INTRODUCTION
The global burden of non-communicable diseases is increasing at an alarming rate, with diabetes ranking as the fourth most prevalent condition, and obesity serving as a major risk factor for these diseases[1,2]. In 2022, the global prevalence of diabetes was reported to affect approximately 830 million individuals, with projections indicating this figure will rise to 1.3 billion by 2050[3]. Similarly, data from the World Obesity Federation[4] estimate that 820 million people are currently living with obesity, and this number is expected to increase to 1.53 billion by 2035 unless effective prevention measures are implemented.
Obesity is characterized by excessive accumulation of adipose tissue, which impairs metabolic homeostasis, and promotes systematic inflammation, insulin resistance and dyslipidemia[5]. These pathophysiological alterations increase the risk of developing type 2 diabetes (T2D), a chronic condition characterized by impaired insulin activity and hyperglycemia[5]. The relationship between obesity and diabetes is both bidirectional and synergistic. Obesity is a major risk factor for diabetes, while poorly managed diabetes can further contribute to weight gain and metabolic dysfunction. Furthermore, the coexistence of obesity and diabetes exacerbate the risk of cardiovascular diseases, non-alcoholic fatty liver disease, certain cancers, and other metabolic disorders[6]. Addressing and understanding the complex interplay between these conditions is therefore essential to reducing their global health impact and preventing associated comorbidities.
Current treatments for obesity and diabetes, including pharmacological interventions, lifestyle modifications, and bariatric surgery, often demonstrate limited long-term efficacy[7]. Pharmacological treatments may cause side effects, and lifestyle interventions frequently lack sustainability. Although Surgical options may be effective for some, they carry inherent risks and are not universally accessible. Additionally, these conventional therapies primarily focus on symptom management rather than addressing the underlying metabolic mechanisms driving these conditions and fail to account for the interconnected nature of obesity, diabetes, and related comorbidities. As a result, there is a growing need for naturally derived treatments that can effectively target mechanisms behind obesity and diabetes, offering a safer, sustainable, and comprehensive approach to managing obesity and diabetes.
In recent years, there has been an increasing interest in marine natural products as a promising source of novel therapeutic agents for the treatment of obesity and diabetes and their related complications. Among the wide array of marine organisms, seaweeds have garnered attention due to their distinctive chemical composition and potential medicinal properties. They have been reported to produce a variety of compounds including sulphated polysaccharides, phenols, carotenoids, sterols, vitamins, and amino acids[8,9]. Numerous studies have highlighted the potential of seaweeds in mitigating obesity and diabetes through various mechanisms. These include regulation of adipogenesis, appetite control, modulation of gut microbiota, enhancement of insulin sensitivity, and reduction of inflammation, oxidative stress, and pancreatic β-cell dysfunction, as demonstrated in vitro, ex vivo, in vivo, and clinical studies.
Seaweeds have demonstrated the ability to inhibit adipogenesis by suppressing lipogenic gene expression and key regulatory factors in white adipose tissue while enhancing thermogenic factors[10-12]. A clinical trial conducted by Zaharudin et al[13] revealed that supplementation with two brown seaweeds, Laminaria digitata and Undaria pinnatifida, improved postprandial glucose levels and appetite control in healthy adults. This effect may be attributed to the high fiber content of seaweeds, which slows digestion, thereby regulating appetite and blood glucose levels. Supporting this, a meta-analysis by Kim et al[14] indicated that seaweed supplementation effectively reduces postprandial blood glucose, hemoglobin A1c, and HOMA-IR levels, improving glycemic control and lowering the risk of T2D.
Additionally, seaweed exhibits anti-inflammatory properties by downregulating inflammatory markers and cytokines and suppressing inflammatory protein expression[15,16]. They also possess antioxidant activities, demonstrated through radical scavenging and restoration of antioxidant defense systems in oxidative injury[17]. He et al[18] further showed that the sulphated lipopolysaccharide derived from seaweed mitigated inflammatory responses and restored the gut barrier in lipopolysaccharide-induced mice. Moreover, a study by Shannon et al[19] highlighted the potential of seaweeds to improve gut microbiota by enhancing the abundance of commensal bacteria and promoting the production of short-chain fatty acids (SCFAs). These biological effects are primarily attributed to the phytochemical constituents of seaweed. In this study, we present a comprehensive review of the molecular mechanisms underlying these actions, aiming to advance understanding of their mode of actions. This could provide a basis for developing therapeutic strategies targeting these pathways to address obesity and diabetes.
PHYTOCHEMICAL COMPOSITION OF SEAWEEDS AND THEIR IMPORTANCE IN THERAPEUTIC APPLICATIONS
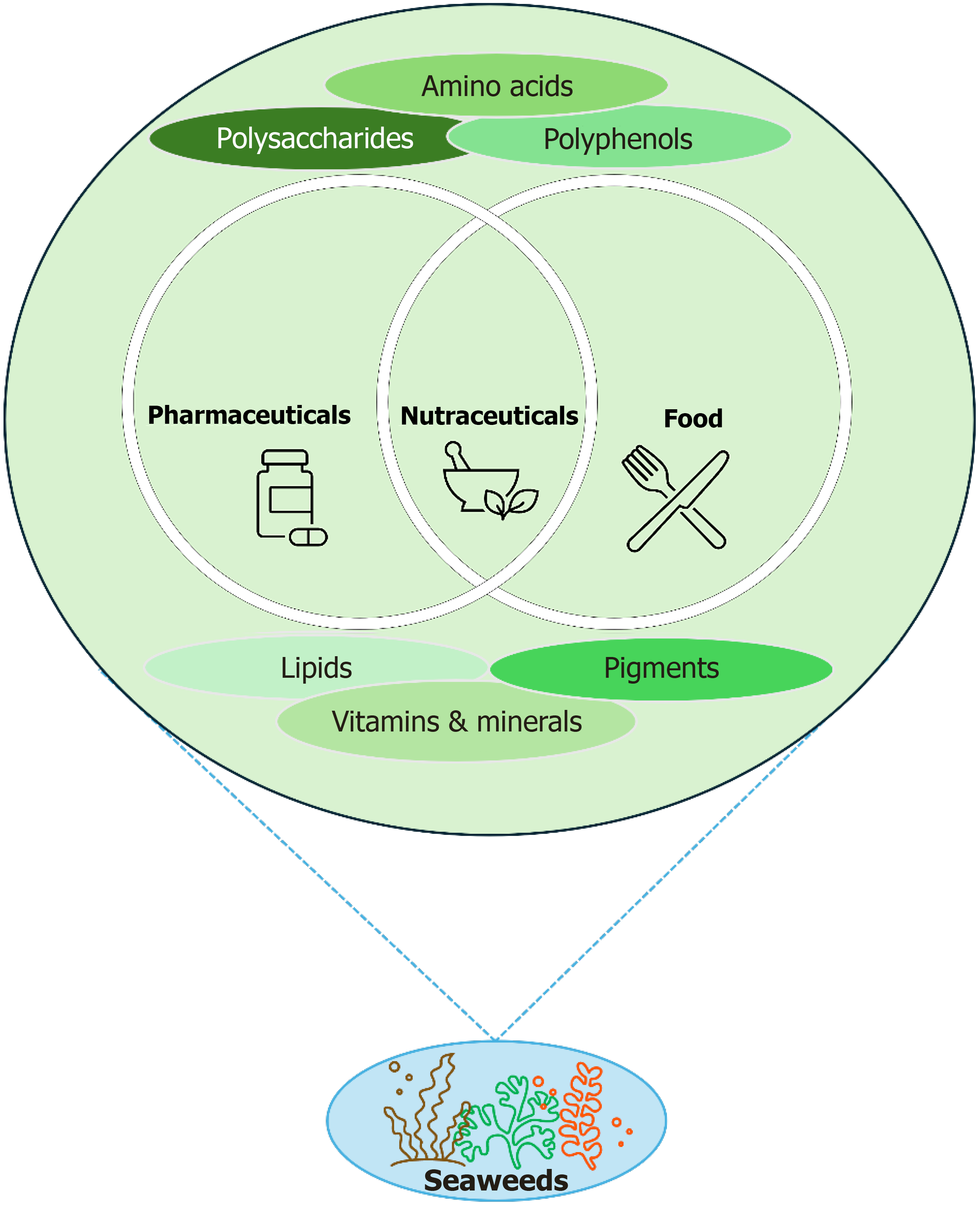
Seaweeds are a rich source of bioactive phytochemicals, which have been extensively studied for their potential therapeutic applications, making them valuable resources for functional foods, nutraceuticals, and pharmaceuticals (Figure 2)[8]. These compounds, including polysaccharides, pigments, terpenes, polyphenols, peptides, and other bioactives, play a vital role in promoting health and preventing disease[8,9,23]. The diverse composition of these phytochemicals varies across seaweed species and classifications (red, brown, and green), contributing to their broad-spectrum biological activities. Their natural origin and multifaceted biological activities make them great alternatives to synthetic drugs, offering safer and potentially more sustainable solutions for preventing and managing various diseases.
Figure 2
Seaweeds as a rich source of bioactive phytochemicals and their potential applications in functional foods, nutraceuticals, and pharmaceuticals.
Polysaccharides
Polysaccharides are among the most abundant and biologically active compounds in seaweed, with sulphated polysaccharides being particularly notable. They are long-chain carbohydrates composed of repeating sugar units and vary by seaweed type: Brown seaweeds contain alginates, laminarins, and fucoidans; red seaweeds are rich in carrageenan and agar; and green seaweeds contain Ulvans[8]. These polysaccharides exhibit a wide range of therapeutic effects, including antioxidant, anticoagulant, antiviral, and anti-inflammatory properties[24]. Fucoidans have been shown to enhance immune responses, regulate blood sugar levels, and exhibit antitumor activities[25,26]. Similarly, carrageenan is recognized for their ability to inhibit viral replication and modulate inflammatory pathways[27]. The high dietary fiber content of seaweeds also contributes to gut health, as these polysaccharides act as prebiotics, supporting the growth of beneficial gut microbiota[19]. A recent systematic review and meta-analysis have highlighted the potential of sulphated polysaccharides as a therapeutic approach for obesity, suggesting that additional studies, including human clinical trials, are necessary to establish conclusive evidence of their efficacy[28].
Polyphenols
Polyphenols are a diverse group of naturally occurring compounds characterized by multiple phenol units. Seaweed polyphenols, such as phlorotannins in brown algae, are antioxidants that provide protection against oxidative stress and related disorders. Phlorotannins have demonstrated efficacy in scavenging free radicals, inhibiting lipid peroxidation, and reducing inflammation[29]. These activities make them valuable in mitigating the effects of chronic diseases such as cardiovascular diseases, diabetes, and neurodegenerative disorders. Additionally, polyphenols can inhibit digestive enzymes like α-amylase and α-glucosidase, contributing to postprandial glucose regulation[30]. The UV-absorbing properties of certain polyphenols also highlight their potential for use in skincare products as natural photoprotective agents[31].
Peptides
Seaweed-derived peptides are short chains of amino acids obtained from the enzymatic hydrolysis of seaweed proteins. They exhibit various biological activities, including antihypertensive, antimicrobial, and antidiabetic effects[32]. For example, certain seaweed-derived peptides inhibit angiotensin-converting enzyme, making them effective in managing hypertension. Others enhance insulin sensitivity and glucose uptake, supporting their role in diabetes management[33]. The antimicrobial properties of these peptides also hold promise for combating antibiotic-resistant pathogens[32].
Vitamins and minerals
Seaweeds serve as a significant source of essential vitamins and minerals, playing a crucial role in human nutrition and health[34]. They are particularly rich in vitamins A, C, E, and various B-complex vitamins, which are integral to cellular metabolism, antioxidant mechanisms, and immune system regulation[8]. Moreover, seaweeds provide an abundant supply of essential minerals, including iodine, calcium, magnesium, iron, and zinc[34], all of which are critical for maintaining thyroid activity, bone integrity, oxygen transport, and enzymatic functions. Due to the high bioavailability of these micronutrients, seaweeds have considerable potential as a dietary resource as a supplement in micronutrient deficiencies and supporting overall physiological well-being.
Lipids
Lipids in seaweeds are present in lower concentrations than those found in terrestrial plants[35]. They exhibit notable bioactive properties. Seaweed-derived polyunsaturated fatty acids, including omega-3 and omega-6 fatty acids, play a crucial role in maintaining cardiovascular health, modulating inflammatory responses, and supporting cognitive function. In addition, seaweeds contain glycolipids and phospholipids, which have demonstrated antimicrobial, antiviral, and anticancer properties[35]. The bioactivity of these lipid compounds has attracted significant scientific attention, highlighting their potential applications in functional foods and pharmaceutical formulations for the prevention and management of chronic diseases, thereby contributing to overall human health.
Pigments
Pigments are naturally occurring colored compounds found in plants and algae that play essential roles in light absorption and photosynthesis. Seaweeds contain a diverse range of pigments, including chlorophylls, carotenoids, and phycobiliproteins[36], which not only determine their characteristic coloration but also exhibit significant biological functions. Chlorophylls are known for their antioxidant and detoxification properties, while carotenoids such as fucoxanthin have been shown to exert anti-obesity, anti-inflammatory, and anticancer effects[36]. Phycobiliproteins, primarily present in red and blue-green algae, possess potent antioxidant and immunomodulatory activities[37]. Due to their ability to regulate oxidative stress and enhance immune function, these bioactive pigments have great potential applications in nutraceuticals, functional foods, and therapeutic interventions.
Other bioactive compounds
Seaweeds also contain other bioactive molecules that contribute to their therapeutic potential. These include carotenoids (e.g., fucoxanthin), sterols, and alkaloids. Fucoxanthin, a carotenoid found in brown seaweeds, has been shown to promote weight loss, reduce inflammation, and improve lipid metabolism, making it beneficial in the management of obesity and metabolic syndrome[8]. Sterols, such as fucosterol, possess anti-inflammatory and cholesterol-lowering properties[8]. Additionally, certain alkaloids from seaweeds exhibit neuroprotective and antimicrobial activities[15], further expanding their therapeutic applications.
ANTI-DIABETES EFFECTS OF SEAWEEDS
The bioactive compounds found in seaweeds, as previously highlighted, have demonstrated multiple mechanisms that regulate glycemic control and enhance insulin sensitivity and secretion (Figure 3). They also reduce inflammation and oxidative stress, which are key factors involved in the pathogenesis of diabetes. This section of the review explores the mechanisms by which seaweeds and their bioactive compounds mitigate diabetes, with a particular focus on glucose metabolism, inflammation, and oxidative stress. The potential mechanisms of action of these seaweeds and their bioactive compounds are to be discussed in detail.
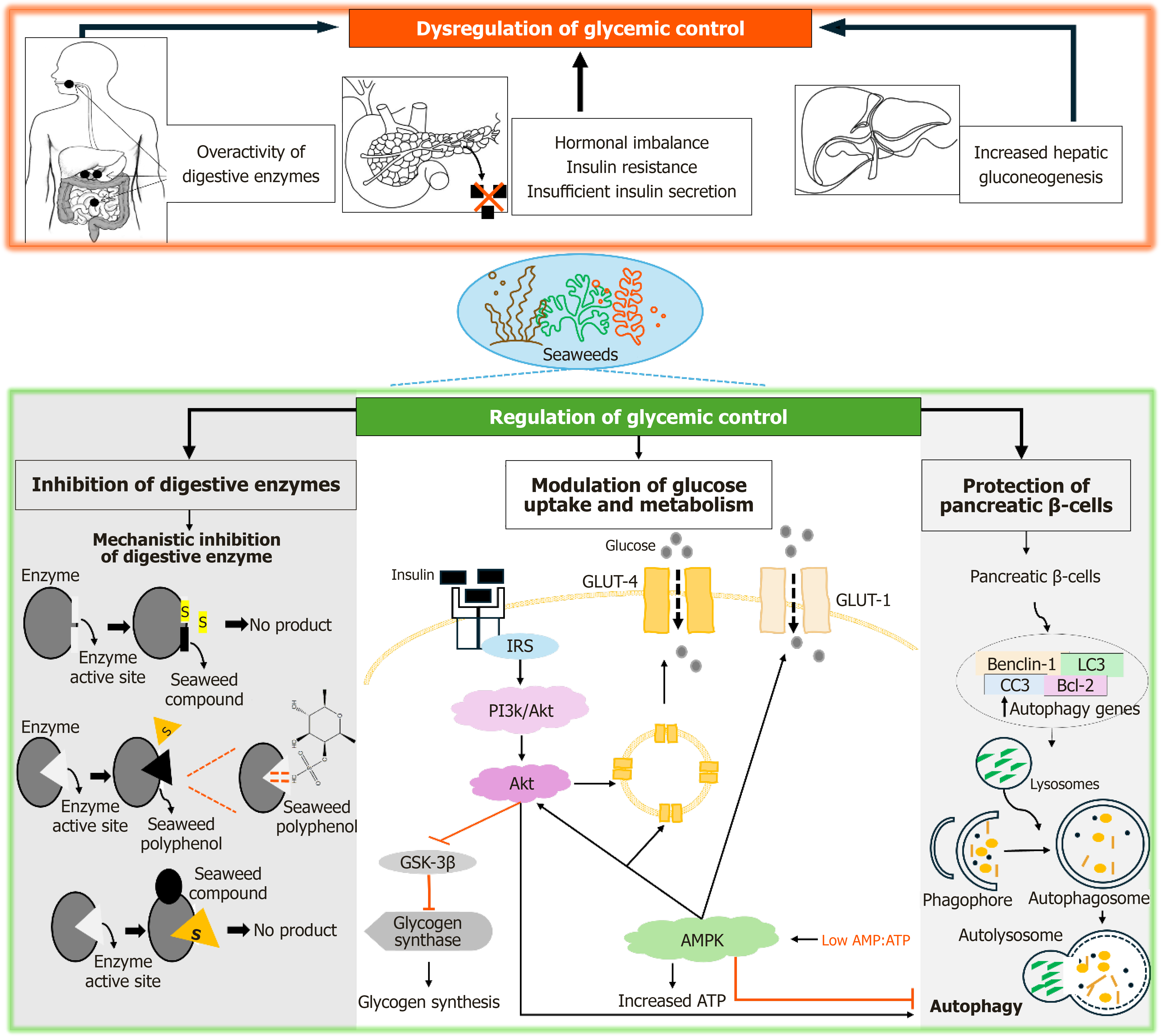
Figure 3 The dysregulation of glycemic control and the antidiabetic effects of seaweeds and their compounds, involving molecular mechanisms of glycemic control regulation through inhibition of digestive enzymes, modulation of glucose uptake, insulin sensitivity and secretion, and protection of pancreatic beta cells.
GLUT: Glucose transporter; IRS: Insulin receptor substrates; AMPK: Adenosine monophosphate-activated protein kinase; ATP: Adenosine triphosphate; PI3K: Phosphatidylinositol 3-kinase; Akt: Protein kinase B; AMP: Adenosine monophosphate.
Regulation of glycemic control
The regulation of glycemic control is essential for metabolic homeostasis, maintaining normal blood glucose levels, and preventing complications such as cardiovascular disease, neuropathy, nephropathy, and retinopathy in individuals with or at risk of diabetes[38]. Following food consumption, digestive enzymes, including alpha-amylase and alpha-glucosidase, facilitate the breakdown of dietary carbohydrates into absorbable glucose[39]. Hormones such as insulin and glucagon, secreted by pancreatic β and α cells, respectively, play key roles in glucose homeostasis[40]. Insulin enhances glucose uptake in muscle and adipose tissue via glucose transporter-4 (GLUT-4), promotes glycogen synthesis in the liver, and suppresses gluconeogenesis. Conversely, glucagon stimulates hepatic glucose production during fasting states[40]. These processes collectively sustain metabolic homeostasis and energy demands. However, in diabetes and obesity, the regulation of glycemic control becomes disrupted, leading to chronic hyperglycemia and impaired glucose homeostasis (Figure 3). Overactivity of digestive enzymes, such as alpha-amylase and alpha-glucosidase, increases the breakdown of dietary carbohydrates, contributing to excessive postprandial glucose levels[39]. Hormonal imbalances, including insulin resistance and insufficient insulin secretion, impair glucose uptake in peripheral tissues, while persistent glucagon hypersecretion exacerbates hepatic gluconeogenesis[41]. These dysregulations create a metabolic imbalance, increasing the risk of complications associated with prolonged hyperglycemia.
Inhibition of carbohydrate digestive enzymes
The inhibition of digestive enzymes such as alpha-amylase and alpha-glucosidase, serves as a therapeutic strategy for diabetes by reducing the breakdown and absorption of carbohydrates, thus slowing the rise in postprandial blood glucose levels[39,42]. Seaweeds and their bioactive compounds such as polyphenols, phlorotannin, and sulphated polysaccharides has been shown to effectively reduce the activity these digestive enzymes, highlighting their potential as therapeutic agents for diabetes management[33]. Bioactive compounds derived from seaweeds exert inhibitory effects on digestive enzymes through specific molecular interactions (Figure 3)[43]. These compounds interact with enzyme active sites, inducing conformational changes that disrupt enzyme-substrate binding, thereby preventing the enzymatic hydrolysis of carbohydrates into glucose. Polyphenols which are particularly abundant in brown seaweeds such Laminaria, Undaria, Ecklonia, and Sargassum contain hydroxyl groups capable of forming hydrogen bonds with polar amino acid residues at the active site of an enzyme[44]. These interactions induce a conformational change in the enzyme, which reduces the accessibility of its active site and impairs its ability to catalyze reactions. Additionally, the hydroxylated aromatic rings of phenolic compounds may interact with the hydrophobic regions of the enzyme[33], further stabilizing the binding and inhibiting its function. As a result, the phenolic compounds act through non-competitive inhibition. They do not directly compete with the substrate for the active site; instead, they alter the structure of the enzyme, reducing its capacity to break down carbohydrates into glucose.
Seaweeds also contain sulphated polysaccharides, such as carrageenan and agar, which possess sulfate groups that facilitate electrostatic interactions with positively charged residues at the active site of an enzyme[45]. These interactions prevent the enzyme from effectively binding to its substrate by occupying the active site or allosteric sites. Additionally, these compounds exhibit mixed inhibition, binding to both the active site and allosteric sites on the enzyme, inducing conformational changes that further impair enzyme activity. The high negative charge density of sulphated polysaccharides also disrupts enzyme-substrate complex formation[46], reducing the efficiency of the enzyme to hydrolyze carbohydrates.
Molecular docking and computational modeling studies have provided better insights into these interactions, demonstrating how these bioactive compounds bind strongly to the active site of enzymes, effectively inhibiting normal catalytic function[47,48]. These studies demonstrate that bioactive compounds from seaweeds, exhibit strong binding affinity for the active sites of alpha-amylase and alpha-glucosidase. The outcomes of molecular docking simulations identify specific amino acid residues, and hydrophobic residues that play a critical role in facilitating the binding and subsequent inhibition of the enzymes. Furthermore, Kinetic studies demonstrate that the binding of seaweeds’ bioactive compounds leads to a dose-dependent decrease in enzyme activity[49], hindering the ability of an enzyme to break down carbohydrates effectively. Comparative studies have examined the enzyme inhibitory effects of different seaweed species, including brown (Sargassum elegans), red (Callophyllis variegata), and green (Bryopsis myosuroides) seaweeds, revealing varying degrees of enzyme inhibition[48]. Sulphated polysaccharide extracted from a red seaweed, Bangia fusco-purpurea exhibited notable alpha-amylase and alpha-glucosidase inhibitory effects[50]. These findings suggest that the enzyme inhibitory properties of seaweeds differ between species and may be influenced by their phytochemical composition.
The inhibition of digestive enzymes by bioactive compounds in seaweeds occurs through a combination of molecular interactions, including hydrogen bonding, hydrophobic interactions, and electrostatic forces. These interactions lead to changes in enzyme structure and function. These mechanisms lead to a reduction in carbohydrate digestion and glucose absorption. This provides a promising natural strategy for controlling postprandial glucose levels and improving glycemic regulation in individuals with diabetes. However, further research, including clinical trials, is necessary to confirm the therapeutic potential of seaweed bioactive compounds in the prevention and management of diabetes.
Modulation of glucose uptake and metabolism
Another promising approach to reverse diabetes and obesity is the regulation of glucose uptake and metabolic pathways. Targeting key mechanisms involved in glucose transport, storage, and utilization offers an effective approach to mitigating hyperglycemia and promoting sustainable weight management. Seaweeds and their bioactive compounds have shown potential to modulate glucose uptake by enhancing the activity or expression of glucose transporters like GLUT-4 in peripheral tissues[51,52]. For instance, studies have shown that seaweed-derived extracts can enhance glucose uptake in adipocytes and myotubes, thereby improving glucose homeostasis (Figure 3)[9,53]. Additionally, research on Anthophycus longifolius (A. longifolius) has highlighted its capacity to promote glucose uptake in adipose tissue, while recent findings indicate that Ulvan extracts from green seaweeds stimulate glucose uptake in rat muscle tissues[17,47].
A key mechanism in glucose homeostasis is the translocation of GLUT-4 to the plasma membrane, which enables glucose entry into cells[54]. Seaweed-derived polysaccharides, including fucoidan can modulate glucose uptake by regulating the expression and translocation of GLUT-4, a transporter critical for insulin-mediated glucose uptake in skeletal muscle and adipose tissues[55,56]. Furthermore, fucoidan has been shown to activate signaling pathways that promote GLUT-4 translocation to the cell membrane, increasing GLUT-4 expression and enhancing glucose uptake in skeletal muscle cells in response to insulin[57]. Similarly, a brown seaweed, Undaria pinnatifida was shown facilitate glucose uptake in muscle cells by promoting GLUT-4 translocation, through the activation of insulin signaling pathways and the upregulation of GLUT-4 gene expression in mice induced with glucose intolerance. Another study demonstrated enhanced glucose uptake in adipose tissue following treatment with the brown seaweed A. longifolius. Molecular docking analysis revealed a binding affinity between demethylalangiside, a compound identified in this seaweed, and GLUT-4[47].
The primary mechanism for GLUT-4 translocation in response to insulin is the insulin signaling pathway. Upon insulin binding to the insulin receptor (IR), a receptor tyrosine kinase on the cell membrane, the receptor's intrinsic kinase activity is activated. This results in the phosphorylation of IR substrates (IRS), including IRS-1 and IRS-2[58]. The activation of IRS initiates the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway, which plays a vital role in glucose uptake and storage. This pathway activates several downstream proteins, including Akt (protein kinase B), which promotes GLUT4 translocation and enhances glucose uptake into peripheral tissues[58]. Fucoidan has been demonstrated to enhance insulin signaling by increasing IRS phosphorylation and activating the PI3K/Akt pathway, leading to improved insulin sensitivity[57,59]. This, in turn, promotes increased GLUT-4 translocation to the plasma membrane, facilitating glucose uptake in muscle and adipose tissues.
Adenosine monophosphate-activated protein kinase (AMPK) plays an important role in regulating cellular energy balance and glucose metabolism[60]. It is activated when the cellular energy status is low, as shown by an increased Adenosine monophosphate/Adenosine triphosphate (AMP/ATP) ratio[60]. Once activated, AMPK initiates processes that restore energy balance, such as facilitating glucose uptake and inhibiting glucose production in the liver. Seaweed extracts from Undaria pinnatifida sporophyll, Codium fragile, and Gracilaria verrucosa activate AMPK, thereby enhancing glucose uptake and improving overall metabolic regulation[53]. A study by Deng et al[61] reported that fucoidan and fucoxanthin from Saccharina japonica activated AMPK in liver cells, promoting glucose uptake by increasing the translocation of GLUT4 to the plasma membrane. Additionally, fucoxanthin-induced activation of AMPK inhibits hepatic glucose production by downregulating the expression of key enzymes involved in gluconeogenesis, such as phosphoenolpyruvate carboxykinase, without significantly altering glucose-6-phosphatase[62]. Furthermore, there was an increased level of glycogen content and expression of GLUT-4[62]. This leads to lower blood glucose levels and enhanced glucose metabolism. The synthesis of glycogen in the liver and muscle is a vital process for maintaining glucose homeostasis. Seaweed-derived compounds can modulate glycogen metabolism by inhibiting glycogen synthase kinase-3 beta (GSK-3β). GSK-3β normally inhibits glycogen synthase, the enzyme responsible for glycogen formation. By inhibiting GSK-3β, compounds like fucoxanthin from seaweeds enhance glycogen storage in muscle and liver tissues. This helps lower circulating glucose levels[62]. This process aids in glucose metabolism by promoting the storage of excess glucose as glycogen, thus reducing blood glucose levels
In addition to activation of AMPK, fucoxanthin is also reported to play a role in activating peroxisome proliferator-activated receptor gamma (PPARγ), both of which are key regulators of fatty acid oxidation and lipid metabolism[62]. By promoting β-oxidation of fatty acids in muscle and liver cells, fucoxanthin reduces lipid accumulation, thus enhancing glucose metabolism and insulin sensitivity. Moreover, fucoxanthin upregulates the expression of genes involved in fatty acid oxidation, such as carnitine palmitoyltransferase-1 and uncoupling protein-1 (UCP1)[62-64]. This enhanced fatty acid oxidation reduces ectopic lipid deposition, improves insulin sensitivity, and aids in the regulation of blood glucose levels[62,63]. These mechanisms collectively contribute to increased glucose uptake, lowered blood glucose levels, and improved metabolic health, indicating seaweed-derived compounds as a promising strategy for managing diabetes and obesity.
Insulin sensitivity and secretion
Insulin sensitivity refers to the efficiency in which the body cells respond to insulin, a hormone that regulates blood glucose levels by promoting glucose uptake into cells for energy and storage[65]. In individuals with optimal insulin sensitivity, even low levels of insulin effectively facilitate glucose uptake, thereby maintaining stable blood glucose levels. Proper insulin secretion is essential for maintaining glucose homeostasis. Adequate insulin sensitivity and secretion are crucial for metabolic health, as they help prevent hyperglycemia and reduce the risk of developing metabolic disorders such as insulin resistance, T2D, and obesity[66]. In diabetes and obesity, insulin sensitivity is often impaired, leading to reduced cellular response to insulin and requiring higher insulin levels for glucose uptake. Over time, the pancreas compensates by increasing insulin secretion, but its capacity diminishes, worsening hyperglycemia[67]. In obesity, excess visceral fat triggers chronic inflammation, which further impairs insulin signaling. Together, these factors disrupt glucose homeostasis, resulting in chronically elevated blood glucose levels, a hallmark of T2D[68]. Effective management of these conditions requires improving both insulin sensitivity and secretion to maintain normal blood glucose levels and reduce the risk of long-term complications. Seaweeds and their bioactive compounds, have been shown to enhance insulin sensitivity and secretion, offering potential benefits for managing T2D and obesity. These compounds improve insulin signaling through pathways like PI3K/Akt and AMPK, facilitating glucose uptake and metabolism (Figure 3).
Protection of pancreatic β-cells
In addition to enhance insulin sensitivity, seaweeds and their compounds can also protect pancreatic β-cells, which are responsible for insulin secretion. These cells are particularly susceptible to lipotoxicity and glucotoxicity, common in conditions of chronic hyperglycemia and fat accumulation, leading to β-cell dysfunction and impaired insulin secretion[69]. A flavonoid fraction from Acanthophora spicifera reversed the damage on the Islets of Langerhans in diabetic rats[70]. Seaweeds exert cytoprotective effects by mitigating oxidative stress and inflammation[15] (discussed in the following section of the review), both of which are major contributors to β-cell apoptosis[71]. For instance, a study by Lee et al[72] showed protective effects of Sargassum sagamianum extracts on INS-1 pancreatic β-cells exposed to high glucose-induced oxidative stress and apoptosis. In contrast, fucoidan demonstrated anti-tumor effects by inhibiting the PI3K/Akt pathway. This was achieved through the downregulation of PI3K gene transcription and protein expression, alongside the suppression of Akt phosphorylation in cancer cells, while inducing apoptosis[59]. Additionally, fucoidan mitigated pancreatic β-cell death and stimulates insulin biosynthesis in streptozotocin-treated β-cells[73]. Moreover, phlorotannins further protect β-cells through their potent antioxidant properties, reducing reactive oxygen species (ROS) levels that are implicated in β-cell dysfunction[74]. By alleviating oxidative stress and enhancing insulin secretion, these seaweeds and their compounds play a crucial role in maintaining glucose homeostasis and preventing the progression of type T2D (Figure 3).
Seaweed-derived bioactive compounds may enhance autophagy in pancreatic β-cells, promoting cell survival and function under diabetic conditions. Zhu et al[75] demonstrated that fucoxanthin promotes autophagy in gastric cancer cells by increasing the expression of key autophagy-related proteins, including Beclin-1, microtubule-associated protein 1A/1B-light chain 3, and cleaved caspase-3, while concurrently downregulating the anti-apoptotic protein Bcl-2 (Figure 3). By restoring autophagic activity, seaweed compounds protect β-cells from apoptosis, support insulin secretion, and maintain glucose homeostasis, offering potential therapeutic benefits for diabetes management. These findings suggest that seaweed-derived compounds protect pancreatic β-cells through multiple mechanisms, including attenuation of oxidative stress, reduction of inflammation, inhibition of apoptosis, enhancement of insulin secretion, restoration of insulin signaling, and induction of autophagy. These molecular actions work synergistically to maintain β-cell viability and functionality.
Reducing inflammation and oxidative stress
Under normal physiological conditions, inflammation and oxidative stress are carefully regulated processes that are vital for cellular homeostasis and immune defense. Acute inflammation functions as a protective mechanism in response to injury or infection, facilitating tissue repair and the clearance of pathogens[76]. Similarly, oxidative stress, driven by ROS, acts as a critical signaling pathway in cellular processes such as proliferation, apoptosis, and stress response. The production of ROS is balanced by antioxidant systems, preventing excessive oxidative damage[76]. This balance is essential for maintaining the integrity of metabolic pathways, protecting tissues, and ensuring overall metabolic health and stability.
In disease conditions, both inflammation and oxidative stress become dysregulated, contributing to the onset and progression of various chronic diseases, such as diabetes, cardiovascular disorders, and neurodegenerative conditions[76]. Chronic inflammation, often triggered by factors like obesity, infection, or environmental stress, leads to the sustained release of pro-inflammatory cytokines, which disrupt normal metabolic and immune functions[77,78]. This prolonged inflammatory response can reduce insulin sensitivity, cause tissue damage, and accelerate disease progression[76,78]. Simultaneously, excessive oxidative stress occurs when the production of ROS surpasses the antioxidant defenses in the body. Elevated ROS levels result in cellular damage, including lipid peroxidation, protein oxidation, and DNA damage, further impairing tissue function[79]. The interplay of chronic inflammation and oxidative stress creates a self-perpetuating cycle that accelerates disease development and impairs normal physiological processes, ultimately promoting metabolic dysfunction[76]. Thus, investigating inflammation and oxidative stress is essential for understanding the pathogenesis of diabetes and obesity, as well as for identifying potential therapeutic approaches.
Seaweeds and their bioactive compounds, including carotenoids, polyphenols, phlorotannins, sterols, and polysaccharides, exhibit potent antioxidant and anti-inflammatory properties that help mitigate cellular damage induced by ROS and pro-inflammatory cytokines[80] (Figure 4). Fucoidan, for example, has been shown to alleviate oxidative stress by upregulating antioxidant enzyme activity and inhibiting inflammatory mediator production[81]. Similarly, phlorotannins offer protective effects by scavenging free radicals and reducing inflammatory responses[82], thus preventing the onset and progression of conditions such as insulin resistance and other metabolic disorders. The following section reviews the molecular mechanisms by which seaweeds and their bioactive compounds modulate inflammation and oxidative stress, contributing to the reduction of related diseases.
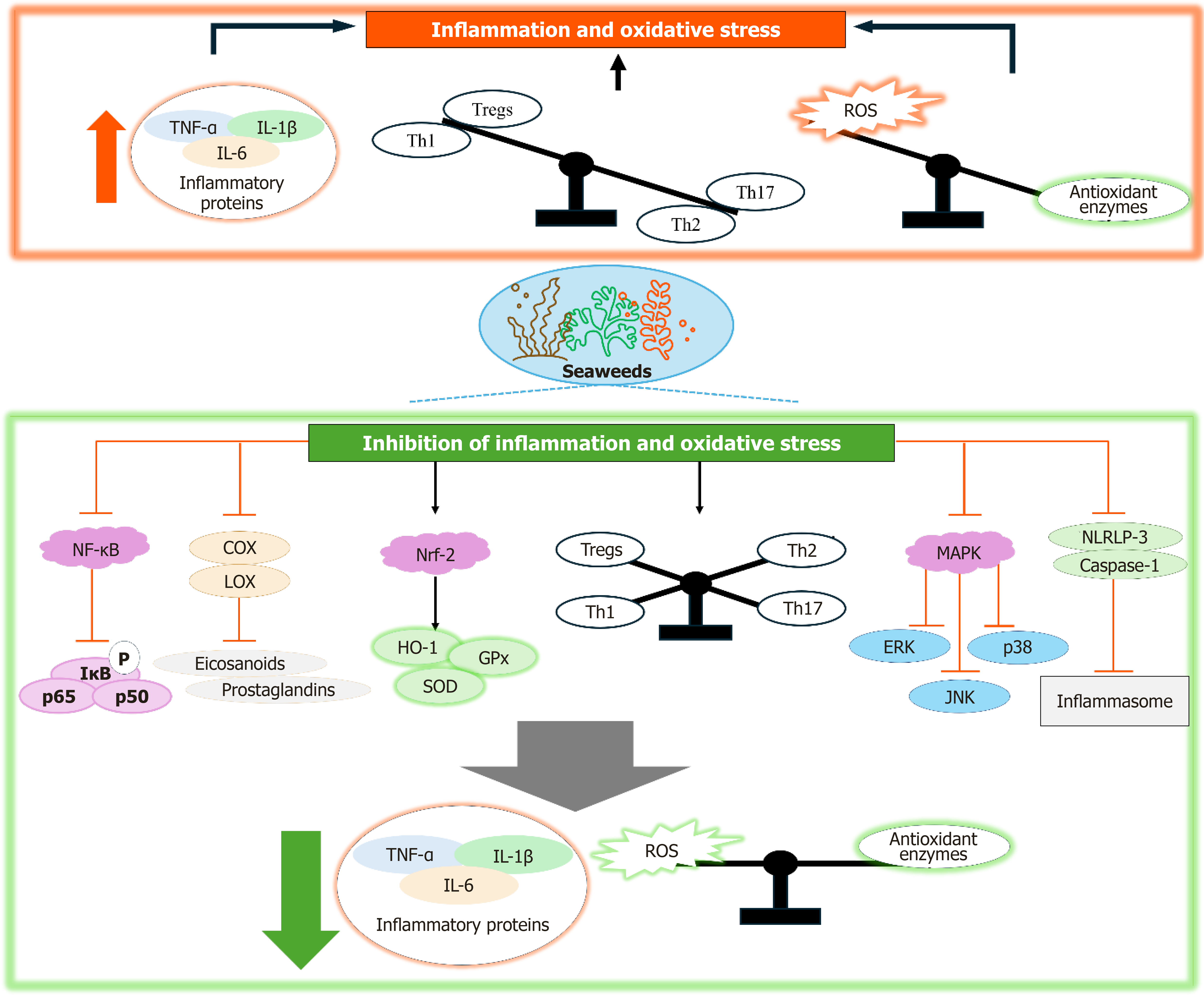
Figure 4 Inflammation and oxidative stress in diabetes and the inhibition of these processes by seaweeds and their compounds, involving mechanisms of action that modulate inflammatory pathways such as inhibition of nuclear factor kappa-B, cyclooxygenase, lipoxygenase, MAPK, NRLP-3, and caspase-1, and enhance antioxidant defense systems including nuclear factor erythroid 2-related factor 2 pathway.
NF-κB: Nuclear factor kappa-B; COX: Cyclooxygenase; LOX: Lipoxygenase; TNF-α: Tumor necrosis factor α; IL: Interleukin-6; Treg: Regulatory T cell; ROS: Reactive oxygen species; HO-1: Heme oxygenase-1; GPx: Glutathione peroxidase; SOD: Superoxide dismutase; ERK: Extracellular signal-regulated kinase; JNK: C-Jun N-terminal kinase; Nrf2: Nuclear factor erythroid 2-related factor 2.
Inhibition of inflammation
Inhibition of inflammation plays an important role in alleviating obesity, diabetes, and related complications. Reducing the chronic inflammatory response helps improve insulin sensitivity and supports normal metabolic processes[78]. Modulating inflammatory pathways can help restore metabolic equilibrium, enhance insulin function, and prevent the progression of insulin resistance and associated comorbidities. Seaweeds and their bioactive compounds have demonstrated significant anti-inflammatory properties, primarily through the modulation of inflammatory pathways and the suppression of pro-inflammatory cytokines. These effects are mediated by various molecular mechanisms, involving key signaling pathways and molecules that regulate inflammation. The nuclear factor kappa B (NF-κB) pathway plays a critical role in regulating inflammation. During an inflammatory response, NF-κB activation triggers the transcription of genes encoding pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β)[83]. Sharma et al[84] demonstrated that the brown seaweed Padina tetrastromatica exhibits anti-inflammatory properties by suppressing NF-κB expression. Similar activities were observed in Sargassum species[85]. Moreover, seaweed-derived compounds like phlorotannins and bromophenols have also been found to inhibit NF-κB expression[86]. Additionally, fucoidan has been shown to inhibit NF-κB activation and reduce TNF-α and IL-6 production in macrophages and other immune cells. This is achieved by preventing the phosphorylation and degradation of IκB proteins, which act as inhibitors of NF-κB, thus blocking the translocation of NF-κB dimers (p65 and p50) to the nucleus. As a result, the expression of pro-inflammatory cytokines is downregulated (Figure 4)[81].
Another method in which seaweeds and their bioactive compounds alleviate inflammation is through modulation of MAPK signaling pathways[86]. The MAPK pathways, including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK, play a significant role in the initiation of inflammation. These pathways are activated by various stressors, resulting in the phosphorylation and activation of transcription factors such as AP-1, which drive the expression of pro-inflammatory cytokines[87]. Seaweed and their compounds can inhibit the activation of these MAPK pathways, thereby reducing the production of inflammatory mediators. For instance, fucoidan and fucoxanthin have been shown to suppress the JNK and p38 MAPK pathways, leading to reduce levels of inflammatory cytokines (Figure 4)[88-90].
The nuclear factor erythroid 2-related factor 2 (Nrf2) pathway is an important regulator of cellular antioxidant defenses[91]. Inflammatory conditions are frequently accompanied by oxidative stress, which worsens the inflammatory response. Seaweeds and their compounds can activate the Nrf2 pathway, leading to the upregulation of antioxidant enzymes, including heme oxygenase-1 (HO-1) and glutathione peroxidase (GPx). These enzymes counteract oxidative stress by neutralizing ROS and minimizing cellular damage, thereby reducing the activation of pro-inflammatory pathways (Figure 4)[80]. For example, studies show that seaweeds and their compounds facilitate Nrf2 translocation to the nucleus, promoting the expression of antioxidants that alleviate ROS-induced inflammation[86,92,93].
The cyclooxygenase (COX) and lipoxygenase (LOX) pathways are responsible for the production of pro-inflammatory eicosanoids, such as prostaglandins and leukotrienes[94]. Seaweed and their products compounds demonstrated to inhibit COX and LOX enzymes, thereby reducing the synthesis of these inflammatory mediators[81,86]. By suppressing COX-2 and LOX activity, seaweed compounds help mitigate inflammation in tissues and organs, contributing to the alleviation of inflammatory conditions like obesity and diabetes.
The inflammasome is a protein complex that triggers the activation of caspase-1, which subsequently activates pro-inflammatory cytokines such as IL-1β and IL-18 into their active forms[95]. In conditions like obesity and diabetes, inflammasome activation contributes to chronic inflammation. Seaweed-derived compounds have been shown to directly suppress the production and secretion of pro-inflammatory cytokines, such as TNF-α, IL-6, IL-1β, and interleukin-18 (IL-18), by immune cells, including macrophages[80,81]. Seaweeds can attenuate inflammasome activation by inhibiting the expression and activation of NOD-like receptor protein 3 (NLRP3), a central component of the inflammasome[90]. By downregulating NLRP3 and caspase-1, these compounds reduce the processing of pro-inflammatory cytokines, thereby mitigating inflammation.
Chronic inflammation is often characterized by an imbalance between pro-inflammatory Th17 cells and anti-inflammatory regulatory T (Treg) cells. The Th1/Th2 ratio is essential for maintaining immune homeostasis, with imbalances, such as a Th1-dominant response, frequently associated with the development of chronic inflammatory disorders. Likewise, a decreased Tregs/Th1 ratio impairs the regulation of inflammatory processes, thereby perpetuating inflammation[96]. Fucoidan has been found to enhance the balance between Th1/Th2 helper T cells and Tregs/Th17 cells, contributing to a reduction in airway inflammation in individuals with asthma[97]. This modulation of immune cell populations helps restore immune balance and reduces inflammation, which is essential for alleviating conditions such as obesity and diabetes. Seaweed and their compounds exhibit strong anti-inflammatory properties by inhibiting key signaling pathways, including NF-κB, MAPK, COX/LOX, and inflammasome activation. Furthermore, these compounds activate antioxidant defense mechanisms, particularly the Nrf2 pathway, which strengthens the cellular response to oxidative stress. These mechanisms collectively reduce the production of pro-inflammatory cytokines, mitigate oxidative stress, and improve metabolic health, making seaweeds a promising natural source for managing inflammation-related diseases like diabetes and obesity.
Antioxidant properties and mitigation of oxidative damage
Antioxidant properties play a crucial role in mitigating oxidative damage by neutralizing ROS and preventing cellular injury, which are key contributors to the pathogenesis of various diseases[98]. By alleviating oxidative stress, antioxidants maintain cellular integrity, safeguard tissue functionality, and slow the progression of conditions such as cancer, cardiovascular diseases, and neurodegenerative disorders. One of the mechanisms in which seaweeds and their compounds reduce oxidative stress is through activation of the Nrf2 pathway, a central regulator of cellular antioxidant defenses (Figure 4)[99]. Under normal conditions, Nrf2 promotes the transcription of antioxidant enzymes, including HO-1, superoxide dismutase (SOD), and GPx, by binding to antioxidant response elements in their gene promoters[80]. Compounds such as fucoidan, astaxanthin, and fucoxanthin from seaweed enhance Nrf2 activation[100-102], facilitating its nuclear translocation and subsequent induction of these enzymes. Therefore, this boosts the cellular capacity to neutralize ROS and mitigate oxidative damage, particularly in metabolically active tissues such as the pancreas, liver, and adipose tissue.
Seaweed also directly scavenge ROS, including superoxide anions, hydrogen peroxide, and hydroxyl radicals, effectively protecting cellular components such as lipids, proteins, and DNA from oxidative damage. In vitro and ex vivo studies have demonstrated that seaweed extracts can effectively scavenge radicals such as 2,2-diphenyl-1-picrylhydrazyl, nitric oxide, and hydroxyl radicals, while also exhibiting ferric reducing power[48]. Ex vivo experiments further reveal the potential of seaweeds to mitigate tissue oxidative damage, as evidenced by reduced lipid peroxidation levels and enhanced antioxidant defense systems, including increased activities of GPx, SOD, and catalase[17,47]. By neutralizing ROS, seaweeds help preserve cellular function and enhance metabolic homeostasis.
Another significant molecular mechanism of seaweed compounds in combating oxidative stress is the modulation of mitochondrial function. Mitochondria are a major source of ROS, and mitochondrial dysfunction contributes to oxidative stress and metabolic diseases like diabetes and obesity. Seaweed and their compounds been shown to enhance mitochondrial function by promoting mitochondrial biogenesis and improving the efficiency of cellular respiration through enhancing PPAR-γ coactivator 1-α (PGC-1α), AMPK, Nrf-2, and reducing ROS production[103,104]. By maintaining mitochondrial integrity and reducing ROS production, seaweeds help prevent insulin resistance, preserving cellular energy metabolism and improving metabolic outcomes in tissues affected by oxidative stress.
Through mitigating inflammation, seaweed and their compounds reduce the subsequent oxidative stress, preventing further tissue damage and the progression of insulin resistance in key metabolic tissues such as muscle, liver, and adipose tissue. The antioxidant effects of seaweed-derived compounds are mediated through the activation of key antioxidant pathways like Nrf2, direct scavenging of ROS, inhibition of lipid peroxidation, modulation of mitochondrial function, and reduction of inflammation. These mechanisms collectively protect cells from oxidative damage, improve insulin sensitivity, and help restore metabolic balance, making seaweeds a promising natural source for alleviating oxidative stress-related complications in diabetes and obesity.
ANTI-OBESITY EFFECTS OF SEAWEEDS
Seaweeds and their bioactive compounds exert anti-obesity effects through multiple biological mechanisms, including the regulation of adipogenesis, modulation of appetite and energy homeostasis, and modulation of gut microbiota composition. By targeting these interconnected pathways, seaweed-derived compounds represent a promising therapeutic approach for obesity control. In this section of the review, we provide detailed molecular mechanisms underlying the anti-obesity effects of seaweeds and their active compounds.
Regulation of adipogenesis
Adipogenesis, the differentiation of preadipocytes into mature adipocytes, is essential for maintaining energy balance under normal physiological conditions. This highly regulated process is driven by a network of transcription factors, with PPARγ and CCAAT/enhancer-binding proteins (C/EBPs) functioning as central regulators[105]. Under normal conditions, adipogenesis supports the proper expansion and function of adipose tissue, facilitating energy storage and the secretion of adipokines critical for metabolic regulation. However, in pathological conditions such as obesity and diabetes, the regulation of adipogenesis becomes disrupted[106]. Both excessive and impaired adipogenesis contribute to adipose tissue dysfunction, leading to chronic inflammation, insulin resistance, and ectopic lipid accumulation. In obesity, adipocyte hypertrophy predominates, resulting in cellular stress and metabolic disturbances. In diabetes, defective adipogenesis further aggravates metabolic dysregulation and systemic insulin resistance[106]. Elucidating the molecular mechanisms that regulate adipogenesis under both physiological and pathological conditions is crucial for developing therapeutic approaches to improve metabolic health.
Seaweeds and their bioactive compounds have gained recognition as potential natural regulators of adipogenesis by modulating key signaling pathways involved in adipocyte differentiation and lipid metabolism. Their well-documented antioxidant and anti-inflammatory properties play a crucial role in preventing adipose tissue dysfunction during the adipogenic process[15,80]. Since chronic low-grade inflammation is a hallmark of impaired adipogenesis in obesity, seaweed-derived compounds help alleviate inflammation, thereby promoting healthy adipose tissue expansion and function. Additionally, these compounds regulate adipokine secretion, enhance insulin sensitivity, and reduce ectopic lipid accumulation, contributing to improved metabolic health.
Compounds derived from seaweed have been demonstrated to modulate adipogenesis through various molecular mechanisms, including the suppression of lipogenic gene expression and the stimulation of thermogenic factors[107]. These actions collectively contribute to the reduction of excessive fat accumulation and the promotion of increased energy expenditure. A primary mechanism by which seaweeds and their bioactive compounds regulate adipogenesis is through the inhibition of lipogenesis, the process of fat cell formation and lipid accumulation (Figure 5)[9]. Key lipogenic genes, such as sterol regulatory element-binding protein 1c (SREBP-1c), fatty acid synthase (FAS), and acetyl-CoA carboxylase (ACC), are critical in the synthesis of fatty acids and triglycerides within adipocytes[108]. Seaweed-derived bioactive compounds, including fucoxanthin (a carotenoid) and sulfated polysaccharides, have been shown to downregulate the expression of these lipogenic genes[9]. For example, fucoxanthin inhibits SREBP-1c activity, which directly regulates the transcription of genes involved in fatty acid synthesis, including FAS and ACC[109]. This reduction in lipogenic gene expression results in lower lipid biosynthesis, which reduces lipid accumulation in adipocytes and prevents excessive fat storage. Furthermore, another carotenoid like fucoxanthin, xanthin from brown seaweeds also regulates AMPK, a critical regulator of cellular energy metabolism[110]. Activation of AMPK inhibits acetyl-CoA carboxylase and FAS, thus decreasing lipogenesis and promoting lipid oxidation. This molecular cascade not only hampers the formation of new adipocytes but also accelerates the breakdown of stored lipids, contributing to a reduction in fat mass.
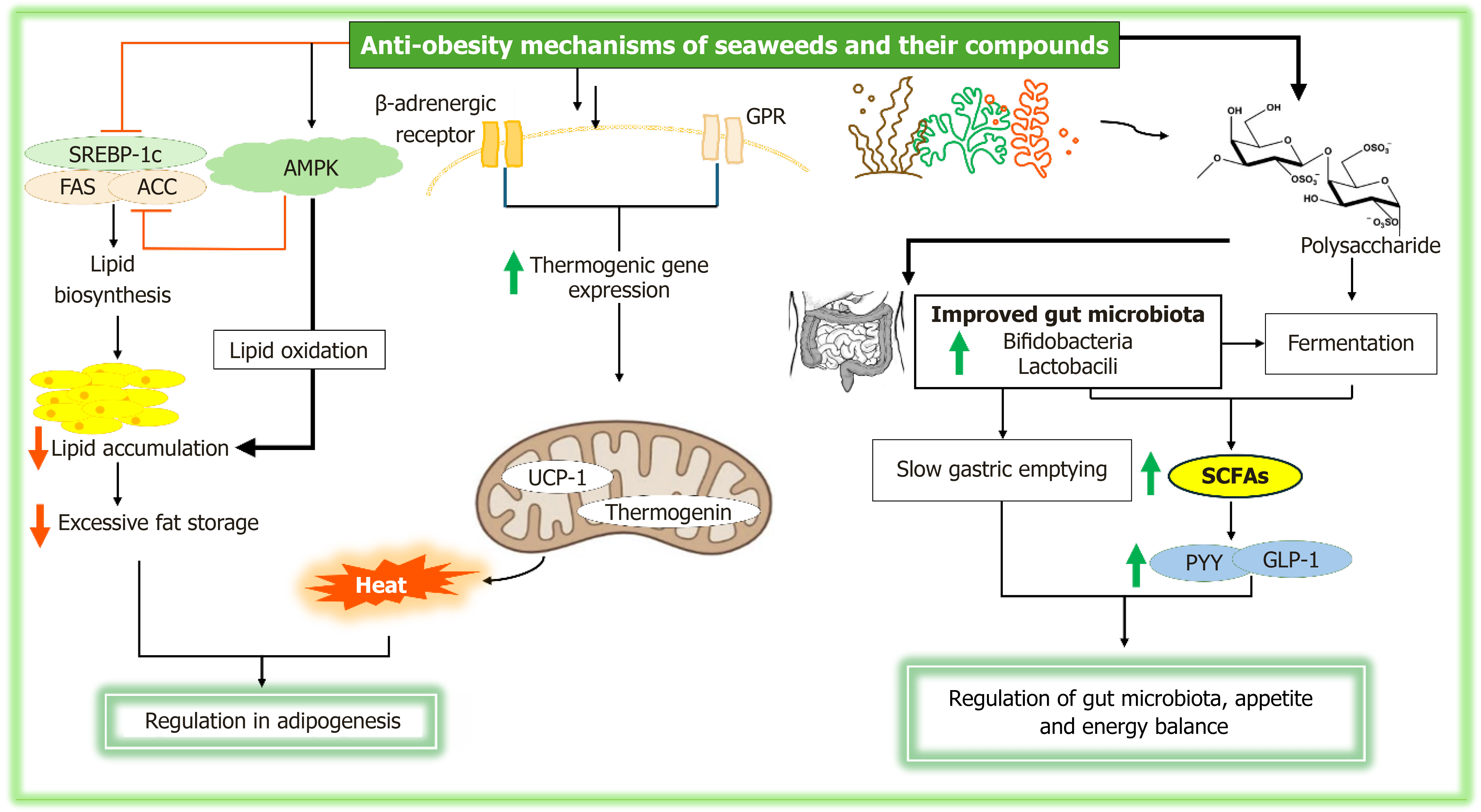
Figure 5 The anti-obesity effects of seaweeds and their compounds, showing their role in regulating adipogenesis, appetite, and energy balance, as well as modulating gut microbiota to promote weight management and overall metabolic health.
AMPK: Adenosine monophosphate-activated protein kinase; SREBP: Sterol regulatory element-binding protein 1c; FAS: Fatty acid synthase; ACC: Acetyl-CoA carboxylase; UCP-1: Uncoupling protein-1; SCFAs: Short-chain fatty acids; PYY: Peptide YY; GLP: Glucagon-like peptide-1.
In addition to inhibiting lipogenic processes, bioactive compounds derived from seaweeds also promote thermogenesis, a process in which energy is expended as heat instead of being stored as fat. Thermogenic proteins, such as uncoupling proteins (UCP), especially UCP1 in brown adipocytes, play a critical role by uncoupling mitochondrial respiration from ATP production, thereby increasing energy expenditure and reducing fat accumulation[63]. Fucoxanthin, a well-studied bioactive compound from seaweeds, has been shown to enhance UCP1 expression in brown adipose tissue, thereby promoting thermogenesis[63,111]. Seaweeds and their compounds also stimulate thermogenic gene expression through the activation of β-adrenergic receptors and the subsequent signaling pathways (Figure 5)[112]. Activation of these receptors triggers the expression of PGC-1α, a central regulator of mitochondrial biogenesis and thermogenesis. PGC-1α, in turn, enhances the expression of thermogenic proteins, including UCP1 and thermogenin, which further facilitate energy dissipation and reduce fat deposition.
Bioactive compounds from seaweeds also influence various signaling pathways involved in adipogenesis and lipid metabolism. The Wnt/β-catenin signaling pathway, which plays a crucial role in regulating adipocyte differentiation and maturation, is modulated by compounds such as flavonoids and polyphenols from seaweeds[113,114]. This mechanism is especially significant in regulating adipose tissue expansion in response to excess energy intake. Additionally, seaweed-derived compounds also affect MAPK pathways, which regulate cell proliferation, differentiation, and survival. For instance, fucoidan activates the ERK pathway[115], which has been associated with the inhibition of adipogenesis through the suppression of key adipogenic transcription factors, such as PPARγ and C/EBPs.
Seaweeds and their bioactive compounds regulate adipogenesis through multiple mechanisms, including the inhibition of lipogenic gene expression, stimulation of thermogenic factors, and modulation of key signaling pathways. By targeting transcription factors such as PPARγ and C/EBPs, and pathways like AMPK, Wnt/β-catenin, and MAPK, these compounds reduce adipocyte differentiation, limit lipid accumulation, and enhance energy expenditure. Additionally, their antioxidants, anti-inflammatory, and prebiotic properties offer further benefits in addressing adipose tissue dysfunction linked to obesity. The collective evidence highlights the potential of seaweed-derived compounds as therapeutic agents for obesity and related metabolic disorders.
Appetite and energy balance
Appetite and energy balance are essential for maintaining body weight and metabolic health, involving complex interactions between hormones, the central nervous system, and peripheral tissues. In a healthy condition, hormones such as leptin, ghrelin, insulin, and peptide YY (PYY) regulate hunger, satiety, and energy storage, ensuring an equilibrium between energy intake and expenditure[116]. Leptin suppresses appetite when energy stores are sufficient, while ghrelin stimulates hunger, especially in negative energy balance[116]. Insulin signals satiety after food intake, and PYY promotes satiety postprandially. In contrast, in obesity and diabetes, this regulation is disrupted[117]. Obesity leads to leptin resistance and insulin resistance, impairing hunger and satiety signaling, which promotes overeating and fat storage[118]. In type 2 diabetes, insulin resistance and abnormal glucose uptake further disrupt appetite regulation, contributing to excessive hunger and weight gain[119]. These metabolic dysfunctions, often exacerbated by chronic inflammation, make appetite control and energy balance challenging, highlighting the need for targeted therapeutic approaches to restore proper regulation in metabolic diseases.
The dietary fiber in seaweeds, particularly soluble fibers such as sulfated polysaccharides and alginates, plays a significant role in appetite regulation. These fibers slow gastric emptying, which prolongs satiety and enhances the release of satiety hormones like PYY and glucagon-like peptide-1 (GLP-1) (Figure 5)[120]. Additionally, the fermentation of seaweed fiber in the gut produces SCFAs that further promote satiety and regulate hunger through the gut-brain axis[19]. Seaweed compounds also enhance energy expenditure and thermogenesis by activating key metabolic pathways[63].
Seaweed-derived bioactive compounds regulate appetite and energy balance through a variety of molecular mechanisms, including the modulation of appetite-regulating hormones, stimulation of thermogenesis, and inhibition of lipogenesis. The dietary fiber in seaweed contributes to satiety and reduced food intake by influencing the release of gut hormones and modulating gut microbiota. Moreover, bioactive compounds such as fucoxanthin activate essential metabolic pathways, including AMPK, which enhances thermogenesis through UCP1 expression, promotes fat oxidation, and decreases fat accumulation. These processes highlight the potential of seaweed-derived bioactive compounds in aiding weight management and improving metabolic health by restoring energy balance and enhancing thermogenic activity.
Gut microbiota modulation
The gut microbiota plays a pivotal role in maintaining metabolic health by influencing digestion, nutrient absorption, immune function, and energy balance. In individuals with a healthy microbiome, optimal digestion is facilitated, the synthesis of SCFAs is enhanced, and overall metabolic functions are supported[121]. Short chain fatty acids, generated through the fermentation of dietary fibers by gut bacteria, play a key role in regulating energy balance, controlling appetite, and modulating inflammation[122]. In contrast, obesity and diabetes are often associated with alterations in the composition and diversity of gut microbiota, leading to dysbiosis. This imbalance is linked to impaired metabolic processes, insulin resistance, and chronic low-grade inflammation[123]. Dysbiosis can further exacerbate metabolic dysfunction by increasing the production of pro-inflammatory cytokines and disrupting glucose and lipid metabolism. Additionally, in obesity, gut dysbiosis may impact the gut-brain axis, potentially enhance appetite and contributing to overeating[124]. Interventions aimed at restoring healthy gut microbiota, such as dietary modifications, probiotics, or prebiotics, offer promising therapeutic strategies for addressing metabolic disturbances associated with obesity and diabetes[125].
Seaweed-derived fibers, including polysaccharides like fucoidan and alginate, act as prebiotics that promote the growth of beneficial gut bacteria, thereby enhancing microbial diversity and supporting a healthy gut microbiome (Figure 5)[126]. The consumption of seaweed has been shown to stimulate the production of SCFAs through the fermentation of dietary fibers by gut microbiota[126]. This suggests that seaweed contributes to a balanced gut microbiota, which is vital for optimal metabolic function and the prevention of chronic diseases such as obesity and diabetes.
Seaweed-derived polysaccharides serve as prebiotics that are not digestible by human enzymes but are fermented by gut microbiota, particularly by beneficial bacteria such as Bifidobacteria and Lactobacilli[127]. This fermentation process results in the production of SCFAs, including acetate, propionate, and butyrate, which are essential for maintaining intestinal health. SCFAs act as energy sources for colonocytes, help regulate gut barrier function, modulate immune responses, and reduce inflammation[128]. By selectively promoting the growth of beneficial bacteria capable of fermenting these fibers, seaweed polysaccharides enhance microbial diversity and stability, contributing to a balanced gut microbiome. Furthermore, seaweeds also have antimicrobial properties that inhibits the growth of harmful gut bacteria like Clostridium and Escherichia coli[127]. These antimicrobial effects stem from the sulfation of polysaccharides, which disrupts the cellular integrity of pathogenic microbes and prevents their adherence to the gut mucosa. This reduction in pathogenic bacteria supports healthy gut microbiota, enhancing gut health and immune function. This shift plays a key role in preventing dysbiosis, a microbial imbalance commonly associated with metabolic diseases like obesity and diabetes.
Furthermore, the production of SCFAs influences the gut microbiome by supporting the growth of beneficial microbes, inhibiting pathogenic bacteria, and maintaining intestinal epithelial cell integrity. Butyrate, in particular, serves as a primary energy source for colonocytes, reinforcing the gut barrier and promoting anti-inflammatory effects[129]. Short chain fatty acids also activate G-protein coupled receptors, specifically GPR41 and GPR43, which trigger the secretion of gut hormones such as PYY and GLP-1[130], which are involved in appetite regulation and insulin sensitivity. Additionally, SCFAs activate AMPK, a key regulator of energy metabolism, which helps improve lipid metabolism and insulin sensitivity, reducing the risk of metabolic disorders[131].
In addition to these effects, seaweed bioactive compounds, particularly polysaccharides like alginate, improve gut barrier function by modulating tight junction proteins such as occludin and claudins[132]. This strengthens intestinal permeability, preventing the translocation of endotoxins and pathogens into the bloodstream, reducing systemic inflammation, and enhancing metabolic health. In metabolic disorders, where intestinal permeability is often increased, seaweed compounds may help counteract "leaky gut" and mitigate associated inflammation and insulin resistance. Finally, seaweed-derived fibers impact bile acid metabolism, which plays a key role in fat digestion and absorption[133]. Seaweed fibers bind to bile acids, promoting their excretion and reducing their reabsorption in the intestines. This process facilitates the conversion of primary bile acids to secondary bile acids by gut microbiota, influencing lipid metabolism and glucose homeostasis.
CHALLENGES AND FUTURE PERSPECTIVES
Seaweeds and their bioactive compounds have demonstrated significant potential in managing obesity and diabetes by influencing key molecular pathways related to metabolism, energy expenditure, and insulin sensitivity. However, despite these promising findings, there are several challenges that need to be addressed to fully harness their therapeutic potential. These challenges include variability in bioactive compound composition, limited bioavailability[134], and a lack of clinical evidence. Identifying solutions to these limitations and focusing on future research directions will be essential for advancing the development of seaweed-based interventions for metabolic disorders.
One of the main challenges is due to the variability of seaweeds composition. The concentration of bioactive compounds, such as polysaccharides, polyphenols, and carotenoids, can differ based on the species, geographic location, and environmental conditions. This variability complicates efforts to standardize seaweed extracts for clinical use, as consistency in the therapeutic dose is necessary for reliable outcomes. Another critical issue is the low bioavailability of certain compounds, which limits their absorption and reduces their biological activity. Compounds such as fucoxanthin are rapidly metabolized, resulting in reduced systemic availability[135]. Strategies to enhance bioavailability, such as nanoencapsulation or the use of delivery systems, may help overcome this limitation.
Furthermore, the molecular mechanisms through which seaweed compounds exert their anti-obesity and anti-diabetes effects are complex and involve multiple overlapping pathways. These mechanisms include AMPK activation, modulation of gut hormones like GLP-1 and PYY, and the regulation of adipogenesis and thermogenesis. Understanding the intricate interactions between these pathways is critical for identifying key therapeutic targets. While preclinical studies have provided valuable insights, human clinical trials are still limited, and the available data are often inconsistent due to differences in study design, treatment duration, and dosage. Large-scale clinical trials are necessary to confirm the therapeutic efficacy of seaweed compounds in human populations. In addition, more tissue-specific studies are needed in order to fully elucidate the distinct roles of seaweed-derived compounds in metabolic regulation.
Safety and regulatory concerns also present obstacles to the widespread use of seaweed-derived products. Seaweeds are known to accumulate heavy metals such as arsenic, cadmium, and lead, which may pose potential health risks, particularly with long-term use or at high doses. Therefore, rigorous safety evaluations, including assessments of heavy metal content and other contaminants, are essential. Future studies should not only focus on establishing efficacy but also on confirming the long-term safety of seaweed-derived compounds in order to support their safe translation into clinical and therapeutic applications. Regulatory frameworks for seaweed products, especially when used as functional foods or nutraceuticals, are still evolving and require greater clarity. Future research should focus on the identification and characterization of novel bioactive compounds from underexplored seaweed species using advanced technologies such as metabolomics and high-throughput screening. Optimizing extraction methods to preserve the bioactivity of these compounds will be crucial, with green extraction techniques and innovative delivery systems offering promising solutions. Moreover, the growing understanding of the gut microbiome’s role in metabolic health highlights the need for studies that explore how seaweed compounds reshape the gut microbial environment.
Developing combination therapies that integrate seaweed compounds with other nutraceuticals or pharmaceuticals may provide a more comprehensive approach to treating obesity and diabetes. Additionally, incorporating seaweed compounds into functional foods and dietary supplements could offer a practical and accessible way to promote metabolic health. Future clinical trials should prioritize long-term safety and efficacy, while also addressing population-specific responses based on factors such as age, sex, and metabolic status. Although seaweed and its bioactive compounds offer great promise in the prevention and treatment of metabolic disorders, significant challenges remain. Addressing these limitations through ongoing research and clinical studies will pave the way for the successful translation of seaweed-based interventions into effective therapeutic solutions for obesity and diabetes.