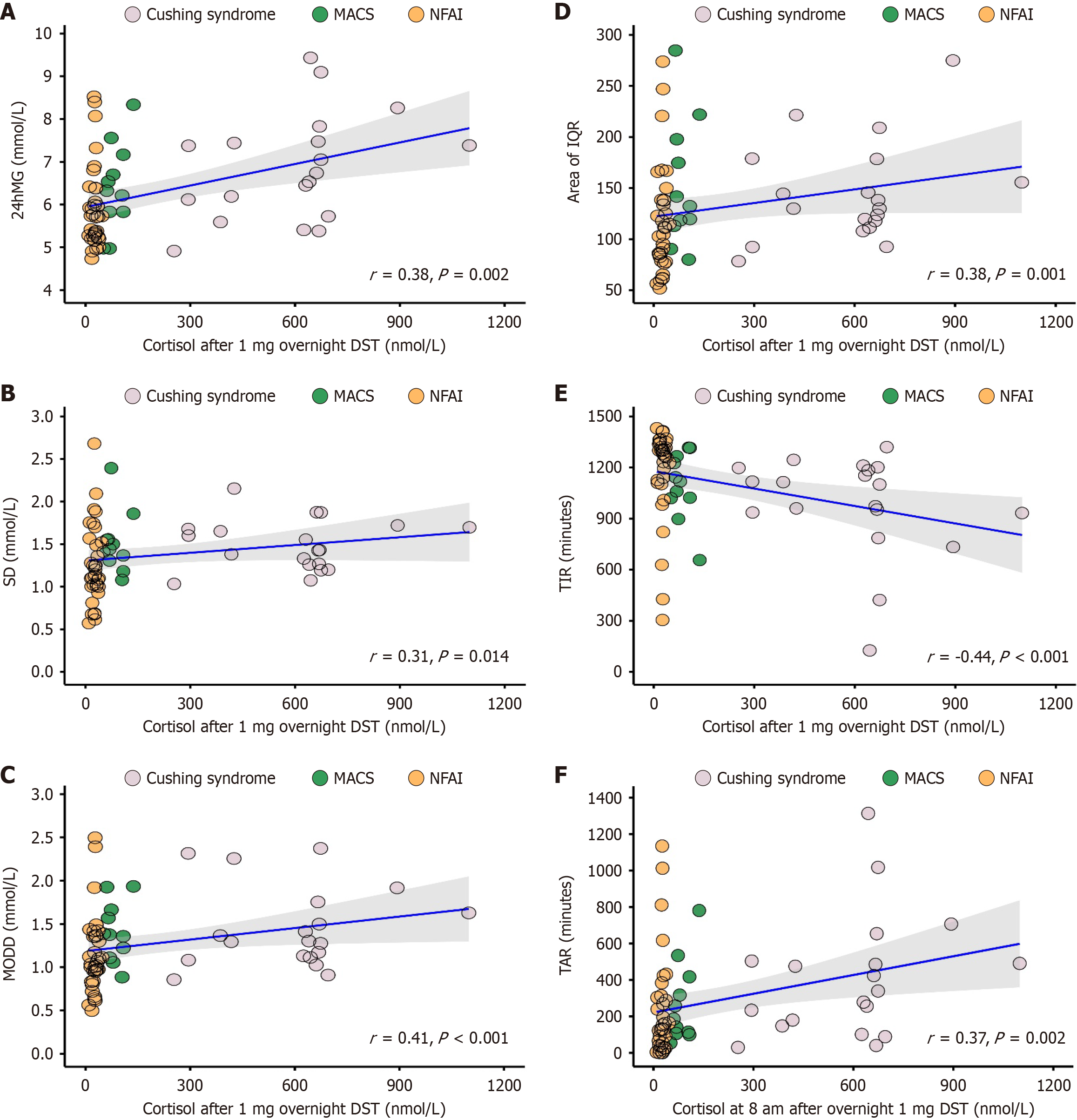
Cortisol after 1 mg overnight DST and parameters derived from CGMS or DXA: Based on CGMS data, significant correlation was identified in parameters of glucose variability and glycemic status with cortisol after 1 mg overnight DST (Figure 4). Wherein, positive correlation was identified in parameters of 24hMG (r = 0.38, P = 0.002), SD (r = 0.31, P = 0.014), MODD (r = 0.41, P < 0.001), area of IQR (r = 0.38, P = 0.001), and TAR (r = 0.37, P = 0.002), along with a negative correlation in TIR (r = -0.44, P < 0.001). However, CV and MAGE showed no significant correlation with cortisol after 1 mg overnight DST (Supplementary Table 2).
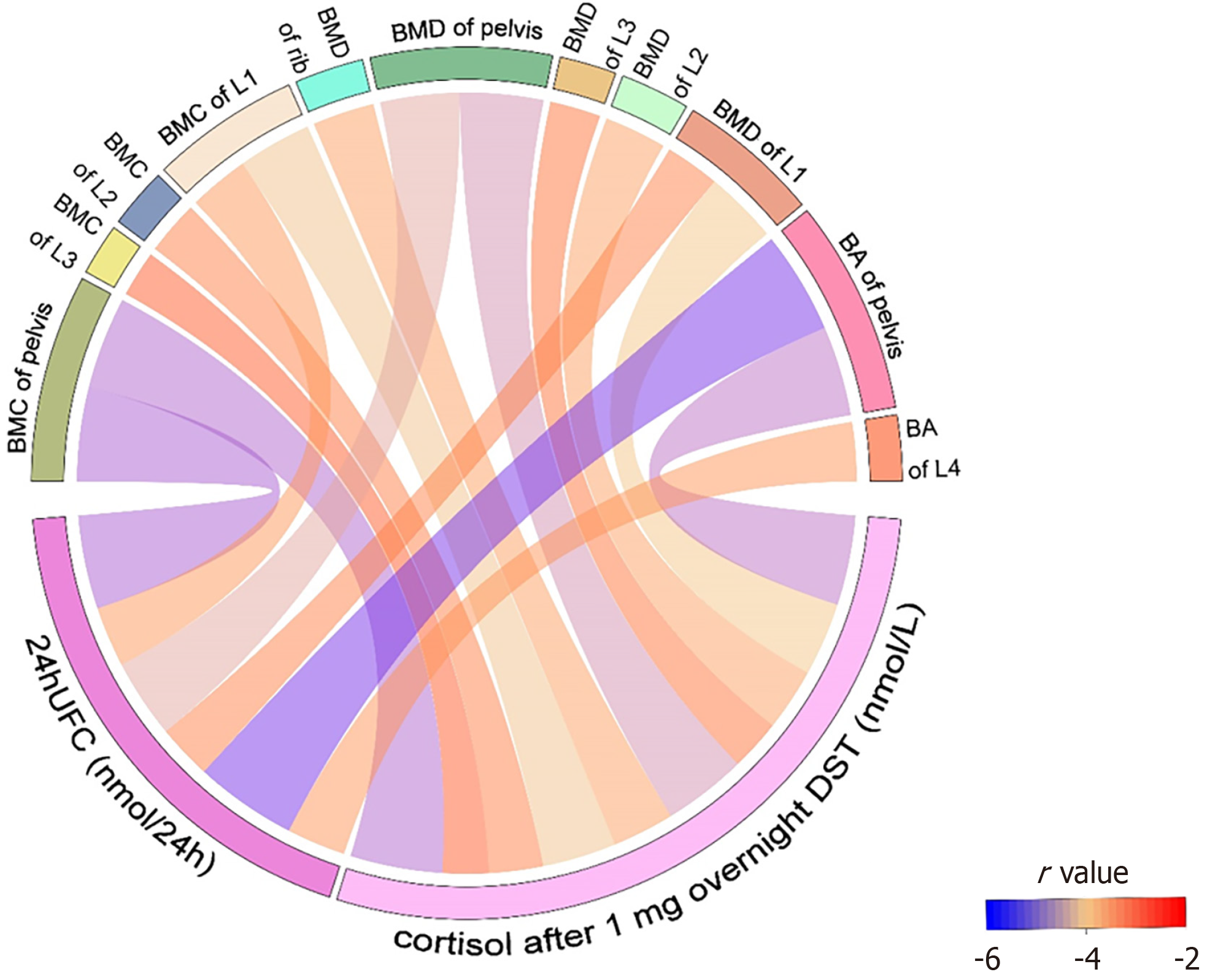
As regard to DXA data, a significant negative correlation was found in BMC, BMD, and BA of specific regions with cortisol after 1 mg overnight DST (Figure 5), including BMC in the pelvis (r = -0.50, P < 0.001), L1 (r = -0.41, P = 0.005), L2 (r = -0.30, P = 0.047), L3 (r = -0.25, P = 0.042), BMD in the rib (r = -0.34, P = 0.019), pelvis (r = -0.46, P = 0.001), L1 (r = -0.40, P = 0.006), L2 (r = -0.34, P = 0.021), L3 (r = -0.28, P = 0.042), and BA in the pelvis (r = -0.49, P < 0.001). In addition to these parameters, others did not identify a significant correlation with cortisol after 1 mg overnight DST (Supplementary Table 2).
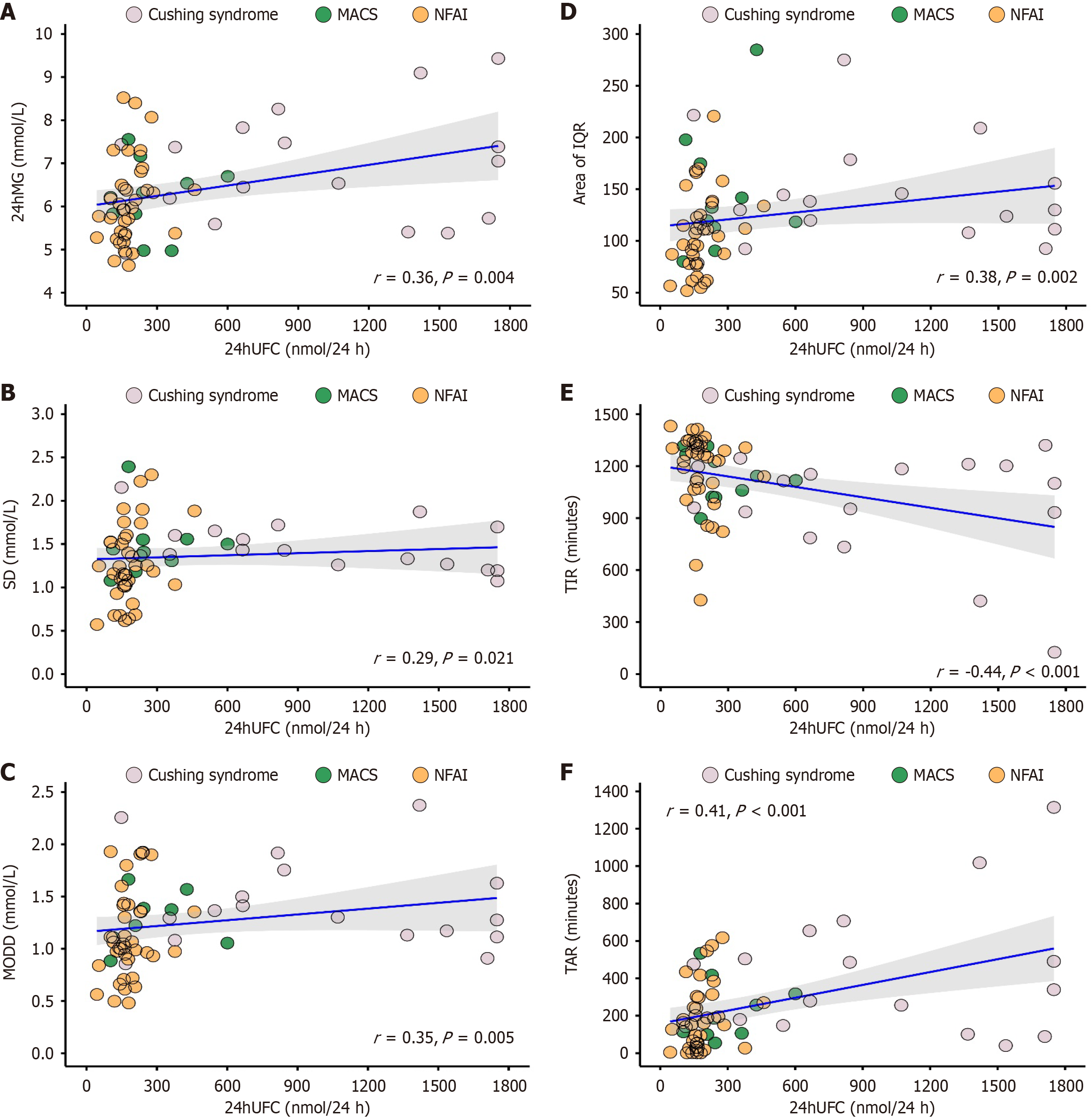
24h UFC and parameters derived from CGMS or DXA: On the basis of CGMS data, a significant correlation was identified between parameters derived from CGMS and 24h UFC (Figure 6). Wherein, a positive correlation was identified in parameters of 24hMG (r = 0.36, P = 0.004), SD (r = 0.29, P = 0.021), MODD (r = 0.35, P = 0.005), area of IQR (r = 0.38, P = 0.002), and TAR (r = 0.41, P < 0.001), along with a negative correlation in TIR (r = -0.44, P < 0.001). However, CV and MAGE did not show significant correlation with 24h UFC (Supplementary Table 3).
As regard to DXA data, a significant negative correlation was found in BMC, BMD, and BA of specific regions with 24h UFC (Figure 5), including BMC in the pelvis (r = -0.51, P < 0.001), and L1 (r = -0.33, P = 0.037), BMD in the pelvis (r = -0.43, P = 0.004), and L1 (r = -0.30, P = 0.040), and BA in the pelvis (r = -0.56, P < 0.001), and L4 (r = -0.32, P = 0.041). In addition to these parameters, others did not identify a significant correlation with 24h UFC (Supplementary Table 3).
Patients with ACS were found to be at risk of metabolic disorders and cardiovascular event, which contribute to higher mortality rates than the general population[1-3]. The relationship between ACS and its associated complications is now an emerging area of intense scientific research.
Many years elapsed before glucose and bone metabolic disorders in ACS were studied and qualified in the both clinical and experimental studies. Endogenous exposure to cortisol affects all the peripheral tissues involved in glucose metabolism, leading to increased glucose production and decreased glucose consumption[20]. Additionally, cortisol disrupts the function of osteoblasts, osteoclasts, and osteocytes, bringing about increased bone resorption and decreased bone formation, and finally culminating in compromised bone mass and quality[21].
Nearly 70% of patients with Cushing syndrome experienced pathoglycemia on a continuum from impaired glucose tolerance to severe diabetes[20]. In patients with mild or subtle cortisol excess, such as those with MACS and NFAI, long-term exposure to cortisol increases the risk of developing glucose abnormalities[22]. In parallel, new evidence has emerged revealing the potential association between degrees of cortisol secretion and indicators reflecting glucose disturbance, for example, HbA1c, fasting blood glucose, and insulin resistance[7,23-25].
Our preliminary study revealed increased glucose variability and decreased TIR in patients with ACS compared to healthy controls[19]. In this study, attempts were made to further investigate glucose variability and glycemic status across subgroups of ACS and their correlation with varying degrees of ACS. Our promising exploration has yielded very encouraging results. 24hMG, SD, MODD, and area of IQR along with TAR and TIR were significantly different among subtypes of ACS, however, no significant difference was identified in CV and MAGE. Additionally, except for CV and MAGE, significant correlation was observed between all parameters of glucose variability or glycemic status and varying degrees of ACS.
Accordingly, we hypothesized that CV and MAGE were less likely to be affected by increasing ACS, while other parameters, such as, 24hMG, SD, MODD, area of IQR, TIR, and TOR, merit special attention when assessing glucose metabolism across ACS subtypes. It is worth noting that SD and MODD were the only two parameters identifying significant difference between groups of MACS and NFAI, suggesting that SD and MODD were susceptible to minor changes of ACS. Paying attention to these two parameters makes sense in patients with MACS and NFAI. No parameters with significance were identified between groups of Cushing syndrome and MACS, indicating that patients with MACS appear to have similar level of metabolic impairment as patients with Cushing syndrome. We assumed that even mild hormone secretion can exert obviously adverse influence on metabolism.
Glucose metabolism impairment was most severe in Cushing syndrome, followed by MACS, and least severe in NFAI. Moreover, a higher degree of ACS was associated with greater impairment of glucose metabolism. There are consistent data across many studies, documenting a similar conclusion with ours[7,23-25]. Nevertheless, several studies have reported conflicting results[26,27].
As stated in previous studies[28,29], occurrence of cardiovascular events varies with degree of ACS. In addition, there is growing evidence supporting the notion that glucose variability and TIR present significant influence on the development of cardiovascular diseases[30,31]. It appears that the continuum effect of ACS on glucose variability and TIR, demonstrated in our study, might contribute to a gradient progression of cardiovascular diseases.
In investigating bone metabolism in patients with ACS, this study focused on BMC, BMD, and BA in various regions assessed by DXA. Nevertheless, the most compelling evidence for the adverse impact of cortisol excess on bone metabolism is from studies examining bone quality indicators or fracture risks[17,32,33]. The literature indicates that patients with ACS exhibit a higher prevalence of fragility fracture and compromised bone quality compared to the general population.
In this study, areal BMC, BMD, and BA in the pelvis, rib, and lumbar vertebrae were significantly different between groups of Cushing syndrome and NFAI, but not between groups of Cushing syndrome and MACS, or MACS and NFAI. In addition, a negative correlation was identified between varying degrees of cortisol secretion and areal BMC, BMD, and BA in the pelvis, rib, and lumbar vertebrae.
More than two decades ago, Godang et al[13] reported significantly reduced BMC, BMD, and BA in specific regions in patients with Cushing syndrome compared with age-, sex-, and BMI-matched healthy controls. Similar results were also pronounced in subsequent studies[14-17]. However, our study differs by suggesting a continuum of reduction in areal BMC, BMD, and BA from NAFI to MACS and Cushing syndrome.
There was a trend of stepwise reduction of areal BMC, BMD, and BA with increasing degrees of cortisol secretion based on our data. Summarizing the published information about this issue yielded inconclusive results, revealing either a negative or no correlation[16,34,35]. It is important to note that DXA measures areal but not volumetric bone mass, resulting in a general and approximate assessment of BMC, BMD, and BA[36]. Furthermore, several factors, such as gonadal status, testosterone levels, and glucose metabolism condition, may influence bone metabolism[17]. Under these circumstances, some studies have failed to identify correlation between cortisol level and bone metabolism indices.
The pelvis, rib, and lumbar vertebrae were mostly influenced by increasing levels of cortisol in patients with ACS based on our results. These regions are rich in cancellous bone. Therefore, we concluded that cancellous bone appeared more susceptible to ACS than cortical bone, aligning with the classical view of site-specific effect of cortisol on bone[37,38]. In clinical practice, we should attach great importance on bones from regions of pelvis, backbone, hip joint, and head of femurs, in patients with ACS.
Unlike Cushing syndrome, MACS and NFAI have an insidious presentation, allowing the disease progression to go undetectable for years. Furthermore, NFAI may progress to MACS over time during follow-up[39-41]. Special attention is necessarily being paid to treatment decision-making for patients with ACS in clinical practice. Traditionally, clinicians have favored conservative therapy for patients with NFAI or MACS. However, results from our study and others have highlighted the significant metabolic impact of ACS and its associated adverse outcomes, casting doubt on the appropriateness of this approach. Consequently, careful consideration is required when choosing a suitable treatment for patients with ACS.
This study had several limitations that should be highlighted. A running debate has posited the potential interaction between glucose metabolism and bone metabolism. Accumulated to data to date suggest that patients with diabetes may have either normal or increased fracture rates[36,42]. Similarly, equivocal evidence existed that those with bone metabolism disorders are at risk of developing diabetes[43,44]. In this context, we conceived that ACS was the primary factor contributing to derangement of glucose or bone metabolism. The interplay between these factors was not accounted for during data analysis. In addition, gonadal status was not considered in this study. The relationship between sex hormone and bone metabolism is well-established. In patients with ACS, research into the impact of sex hormone on bone metabolism has produced substantial advances and an insignificant correlation was observed in between, suggesting that ACS predominated over sex hormone in determining bone status[12,45]. The relatively small sample size was also a limitation of this study. Future studies with expanding sample sizes are needed to strengthen our findings.
The current study highlighted a continuum of glucose and bone metabolism impairment from NFAI to MACS and Cushing syndrome using CGMS and DXA. Those patients with MACS should receive prompt attention due to the adverse influence of mild hormone secretion on metabolism. Parameters of glucose variability and glycemic status along with bone condition in regions rich in cancellous bone will provide valuable information. Considering the significant impact of ACS on the development of bone and glucose metabolism disorders, a multidisciplinary approach is needed to implement a patient-tailored treatment and develop strategies to mitigate adverse outcomes.
| 1. | Park J, De Luca A, Dutton H, Malcolm JC, Doyle MA. Cardiovascular Outcomes in Autonomous Cortisol Secretion and Nonfunctioning Adrenal Adenoma: A Systematic Review. J Endocr Soc. 2019;3:996-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Prete A, Subramanian A, Bancos I, Chortis V, Tsagarakis S, Lang K, Macech M, Delivanis DA, Pupovac ID, Reimondo G, Marina LV, Deutschbein T, Balomenaki M, O'Reilly MW, Gilligan LC, Jenkinson C, Bednarczuk T, Zhang CD, Dusek T, Diamantopoulos A, Asia M, Kondracka A, Li D, Masjkur JR, Quinkler M, Ueland GÅ, Dennedy MC, Beuschlein F, Tabarin A, Fassnacht M, Ivović M, Terzolo M, Kastelan D, Young WF Jr, Manolopoulos KN, Ambroziak U, Vassiliadi DA, Taylor AE, Sitch AJ, Nirantharakumar K, Arlt W; ENSAT EURINE-ACT Investigators*; ENSAT EURINE-ACT Investigators. Cardiometabolic Disease Burden and Steroid Excretion in Benign Adrenal Tumors : A Cross-Sectional Multicenter Study. Ann Intern Med. 2022;175:325-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 3. | Dekkers OM, Horváth-Puhó E, Jørgensen JO, Cannegieter SC, Ehrenstein V, Vandenbroucke JP, Pereira AM, Sørensen HT. Multisystem morbidity and mortality in Cushing's syndrome: a cohort study. J Clin Endocrinol Metab. 2013;98:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 291] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 4. | Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526-1540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1971] [Cited by in RCA: 1732] [Article Influence: 96.2] [Reference Citation Analysis (1)] |
| 5. | Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, Pelsma I, Marina L, Lorenz K, Bancos I, Arlt W, Dekkers OM. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2023;189:G1-G42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 380] [Reference Citation Analysis (0)] |
| 6. | Arlt W, Biehl M, Taylor AE, Hahner S, Libé R, Hughes BA, Schneider P, Smith DJ, Stiekema H, Krone N, Porfiri E, Opocher G, Bertherat J, Mantero F, Allolio B, Terzolo M, Nightingale P, Shackleton CH, Bertagna X, Fassnacht M, Stewart PM. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab. 2011;96:3775-3784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 7. | Papanastasiou L, Alexandraki KI, Androulakis II, Fountoulakis S, Kounadi T, Markou A, Tsiavos V, Samara C, Papaioannou TG, Piaditis G, Kaltsas G. Concomitant alterations of metabolic parameters, cardiovascular risk factors and altered cortisol secretion in patients with adrenal incidentalomas during prolonged follow-up. Clin Endocrinol (Oxf). 2017;86:488-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, Mosconi C, Golfieri R, Paccapelo A, Pagotto U, Pasquali R. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing's syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 9. | Morelli V, Eller-Vainicher C, Salcuni AS, Coletti F, Iorio L, Muscogiuri G, Della Casa S, Arosio M, Ambrosi B, Beck-Peccoz P, Chiodini I. Risk of new vertebral fractures in patients with adrenal incidentaloma with and without subclinical hypercortisolism: a multicenter longitudinal study. J Bone Miner Res. 2011;26:1816-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Chiodini I, Morelli V, Masserini B, Salcuni AS, Eller-Vainicher C, Viti R, Coletti F, Guglielmi G, Battista C, Carnevale V, Iorio L, Beck-Peccoz P, Arosio M, Ambrosi B, Scillitani A. Bone mineral density, prevalence of vertebral fractures, and bone quality in patients with adrenal incidentalomas with and without subclinical hypercortisolism: an Italian multicenter study. J Clin Endocrinol Metab. 2009;94:3207-3214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Koh JM, Song K, Kwak MK, Suh S, Kim BJ, Sung TY, Hong JH, Jeong BC, Kim JH, Lee SH. Adrenalectomy Improves Body Weight, Glucose, and Blood Pressure Control in Patients With Mild Autonomous Cortisol Secretion: Results of an Randomized Controlled Trial by the Co-work of Adrenal Research (COAR) Study. Ann Surg. 2024;279:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Vinolas H, Grouthier V, Mehsen-Cetre N, Boisson A, Winzenrieth R, Schaeverbeke T, Mesguich C, Bordenave L, Tabarin A. Assessment of vertebral microarchitecture in overt and mild Cushing's syndrome using trabecular bone score. Clin Endocrinol (Oxf). 2018;89:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Godang K, Ueland T, Bollerslev J. Decreased bone area, bone mineral content, formative markers, and increased bone resorptive markers in endogenous Cushing's syndrome. Eur J Endocrinol. 1999;141:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Valassi E, Santos A, Yaneva M, Tóth M, Strasburger CJ, Chanson P, Wass JA, Chabre O, Pfeifer M, Feelders RA, Tsagarakis S, Trainer PJ, Franz H, Zopf K, Zacharieva S, Lamberts SW, Tabarin A, Webb SM; ERCUSYN Study Group. The European Registry on Cushing's syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol. 2011;165:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 15. | Kristo C, Jemtland R, Ueland T, Godang K, Bollerslev J. Restoration of the coupling process and normalization of bone mass following successful treatment of endogenous Cushing's syndrome: a prospective, long-term study. Eur J Endocrinol. 2006;154:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Füto L, Toke J, Patócs A, Szappanos A, Varga I, Gláz E, Tulassay Z, Rácz K, Tóth M. Skeletal differences in bone mineral area and content before and after cure of endogenous Cushing's syndrome. Osteoporos Int. 2008;19:941-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Boro H, Mannar V, Malhotra R, Alam S, Khatiwada S, Kubihal S, Dogra V, Golla KK, Mathew UE, Halebidu T, Attri B. Trabecular bone score and bone mineral density as indices of skeletal fragility in endogenous Cushing's syndrome. Clin Endocrinol (Oxf). 2023;99:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Gómez AM, Umpierrez GE, Muñoz OM, Herrera F, Rubio C, Aschner P, Buendia R. Continuous Glucose Monitoring Versus Capillary Point-of-Care Testing for Inpatient Glycemic Control in Type 2 Diabetes Patients Hospitalized in the General Ward and Treated With a Basal Bolus Insulin Regimen. J Diabetes Sci Technol. 2015;10:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Han M, Cao X, Zhao C, Yang L, Yin N, Shen P, Zhang J, Gao F, Ren Y, Liang D, Yang J, Zhang Y, Liu Y. Assessment of Glycometabolism Impairment and Glucose Variability Using Flash Glucose Monitoring System in Patients With Adrenal Diseases. Front Endocrinol (Lausanne). 2020;11:544752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Scaroni C, Zilio M, Foti M, Boscaro M. Glucose Metabolism Abnormalities in Cushing Syndrome: From Molecular Basis to Clinical Management. Endocr Rev. 2017;38:189-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Henneicke H, Gasparini SJ, Brennan-Speranza TC, Zhou H, Seibel MJ. Glucocorticoids and bone: local effects and systemic implications. Trends Endocrinol Metab. 2014;25:197-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Reimondo G, Castellano E, Grosso M, Priotto R, Puglisi S, Pia A, Pellegrino M, Borretta G, Terzolo M. Adrenal Incidentalomas are Tied to Increased Risk of Diabetes: Findings from a Prospective Study. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 23. | Krzyżewska K, Niemczuk E, Myśliwiec BJ, Junik R. Glucose metabolism disorders in patients with non-functioning adrenal adenomas - single-centre experience. Endokrynol Pol. 2017;68:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Araujo-Castro M, Mínguez Ojeda C, Sánchez Ramírez MN, Gómez Dos Santos V, Pascual-Corrrales E, Fernández-Argüeso M. Adrenalectomy improves blood pressure control in nonfunctioning adrenal incidentalomas and glycemic and lipid control in patients with autonomous cortisol secretion. Endocrine. 2022;78:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Lopez D, Luque-Fernandez MA, Steele A, Adler GK, Turchin A, Vaidya A. "Nonfunctional" Adrenal Tumors and the Risk for Incident Diabetes and Cardiovascular Outcomes: A Cohort Study. Ann Intern Med. 2016;165:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 26. | Pecori Giraldi F, Moro M, Cavagnini F; Study Group on the Hypothalamo-Pituitary-Adrenal Axis of the Italian Society of Endocrinology. Gender-related differences in the presentation and course of Cushing's disease. J Clin Endocrinol Metab. 2003;88:1554-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Biering H, Knappe G, Gerl H, Lochs H. [Prevalence of diabetes in acromegaly and Cushing syndrome]. Acta Med Austriaca. 2000;27:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 28. | Szychlińska M, Rzeczkowska M, Matuszewski W, Bandurska-Stankiewicz E. Could a nonfunctional adrenal incidentaloma be a risk factor for increased carotid intima-media thickness and 10-year cardiovascular mortality based on the SCORE algorithm? A study from a single centre in Poland. Endokrynol Pol. 2023;74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Androulakis II, Kaltsas GA, Kollias GE, Markou AC, Gouli AK, Thomas DA, Alexandraki KI, Papamichael CM, Hadjidakis DJ, Piaditis GP. Patients with apparently nonfunctioning adrenal incidentalomas may be at increased cardiovascular risk due to excessive cortisol secretion. J Clin Endocrinol Metab. 2014;99:2754-2762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Lu J, Wang C, Shen Y, Chen L, Zhang L, Cai J, Lu W, Zhu W, Hu G, Xia T, Zhou J. Time in Range in Relation to All-Cause and Cardiovascular Mortality in Patients With Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care. 2021;44:549-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 31. | Tang X, Li S, Wang Y, Wang M, Yin Q, Mu P, Lin S, Qian X, Ye X, Chen Y. Glycemic variability evaluated by continuous glucose monitoring system is associated with the 10-y cardiovascular risk of diabetic patients with well-controlled HbA1c. Clin Chim Acta. 2016;461:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Favero V, Eller-Vainicher C, Morelli V, Cairoli E, Salcuni AS, Scillitani A, Corbetta S, Casa SD, Muscogiuri G, Persani L, Chiodini I. Increased Risk of Vertebral Fractures in Patients With Mild Autonomous Cortisol Secretion. J Clin Endocrinol Metab. 2024;109:e623-e632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 33. | Zavatta G, Vicennati V, Altieri P, Tucci L, Colombin G, Coscia K, Mosconi C, Balacchi C, Fanelli F, Malagrinò M, Magagnoli M, Golfieri R, Pagotto U, Di Dalmazi G. Mild autonomous cortisol secretion in adrenal incidentalomas and risk of fragility fractures: a large cross-sectional study. Eur J Endocrinol. 2023;188:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 34. | Dennison E, Hindmarsh P, Fall C, Kellingray S, Barker D, Phillips D, Cooper C. Profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men. J Clin Endocrinol Metab. 1999;84:3058-3063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Acharya SV, Gopal RA, Lila A, Menon PS, Bandgar TR, Shah NS. Bone age and factors affecting skeletal maturation at diagnosis of paediatric Cushing's disease. Pituitary. 2010;13:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | dos Santos CV, Vieira Neto L, Madeira M, Alves Coelho MC, de Mendonça LM, Paranhos-Neto Fde P, Lima IC, Gadelha MR, Farias ML. Bone density and microarchitecture in endogenous hypercortisolism. Clin Endocrinol (Oxf). 2015;83:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Chen Y, Zhong Z, Chen W, Lv X, Luo SY. Glucocorticoid-induced dose-related and site-specific bone remodelling, microstructure, and mechanical changes in cancellous and cortical bones. Clin Exp Pharmacol Physiol. 2021;48:1421-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Ma CC, Xu SQ, Gong X, Wu Y, Qi S, Liu W, Xu JH. Prevalence and risk factors associated with glucocorticoid-induced osteoporosis in Chinese patients with rheumatoid arthritis. Arch Osteoporos. 2017;12:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Morelli V, Reimondo G, Giordano R, Della Casa S, Policola C, Palmieri S, Salcuni AS, Dolci A, Mendola M, Arosio M, Ambrosi B, Scillitani A, Ghigo E, Beck-Peccoz P, Terzolo M, Chiodini I. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014;99:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 40. | Vassilatou E, Vryonidou A, Michalopoulou S, Manolis J, Caratzas J, Phenekos C, Tzavara I. Hormonal activity of adrenal incidentalomas: results from a long-term follow-up study. Clin Endocrinol (Oxf). 2009;70:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Comlekci A, Yener S, Ertilav S, Secil M, Akinci B, Demir T, Kebapcilar L, Bayraktar F, Yesil S, Eraslan S. Adrenal incidentaloma, clinical, metabolic, follow-up aspects: single centre experience. Endocrine. 2010;37:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, Link TM. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013;28:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 334] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 43. | Lu C, Ivaska KK, Alen M, Wang Q, Törmäkangas T, Xu L, Wiklund P, Mikkola TM, Pekkala S, Tian H, Väänänen HK, Cheng S. Serum osteocalcin is not associated with glucose but is inversely associated with leptin across generations of nondiabetic women. J Clin Endocrinol Metab. 2012;97:4106-4114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Hwang YC, Jee JH, Jeong IK, Ahn KJ, Chung HY, Lee MK. Circulating osteocalcin level is not associated with incident type 2 diabetes in middle-aged male subjects: mean 8.4-year retrospective follow-up study. Diabetes Care. 2012;35:1919-1924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Giuliodori A, Soudah E, Malouf J, Martel-Duguech L, Amodru V, Gil J, Hernández JA, Domingo MP, Webb SM, Valassi E. Evaluation of bone-related mechanical properties in female patients with long-term remission of Cushing's syndrome using quantitative computed tomography-based finite element analysis. Eur J Endocrinol. 2024;190:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/