Published online Feb 15, 2025. doi: 10.4239/wjd.v16.i2.101501
Revised: November 10, 2024
Accepted: December 2, 2024
Published online: February 15, 2025
Processing time: 104 Days and 16.9 Hours
Type 2 diabetes mellitus (T2DM) often leads to vascular complications, such as albuminuria. The role of insulin autoantibodies (IAA) and their interaction with D-dimer in this context remains unclear.
To investigate the characteristics of IAA and its effect on albuminuria in T2DM patients.
We retrospectively analyzed clinical data from 115 T2DM patients with positive IAA induced by exogenous insulin, and 115 age- and sex-matched IAA-negative T2DM patients as controls. Propensity scores were calculated using multivariate logistic regression. Key variables were selected using the least absolute shrinkage and selection operator (LASSO) algorithm. We constructed a prediction model and analyzed the association between IAA and albuminuria based on demo
The IAA-positive group had significantly higher D-dimer levels [0.30 (0.19-0.55) mg/L vs 0.21 (0.19-0.33) mg/L, P = 0.008] and plasma insulin levels [39.1 (12.0-102.7) μU/mL vs 9.8 (5.5-17.6) μU/mL, P < 0.001] compared to the IAA-negative group. Increases in the insulin dose per weight ratio, diabetes duration, and urinary albumin-to-creatinine ratio (UACR) were observed but did not reach statistical significance. The LASSO model identified plasma insulin and D-dimer as key factors with larger coefficients. D-dimer was significantly associated with UACR in the total and IAA-positive groups but not in the IAA-negative group. The odds ratio for D-dimer elevation (> 0.5 g/L) was 2.88 (95% confidence interval: 1.17-7.07) in the IAA-positive group (P interaction < 0.05).
D-dimer elevation is an independent risk factor for abnormal albuminuria and interacts with IAA in the development of abnormal albuminuria in T2DM patients.
Core Tip: This study explores the role of insulin autoantibodies (IAA) and their impact on albuminuria in patients with type 2 diabetes mellitus (T2DM). Our findings reveal that elevated D-dimer levels are a significant risk factor for abnormal albuminuria, particularly in the presence of IAA. This highlights a complex interaction between autoimmune responses to insulin and coagulation abnormalities, emphasizing the importance of monitoring D-dimer levels in T2DM patients with IAA for early detection and management.
- Citation: Zhang LS, Yu P, Yao F, Lu ZQ, Li XM, Chen H. Insulin autoantibodies, D-dimer and microalbuminuria: A cross-sectional, case-control study of type 2 diabetes. World J Diabetes 2025; 16(2): 101501
- URL: https://www.wjgnet.com/1948-9358/full/v16/i2/101501.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i2.101501
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by hyperglycemia, which can lead to long-term vascular complications affecting various organs, including the kidneys. One of the earliest indicators of diabetic nephropathy (DN) is microalbuminuria, which is an early marker of renal damage and a predictor of cardiovascular disease in diabetic patients[1,2]. D-dimer is a fibrin degradation product present in the blood after a blood clot dissolves, and it is commonly used as a biomarker for thrombotic activity. D-dimer is associated with microalbuminuria in patients with diabetes and this suggests that glomerular dysfunction is in part mediated by hypercoagulability[3]. High level of D-dimer is observed in patients with DN especially in T2DM than it in type 1 diabetes mellitus[4].
Insulin autoantibodies (IAA) could develop with exogenous insulin administration. Most do not produce clinical significance. However, at the sufficient concentration and affinity, the presence of IAA can interfere with insulin therapy by altering insulin pharmacokinetics, leading to variations in insulin absorption, distribution, and clearance. This can affect the efficacy and safety of insulin therapy and potentially impact broader clinical outcomes, including metabolic control and complications related to diabetes[5]. Several studies have investigated the role of IAA in type 1 diabetes, but there is limited research on the impact of IAA in type 2 diabetic patients[6,7]. However, in T2DM, which is mainly characterized by insulin resistance and not by autoimmunity, the implications of IAA are less clear.
Although there is no solid data supporting that IAAs are a direct cause of high coagulation, previous studies have confirmed that the autoimmune and inflammatory processes they are involved in might indirectly contribute to such a state[8]. Based on these, we hypothesize that the presence of IAA may be associated with an increased risk of albuminuria and that certain clinical parameters, such as inflammatory markers or D-dimer levels, may interact with IAA to influence the development of albuminuria. By investigating these interactions, we hope to elucidate the complex mechanisms underlying renal complications in T2DM and provide insights into potential therapeutic targets for preventing DN.
This study aims to fill this gap by analyzing the clinical data of T2DM patients with positive IAA induced by exo
The clinical data of patients with T2DM were retrospectively analyzed who were admitted to the Department of Endocrinology, Zhongshan Hospital Fudan University from January 2018 to March 2020. This study was approved by the Ethics Committee of Zhongshan Hospital, with approval number: B2020-201. Although this is a retrospective study, written informed consent was obtained from all participants prior to study enrollment. This aligns with our institution's standard protocol to ensure that participants are fully informed about the use of their clinical data for research purposes, thereby respecting their autonomy and maintaining transparency.
A total of 115 patients with positive IAA induced by exogenous insulin application were included in the study. Additionally, 115 T2DM patients with negative IAA treated with insulin, matched for age and sex, were included as the control group. This matching was done to control for potential confounding factors related to age and sex.
Patients were excluded: (1) With chronic kidney disease stages 3 or higher (estimated glomerular filtration rate < 60 mL/min/1.73 m²), as this could independently affect albuminuria and D-dimer levels; (2) With significant liver disease or liver enzyme abnormalities; (3) With use of medications that could induce IAA, such as methimazole, lipoic acid, captopril, fosinopril, irbesartan, glutathione, imipenem; (4) With recent thrombotic events; and (5) With inflammatory diseases, such as active inflammatory or autoimmune diseases.
T2DM is diagnosed based on the patient's self-reported history or on the results of fasting plasma glucose (FPG), hemoglobulin A1C (HbA1c), and symptoms according to American Diabetes Association criteria for the diagnosis of diabetes[9].
Hypertension is defined based on patients’ self-reported history or on repeated measurement of blood pressure. Diabetes duration was defined as the time since diagnosis. Urinary albumin-to-creatinine ratio (UACR) was used to define albuminuria, with abnormal albuminuria defined as UACR ≥ 30 mg/g.
Hypoglycemia is defined as blood glucose < 70 mg/dL (3.9 mmol/L) for the conscious individual with T2DM, either reported at home or during hospitalization[10].
Basic information including medical history, age, sex, and body mass index (BMI) was collected. Blood samples were taken after 12-hour fasting to measure FPG, fructosamine, HbA1c, glycated albumin, total cholesterol, triglyceride, low-density lipoprotein-cholesterol, high--density lipoprotein-cholesterol. Creatinine and uric acid were also measured. Inflammatory markers, including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin were measured. Serum insulin and C-peptide concentrations were detected using a chemiluminescence immunoassay (Roche Medical Solutions Diagnostics, Tarrytown, NY, United States). The IAA detection method was via Chemiluminescence Immunoassay. If the insulin level was high, polyethylene glycol precipitation (PEG) was performed. The qualitative assessment of circulating immune complexes was carried out via precipitation with polyethylene glycol followed by insulin assay of the supernatant. As previously described[11], circulating immune complexes were quantified by precipitation with PEG, followed by an insulin assay in the supernatant. Changes in serum insulin levels after PEG precipitation in healthy individuals were used as the control samples.
Propensity scores were calculated using multivariate logistic regression by setting the entry method, adjusting for demographic (age, sex) and laboratory parameters (HbA1c, BMI, blood pressure). The propensity score matching aimed to balance the baseline characteristics between the IAA-positive and IAA-negative groups.
The least absolute shrinkage and selection operator (LASSO) algorithm was used to select important variables for the prediction model. LASSO regression is a form of regularized regression that penalizes the absolute size of the regression coefficients, driving some coefficients to zero and thus performing variable selection.
STATA 18.0 for windows (Stata-Corp, College Station, TX, United States) was used for statistical analysis. All continuous variables were tested for normality using the Shapiro-Wilk test. Descriptive data are expressed as means ± SD or medians and interquartile ranges, according to the normal test. Variables that did not follow a normal distribution were analyzed using non-parametric tests (e.g., Mann-Whitney U test for comparisons between groups). For normally distributed variables, independent t-tests were used. Logistic regression was used to analyze the association of IAA and selected parameters with albuminuria. Odds ratios (ORs) and 95% confidence intervals (95%CIs) were calculated to quantify the strength of the associations. P values < 0.05 were considered statistically significant.
The baseline characteristics of the study population were summarized in Table 1. Age, gender distribution, BMI, blood pressure, HbA1c, and lipid profiles were similar between the two groups. Although the differences did not reach statistical significance, the IAA-positive group exhibited higher values for the insulin dose per weight ratio, diabetes duration, and UACR. Compared with the IAA-negative group, the IAA-positive group had significantly higher levels of D-dimer and plasma insulin. Specifically, the mean D-dimer level was 0.30 (0.19-0.55) mg/L in the IAA-positive group compared to 0.21 (0.19-0.33) mg/L in the IAA-negative group (P = 0.008). The median plasma insulin level was 39.1 (12.0-102.7) μU/mL in the IAA-positive group compared to 9.8 (5.5-17.6) μU/mL in the IAA-negative group (P < 0.001).
| IAA-negative (n = 115) | IAA-positive (n = 115) | Total (n = 230) | P value | |
| Age (years) | 62.13 ± 10.43 | 63.23 ± 9.72 | 62.68 ± 10.07 | 0.41 |
| Gender (male), n (%) | 66 (57.4) | 65 (56.5) | 131 (57.0) | 0.89 |
| BMI (kg/m2) | 26.00 ± 3.54 | 25.81 ± 3.57 | 25.91 ± 3.55 | 0.69 |
| Hypertension, n (%) | 61 (53.0) | 63 (54.8) | 124 (53.9) | 0.79 |
| Smoking, n (%) | 25 (21.74) | 32 (27.83) | 57 (24.78) | 0.29 |
| Uric acid (μmol/L) | 327.06 ± 91.31 | 309.04 ± 111.72 | 318.05 ± 102.21 | 0.18 |
| TC (mmol/L) | 4.27 ± 1.24 | 3.99 ± 1.28 | 4.13 ± 1.26 | 0.10 |
| LDL-C (mmol/L) | 2.26 ± 0.98 | 2.11 ± 1.01 | 2.19 ± 1.00 | 0.24 |
| HDL-C (mmol/L) | 1.13 ± 0.35 | 1.22 ± 0.49 | 1.18 ± 0.43 | 0.11 |
| Triglycerides (mmol/L) | 2.03 ± 1.55 | 2.04 ± 2.53 | 2.03 ± 2.09 | 0.96 |
| Homocysteine (μmol/L) | 11.10 ± 7.86 | 10.76 ± 7.77 | 10.93 ± 7.80 | 0.74 |
| WBC (× 109/L) | 6.17 ± 1.66 | 5.96 ± 1.71 | 6.06 ± 1.69 | 0.34 |
| ESR (mm/Hr) | 15.92 ± 16.04 | 18.84 ± 25.31 | 17.38 ± 21.20 | 0.29 |
| CRP (mg/L) | 4.30 ± 13.89 | 4.70 ± 22.89 | 4.50 ± 18.89 | 0.87 |
| PCT (ng/mL) | 0.03 (0.02-0.04) | 0.03 (0.02-0.05) | 0.03 (0.02-0.05) | 0.28 |
| D-dimer (mg/L) | 0.21 (0.19-0.33) | 0.30 (0.19-0.55) | 0.24 (0.19-0.41) | 0.008 |
| UACR (mg/g) | 12.7 (4.5-103.9) | 20.8 (3.9-285.5) | 15.8 (4.3-163.0) | 0.21 |
| eGFR (mL/min/1.73 m2) | 90.16 ± 16.27 | 87.64 ± 27.20 | 88.90 ± 22.39 | 0.39 |
| Diabetes duration (years) | 13.03 ± 6.79 | 14.88 ± 7.76 | 13.95 ± 7.33 | 0.056 |
| Insulin dosage (U/day) | 32 (24-40) | 36 (28-44) | 34 (26-44) | 0.12 |
| Insulin use duration (years) | 5 (1-9) | 5 (2-9) | 5 (2-9) | 0.99 |
| Insulin types | ||||
| Human insulin, n (%) | 41 (35.7) | 53 (46.1) | 94 (40.9) | 0.10 |
| Insulin analogs, n (%) | 77 (67.0) | 65 (56.5) | 142 (61.7) | 0.10 |
| Hypoglycemia, n (%) | 13 (11.3) | 26 (22.6) | 39 (17.0) | 0.02 |
| HbA1c (%) | 8.63 ± 1.70 | 8.90 ± 1.84 | 8.77 ± 1.77 | 0.24 |
| Fructosamine (%) | 22.69 ± 6.08 | 23.02 ± 5.86 | 22.86 ± 5.96 | 0.68 |
| FPG (mmol/L) | 8.30 ± 2.84 | 7.86 ± 3.20 | 8.08 ± 3.02 | 0.28 |
| Plasma insulin (μU/mL) | 9.8 (5.5-17.6) | 39.1 (12.0- 102.7) | 16.1 (7.6-48.5) | < 0.001 |
| Plasma C-peptide (ng/mL) | 1.39 (0.89-2.45) | 1.50 (0.94-2.39) | 1.46 (0.90-2.40) | 0.47 |
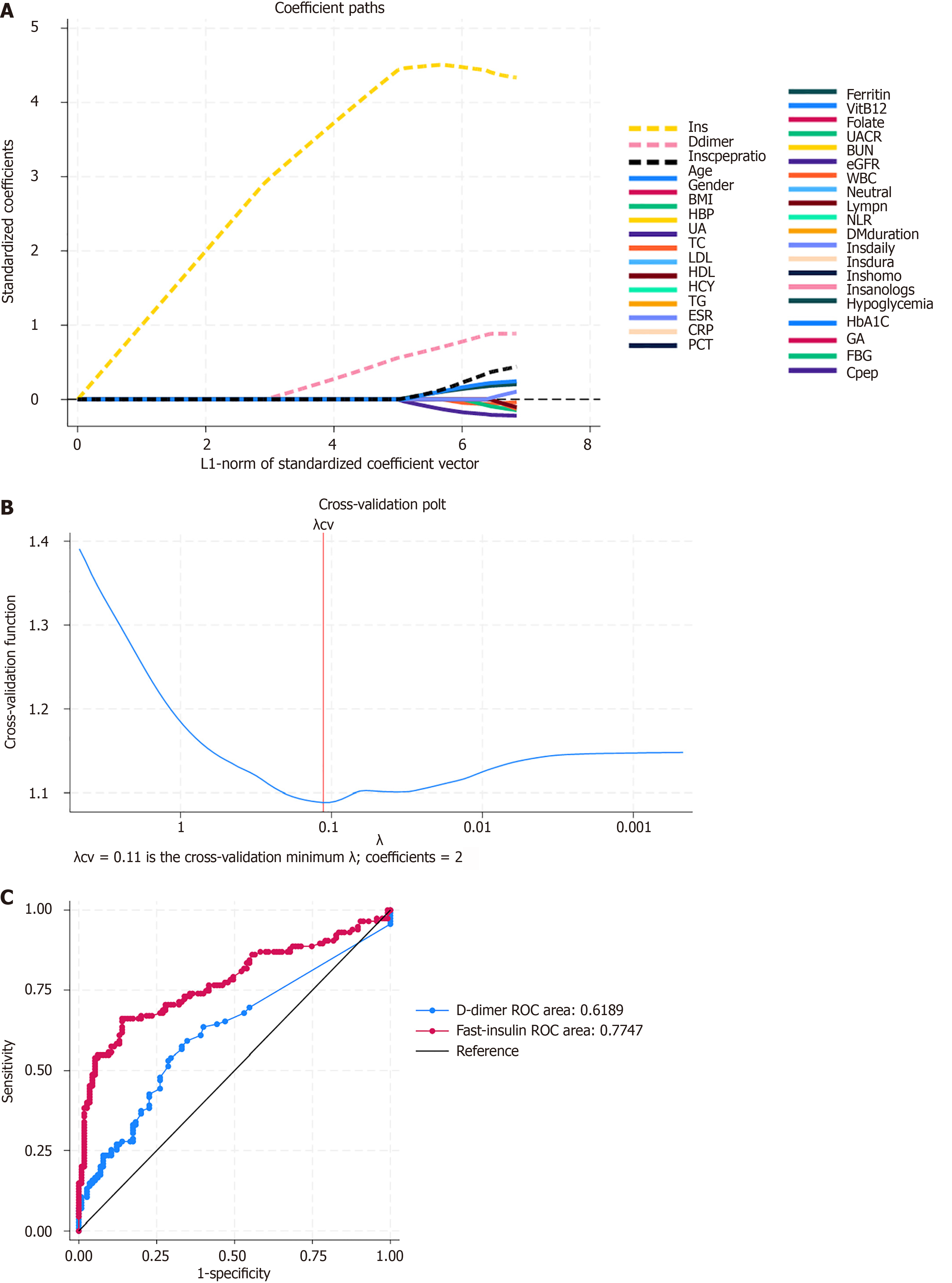
Using LASSO regression analysis, several key variables were selected for the model. In Figure 1A, the variable 'fasting insulin' (yellow dashed line) shows a significant increase in its coefficient as regularization decreases, highlighting its strong influence in predicting the presence of IAA in the model. Similarly, the 'D-dimer' variable (blue dashed line) also showed an increase, though to a lesser extent. Other variables have coefficients that remain small and approach zero as regularization increases, indicating lower importance. This selection process ensured that only the variables with the most predictive power were included in the final model.
The optimal value of the regularization parameter λ was determined through cross-validation, with λ = 0.11 minimizing the cross-validation error and selecting the most important coefficients (Figure 1B). The blue line shows the error decreasing as λ decreases, reaching a minimum before increasing again. The optimal λ value, marked by the red vertical line at λ = 0.11, minimizes the cross-validation error and includes two coefficients in the model.
The model's predictive performance was evaluated using receiver operating characteristic (ROC) curves for the key variables. The ROC curve for D-dimer (blue line) has an area of 0.6189, indicating moderate discriminative ability. In contrast, the fast-insulin ROC curve (red line) has a higher area of 0.7747, demonstrating superior discriminative performance (Figure 1C). The black diagonal line represents the performance of a random classifier with an area of 0.5. The comparison of ROC curves reveals that the model based on fast-insulin has superior predictive ability compared to the model based on D-dimer.
By following these procedures, the LASSO model was effectively developed, leveraging key variables such as D-dimer and fast-insulin to achieve optimal predictive performance. The model demonstrated excellent performance in terms of both discrimination and calibration, suggesting it is a reliable tool for predicting the presence of IAA in patients with T2DM.
To further understand the association between D-dimer levels and UACR, we performed linear regression analyses stratified by IAA, as shown in Table 2. D-dimer levels were significantly correlated with UACR in the total cohort and in the IAA-positive patient group. However, this correlation was not present in IAA-negative patients. In patients with IAA-positive, D-dimer levels had a significant positive correlation with UACR (β = 0.643, P < 0.01), while in the IAA-negative group, this correlation was not observed (β = 0.415, P > 0.05).
To explore the impact of D-dimer elevation on the risk of abnormal albuminuria in patients with and without IAA, the OR for D-dimer elevation (> 0.5 g/L) was 2.88 (95%CI: 1.17-7.07) in the IAA-positive group, compared to 0.48 (95%CI: 0.14-1.66) in the IAA-negative group, with a significant interaction effect (P interaction < 0.05), as shown in Table 3. These results suggest that D-dimer elevation is a significant risk factor for abnormal albuminuria, particularly in the presence of IAA, highlighting the importance of considering IAA status in the assessment of albuminuria risk.
| IAA-negative | IAA-positive | Total | Interaction P value | |
| D-dimer | ||||
| Model 1 | 1.24 (0.28-5.49) | 2.88 (1.25-6.6) | 2.48 (1.26-4.88) | 0.334 |
| Model 2 | 1.32 (0.29-5.97) | 2.68 (1.16-6.18) | 2.47 (1.25-4.89) | 0.439 |
| Model 3 | 1.82 (0.37-9.01) | 3.39 (1.34-8.59) | 2.90 (1.36-6.15) | 0.494 |
| Elevated D-dimer | ||||
| Model 1 | 0.41 (0.13-1.32) | 3.20 (1.36-7.53) | 1.53 (0.82-2.87) | 0.005 |
| Model 2 | 0.41 (0.13-1.36) | 2.94 (1.23-6.99) | 1.53 (0.82-2.88) | 0.009 |
| Model 3 | 0.48 (0.14-1.66) | 2.88 (1.17-7.07) | 1.60 (0.84-3.07) | 0.015 |
In this study, we found that presence of IAA is associated with higher levels of D-dimer and insulin, and increased D-dimer is a significant risk factor for abnormal albuminuria, suggesting a potential link between autoimmune responses to exogenous insulin and increased vascular inflammation and coagulation. These findings indicate the importance of considering IAA status in assessing albuminuria risk. This study provides novel insights into the relationship between IAA and microalbuminuria in T2DM patients. the elevated D-dimer levels in IAA-positive patients underscore its importance as a marker for vascular complications, especially diabetic nephrology. Despite this, our study highlights that IAA may play a significant role in the pathophysiology of T2DM.
IAA was first described in patients receiving exogenous insulin[12]. It could be developed as early as 7-10 days after initiation of insulin therapy[13]. however, most do not produce clinically significant derangement of insulin kinetics[14]. for several patients with positive IAA, IAA has low capacity and high affinity and can bind endogenous and exogenous insulin to cause poor glycemic control and hypoglycemia[15,16]. Patients with induced positive IAA usually presented with fasting and nocturnal hypoglycemia. This is attributed to the dissociation of insulin and IAA as the internal environment changes at night[17]. The generation of IAA may be related to many factors, such as insulin formulation. This autoimmune reaction may be associated with an inflammatory state that could contribute to a heightened coagu
The hypercoagulable state is characterized by increased levels of various pro-thrombotic markers, among which plasma D-dimer is the gold standard. Currently, hypercoagulability plays a major role in DN progression[18]. DN patients with higher levels of plasma D-dimer had higher 24-hour proteinuria, lower e-GFR, a higher glomerular lesion class[4]. In our study, the significant interaction between D-dimer elevation and IAA on abnormal albuminuria further supports the hypothesis that IAA may exacerbate vascular complications in diabetes. This interplay underscores the complex relationship between autoimmune responses induced by insulin therapy and coagulation abnormalities in diabetes management.
We recognize that the relationship between elevated D-dimer and abnormal albuminuria in T2DM may be influenced by other clinical factors commonly present in diabetic patients, such as systemic inflammation and hypertension. Elevated inflammatory markers (e.g., ESR, high-sensitivity-CRP) and uncontrolled blood pressure could contribute to both hypercoagulability and renal impairment, potentially strengthening the observed association between D-dimer and albuminuria. We suggest future studies consider these factors to better clarify their roles in this relationship.
The LASSO-derived model identified plasma insulin and D-dimer as key predictors of IAA presence, suggesting that these variables play a significant role in the pathophysiology of IAA in type 2 diabetes. The strong association between D-dimer levels and UACR, particularly in IAA-positive patients, underscores the importance of D-dimer as a marker of vascular complications in diabetes. The odds ratio for D-dimer elevation was markedly higher in the IAA-positive group compared to the IAA-negative group, indicating that the presence of IAA amplifies the risk associated with elevated D-dimer levels.
Our study has several limitations. Firstly, this retrospective design may introduce selection bias, and the sample size, while adequate, could be larger to increase the power of the findings. Secondly, the study population was limited to patients from a single hospital, which may limit the generalizability of the results. Thirdly, the lack of a control group with non-diabetic kidney diseases limits our ability to determine whether the observed associations between IAA, D-dimer, and albuminuria are specific to T2DM or reflect broader patterns that may be present in other forms of renal impairment. Including a positive control group with other kidney diseases, such as hypertensive nephropathy or glomerulonephritis, could provide additional insight into whether elevated D-dimer and its association with albuminuria is unique to DN. Future studies incorporating such controls would help clarify the specificity of our findings and strengthen the generalizability of the conclusions drawn about IAA’s role in diabetic kidney disease. Lastly, our study’s cross-sectional design captured D-dimer levels at a single time point, so we were unable to assess dynamic changes in D-dimer over time. Future longitudinal studies could benefit from monitoring D-dimer levels at multiple intervals to detect patterns or fluctuations in D-dimer, particularly in high-risk IAA-positive T2DM patients. Such data could help clarify when and how D-dimer levels increase, potentially providing a more precise risk assessment for developing albuminuria.
In conclusion, this study highlights the significant role of D-dimer elevation as an independent risk factor for abnormal albuminuria in type 2 diabetic patients with IAA. These findings suggest that monitoring D-dimer levels in IAA-positive patients may help identify those at higher risk for albuminuria and related vascular complications. Further research is needed to elucidate the underlying mechanisms and to explore potential therapeutic interventions targeting these pathways.
We thank all the participants and staff involved in this study for their valuable contributions.
| 1. | Basi S, Fesler P, Mimran A, Lewis JB. Microalbuminuria in type 2 diabetes and hypertension: a marker, treatment target, or innocent bystander? Diabetes Care. 2008;31 Suppl 2:S194-S201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Liu Y, Chai S, Zhang X. Effect of MAFLD on albuminuria and the interaction between MAFLD and diabetes on albuminuria. J Diabetes. 2024;16:e13501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Wakabayashi I, Masuda H. Association of D-dimer with microalbuminuria in patients with type 2 diabetes mellitus. J Thromb Thrombolysis. 2009;27:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Yu Y, Zhu C, Lin Y, Qian Q, Shen X, Zou W, Wang M, Gong J, Chen M, Liu L, Yu R, Shen Q, Shao L, Zhu B. Plasma D-dimer levels are associated with disease progression in diabetic nephropathy: a two-center cohort study. Ren Fail. 2023;45:2285868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 5. | Liu Y, Ping F, Yu J, Lv L, Zhao Y, Qi M, Li W, Xu L, Yu M, Li M, Zhang H, Li Y. Hypoglycemia Caused by Exogenous Insulin Antibody Syndrome: A Large Single-Center Case Series From China. J Clin Endocrinol Metab. 2023;108:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 6. | Smith MJ, Simmons KM, Cambier JC. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat Rev Nephrol. 2017;13:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Ates DC, Akinci A, Dundar I. The relationship between autoimmunity and HbA1c in type 1 diabetes mellitus patients. Med Sci. 2021;10:1505-1010. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Wilhelm G, Mertowska P, Mertowski S, Przysucha A, Strużyna J, Grywalska E, Torres K. The Crossroads of the Coagulation System and the Immune System: Interactions and Connections. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 74] [Reference Citation Analysis (0)] |
| 9. | American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S17-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1616] [Article Influence: 404.0] [Reference Citation Analysis (1)] |
| 10. | American Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S83-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 457] [Article Influence: 114.3] [Reference Citation Analysis (1)] |
| 11. | Nakagawa S, Nakayama H, Sasaki T, Yoshino K, Yu YY. A simple method for the determination of serum free insulin levels in insulin-treated patients. Diabetes. 1973;22:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 248] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Berson SA, Yalow RS, Bauman A, Rothschild MA, Newerly K. Insulin-I131 metabolism in human subjects: demonstration of insulin binding globulin in the circulation of insulin treated subjects. J Clin Invest. 1956;35:170-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 536] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54 Suppl 2:S52-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Holmberg H, Mersebach H, Kanc K, Ludvigsson J; Insulin Aspart Study Group on Immunogenicity. Antibody response to insulin in children and adolescents with newly diagnosed Type 1 diabetes. Diabet Med. 2008;25:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Cappellani D, Macchia E, Falorni A, Marchetti P. Insulin Autoimmune Syndrome (Hirata Disease): A Comprehensive Review Fifty Years After Its First Description. Diabetes Metab Syndr Obes. 2020;13:963-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Tamura Y, Kimbara Y, Funatsuki S, Mabuchi S, Kodera R, Yoshimoto A, Chiba Y, Mori S, Ito H, Araki A. A case of insulin antibody-induced glucose instability in an elderly woman with type 2 diabetes on hemodialysis, successfully ameliorated with liraglutide. Diabetol Int. 2013;4:71-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Censi S, Mian C, Betterle C. Insulin autoimmune syndrome: from diagnosis to clinical management. Ann Transl Med. 2018;6:335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 18. | Domingueti CP, Dusse LM, Carvalho Md, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/