Published online Feb 15, 2025. doi: 10.4239/wjd.v16.i2.99053
Revised: October 26, 2024
Accepted: November 26, 2024
Published online: February 15, 2025
Processing time: 171 Days and 5.8 Hours
Plantamajoside (PMS) has shown potential in mitigating cell damage caused by high glucose (HG) levels. Despite this, the precise therapeutic effects of PMS on type 2 diabetes mellitus (T2DM) and the underlying regulatory mechanisms require further exploration.
To investigate PMS therapeutic effects on T2DM in mice and elucidate its mechanisms of action through in vivo and in vitro experiments.
An in vitro damage model of MIN6 cells was established using HG and palmitic acid (PA). PMS's protective effect on cell damage was assessed. Next, transcriptomics was employed to examine how PMS treatment affects gene expression of MIN6 cells. Furthermore, the effect of PMS on protein processing in endoplasmic reticulum and apoptosis pathways was validated. A T2DM mouse model was used to validate the therapeutic effects and mechanisms of PMS in vivo.
PMS intervention ameliorated cell injury in HG + PA-induced MIN6 cell damage. Transcriptomic analysis revealed that protein processing in the endoplasmic reticulum and apoptosis pathways were enriched in cells treated with PMS, with significant downregulation of the gene Dnajc1. Further validation indicated that PMS significantly inhibited the expression of apoptosis-related factors (Bax, CytC) and endoplasmic reticulum stress (ERS)-related factors [ATF6, XBP1, Ddit3 (CHOP), GRP78], while promoting the expression of Bcl-2 and Dnajc1. Additionally, the inhibitory effects of PMS on ERS and apoptosis were abolished upon Dnajc1 silencing. Furthermore, in vivo experiments demonstrated that PMS intervention effectively improved pancreatic damage, suppressed the expression of apoptosis-related factors (Bax, CytC), and ERS-related factors [ATF6, XBP1, Ddit3 (CHOP), GRP78], while promoting the expression of Bcl-2 and Dnajc1 in a T2DM model mice.
PMS intervention could alleviate pancreatic tissue damage effectively. The mechanism of action involves Dnajc1 activation, which subsequently inhibits apoptosis and ERS, ameliorating damage to pancreatic β-cells.
Core Tip: Plantamajoside (PMS) exhibits therapeutic effects in rats with type 2 diabetes mellitus pancreatic β-cell damage. The mechanism of action of PMS appears to involve Dnajc1 activation, which subsequently inhibits apoptosis and endoplasmic reticulum stress, ultimately ameliorating damage to pancreatic β-cells.
- Citation: Wang D, Wang YS, Zhao HM, Lu P, Li M, Li W, Cui HT, Zhang ZY, Lv SQ. Plantamajoside improves type 2 diabetes mellitus pancreatic β-cell damage by inhibiting endoplasmic reticulum stress through Dnajc1 up-regulation. World J Diabetes 2025; 16(2): 99053
- URL: https://www.wjgnet.com/1948-9358/full/v16/i2/99053.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i2.99053
Type 2 diabetes mellitus (T2DM) is a complex metabolic syndrome primarily characterized by reduced insulin sensitivity in target tissues or organs and relative insulin deficiency[1]. The latest report from the International Diabetes Federation estimates that approximately 537 million people worldwide have diabetes, with T2DM being the most prevalent form[2]. As T2DM progresses, it can lead to complications such as kidney disease, retinopathy, and coronary heart disease, affecting multiple systems[3]. The disease is increasingly common among younger individuals, has a prolonged duration, numerous complications, and poses severe health risks, making it a serious global public health issue.
Current treatment strategies primarily involve metformin and insulin injections, but monotherapy often results in inadequate blood sugar control, significant economic burdens, and potential hepatic and renal side effects[4]. Recent studies have increasingly shown that natural products offer advantages in managing T2DM, including good safety profiles and stable efficacy[5]. Numerous animal experiments have demonstrated that natural products, such as arte
The primary pathological features of T2DM include insulin resistance and insufficient insulin secretion[10]. Insulin is mainly produced and secreted by β-cells located within the islets of Langerhans in the pancreas[11]. Autopsy studies of T2DM patients have revealed progressive pancreatic damage[12]. Pathological examination of the pancreas in T2DM mice has shown unclear islet boundaries and islet atrophy[13]. Metabolic disturbances in glucose and lipids in T2DM can lead to damage of pancreatic β-cells, resulting in reduced insulin secretion[13]. Exposure of pancreatic β-cells to high concentrations of glucose and palmitic acid (PA) causes significant damage and reduces insulin secretion levels[14]. Alleviating damage to pancreatic β-cells is a critical mechanism through which natural products improve T2DM[15]. Dendrobium polysaccharides[13], Polygonatum polysaccharides[14], Schisandra polysaccharides[16,17], and quercetin[18] have all been shown to alleviate pancreatic β-cell damage in T2DM models.
Plantamajoside (PMS) is a key bioactive compound derived from the traditional Chinese medicinal herb Plantago asiatica, known for its anti-inflammatory, antioxidant, and anti-apoptotic properties[19,20]. Studies have shown that PMS can protect cells from damage induced by high glucose (HG) levels[21]. In addition, PMS has a strong glycation inhibitory activity[22]. However, further exploration is needed to fully understand the therapeutic effects of PMS on T2DM and its underlying mechanisms.
In this study, we initially cultured MIN6 cells to investigate the protective effects of PMS against HG and PA-induced cell damage. We subsequently employed transcriptomic analysis to identify the critical pathways and core genes through which PMS protects MIN6 cells. Building upon these transcriptomic findings, we examined the effects of PMS on apoptosis and endoplasmic reticulum stress (ERS) in vitro. Finally, we conducted in vivo experiments using a T2DM mouse model and administered different doses of PMS to evaluate its therapeutic effects on T2DM. We also assessed the impact of PMS on the expression of Dnajc1, apoptosis, and ERS in pancreatic tissue.
The experimental reagents, pharmaceuticals, and other materials necessary for this study are described in detail in the supplementary materials.
Cell culture: The MIN6 mouse insulinoma cell line was obtained from Shanghai Fuheng Biotechnology Co., Ltd. (Xi’an, China) MIN6 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (double antibiotics). The cells were incubated in a cell culture incubator (37 °C, 5% CO2) for 24 hours to observe cell growth and morphology. Cells at 70% to 80% confluence were chosen for subsequent experiments.
Induction of diabetic model: Hyperglycemia and hyperlipidemia, conditions frequently observed in patients with T2DM, substantially contribute to the damage of pancreatic β-cells. To replicate the process of pancreatic β-cell injury in T2DM, a combination of high concentrations of glucose and PA was employed, as documented in previous studies[23]. Briefly, MIN6 cells were treated with 40 mmol/L glucose and 0.4 mmol/L PA for 24 hours following attachment to induce the diabetic model.
Gene silencing: MIN6 cells in the logarithmic growth phase were adjusted to a density of 2 × 105 cells/mL and plated into 6-well plates. Upon reaching approximately 70% confluence, cells were transfected with Dnajc1-specific siRNA sequences and a negative control sequence (si-NC) using Lipo2000 transfection reagent at the manufacturer's recommended dosage. The cells were then cultured for an additional 6 hours. The sequence for siDnajc1 was GCAAGA
MTT assay: MIN6 cells were seeded at a density of 1 × 104 cells per well in a 96-well plate. After incubating the cells for 24 hours in a 5% CO2, 37 °C incubator, they were subjected to drug intervention according to their designated groups. Subsequently, 10 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) solution was added to each well. The plate was incubated at 37 °C for an additional 4 hours. To halt the reaction, 100 μL of DMSO was added to each well. Absorbance was measured at a wavelength of 550 nm, and cell viability was calculated based on the absor
Detection of insulin, reactive oxygen species, and lactate dehydrogenase levels: MIN6 cells were plated at a density of 2 × 105 cells per well in 6-well plates. After 24 hours of drug intervention, the supernatant was collected. Insulin levels were measured by ELISA. The activity of lactate dehydrogenase (LDH) was measured using a commercial LDH assay kit. Additionally, the levels of reactive oxygen species (ROS) was measured using a ROS detection assay kit.
Transcriptomics analysis: Total RNA was extracted from cells of each experimental group using Trizol. mRNA was enriched by magnetic beads with Oligo (dT) and subsequently fragmented. Complementary DNA (cDNA) was synthesized using mRNA as a template. After purification with magnetic beads, cDNA underwent end repair, adenylation of 3' ends, and ligation of sequencing adapters. The final cDNA library was enriched by PCR. We assessed the quality and quantity of the cDNA library using the Agilent 2100 Bioanalyzer microfluidic chip analysis system and the ABI 7500 real-time fluorescent quantitative PCR instrument.
Flow cytometry for apoptosis detection: Cells were collected from each experimental group and counted using a cell counting chamber. A total of 2 × 105 cells were resuspended, and the supernatant was discarded after centrifugation. The cells were washed once with PBS and then resuspended in 500 μL of diluted 1 × annexin V binding buffer. A total of 5 μL Annexin-V-FITC and 5 μL propidium iodide staining solution was added to the cell suspension. After gentle vortex mixing, the cells were incubated at room temperature in the dark for 20 minutes, and the apoptosis rate was measured by flow cytometry.
Animals: Specific pathogen-free grade, 6-8-week-old healthy male C57BL/6 mice weighing 20 ± 1 g were procured from Beijing Huafukang Bio-technology Co., Ltd. [Production License Number: SYXK (Jing) 2019-0030; Beijing, China]. Mice were housed in groups of five per cage at a temperature of 24 ± 2 °C, with a relative humidity of 55% ± 5%, and a 12-hour light/dark cycle, with ad libitum access to food and water. All experimental procedures followed the Guidelines for Animal Ethics and were approved by Cangzhou Hospital of Integrated Traditional Chinese Medicine and Western Medicine of Hebei Province (Approval Number: CZX2024-KY-052). Details regarding materials and reagent kits used in this study are provided in the Supplementary Materials.
Modeling, grouping, and drug administration: High-fat diet combined with intraperitoneal injection of streptozotocin (STZ) was utilized to replicate T2DM model in mice. Briefly, mice were fed a high-fat/high-sugar diet (HFD) for 8 weeks. At the end of the 8th week, a single intraperitoneal injection of STZ was administered at a dose of 30 mg/kg. After STZ injection, the mice were maintained on the HFD for an additional 4 weeks. Successful establishment of the T2DM model was confirmed by a random blood glucose level of ≥ 16.7 mmol/L.
For animal grouping, 50 C57BL/6J mice were used. After acclimatizing the mice for 1 week, they were randomly divided into five groups: Control, T2DM, metformin group, PMS low-dose group (PMS-L), and PMS high-dose group (PMS-H). The Control group received a standard diet, while the other groups received HFD and STZ injection to induce T2DM. After STZ injection, mice in the Control and T2DM groups received 0.2 mL of physiological saline daily via oral gavage, whereas those in the PMS-L and PMS-H groups received 50 mg/kg and 100 mg/kg of PMS daily via oral gavage. All drug treatments were 4 weeks long.
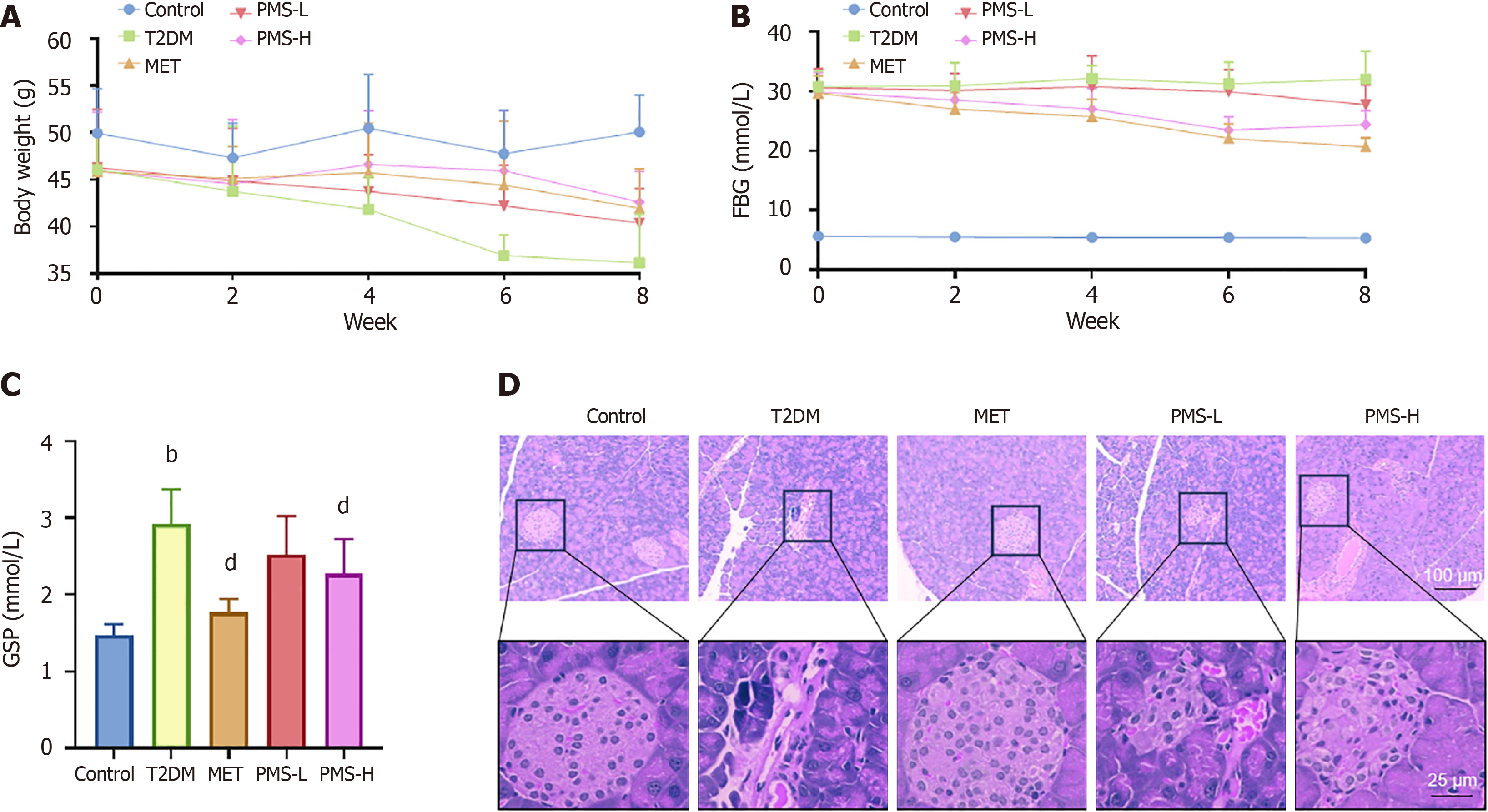
Changes in body weight and Fasting Blood Glucose (FBG) levels were monitored weekly throughout the 8-week treatment period. At the end of the treatment period, mice underwent an overnight fast with access to water. Following anesthesia with sodium pentobarbital (50 mg/kg), blood samples were collected from the abdominal aorta, centrifuged to obtain the supernatant, and stored at -80 °C. Subsequently, pancreatic tissue samples were collected, with a portion fixed in formalin solution and the remainder stored in cryovials at -80 °C for further analysis.
Detection of glycated serum protein: After allowing the serum from each group of mice to equilibrate to room temperature, glycated serum protein (GSP) levels were measured by ELISA.
Paraffin sections were prepared from fixed pancreatic tissues. General pathological changes and inflammatory conditions of the pancreatic tissues from each group were analyzed by hematoxylin and eosin (HE) staining. Additionally, apoptosis was assessed by TUNEL staining.
For in vitro experiments, 1 × 106 cells per sample were collected, and for in vivo experiments, 30 mg of pancreatic tissue was collected per sample. Samples were placed in ice-cold RIPA buffer and total protein was extracted using ultrasonic homogenization. The protein concentration in the supernatant was determined by BCA assay. After adding protein loading buffer, the samples were heated at 99 °C for 5 minutes to fully denature the proteins. Denatured proteins were separated by SDS-polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene fluoride membrane. The membrane was blocked with 5% skim milk powder for 1 hour, followed by overnight incubation with the primary antibody against the target protein at 4 °C. After washing the membrane three times with Tris-buffered saline with Tween-20, it was incubated with HRP-labeled secondary antibody for 1 hour at room temperature. Following further washing, the membrane was developed using enhanced chemiluminescence and the bands were quantitatively analyzed using Image J software.
Statistical analysis was performed using SPSS 22.0 software (IBM Corp., Armonk, NY, United States). All data are presented as mean ± SD. Inter-group differences were assessed using one-way analysis of variance, followed by Tukey's Honestly Significant Difference test. A P value less than 0.05 was considered statistically significant.
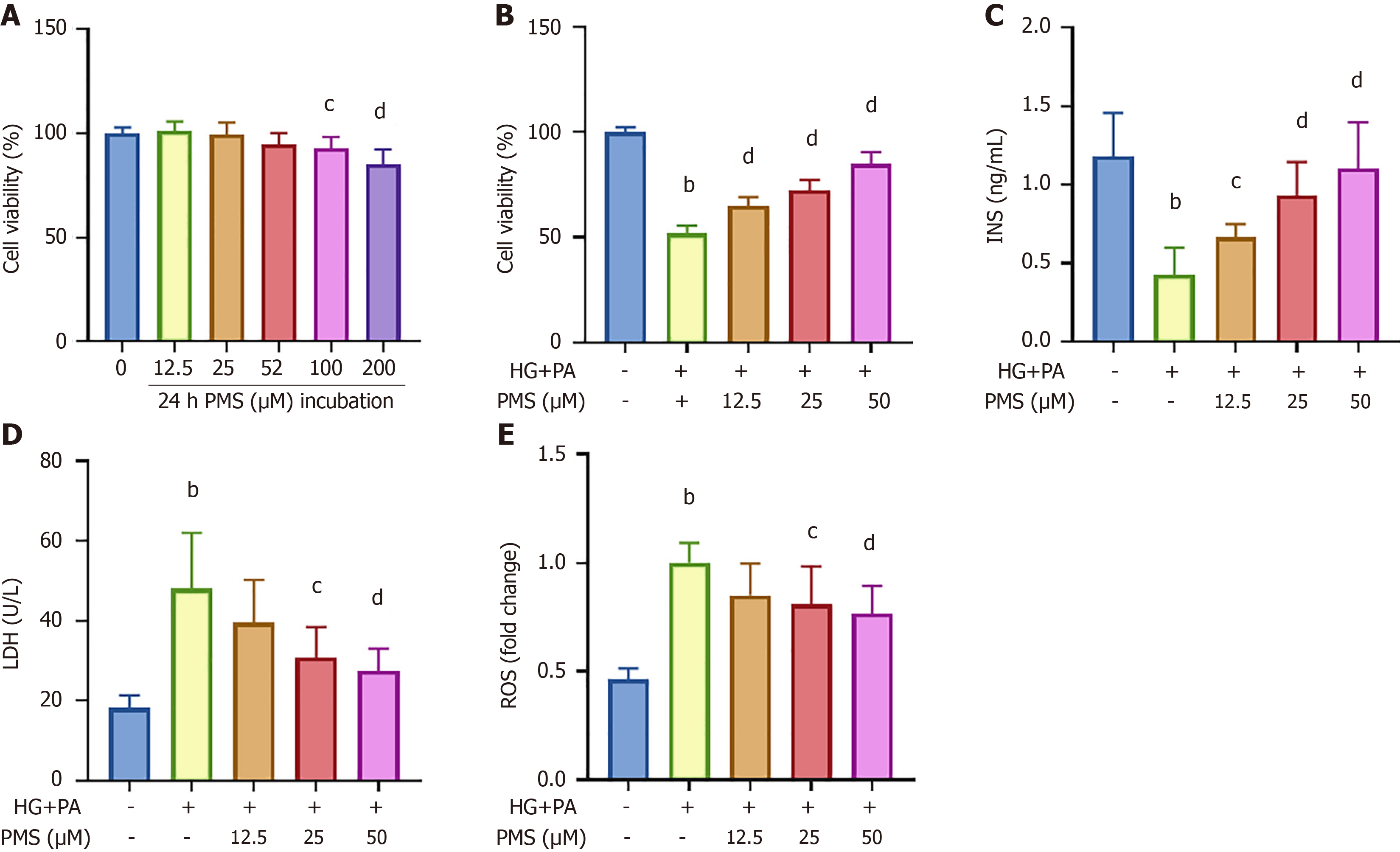
We initially conducted MTT assays using varying concentrations of PMS (0 μM, 12.5 μM, 25 μM, 50 μM, 100 μM and 200 μM) to assess its cytotoxic effects on MIN6 cells. The results (Figure 1A) demonstrated that 12.5 μM, 25 μM, and 50 μM PMS did not significantly impact MIN6 cell viability. Therefore, these concentrations were chosen for further investigation. Subsequently, we induced damage in MIN6 cells using HG + PA and treated them with different PMS concentrations. We then measured cell activity by MTT assay (Figure 1B). Meanwhile, we evaluated the effects of PMS on pancreatic β-cell damage by measuring insulin content in the cell supernatant, intracellular ROS levels, and LDH activity in the supernatant (Figure 1C-E). Our findings indicated that HG + PA treatment resulted in reduced insulin content in the MIN6 cell supernatant, increased intracellular ROS levels, and enhanced LDH activity in the supernatant, confirming successful cell damage induction. Intervention with PMS mitigated these effects, thus ameliorating cell damage, with the most pronounced improvement observed at 50 μM PMS. Therefore, 50 μM PMS was used for all subsequent mechanistic studies on MIN6 cells.
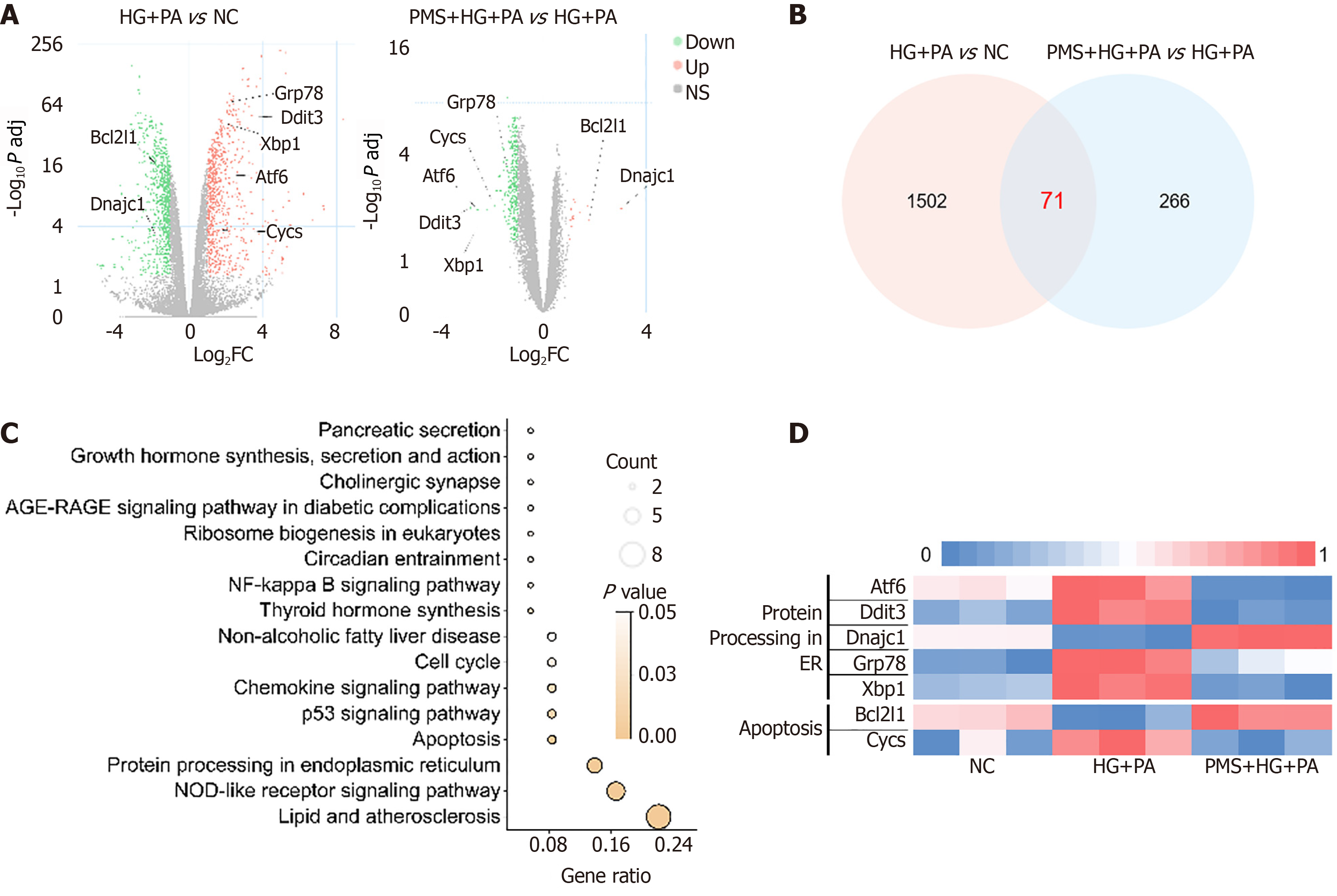
We employed transcriptome sequencing to examine how PMS intervention affects mRNA expression in the HG + PA-induced MIN6 damage model. Using the criteria of P adj < 0.05 and |Log2 (Fold change) |> 1, we identified differentially expressed genes between the HG + PA and normal control (NC) groups, as well as between the PMS + HG + PA and HG + PA groups. The results revealed 1573 differentially expressed genes between the HG + PA and NC groups, and 337 genes between the PMS + HG + PA and HG + PA groups, with 71 genes common to both comparisons (Supplementary Table 1). Interestingly, KEGG pathway analysis revealed enrichment of pathways related to the endoplasmic reticulum, including Protein processing in the endoplasmic reticulum (mmu04141). Additionally, the Apoptosis pathway (mmu04215) was also enriched, and apoptosis is widely associated with abnormal endoplasmic reticulum function. ERS-induced apoptosis is a significant factor contributing to pancreatic β-cell damage in T2DM. Therefore, we proceeded to investigate the effects of PMS on endoplasmic reticulum function, particularly ERS, and apoptosis (Figure 2).
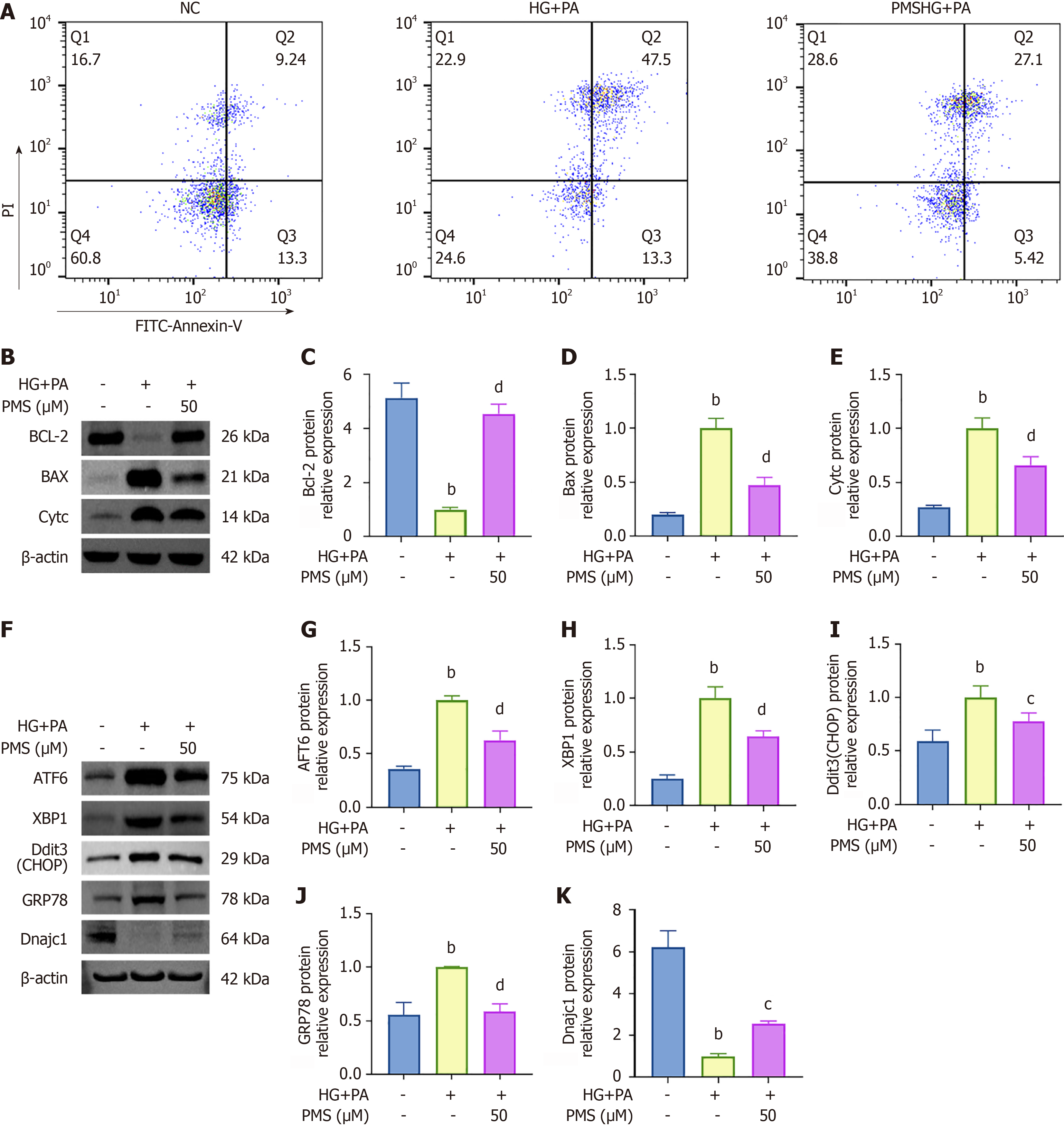
We employed flow cytometry to quantify the apoptosis rate, and Western blot analysis was utilized to assess the levels of apoptosis-related proteins Bcl-2, Bax, and CytC. Flow cytometry results (Figure 3A) demonstrated an increase in the apoptosis rate following HG + PA intervention, which decreased after PMS intervention. Western blot analysis (Figure 3B-E) revealed that following HG + PA intervention, the expression of the anti-apoptotic protein Bcl-2 decreased, while the expression of the pro-apoptotic proteins Bax and CytC increased. However, after PMS intervention, the expression of Bcl-2 was upregulated, while the expression of Bax and CytC was downregulated.
Moreover, PMS intervention exerted regulatory effects on genes implicated in protein processing in the endoplasmic reticulum, including ATF6, GRP78, Dnajc1, Sec24a, Sec62, Ssr3, Hsph1, Sar1b, Txndc5, Ddit3, and XBP1. Aberrant expression of these genes is closely associated with ERS. ERS emerges from the accumulation of unfolded proteins in the endoplasmic reticulum, leading to the activation of GRP78 (also known as BIP), which subsequently triggers downstream factors such as ATF6, XBP1, and CHOP, thereby exacerbating ERS. Dnajc1, a co-chaperone molecule of GRP78, inhibits GRP78 activation, thereby suppressing ERS[24,25]. Therefore, we focused on key factors in the ERS pathway, including ATF6, XBP1, Ddit3 (CHOP), GRP78, and Dnajc1, for validation. Western blot analysis was employed to assess the expression of ERS-related proteins ATF6, XBP1, Ddit3 (CHOP), GRP78, and Dnajc1 in the control group, HG + PA group, and PMS-treated group. The results revealed that in the HG + PA group, the expression of ATF6, XBP1, Ddit3 (CHOP), and GRP78 increased, whereas the expression of Dnajc1 decreased. Conversely, PMS treatment downregulated the expression of ATF6, XBP1, Ddit3 (CHOP), and GRP78, while upregulating the expression of Dnajc1 (Figure 3F-K).
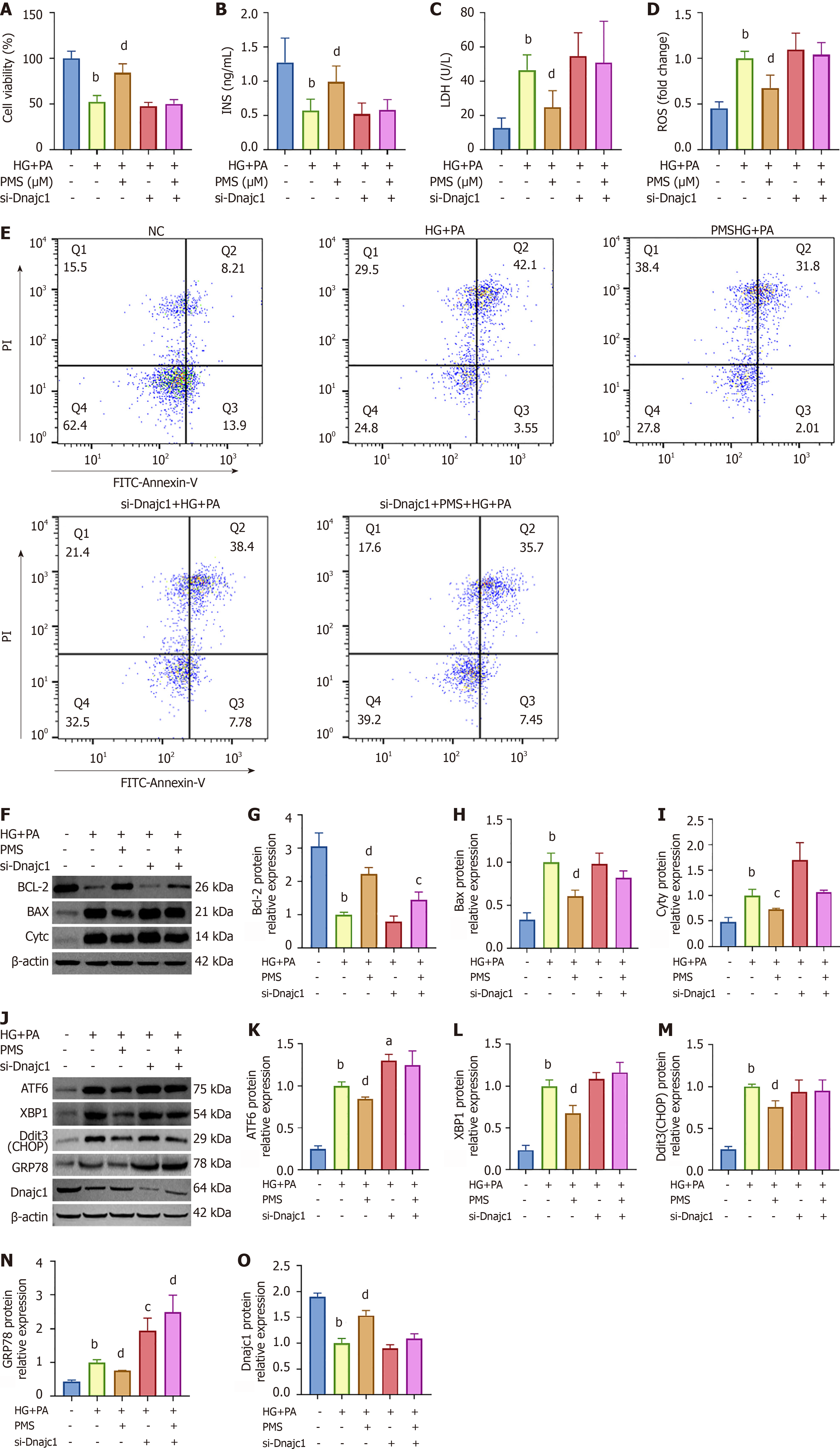
To validate the impact of PMS on the key ERS factor Dnajc1, we employed siRNA to silence the Dnajc1 gene in MIN6 cells. We investigated the effects of PMS on MIN6 cell damage following Dnajc1 silencing. The experiment included five groups: NC group, HG + PA group, PMS-H + HG + PA group, si-Dnajc1 + HG + PA group, and si-Dnajc1 + PMS-H + HG + PA group. We initially conducted MTT assays the viability of MIN6 cells (Figure 4A). Subsequently, we observed that after Dnajc1 silencing, the effects of PMS on insulin content in the supernatant, LDH activity, and intracellular ROS levels in the HG+PA-induced MIN6 damage model were attenuated or abolished (Figure 4B-D).
Additionally, we examined the impact of PMS on apoptosis and ERS in MIN6 cells after Dnajc1 silencing. We found that following Dnajc1 silencing, the effects of PMS on the apoptosis rate and the expression of apoptosis-related proteins Bcl-2, Bax, and CytC in HG + PA-induced MIN6 cells were not significantly different from those observed in MIN6 cells induced by HG + PA alone (Figure 4E-I). Similarly, after Dnajc1 silencing, the regulatory effects of PMS on the expression of ERS-related proteins ATF6, XBP1, Ddit3 (CHOP), and GRP78 disappeared (Figure 4J-O).
We next conducted in vivo experiments to investigate the therapeutic effects of PMS on T2DM. The results demonstrated a significant decrease in the body weight of T2DM mice, which partially recovered after PMS treatment (Figure 5A). Moreover, the levels of FBG and GSP in T2DM mice were notably elevated, and both significantly decreased following PMS treatment (Figure 5B and C). Histological examination of the pancreas using HE staining revealed that the islet structure in normal mice was intact and regular, while it appeared disrupted, irregular, and poorly demarcated from surrounding tissues in T2DM mice, making them difficult to identify. Following PMS intervention, these structural abnormalities were improved to varying degrees (Figure 5D). These findings indicate that PMS effectively ameliorates T2DM in mice, with the highest dose of PMS demonstrating the most pronounced effects.
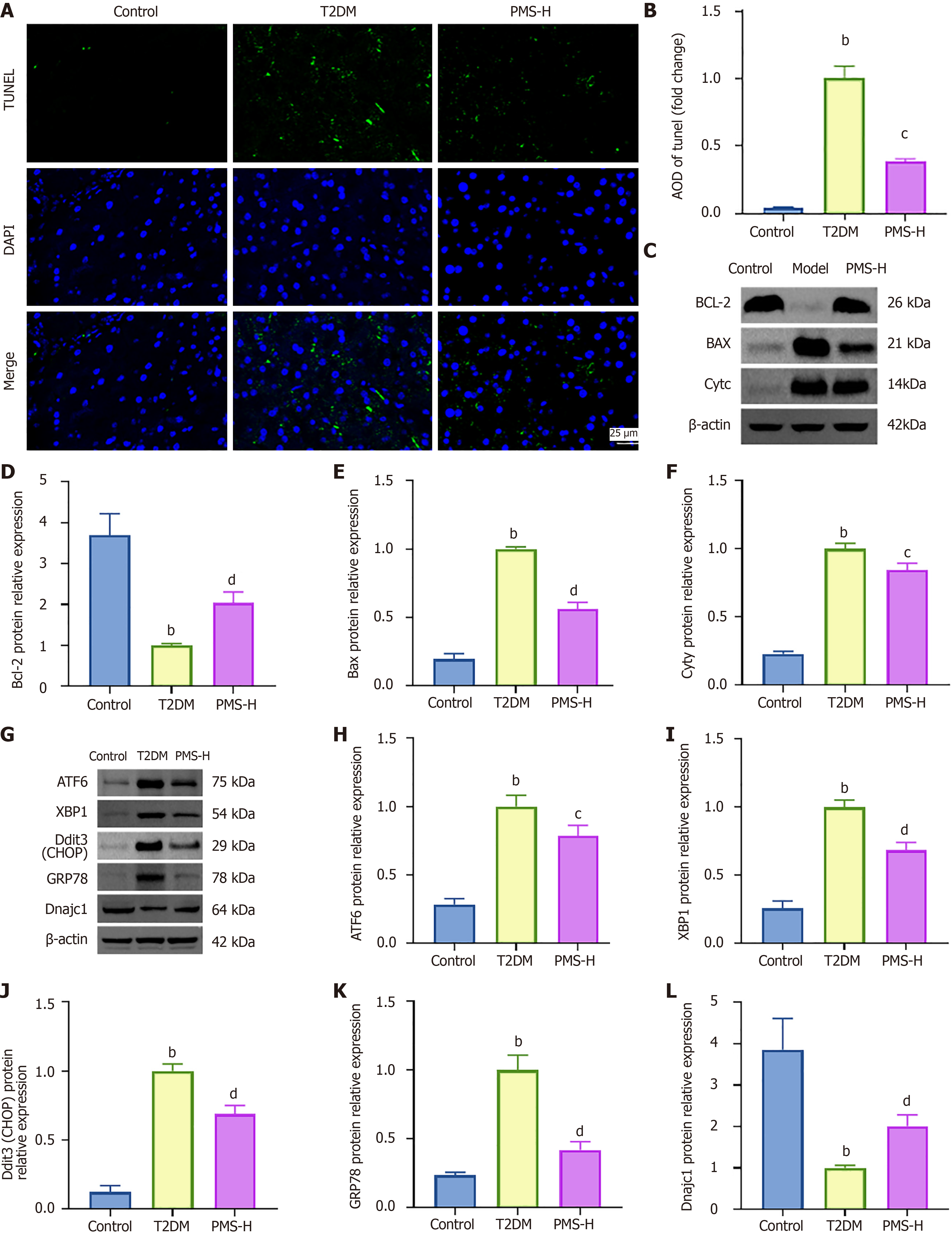
We utilized TUNEL staining and Western blot analysis to evaluate the impact of PMS on the expression of apoptosis-related proteins in the T2DM mouse pancreas. The results revealed (Figure 6A and B) a higher area of TUNEL-positive expression in the pancreatic tissue of T2DM mice compared to the Control group. Following PMS intervention, the area of TUNEL-positive cell expression decreased compared to the T2DM group. Moreover, in comparison to the control group, T2DM mice exhibited reduced expression of the anti-apoptotic protein Bcl-2 and increased expression of the pro-apoptotic proteins Bax and CytC. However, after PMS treatment, the expression level of Bcl-2 increased, while the expression levels of Bax and CytC decreased (Figure 6C-F).
Furthermore, Western blot analysis was employed to investigate the effects of PMS on the expression of ERS-related proteins in the pancreatic tissue of T2DM mice. The results indicated higher protein expression levels of ATF6, XBP1, Ddit3 (CHOP), and GRP78 in the pancreatic tissue of T2DM mice compared to the Control group, while the expression of Dnajc1 was decreased. PMS intervention downregulated the expression of ATF6, XBP1, Ddit3 (CHOP), and GRP78 and upregulated the expression of Dnajc1 (Figure 6G-L).
In this experiment, we induced damage in MIN6 cells using HG + PA. Compared to single-factor treatments that induce acute cell injury, such as high concentrations of glucose or insulin, this method more accurately mimics the pathogenic process in the body[26]. The level of insulin in the cell supernatant serves as a crucial indicator for assessing pancreatic β-cell function integrity[27]. Studies have demonstrated that elevated glucose levels can diminish insulin secretion by MIN6 cells. Additionally, the accumulation of ROS and LDH correlated with the progression of T2DM[28]. Our findings indicated that induction with HG + PA resulted in reduces insulin content in the MIN6 cell supernatant, increased intracellular ROS levels, and enhanced LDH activity in the supernatant, confirming successful cell damage induction. However, following PMS intervention, these indicators exhibited marked improvement. These findings affirm the protective effect of PMS against HG + PA-induced pancreatic β-cell damage, which was further corroborated by in vivo experiments.
Transcriptomics analysis indicates that apoptosis and ERS are important pathways through which PMS improves pancreatic β-cell damage. Our in vitro experimental findings demonstrate that PMS inhibits apoptosis in pancreatic β-cells induced by HG+PA. Specifically, PMS downregulates the expression of Bax and Cytochrome c (Cyc), while upregulating the expression of Bcl-2. The Bcl-2 protein family plays a crucial role in regulating apoptotic cell death. The pro-apoptotic factor Bax is primarily inactive due to its interaction with the anti-apoptotic Bcl-2 protein[29]. Upon activation of an apoptotic signal, Bax translocates to the mitochondria, leading to the release of Cyc and initiation of apoptosis[30]. Furthermore, our in vivo experiments also confirm the inhibitory effect of PMS on apoptosis in the pancreatic tissue of T2DM mice.
Insulin synthesis and modification occur in the rough endoplasmic reticulum of pancreatic β-cells, followed by transportation to the Golgi apparatus and eventual release into the bloodstream[31]. The transit from the endoplasmic reticulum to the Golgi apparatus is a critical step in insulin synthesis and secretion. Dysregulation of this transport process leads to ERS, which significantly contributes to pancreatic β-cell dysfunction and apoptosis. Pancreatic β-cells exhibit high sensitivity to ERS, with factors like HG, high lipids, and cytokines playing pivotal roles in insulin resistance and pancreatic β-cell damage in T2DM[32]. Our in vitro experimental findings indicate that PMS downregulates ERS-related proteins, including ATF6, XBP1, Ddit3 (CHOP), and GRP78. GRP78 serves as the principal regulator of the N-termini of ER transmembrane proteins. Upon accumulation of unfolded proteins in the ER, GRP78 dissociates, activating downstream PERK, IRE1, and ATF6 signaling pathways[33]. CHOP is a critical regulator of the PERK signaling pathway, while XBP-1 plays a key role in the IRE1 signaling pathway by regulating lipid biosynthetic enzymes and ER-associated degradation components[33]. Pathologically, when unfolded proteins accumulate in the ER, GRP78 release senses and transmits unfolded protein response (UPR) signals. Activation of the PERK, IRE1, and ATF6 ERS signaling pathways recruits TRAF2 and ASK1, leading to JNK and NF-kB activation, ultimately resulting in inflammation and apoptosis. Our in vivo experiments also demonstrate that PMS can inhibit the expression of ERS-related factors, such as ATF6, XBP1, Ddit3 (CHOP), and GRP78.
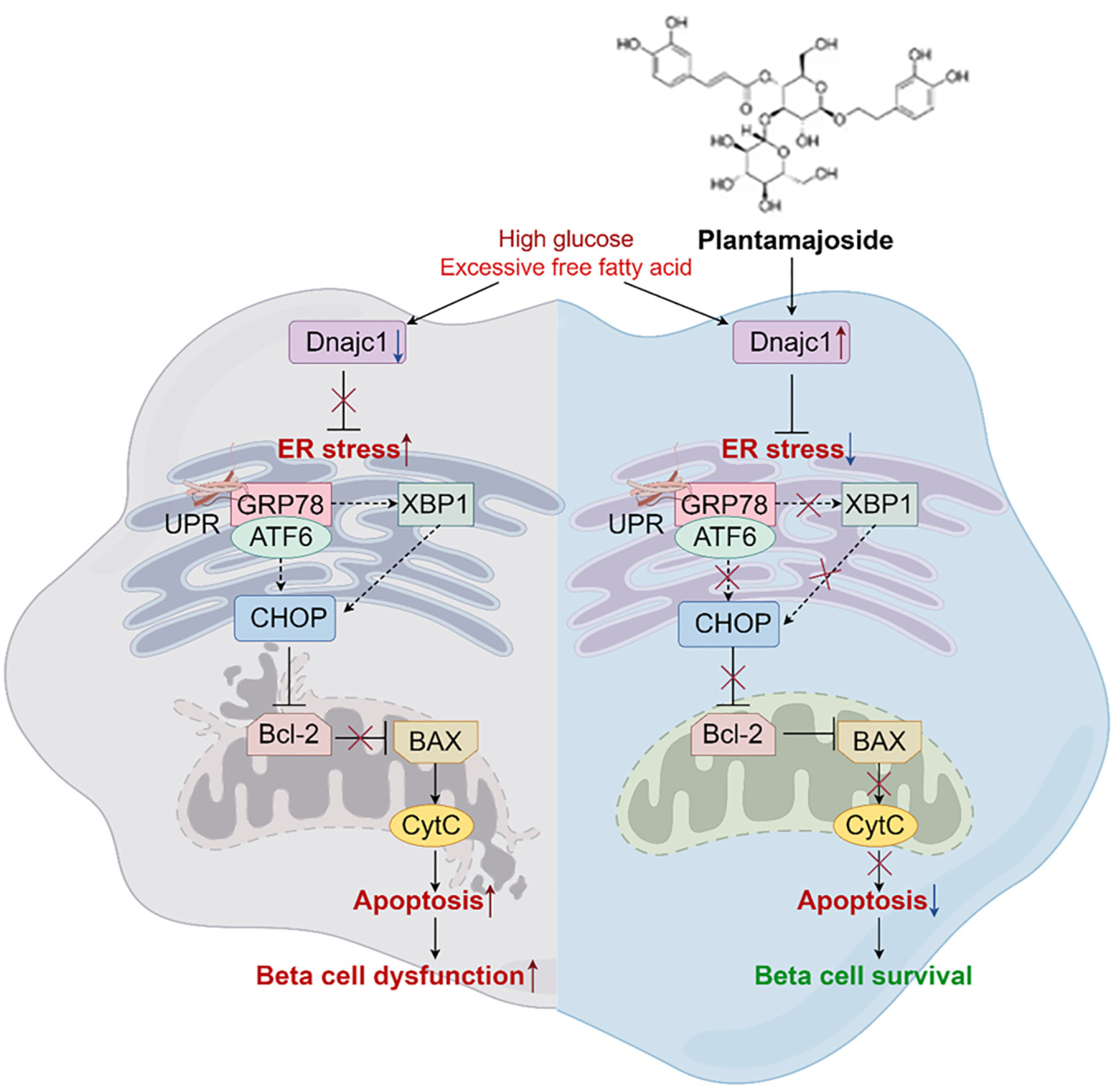
Interestingly, our transcriptomics results revealed a statistically significant change in expression of the ERS-related factor Dnajc1 across groups. Dnajc1 belongs to the C1 member of the heat shock protein DNAJ family[34,35]. The membrane protein encoded by Dnajc1 is a heat shock protein similar to DNAJ, containing two SANT domains that can bind to the molecular chaperone GRP78, forming a stable GRP78/Dnajc1 co-chaperone folding enzyme cycle[24,25]. This cycle promotes the correct folding of proteins and inhibits ERS.
Studies have demonstrated that Dnajc1 promotes the ATPase activity of GRP78 in a dose-dependent manner, and the interaction between Dnajc1 and GRP78 effectively ameliorates ERS[35]. Additionally, research on the association between ERS genes and obesity traits and related metabolic disorders in adults has indicated a strong correlation between Dnajc1 and body mass index[36]. Moreover, silencing Dnajc1 leads to the accumulation of GRP78 in the ER, exacerbating the UPR and promoting ERS[37]. Our findings suggest that PMS intervention promotes Dnajc1 expression, and this promoting effect of PMS on Dnajc1 expression disappears after silencing Dnajc1. This indicates that PMS alleviates ERS and cell apoptosis by enhancing Dnajc1 expression.
In our study, we used of a single strain of db/db mice. However, more animal strains can be used to evaluate the effects of PMS across sex, age, or other diabetes models. Moreover, our results revealed that silencing of Dnajc1 abolished the inhibitory effects of PMS on apoptosis and ERS in vitro. However, our in vitro model (HG + PA-induced β-cell injury) cannot fully reflect the changes during the progression of T2DM in vivo, as too many pathological processes (e.g., oxidative stress, chronic inflammation, the production of cytotoxic metabolites, etc.) occurs in vivo. Many factors could contribute to the β-cell injury independent of excessive glucose and lipids. Therefore, conditional knockout mice using Cre-LoxP technique should be used in future studies to knockout Dnajc1 in β-cells to deeply verify the protective mechanisms of PMS on T2DM. Furthermore, the detailed mechanisms of PMS on enhancing Dnajc1 still need to be studied. Cellular thermal shift assay and drug affinity responsive target stability can be used to find the direct targets of PMS on β-cell.
This study underscores the potential of PMS in treating T2DM. Expanding on this, we have observed that PMS mitigates pancreatic β-cell injury in T2DM mice by enhancing Dnajc1expression, thereby suppressing ERS and apoptosis in these cells. While the precise mechanisms through which PMS regulates Dnajc1 expression necessitate further investigation, this study lays a solid groundwork for such inquiries. In future research, integrating techniques such as knockout mice and single-cell RNA sequencing to delve deeper into the mechanisms of PMS in T2DM treatment will furnish robust support for clinical trials and the application of PMS (Figure 7).
| 1. | Buttermore E, Campanella V, Priefer R. The increasing trend of Type 2 diabetes in youth: An overview. Diabetes Metab Syndr. 2021;15:102253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Ogurtsova K, Guariguata L, Barengo NC, Ruiz PL, Sacre JW, Karuranga S, Sun H, Boyko EJ, Magliano DJ. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183:109118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 523] [Article Influence: 130.8] [Reference Citation Analysis (5)] |
| 3. | Lim LL, Chow E, Chan JCN. Cardiorenal diseases in type 2 diabetes mellitus: clinical trials and real-world practice. Nat Rev Endocrinol. 2023;19:151-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 4. | Ruze R, Liu T, Zou X, Song J, Chen Y, Xu R, Yin X, Xu Q. Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front Endocrinol (Lausanne). 2023;14:1161521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 417] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 5. | Newman DJ. Non-Insulin-Based Drug Entities Used to Treat Diabetes Type 2 Disease (T2DM), Based on Natural Products from All Sources. J Nat Prod. 2024;87:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Ke RQ, Wang Y, Hong SH, Xiao LX. Anti-diabetic effect of quercetin in type 2 diabetes mellitus by regulating the microRNA-92b-3p/EGR1 axis. J Physiol Pharmacol. 2023;74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Chen J, Meng X. Aronia melanocarpa Anthocyanin Extracts Improve Hepatic Structure and Function in High-Fat Diet-/Streptozotocin-Induced T2DM Mice. J Agric Food Chem. 2022;70:11531-11543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Ran Q, Wang J, Wang L, Zeng HR, Yang XB, Huang QW. Rhizoma coptidis as a Potential Treatment Agent for Type 2 Diabetes Mellitus and the Underlying Mechanisms: A Review. Front Pharmacol. 2019;10:805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Jiang YY, Shui JC, Zhang BX, Chin JW, Yue RS. The Potential Roles of Artemisinin and Its Derivatives in the Treatment of Type 2 Diabetes Mellitus. Front Pharmacol. 2020;11:585487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Rubio-Navarro A, Gómez-Banoy N, Stoll L, Dündar F, Mawla AM, Ma L, Cortada E, Zumbo P, Li A, Reiterer M, Montoya-Oviedo N, Homan EA, Imai N, Gilani A, Liu C, Naji A, Yang B, Chong ACN, Cohen DE, Chen S, Cao J, Pitt GS, Huising MO, Betel D, Lo JC. A beta cell subset with enhanced insulin secretion and glucose metabolism is reduced in type 2 diabetes. Nat Cell Biol. 2023;25:565-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 11. | Kerper N, Ashe S, Hebrok M. Pancreatic β-Cell Development and Regeneration. Cold Spring Harb Perspect Biol. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Ni YH, Song LJ, Xiao B. Magnetic resonance imaging for acute pancreatitis in type 2 diabetes patients. World J Clin Cases. 2023;11:7268-7276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Chen X, Wu J, Fu X, Wang P, Chen C. Fructus mori polysaccharide alleviates diabetic symptoms by regulating intestinal microbiota and intestinal barrier against TLR4/NF-κB pathway. Int J Biol Macromol. 2023;249:126038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 14. | Deng S, Yang L, Ma K, Bian W. Astragalus polysaccharide improve the proliferation and insulin secretion of mouse pancreatic β cells induced by high glucose and palmitic acid partially through promoting miR-136-5p and miR-149-5p expression. Bioengineered. 2021;12:9872-9884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Fei Z, Xu Y, Zhang G, Liu Y, Li H, Chen L. Natural products with potential hypoglycemic activity in T2DM: 2019-2023. Phytochemistry. 2024;223:114130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 16. | Du XX, Tao X, Liang S, Che JY, Yang S, Li H, Chen JG, Wang CM. Hypoglycemic Effect of Acidic Polysaccharide from Schisandra chinensis on T2D Rats Induced by High-Fat Diet Combined with STZ. Biol Pharm Bull. 2019;42:1275-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Jiang H, Feng S, Zhang P, Wang J, Jiang Y, Zhang H, Song X, Huang W, Xie Y, Deng C. Petroleum ether extract of Schisandra sphenanthera prevents hyperglycemia and insulin resistance in association with modulation of sweet taste receptors and gut microbiota in T2DM rats. J Ethnopharmacol. 2024;331:118300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 18. | Li D, Jiang C, Mei G, Zhao Y, Chen L, Liu J, Tang Y, Gao C, Yao P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 19. | Liu F, Huang X, He JJ, Song C, Peng L, Chen T, Wu BL. Plantamajoside attenuates inflammatory response in LPS-stimulated human gingival fibroblasts by inhibiting PI3K/AKT signaling pathway. Microb Pathog. 2019;127:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Zeng G, An H, Fang D, Wang W, Han Y, Lian C. Plantamajoside protects H9c2 cells against hypoxia/reoxygenation-induced injury through regulating the akt/Nrf2/HO-1 and NF-κB signaling pathways. J Recept Signal Transduct Res. 2022;42:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Xiao D, Yang R, Gong L, Zhang Y, Xie Y, Ni S. Plantamajoside inhibits high glucose-induced oxidative stress, inflammation, and extracellular matrix accumulation in rat glomerular mesangial cells through the inactivation of Akt/NF-κB pathway. J Recept Signal Transduct Res. 2021;41:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Choi SY, Jung SH, Lee HS, Park KW, Yun BS, Lee KW. Glycation inhibitory activity and the identification of an active compound in Plantago asiatica extract. Phytother Res. 2008;22:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Lu Y, Huang R, Sun Z, Ou Y. A bovine milk-derived peptide ameliorates pancreatic β-cell dedifferentiation through PI3K/Akt/FOXO1 signaling in type 2 diabetes. Food Funct. 2023;14:8018-8029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 24. | Diane A, Mahmoud N, Bensmail I, Khattab N, Abunada HA, Dehbi M. Alpha lipoic acid attenuates ER stress and improves glucose uptake through DNAJB3 cochaperone. Sci Rep. 2020;10:20482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Islam Z, Diane A, Khattab N, Dehbi M, Thornalley P, Kolatkar PR. DNAJB3 attenuates ER stress through direct interaction with AKT. PLoS One. 2023;18:e0290340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | QiNan W, XiaGuang G, XiaoTian L, WuQuan D, Ling Z, Bing C. Par-4/NF-κB Mediates the Apoptosis of Islet β Cells Induced by Glucolipotoxicity. J Diabetes Res. 2016;2016:4692478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Zhou F, Zhang L, Zhu K, Bai M, Zhang Y, Zhu Q, Wang S, Sheng C, Yuan M, Liu Y, Lu J, Shao L, Wang X, Zhou L. SIRT2 ablation inhibits glucose-stimulated insulin secretion through decreasing glycolytic flux. Theranostics. 2021;11:4825-4838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 28. | Stancic A, Saksida T, Markelic M, Vucetic M, Grigorov I, Martinovic V, Gajic D, Ivanovic A, Velickovic K, Savic N, Otasevic V. Ferroptosis as a Novel Determinant of β-Cell Death in Diabetic Conditions. Oxid Med Cell Longev. 2022;2022:3873420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Spitz AZ, Gavathiotis E. Physiological and pharmacological modulation of BAX. Trends Pharmacol Sci. 2022;43:206-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 204] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 30. | Park MY, Ha SE, Vetrivel P, Kim HH, Bhosale PB, Abusaliya A, Kim GS. Differences of Key Proteins between Apoptosis and Necroptosis. Biomed Res Int. 2021;2021:3420168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Lee JH, Lee J. Endoplasmic Reticulum (ER) Stress and Its Role in Pancreatic β-Cell Dysfunction and Senescence in Type 2 Diabetes. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 32. | Chen X, Shi C, He M, Xiong S, Xia X. Endoplasmic reticulum stress: molecular mechanism and therapeutic targets. Signal Transduct Target Ther. 2023;8:352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 566] [Article Influence: 188.7] [Reference Citation Analysis (0)] |
| 33. | Yi X, Cai X, Wang S, Xiao Y. Mechanisms of impaired pancreatic βcell function in highfat dietinduced obese mice: The role of endoplasmic reticulum stress. Mol Med Rep. 2020;21:2041-2050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Kroczynska B, King-Simmons L, Alloza L, Alava MA, Elguindi EC, Blond SY. BIP co-chaperone MTJ1/ERDJ1 interacts with inter-alpha-trypsin inhibitor heavy chain 4. Biochem Biophys Res Commun. 2005;338:1467-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Chevalier M, Rhee H, Elguindi EC, Blond SY. Interaction of murine BiP/GRP78 with the DnaJ homologue MTJ1. J Biol Chem. 2000;275:19620-19627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Martinez JA; MENA Project. DNA methylation signatures at endoplasmic reticulum stress genes are associated with adiposity and insulin resistance. Mol Genet Metab. 2018;123:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Misra UK, Gonzalez-Gronow M, Gawdi G, Pizzo SV. The role of MTJ-1 in cell surface translocation of GRP78, a receptor for alpha 2-macroglobulin-dependent signaling. J Immunol. 2005;174:2092-2097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |