Published online Mar 15, 2025. doi: 10.4239/wjd.v16.i3.100059
Revised: October 28, 2024
Accepted: December 10, 2024
Published online: March 15, 2025
Processing time: 167 Days and 20.5 Hours
The prevalence and clinical characteristics of chronic kidney disease (CKD) among patients with ketosis-onset diabetes (also known as ketosis-prone diabetes) remain unclear. Furthermore, the classification of ketosis-onset diabetes remains controversial and requires further investigation.
To investigate the prevalence and clinical features of CKD in patients with newly diagnosed ketosis-onset diabetes.
This real-world study included 217 patients with type 1 diabetes mellitus (T1DM), 698 with ketosis-onset diabetes, and 993 with non-ketotic T2DM. The prevalence and clinical characteristics of CKD were compared among the three groups. Risk factors associated with CKD were evaluated using binary logistic regression for each group.
After adjusting for age and sex, the prevalence of CKD among patients with ketosis-onset diabetes (17.8%) was significantly higher than that in those with T1DM (8.3%, P = 0.007), but was not statistically different compared to those with non-ketotic T2DM (21.7%, P = 0.214). Furthermore, some risk factors for CKD, including age, and serum uric acid and C-reactive protein levels, in patients with ketosis-onset diabetes were similar to those with T2DM, but significantly different from those with T1DM.
The prevalence, clinical characteristics, and risk factors for CKD among patients with ketosis-onset diabetes were more similar to those with non-ketotic T2DM but considerably different from those with T1DM. These findings further support the classification of ketosis-onset diabetes as a subtype of T2DM rather than idiopathic T1DM.
Core Tip: The classification of ketosis-onset diabetes remains controversial, and the prevalence and clinical features of chronic kidney disease (CKD) among those with diabetes remain unclear. Results of the present study demonstrated that the prevalence, clinical characteristics, and risk factors for CKD in patients with ketosis-onset diabetes were similar to those with non-ketotic type 2 diabetes mellitus (T2DM) but distinct from those with T1DM. Therefore, from the perspective of CKD, ketosis-onset diabetes may be more appropriately classified as a subtype of T2DM than of T1DM.
- Citation: Li MH, Xu MR, Wang YJ, Shen L, Chen MY, Li LX. Prevalence and clinical characteristics of chronic kidney disease among patients with newly diagnosed ketosis-onset diabetes. World J Diabetes 2025; 16(3): 100059
- URL: https://www.wjgnet.com/1948-9358/full/v16/i3/100059.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i3.100059
Chronic kidney disease (CKD) is a serious condition that causes gradual and permanent damage to the kidneys, affecting approximately 13% of the global population[1,2]. Progressive CKD is associated with a range of complications including anemia, weakened bones, nerve damage, and cardiovascular disease(s)[3]. It is widely accepted that diabetes is the leading cause of CKD globally, and almost one-half of kidney failure cases requiring replacement therapy are caused by diabetes[4]. The prevalence of CKD among Chinese patients with type 2 diabetes mellitus (T2DM) has varied from 27.1% to 43.5% across different studies[5-7]. For example, Yang et al[7] reported that the prevalence of CKD, defined by both estimated glomerular filtration rate (eGFR) and albuminuria, was 29.7% in those with T2DM[7]. Additionally, a previous study estimated that approximately one-third of patients with T1DM develop CKD, in whom CKD was clinically defined as impaired kidney function, elevated urinary albumin excretion (UAE), or both[8]. Although multiple studies have investigated the prevalence of CKD among T1DM and T2DM populations, few have explored the prevalence of CKD among populations with ketosis-onset diabetes. In a study from a tertiary diabetes center in China, Du et al[9] reported that 11.7% of 371 patients diagnosed with ketosis-onset diabetes exhibited persistent microalbuminuria at admission. Additionally, a previous study involving a Tunisian population reported that the prevalence of microvascular complications was 30% in patients with ketosis-onset diabetes, with a 7% prevalence of nephropathy[10]. However, to our knowledge, there have not been any studies exploring the prevalence and clinical features of CKD among individuals with ketosis-onset diabetes.
Ketosis-onset diabetes, also known as ketosis-prone diabetes, was first reported in an African American population[11]. Ketosis-onset diabetes has been classified as type 1 B diabetes by the American Diabetes Association, defined by clear insulin deficiency, spontaneous ketoacidosis at diagnosis, and the absence of islet-related auto-antibodies[12,13], which closely resemble the characteristics of T1DM[14]. However, a cross-sectional study involving 37 patients with an average diabetes duration of 4 years found that insulin secretion and sensitivity in patients with ketosis-onset diabetes were similar to those with T2DM[15]. Notably, significant differences in insulin secretion and sensitivity were observed between T1DM and T2DM, as well as between T1DM and ketosis-onset diabetes[15]. Furthermore, our previous studies demonstrated that the prevalence and characteristics of atherosclerosis, non-alcoholic fatty liver disease, hypertension, and metabolic syndrome in ketosis-onset diabetes were similar to those in non-ketotic T2DM rather than in T1DM[16-18], indicating that ketosis-onset diabetes may be more appropriately classified as a subtype of T2DM rather than of T1DM. As such, the classification of ketosis-onset diabetes remains controversial and requires further investigation. Accordingly, this study aimed to evaluate the prevalence of CKD among patients with newly diagnosed ketosis-onset diabetes, and to compare the clinical characteristics of and risk factors for CKD among patients with T1DM, ketosis-onset diabetes, and T2DM. More importantly, we aimed to find further evidence supporting the classification of ketosis-onset diabetes as a subtype of T2DM based on the characteristics of CKD.
This real-world cross-sectional study (clinical trial registration number: ChiCTR1800015893) was based in part on data from our previous studies[16-18]. Briefly, 1908 consecutive patients newly diagnosed with diabetes in our department between January 2003 and December 2012 were identified. Patients were categorized into three groups: T1DM (n = 217); ketosis-onset diabetes (n = 698); and T2DM (n = 993). The clinical characteristics of patients with CKD were analyzed and compared across these groups. From the perspective of CKD, this study aimed to provide further evidence supporting the classification of ketosis-onset diabetes. The present study adhered to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Shanghai, China), approval No. 2018-KY-018(K). All patients were questioned about their history of hypertension, smoking habits, alcohol consumption, and medication use. Written informed consent was obtained from all participants.
All patients underwent comprehensive physical and laboratory examinations. Weight, height, blood pressure, waist circumference, and hip circumference were measured as described previously[16,17]. Body mass index (BMI) and waist-to-hip ratio were calculated using previously published formulas[16]. Fasting plasma glucose, 2 h postprandial glucose, fasting plasma C-peptide, 2 h postprandial C-peptide, glycated hemoglobin, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, triglycerides, total cholesterol (TC), alanine aminotransferase, r-glutamyltransferase, serum uric acid (SUA), serum creatinine, and C-reactive protein (CRP) levels were measured using standard laboratory protocols[16]. Homeostatic model assessment for insulin resistance, homeostasis model assessment for beta cell function, eGFR, and UAE were described in our previous studies[18]. Consistent with our previous study, urine ketone levels were measured using Legal’s test[16]. All measurements were performed by trained personnel following established protocols. Strict quality control measures and standardized procedures were implemented to ensure the reliability and reproducibility of the results.
The diagnostic criteria for T1DM, ketosis-onset diabetes, and non-ketotic T2DM were described in our previous study[17]. Briefly, T1DM was defined as newly diagnosed diabetes with positive glutamic acid decarboxylase (GAD) and/or islet antigen-2 (IA-2) autoantibodies. Ketosis-onset diabetes was defined as newly diagnosed diabetes with diabetic ketosis, but without GAD and IA-2 autoantibodies. Non-ketotic T2DM was defined as newly diagnosed diabetes without GAD/IA-2 autoantibodies and diabetic ketosis[16-18]. CKD was defined as eGFR < 60 mL/minute/1.73 m2 and/or UAE ≥ 300 mg/24 h. Consistent with our previous studies, diabetic ketosis was defined as the presence of hyperglycemia and elevated urine ketone levels (15-150 mg/dL)[16,17].
Statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, United States). Normally distributed data are expressed as mean ± standard deviation, median with interquartile range for non-normally distributed data, and absolute number and percentage for qualitative data. Normally distributed variables were analyzed using one-way analysis of variance with least significant difference, whereas non-normally distributed variables were analyzed using the Kruskal-Wallis test. The χ2 test was used to compare categorical variables among the three groups. Binary logistic regression analysis was used to assess the differences in categorical variables after controlling for sex and age. Generalized linear model univariate analysis was used to estimate differences in quantitative variables after controlling for sex and age. Binary logistic regression analysis was performed to investigate risk factors associated with CKD. Differences with P < 0.05 were considered statistically significant.
The clinical characteristics of the study participants are summarized in Table 1. Patients with non-ketotic T2DM and those with T1DM did not exhibited any statistically significant differences in sex distribution. However, there was a male predominance among patients with ketosis-onset diabetes, even after adjusting for age. The age of the patients in the three groups was significantly different. Patients with ketosis-onset diabetes exhibited higher fasting plasma glucose and 2 h postprandial glucose levels compared to those with T1DM and non-ketotic T2DM (all P < 0.05). Fasting plasma C-peptide and 2-h postprandial C-peptide levels were markedly higher in patients with ketosis-onset diabetes than in those with T1DM but significantly lower than in those with non-ketotic T2DM before and after adjusting for age and sex (all P < 0.001). Additionally, the prevalence of hypertension and diabetic retinopathy, BMI, waist-to-hip ratio, systolic blood pressure (SBP), diastolic blood pressure (DBP), triglycerides, TC, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, alanine aminotransferase, r-glutamyltransferase, glycated hemoglobin, homeostatic model assessment for insulin resistance, homeostasis model assessment for beta cell function, SUA, CRP, and the use of lipid-lowering drugs also exhibited significant differences among the three groups, even after adjusting for age and sex (all P < 0.05).
| Variables | T1DM, n = 217 | Ketosis-onset diabetes, n = 698 | Non-ketotic T2DM, | P value | P value2 |
| Male | 120 (55.3) | 520 (74.5) | 605 (60.9) | < 0.001 | < 0.001 |
| Age in years | 39 ± 19 | 49 ± 15 | 55 ± 14 | < 0.001 | < 0.001 |
| Hypertension | 29 (13.4) | 258 (37.0) | 445 (44.8) | < 0.001 | < 0.001 |
| Smoking | 61 (28.1) | 278 (39.8) | 342 (34.4) | 0.003 | 0.451 |
| Alcohol | 28 (12.9) | 153 (21.9) | 196 (19.7) | 0.014 | 0.305 |
| LLDs | 39 (18.0) | 186 (26.6) | 316 (31.8) | < 0.001 | 0.003 |
| APAs | 40 (18.4) | 190 (27.2) | 345 (34.7) | < 0.001 | 0.640 |
| DR | 10 (4.6) | 59 (8.5) | 106 (10.7) | 0.014 | 0.018 |
| SBP in mmHg | 119 ± 15 | 128 ± 16 | 129 ± 16 | < 0.001 | < 0.001 |
| DBP in mmHg | 75 ± 10 | 82 ± 11 | 81 ± 9 | < 0.001 | < 0.001 |
| BMI in kg/m2 | 21.26 ± 3.95 | 24.99 ± 3.57 | 24.94 ± 3.41 | < 0.001 | < 0.001 |
| WHR | 0.87 ± 0.06 | 0.92 ± 0.06 | 0.92 ± 0.06 | < 0.001 | < 0.001 |
| FPG in mmol/L1 | 8.23 (6.24-11.04) | 9.74 (7.71-12.54) | 8.07 (6.7-10.05) | < 0.001 | 0.002 |
| 2-h PPG in mmol/L1 | 14.95 (10.75-18.67) | 16.28 (12.48-20.24) | 14.01 (10.83-17.31) | < 0.001 | 0.020 |
| HbA1c as % | 11.66 ± 2.86 | 11.44 ± 2.23 | 9.98 ± 2.49 | < 0.001 | < 0.001 |
| FCP in ng/mL1 | 0.62 (0.29-1.05) | 1.36 (0.8-2.05) | 2.09 (1.35-3.04) | < 0.001 | < 0.001 |
| 2-h PCP in ng/mL1 | 1.11 (0.59-2.20) | 2.52 (1.48-3.99) | 4.83 (2.95-6.96) | < 0.001 | < 0.001 |
| HOMA2-IR1 | 0.57 (0.28-0.95) | 1.27 (0.71-2.07) | 1.8 (1.2-2.63) | < 0.001 | < 0.001 |
| HOMA2-B1 | 20.6 (11.2-38.9) | 26.7 (17.7-39.5) | 51.5 (31.63-82.53) | < 0.001 | < 0.001 |
| TG in mmol/L1 | 0.99 (0.71-1.48) | 1.44 (0.98-2.23) | 1.53 (1.11-2.13) | < 0.001 | < 0.001 |
| TC in mmol/L | 4.59 ± 1.12 | 4.95 ± 1.31 | 4.87 ± 1.17 | 0.001 | < 0.001 |
| HDL-C in mmol/L | 1.19 ± 0.37 | 1.06 ± 0.31 | 1.11 ± 0.29 | < 0.001 | < 0.001 |
| LDL-C in mmol/L | 2.90 ± 0.93 | 3.13 ± 0.99 | 3.14 ± 0.95 | 0.004 | < 0.001 |
| ALT1 | 21 (13.25-31.75) | 26 (17-40.25) | 25 (16-42) | < 0.001 | < 0.001 |
| r-GT1 | 17 (13-25) | 29 (20-48) | 32 (21-54.5) | < 0.001 | < 0.001 |
| SUA1 | 255 (202-323.25) | 303 (243-374) | 312 (256-373) | < 0.001 | < 0.001 |
| CRP in mg/L1 | 0.71 (0.25-2.26) | 1.43 (0.6-3.78) | 1.37 (0.65-3.33) | < 0.001 | < 0.001 |
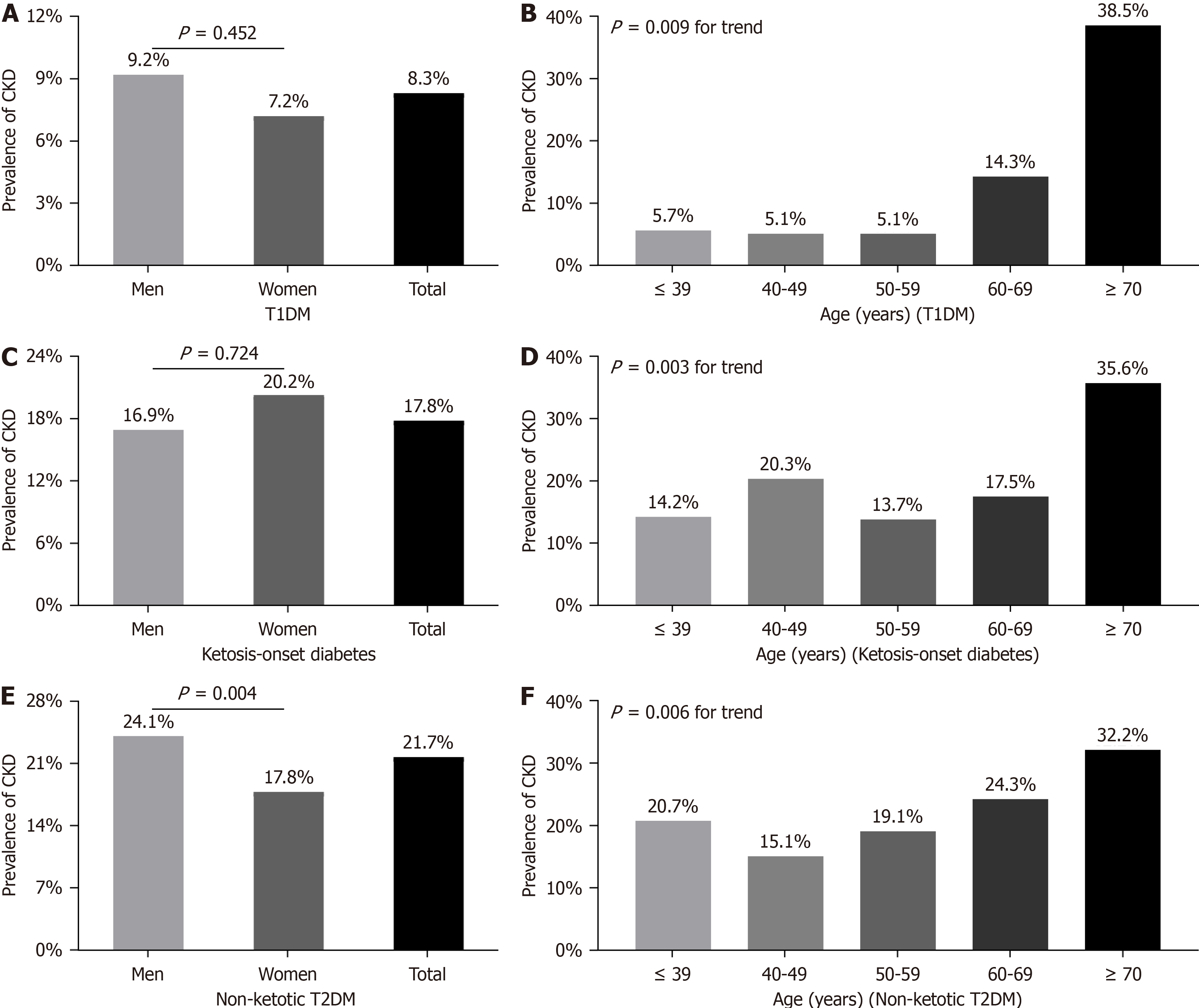
A comparison of CKD prevalence stratified according to sex and age in each group is presented in Figure 1. There was no significant difference in the prevalence of CKD between sex in those with T1DM (9.2% for men, 7.2% for women; P = 0.452) and ketosis-onset diabetes (16.9% for men, 20.2% for women; P = 0.724) after controlling for age (Figure 1A and C). However, the prevalence of CKD was clearly higher among men than in women among patients with non-ketotic T2DM (24.1% vs 17.8%; P = 0.004) (Figure 1E). The prevalence of CKD was similar among the first three age groups but gradually increased from the third to the fifth age group in patients with T1DM (Figure 1B). In contrast, there was no obvious difference in CKD prevalence among the first four age groups, with the highest prevalence in the fifth age group among patients with ketosis-onset diabetes (Figure 1D). Finally, CKD prevalence gradually increased from the second to the fifth age group in patients with non-ketotic T2DM (Figure 1F).
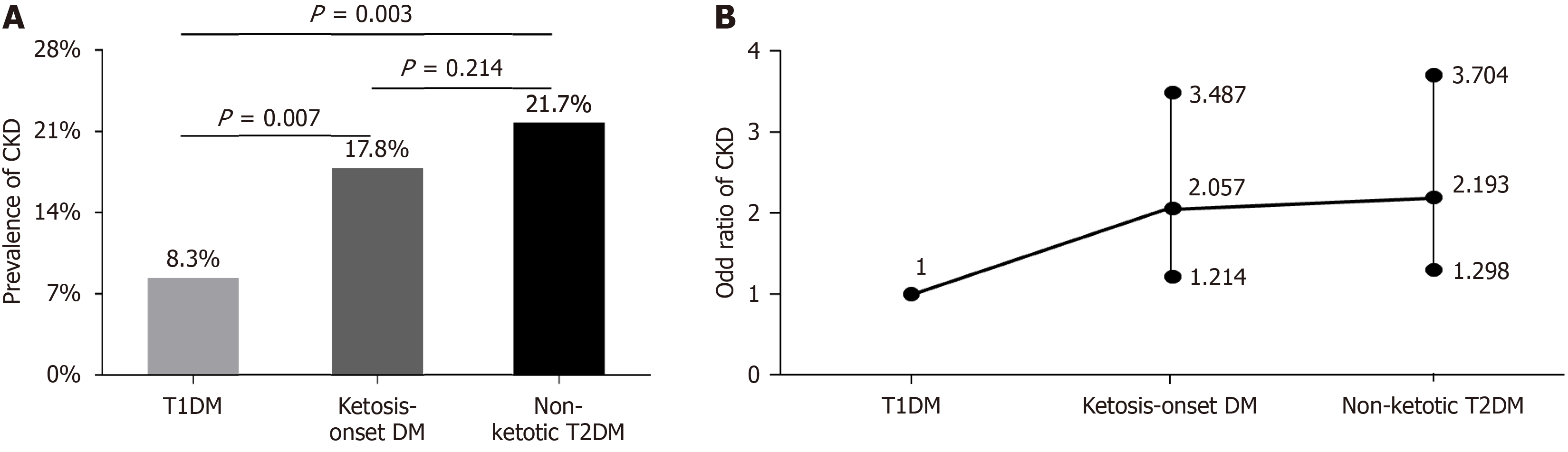
A comparison of CKD prevalence among the three groups is presented in Figure 2. The prevalence of CKD among patients with ketosis-onset diabetes (17.8%) was significantly higher than among those with T1DM (8.3%, P = 0.007); however, no significant difference was observed between the ketosis-onset diabetes and non-ketotic T2DM groups (21.7%, P = 0.214) (Figure 2A). After controlling for age and sex, the risk for CKD among patients with ketosis-onset diabetes [odds ratio (OR) = 2.057; 95% confidence interval (CI): 1.214-3.487] was almost 2.1 times higher than that in patients with T1DM but was not significantly different compared to patients with non-ketotic T2DM (OR = 2.193; 95%CI: 1.298-3.704) (Figure 2B).
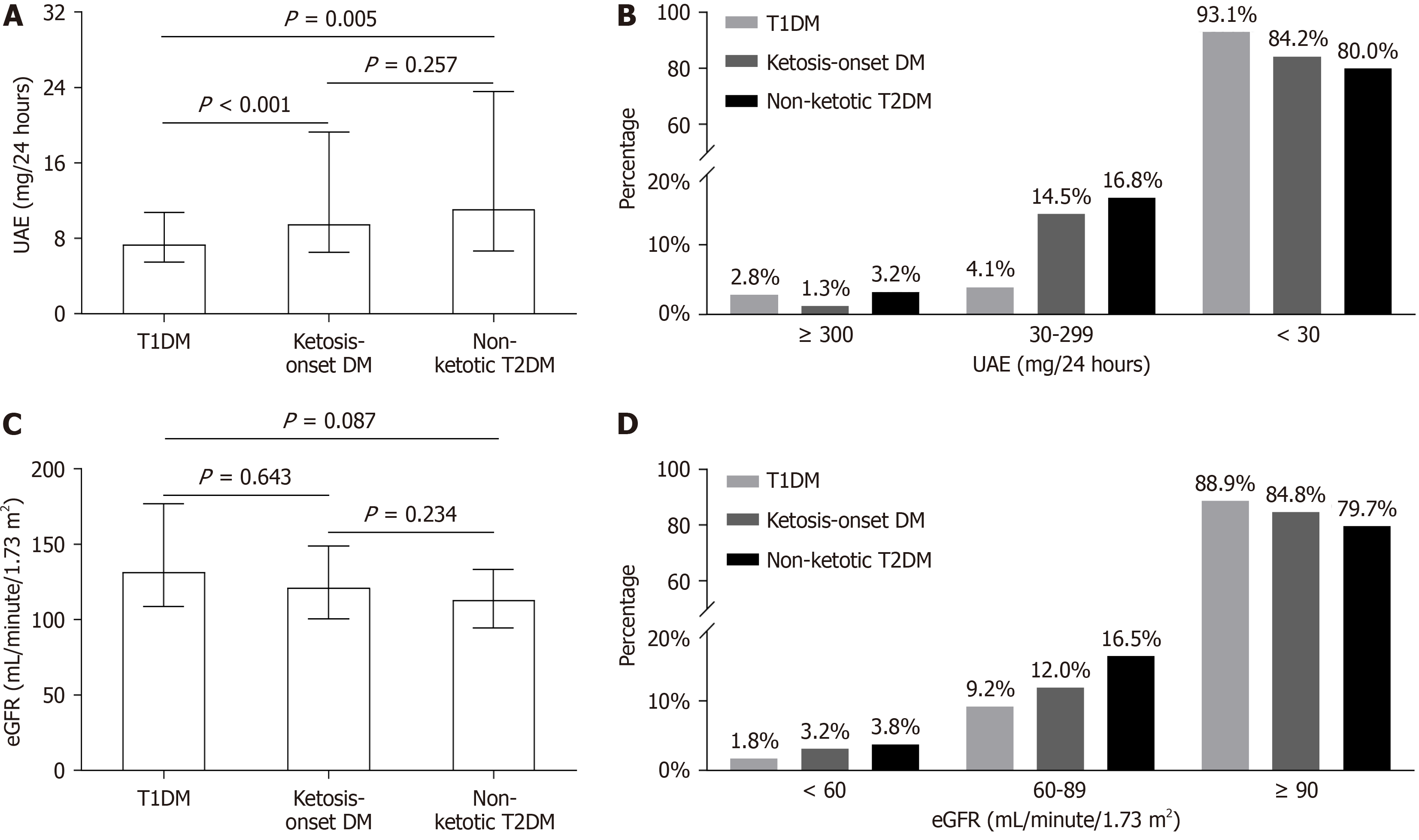
A comparison of UAE and eGFR among the three groups is presented in Figure 3. UAE levels in patients with ketosis-onset diabetes were significantly higher than in those with T1DM (P < 0.001) but were similar to those in patients with non-ketotic T2DM (P = 0.257) (Figure 3A). The percentage of patients with UAE < 30 mg/24 h and 30-299 mg/24 h was similar between ketosis-onset diabetes and non-ketotic T2DM, but exhibited a significant difference compared with T1DM. Patients with ketosis-onset diabetes had the lowest proportion of UAE ≥ 300 mg/24 h among the three groups (1.3% vs 2.8%, 3.2%) (Figure 3B). However, there was no significant difference in eGFR among the three groups (all P > 0.05) (Figure 3C). The percentage of patients with eGFR < 60 mL/min/1.73 m2 and 60-90 mL/min/1.73 m2 in the ketosis-onset diabetes group (3.2% and 12.0%, respectively) was higher than in the T1DM group (1.8% and 9.2%, respectively), but was not significantly different compared to non-ketotic T2DM group (3.8% and 16.5%, respectively) (Figure 3D).
Risk factors associated with CKD are listed in Table 2. In patients with T1DM, SBP (OR = 1.122; 95%CI: 1.023-1.230; P = 0.015) was associated with increased risk for CKD. Interestingly, the risk factors for CKD, including age, SUA, and CRP, in ketosis-onset diabetes were also present in non-ketotic T2DM. Additionally, diabetic retinopathy (OR = 2.943; 95%CI: 1.171-7.397; P = 0.022) and BMI (OR = 1.109; 95%CI: 1.004-1.224; P = 0.041) were linked with a significantly increased risk for CKD in those with ketosis-onset diabetes. DBP (OR = 1.028; 95%CI: 1.001-1.055; P = 0.045) and TC (OR = 1.233; 95%CI: 1.031-1.474; P = 0.022) were independent risk factors for CKD in those with non-ketotic T2DM.
| Group | Variables | B statistic | OR | 95%CI | P value |
| T1DM | SBP | 0.115 | 1.122 | 1.023-1.230 | 0.015 |
| Ketosis-onset diabetes | Age | 0.022 | 1.022 | 1.000-1.045 | 0.054 |
| DR | 1.079 | 2.943 | 1.171-7.397 | 0.022 | |
| BMI | 0.103 | 1.109 | 1.004-1.224 | 0.041 | |
| SUA | 0.338 | 1.402 | 1.017-1.933 | 0.039 | |
| CRP | 0.459 | 1.582 | 1.061-2.360 | 0.025 | |
| Non-ketotic T2DM | Age | 0.019 | 1.019 | 1.000-1.038 | 0.048 |
| DBP | 0.027 | 1.028 | 1.001-1.055 | 0.045 | |
| TC | 0.210 | 1.233 | 1.031-1.474 | 0.022 | |
| SUA | 0.507 | 1.660 | 1.289-2.138 | < 0.001 | |
| CRP | 0.408 | 1.504 | 1.129-2.002 | 0.005 |
Based on our findings, the prevalence and clinical features of CKD in ketosis-onset diabetes were distinctly different from those in T1DM but more closely resembled those in non-ketotic T2DM. These findings provide further evidence to support the classification of ketosis-onset diabetes as a subtype of T2DM rather than of T1DM. In this hospital-based, real-world study, the prevalence of CKD in patients with non-ketotic T2DM was 21.7%, which is very similar to the prevalence of CKD reported in Chinese patients with T2DM (21.8%) in a previous meta-analysis[19]. The CKD criteria used in this study were fully aligned with those used in our previous studies[16-18]. In addition, Guo et al[6] noted that based on criteria from the Kidney Disease Outcomes Quality Initiative, the prevalence of CKD in T2DM was 27.1% in Shanghai, China. Another study reported a CKD prevalence of 15.8% among patients with T2DM in Hong Kong, in which CKD was defined as an eGFR < 60 mL/min/1.73 m2[20]. The differences in CKD prevalence among these studies may be attributed to differences in sample selection, diabetes duration, and diagnostic criteria for CKD.
In our study, the prevalence of CKD among patients with newly diagnosed T1DM was 8.7%, which was significantly lower than that in those with non-ketotic T2DM. One study assessed the burden of DM-related CKD in 204 countries and regions between 1990 and 2019, and reported that the prevalence of CKD caused by T1DM remained stable across all age groups and sexes, while the prevalence of CKD attributed to T2DM increased with age and was higher in males than females in those ≥ 50 years of age[21]. Similarly, we did not find any significant difference in the prevalence of CKD between the sexes in those with T1DM after controlling for age; however, the prevalence of CKD among men was obviously higher than that in women with non-ketotic T2DM. Additionally, in our study, the prevalence of CKD in both patients with T1DM and non-ketotic T2DM was higher among elderly individuals. Moreover, a study including 504 patients with T1DM and 3071 with T2DM demonstrated that those with T2DM were more than twice as likely as those with T1DM to have CKD, as defined by albuminuria and/or decreased eGFR[22]. Therefore, different types of diabetes-related CKD exhibit distinct characteristics in terms of sex and age, and the prevalence of CKD varies among diabetes types.
Few studies have investigated the prevalence of CKD among patients with ketosis-onset diabetes. A previous study by Du et al[9] found that 11.7% of patients with ketosis-onset diabetes exhibited persistent microalbuminuria at admission between January 2011 and July 2015. Another study with a small sample size demonstrated that 7 of 100 patients with ketosis-onset diabetes had nephropathy[10]. The prevalence of CKD in those with ketosis-onset diabetes in the present study was 17.8%, which was similar to the 21.7% in non-ketotic T2DM, but significantly higher than the 8.3% in T1DM. Furthermore, patients with ketosis-onset diabetes exhibited a nearly 2.1-fold increased risk for CKD compared to those with T1DM. However, the OR for CKD between patients with ketosis-onset diabetes and non-ketotic T2DM exhibited no significant difference after correcting for sex and age. Consistent with our findings, another study also observed that patients with T2DM had a significantly increased risk for CKD compared to those with T1DM, with an approximately 1.8-fold increase after adjusting for diabetes duration, age, and sex[22]. Although the risk for CKD differed between T1DM and T2DM across studies, our findings revealed that the prevalence of CKD in ketosis-onset diabetes was similar to that in T2DM but significantly different from that in T1DM, which further suggests that ketosis-onset diabetes should be considered a subtype of T2DM rather than of T1DM.
Proteinuria, a hallmark of diabetic kidney disease, serves as a valuable indicator of disease severity and guides the clinical treatment of patients with CKD[23]. A previous study reported that 14.8% of 27 patients with ketosis-onset diabetes had microalbuminuria on admission[24]. In our study, the proportion of patients with ketosis-onset diabetes with a UAE level between 30 and 299 mg/24 h was 14.5%, which was similar to 16.8% in non-ketotic T2DM, but significantly higher than 4.1% in T1DM. Consistent with our findings, several studies have reported a higher prevalence of albuminuria in patients with T2DM than in those with T1DM[22,25,26]. For example, in a cross-sectional study involving 3575 Japanese patients with diabetes, albuminuria was observed in 36.1% of patients with T2DM compared to 15.9% with T1DM[22]. Therefore, our findings, supported by the clinical characteristics of UAE, further suggest that ketosis-onset diabetes should be classified as a subtype of T2DM rather than of T1DM.
In addition to albuminuria, GFR is another crucial indicator of kidney function in patients with diabetes. A re
The fundamental mechanism of CKD in T1DM is oxidative stress and inflammation induced by hyperglycemia, leading to renal parenchymal damage[29]. A follow-up study by Ku et al[30] reported a negative association between low blood pressure (< 120/70 mmHg) and the risk for adverse renal outcomes in patients with T1DM, independent of glycemic control. Consistent with this study, SBP was also an independent risk factor for CKD in patients with T1DM in our study. In addition to hyperglycemia, other factors, such as insulin resistance, age, and various comorbidities of T2DM, such as obesity and hypertension, accelerate the progression of CKD in T2DM[29]. We also found that age, serum CRP, and DBP were risk factors for CKD in patients with non-ketotic T2DM, despite the presence of other established CKD risk factors, such as SUA and TC, in this group. Although the pathogenesis of CKD in ketosis-onset diabetes has not been extensively studied, we found that the risk factors for CKD in ketosis-onset diabetes resembled those in non-ketotic T2DM. Thus, we speculated that the underlying mechanism of CKD in ketosis-onset diabetes is consistent with that in T2DM, which further supports the classification of ketosis-onset diabetes as a subtype of T2DM rather than of T1DM.
However, the present study had several limitations, the first of which was its cross-sectional design, restricting our ability to establish a causal relationship between diabetic ketosis and CKD development. Further studies are required to investigate the long-term effects of diabetic ketosis on CKD in patients with ketosis-onset diabetes. Second, there are various diagnostic criteria for CKD; however, the present study focused on evaluating the specific criteria adopted in our previous studies[16-18]. Therefore, comparing the prevalence and clinical characteristics of CKD among patients with T1DM, ketosis-onset diabetes, and T2DM based on different diagnostic criteria will contribute to a better understanding of the clinical features of CKD in ketosis-onset diabetes and, thus, help refine its classification from a CKD perspective. Finally, all participants enrolled in this study were Chinese; therefore, the generalizability of our findings to other races and/or populations requires further verification. As such, multicenter studies are required to compare the prevalence and clinical characteristics of CKD among different types of diabetes across races and populations.
In conclusion, the prevalence of CKD among patients with ketosis-onset diabetes was significantly higher than that in patients with T1DM but exhibited no difference compared to those with non-ketotic T2DM. Furthermore, the clinical characteristics of and risk factors for CKD in ketosis-onset diabetes were more similar to those in non-ketotic T2DM but were different from those in T1DM. Therefore, the present study provides further evidence supporting the concept that ketosis-onset diabetes should be classified as a subtype of T2DM rather than of T1DM.
We thank the other investigators, the staff and all the patients of the present study for their invaluable contributions.
| 1. | Ammirati AL. Chronic Kidney Disease. Rev Assoc Med Bras (1992). 2020;66Suppl 1: s03-s09. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 2. | Nagib SN, Abdelwahab S, Amin GEE, Allam MF. What is the prevalence of chronic kidney disease among hypertensive non-diabetic Egyptian patients attending primary healthcare? Clin Exp Hypertens. 2023;45:2203411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care. 2008;35:329-344, vii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 283] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 4. | Tuttle KR, Brosius FC 3rd, Cavender MA, Fioretto P, Fowler KJ, Heerspink HJL, Manley T, McGuire DK, Molitch ME, Mottl AK, Perreault L, Rosas SE, Rossing P, Sola L, Vallon V, Wanner C, Perkovic V. SGLT2 Inhibition for CKD and Cardiovascular Disease in Type 2 Diabetes: Report of a Scientific Workshop Sponsored by the National Kidney Foundation. Diabetes. 2021;70:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 5. | Bailey RA, Wang Y, Zhu V, Rupnow MF. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) staging. BMC Res Notes. 2014;7:415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Guo K, Zhang L, Zhao F, Lu J, Pan P, Yu H, Bao Y, Chen H, Jia W. Prevalence of chronic kidney disease and associated factors in Chinese individuals with type 2 diabetes: Cross-sectional study. J Diabetes Complications. 2016;30:803-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Yang L, Chu TK, Lian J, Lo CW, Lau PK, Nan H, Liang J. Risk factors of chronic kidney diseases in Chinese adults with type 2 diabetes. Sci Rep. 2018;8:14686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, Rossing P, Groop PH, Cooper ME. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 802] [Article Influence: 72.9] [Reference Citation Analysis (1)] |
| 9. | Du S, Yang X, Shi D, Su Q. Characteristics of Type 2 Diabetes with Ketosis in Baoshan, Yunnan of China. J Diabetes Res. 2016;2016:7854294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Chihaoui M, Kanoun F, Tabassi N, Ftouhi B, Yazidi M, Lamine F, Slimane H. Characteristics of Ketosis-onset Diabetes in Tunisian Population. J Diabetes Metab. 2012;3:1-5. [DOI] [Full Text] |
| 11. | Mauvais-Jarvis F, Sobngwi E, Porcher R, Riveline JP, Kevorkian JP, Vaisse C, Charpentier G, Guillausseau PJ, Vexiau P, Gautier JF. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 196] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1853] [Cited by in RCA: 2345] [Article Influence: 293.1] [Reference Citation Analysis (0)] |
| 13. | Sobngwi E, Gautier JF. Adult-onset idiopathic Type I or ketosis-prone Type II diabetes: evidence to revisit diabetes classification. Diabetologia. 2002;45:283-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Balasubramanyam A, Nalini R, Hampe CS, Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev. 2008;29:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Ramos-Román MA, Piñero-Piloña A, Adams-Huet B, Raskin P. Comparison of type 1, type 2, and atypical ketosis-prone diabetes at 4 years of diabetes duration. J Diabetes Complications. 2006;20:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Li LX, Zhao CC, Ren Y, Tu YF, Lu JX, Wu X, Zhang WX, Zhu JA, Li MF, Yu LB, Bao YQ, Jia WP. Prevalence and clinical characteristics of carotid atherosclerosis in newly diagnosed patients with ketosis-onset diabetes: a cross-sectional study. Cardiovasc Diabetol. 2013;12:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Li TT, Wang AP, Lu JX, Chen MY, Zhao CC, Tang ZH, Li LX, Jia WP. Prevalence and clinical characteristics of non-alcoholic fatty liver disease in newly diagnosed patients with ketosis-onset diabetes. Diabetes Metab. 2018;44:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Wang JW, Wang AP, Chen MY, Lu JX, Ke JF, Li LX, Jia WP. Prevalence and clinical characteristics of hypertension and metabolic syndrome in newly diagnosed patients with ketosis-onset diabetes: a cross-sectional study. Diabetol Metab Syndr. 2019;11:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Zhang XX, Kong J, Yun K. Prevalence of Diabetic Nephropathy among Patients with Type 2 Diabetes Mellitus in China: A Meta-Analysis of Observational Studies. J Diabetes Res. 2020;2020:2315607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 20. | Kong AP, So WY, Szeto CC, Chan NN, Luk A, Ma RC, Ozaki R, Ng VW, Ho CS, Lam CW, Chow CC, Cockram CS, Chan JC, Tong PC. Assessment of glomerular filtration rate in addition to albuminuria is important in managing type II diabetes. Kidney Int. 2006;69:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Deng Y, Li N, Wu Y, Wang M, Yang S, Zheng Y, Deng X, Xiang D, Zhu Y, Xu P, Zhai Z, Zhang D, Dai Z, Gao J. Global, Regional, and National Burden of Diabetes-Related Chronic Kidney Disease From 1990 to 2019. Front Endocrinol (Lausanne). 2021;12:672350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 22. | Ohta M, Babazono T, Uchigata Y, Iwamoto Y. Comparison of the prevalence of chronic kidney disease in Japanese patients with Type 1 and Type 2 diabetes. Diabet Med. 2010;27:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74:22-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 24. | Govender P, Elmezughi K, Esterhuizen T, Paruk I, Pirie FJ, Motala AA. Characteristics of subjects with diabetes mellitus diagnosed before 35 years of age presenting to a tertiary diabetes clinic in Durban, South Africa, from 2003 to 2016. J Endocrinol Metab Diabetes S Afr. 2018;23:26-31. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Kahkoska AR, Isom S, Divers J, Mayer-Davis EJ, Dolan L, Shah AS, Afkarian M, Pettitt DJ, Lawrence JM, Marcovina S, Saydah SH, Dabelea D, Maahs DM, Mottl AK; SEARCH for Diabetes in Youth Study Group. The early natural history of albuminuria in young adults with youth-onset type 1 and type 2 diabetes. J Diabetes Complications. 2018;32:1160-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Lee YB, Han K, Kim B, Jun JE, Lee SE, Ahn J, Kim G, Jin SM, Kim JH. Risk of end-stage renal disease from chronic kidney disease defined by decreased glomerular filtration rate in type 1 diabetes: A comparison with type 2 diabetes and the effect of metabolic syndrome. Diabetes Metab Res Rev. 2019;35:e3197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | He X, Luo Y, Hao J, Hu R, Yang X, Ren L. High Atherogenic Risk in Ketosis-Prone Type 2 Diabetic Individuals with Ketosis Episodes: A Cross-Sectional Study. Diabetes Metab Syndr Obes. 2023;16:3085-3094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Wang Y, Lu C, Augusto Monteiro Cardoso Lopes M, Chen L, Luo Y, Wu W, Gu X. A Cross-Sectional Study of Atherosclerosis in Newly Diagnosed Patients with Ketosis-Prone Type 2 Diabetes. Diabetes Metab Syndr Obes. 2022;15:933-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Pyram R, Kansara A, Banerji MA, Loney-Hutchinson L. Chronic kidney disease and diabetes. Maturitas. 2012;71:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Ku E, McCulloch CE, Mauer M, Gitelman SE, Grimes BA, Hsu CY. Association Between Blood Pressure and Adverse Renal Events in Type 1 Diabetes. Diabetes Care. 2016;39:2218-2224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/